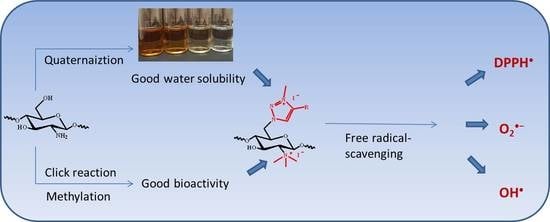
Novel Water Soluble Chitosan Derivatives with 1,2,3-Triazolium and Their Free Radical-Scavenging Activity
Abstract
:1. Introduction
2. Results
2.1. Chemical Synthesis and Characterization
2.2. Solubility and Radical Scavenging Activity
3. Materials and Methods
3.1. Materials
3.2. Analytical Methods
3.3. The synthesis of Chitosam Derivatives
3.3.1. Synthesis of 6-Azido-6-deoxy-N-trimethyl Quaternary Ammonium Chitosan (3)
3.3.2. Synthesis of Chitosan Derivative Bearing 1,2,3-Triazole (4) and 1,2,3-Triazolium (5)
3.4. Antioxidant Assay
3.4.1. DPPH-Radical Scavenging Ability Assay
3.4.2. Superoxide-Radical Scavenging Ability Assay
3.4.3. Hydroxyl-Radical Scavenging Ability Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DPPH | 1,1-Diphenyl-2-picrylhydrazyl |
| DMSO | Dimethyl Sulphoxide |
| DMF | N,N-Dimethylformamide |
| EDTA | Ethylenediaminetetraacetic acid |
| NMP | N-Methyl pyrrolidone |
References
- Mahakunakorn, P.; Tohda, M.; Murakami, Y.; Matsumoto, K.; Watanabe, H. Antioxidant and free radical-scavenging activity of Choto-san and its related constituents. Biol. Pharm. Bull. 2004, 27, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Mazzone, G.; Alvarez-Diduk, R.; Marino, T.; Alvarez-Idaboy, J.R.; Russo, N. Food Antioxidants: Chemical Insights at the Molecular Level. Annu. Rev. Food Sci. Technol. 2016, 7, 223–352. [Google Scholar] [CrossRef] [PubMed]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Fundam. Mol. Mach. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, M.; Jabbari, A. DPPH radical-scavenging activity and kinetics of antioxidant agent hesperidin in pure aqueous micellar solutions. Bull. Chem. Soc. Jpn. 2016, 89, 869–875. [Google Scholar] [CrossRef]
- Nakajima, A.; Yamaguchi, T.; Hattori, G.; Sakurai, Y.; Kawamura, M.; Kawai, K.; Miyake, Y.; Kanaori, K.; Tajima, K. Accuracy and validity of AREC (Alkoxy Radical Elimination Capacity) assay in evaluating the antioxidant abilities of various biosubstances. Bull. Chem. Soc. Jpn. 2017, 90, 223–230. [Google Scholar] [CrossRef]
- Gehrcke, M.; Giuliani, L.M.; Ferreira, L.M.; Barbieri, A.V.; Sari, M.H.M.; da Silveira, E.F.; Azambuja, J.H.; Nogueira, C.W.; Braganhol, E.; Cruz, L. Enhanced photostability, radical scavenging and antitumor activity of indole-3-carbinol-loaded rose hip oil nanocapsules. Mater. Sci. Eng. C 2017, 74, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Zhang, J.; Luan, F.; Wei, L.; Chen, Y.; Dong, F.; Li, Q.; Guo, Z. Design, synthesis of novel chitosan derivatives bearing quaternary phosphonium salts and evaluation of antifungal activity. Int. J. Biol. Macromol. 2017, 102, 704–711. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Wei, L.; Wang, P.; Gao, Z.; Chen, Y.; Dong, F.; Guo, Z. Synthesis, characterization, and antifungal property of starch derivatives modified with quaternary phosphonium salts. Mater. Sci. Eng. C 2017, 76, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, C.; Tan, W.; Gu, G.; Guo, Z. Novel amino-pyridine functionalized chitosan quaternary ammonium derivatives: Design, synthesis, and antioxidant activity. Molecules 2017, 22, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Thomas, L.; Taher, A.; Joseph, A. Impact of high pressure treatment on functional, rheological, pasting, and structural properties of lentil starch dispersions. Carbohydr. Polym. 2016, 152, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Adak, S.; Banerjee, R. A green approach for starch modification: Esterification by lipase and novel imidazolium surfactant. Carbohydr. Polym. 2016, 150, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Yang, J.; Wu, H.; Hu, Z.; Yi, J.; Tong, J.; Zhu, X. Preparation and characterization of quaternary ammonium chitosan hydrogel with significant antibacterial activity. Int. J. Biol. Macromol. 2015, 79, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Liu, H.; Chen, X.; Ji, X.; Li, P. Hydroxyl radicals scavenging activity of N-substituted chitosan and quaternized chitosan. Bioorg. Med. Chem. Lett. 2006, 16, 6348–6350. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Min, L.; Zhu, C.; Rao, Z.; Liu, L.; Xu, W.; Luo, P.; Fan, L. Preparation, characterization and antioxidant activity of silk peptides grafted carboxymethyl chitosan. Int. J. Biol. Macromol. 2017, 104, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Q.; Tan, W.; Dong, F.; Luan, F.; Guo, Z. Synthesis, characterization, and the antioxidant activity of double quaternized chitosan derivatives. Molecules 2017, 22, 501–601. [Google Scholar] [CrossRef] [PubMed]
- Eswaran, S.; Adhikari, A.V.; Shetty, N.S. Synthesis and antimicrobial activities of novel quinoline derivatives carrying 1,2,4-triazole moiety. Eur. J. Med. Chem. 2009, 44, 4637–4647. [Google Scholar] [CrossRef] [PubMed]
- Abuo-Rahma Gel, D.; Abdel-Aziz, M.; Beshr, E.A.; Ali, T.F. 1,2,4-Triazole/oxime hybrids as new strategy for nitric oxide donors: Synthesis, anti-inflammatory, ulceroginicity and antiproliferative activities. Eur. J. Med. Chem. 2014, 71, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.P.; Sun, J.; Pang, Z.H.; Lv, P.C.; Li, D.D.; Yan, L.; Zhang, H.J.; Zheng, E.X.; Zhao, J.; Zhu, H.L. Synthesis and antitumor activity of 1,2,4-triazoles having 1,4-benzodioxan fragment as a novel class of potent methionine aminopeptidase type II inhibitors. Bioorg. Med. Chem. 2011, 19, 5948–5954. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Chen, X.; Piatnitski, E.L.; Kiselyov, A.S.; He, H.Y.; Mao, Y.; Pattaropong, V.; Yu, Y.; Kim, K.H.; Kincaid, J.; et al. Synthesis and structure-activity relationships of 1,2,4-triazoles as a novel class of potent tubulin polymerization inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 5154–5159. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Bahn, Y.J.; Ryu, S.E. Structure-based de novo design and biochemical evaluation of novel Cdc25 phosphatase inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 4330–4334. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua, J.M.; Fratila, R.M.; Monasterio, Z.; Perez-Esnaola, N.; Andreieff, E.; Irastorza, A.; Sagartzazu-Aizpurua, M. Triazolium cations: From the “click” pool to multipurpose applications. New J. Chem. 2014, 38, 474–480. [Google Scholar] [CrossRef]
- Li, Q.; Tan., W.; Zhang, C.; Gu, G.; Guo, Z. Novel triazolyl-functionalized chitosan derivatives with different chain lengths of aliphatic alcohol substituent: Design, synthesis, and antifungal activity. Carbohydr. Res. 2015, 418, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tan., W.; Zhang, C.; Gu, G.; Guo, Z. Synthesis of water soluble chitosan derivatives with halogeno-1,2,3-triazole and their antifungal activity. Int. J. Biol. Macromol. 2016, 91, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Li, Q.; Dong, F.; Feng, Y.; Guo, Z. Phenolic antioxidants-functionalized quaternized chitosan: Synthesis and antioxidant properties. Int. J. Biol. Macromol. 2013, 53, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhang, J.; Yu, C.; Li, Q.; Ren, J.; Wang, G.; Gu, G.; Guo, Z. Synthesis of amphiphilic aminated inulin via ‘click chemistry’ and evaluation for its antibacterial activity. Bioorg. Med. Chem. Lett. 2014, 24, 4590–4593. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.L.; Gong, X.D.; Ju, X.H.; Ji, G.F.; Xiao, H.M. Structures and properties of 1,2,3-triazoles and 1,2,4-triazoles. Chin. J. Struct. Chem. 2006, 25, 582–588. [Google Scholar]
- Sood, R.; Obadia, M.M.; Mudraboyina, B.P.; Zhang, B.; Serghei, A.; Bernard, J.; Drockenmuller, E. 1,2,3-Triazolium-based poly(acrylate ionic liquid)s. Polymer 2014, 55, 3314–3319. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, F.J.; Yun, D.R.; Guo, Y.W.; Ye, Y.C.; Wang, Y.X.; Tan, H.M. Preparation of a C6 quaternary ammonium chitosan derivative through a chitosan schiff base with click chemistry. J. Appl. Polym. Sci. 2013, 129, 3185–3191. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; El-Rafey, E.; Rezki, N.; Abou-Elnaga, H.H.; Bakry, W.M.A.; Boghdadi, Y.M. Evaluation of some functionalized imidazoles and 1,2,4-triazoles as antioxidant additives for industrial lubricating oils and correlating the results with the structures of additives using empirical AM1 calculations. J. Saudi Chem. Soc. 2014, 18, 443–449. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Luan, F.; Wei, L.; Li, Q.; Dong, F.; Guo, Z. Synthesis, characterization, and antifungal evaluation of novel 1,2,3-triazolium-functionalized starch derivative. Int. J. Biol. Macromol. 2017, 101, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Qiu, L.; Tan, W.; Gu, G.; Guo, Z. Novel 1,2,3-triazolium-functionalized inulin derivatives: Synthesis, free radical-scavenging activity, and antifungal activity. RSC Adv. 2017, 7, 42225–42232. [Google Scholar] [CrossRef]
- Tan, W.; Li, Q.; Gao, Z.; Qiu, S.; Dong, F.; Guo, Z. Design, synthesis of novel starch derivative bearing 1,2,3-triazolium and pyridinium and evaluation of its antifungal activity. Carbohydr. Polym. 2017, 157, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Yu, C.; Li, Q.; Dong, F.; Wang, G.; Guo, Z. Synthesis, characterization, and antioxidant properties of novel inulin derivatives with amino-pyridine group. Int. J. Biol. Macromol. 2014, 70, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Liu, J.; Dong, F.; Guo, Z. Highly efficient synthesis and antioxidant activity of O-(aminoethyl)inulin. Carbohydr. Polym. 2011, 83, 1240–1244. [Google Scholar] [CrossRef]
- Liu, J.; Sun, H.; Dong, F.; Xue, Q.; Wang, G.; Qin, S.; Guo, Z. The influence of the cation of quaternized chitosans on antioxidant activity. Carbohydr. Polym. 2009, 78, 439–443. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Sun, X.; Gu, G.; Guo, Z. Novel Water Soluble Chitosan Derivatives with 1,2,3-Triazolium and Their Free Radical-Scavenging Activity. Mar. Drugs 2018, 16, 107. https://doi.org/10.3390/md16040107
Li Q, Sun X, Gu G, Guo Z. Novel Water Soluble Chitosan Derivatives with 1,2,3-Triazolium and Their Free Radical-Scavenging Activity. Marine Drugs. 2018; 16(4):107. https://doi.org/10.3390/md16040107
Chicago/Turabian StyleLi, Qing, Xueqi Sun, Guodong Gu, and Zhanyong Guo. 2018. "Novel Water Soluble Chitosan Derivatives with 1,2,3-Triazolium and Their Free Radical-Scavenging Activity" Marine Drugs 16, no. 4: 107. https://doi.org/10.3390/md16040107
APA StyleLi, Q., Sun, X., Gu, G., & Guo, Z. (2018). Novel Water Soluble Chitosan Derivatives with 1,2,3-Triazolium and Their Free Radical-Scavenging Activity. Marine Drugs, 16(4), 107. https://doi.org/10.3390/md16040107