Biochemical Characterization and Degradation Pattern of a Unique pH-Stable PolyM-Specific Alginate Lyase from Newly Isolated Serratia marcescens NJ-07
Abstract
:1. Introduction
2. Results and Discussions
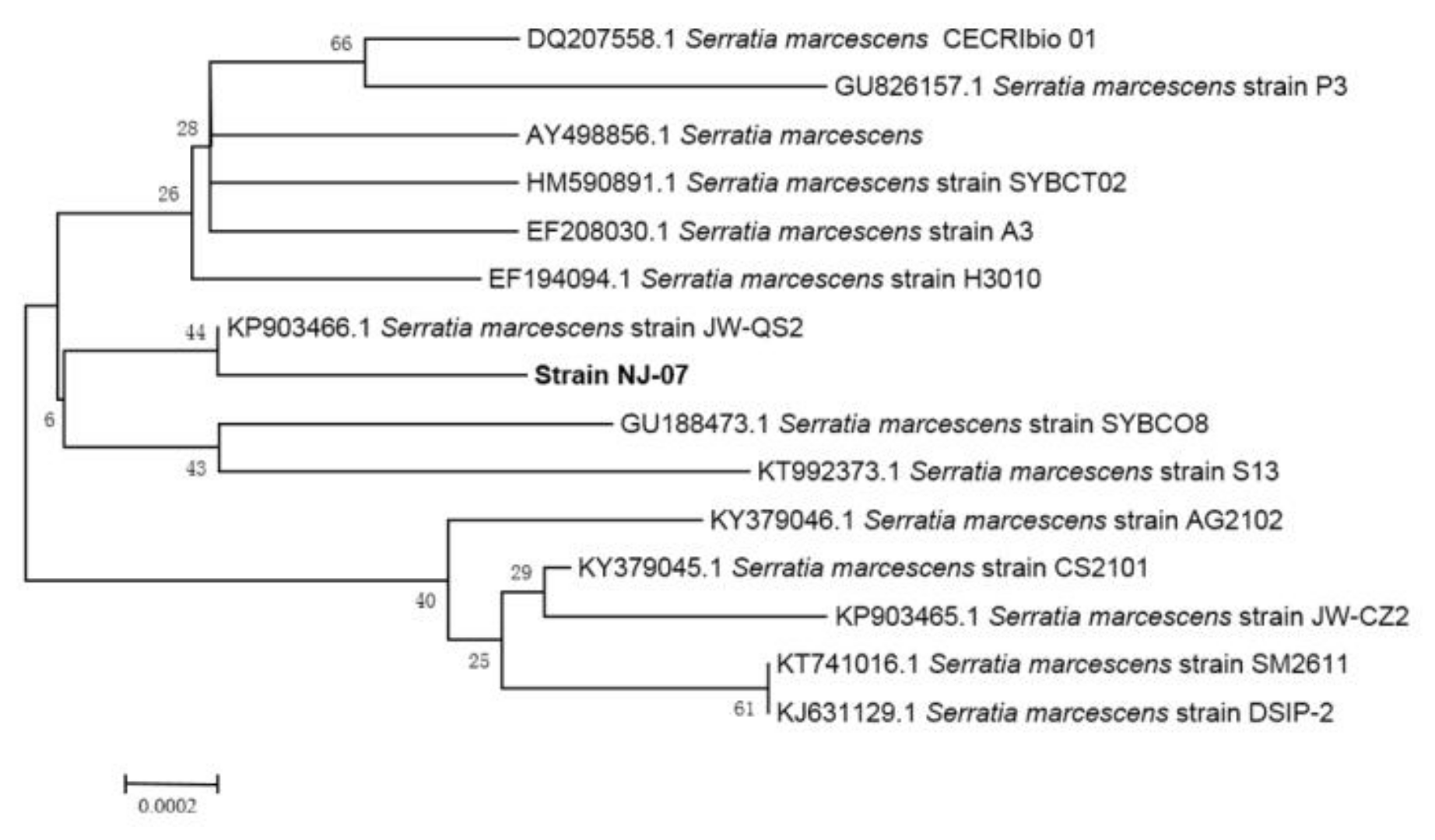
2.1. Screening and Identification of Strain NJ-07
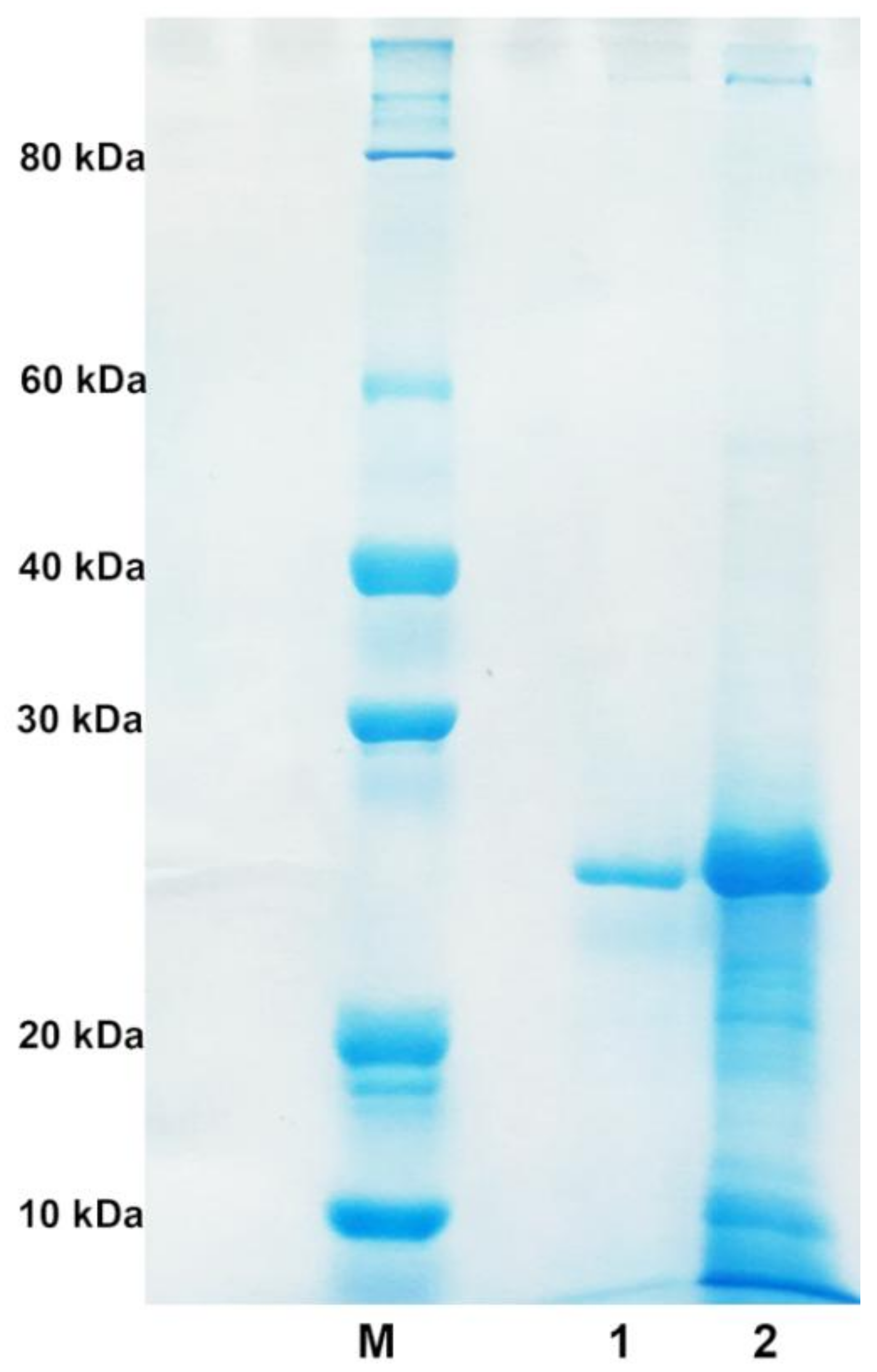
2.2. Purification of Alginate Lyase
2.3. Substrate Specifity and Enzymatic Kinetics of the Enzyme
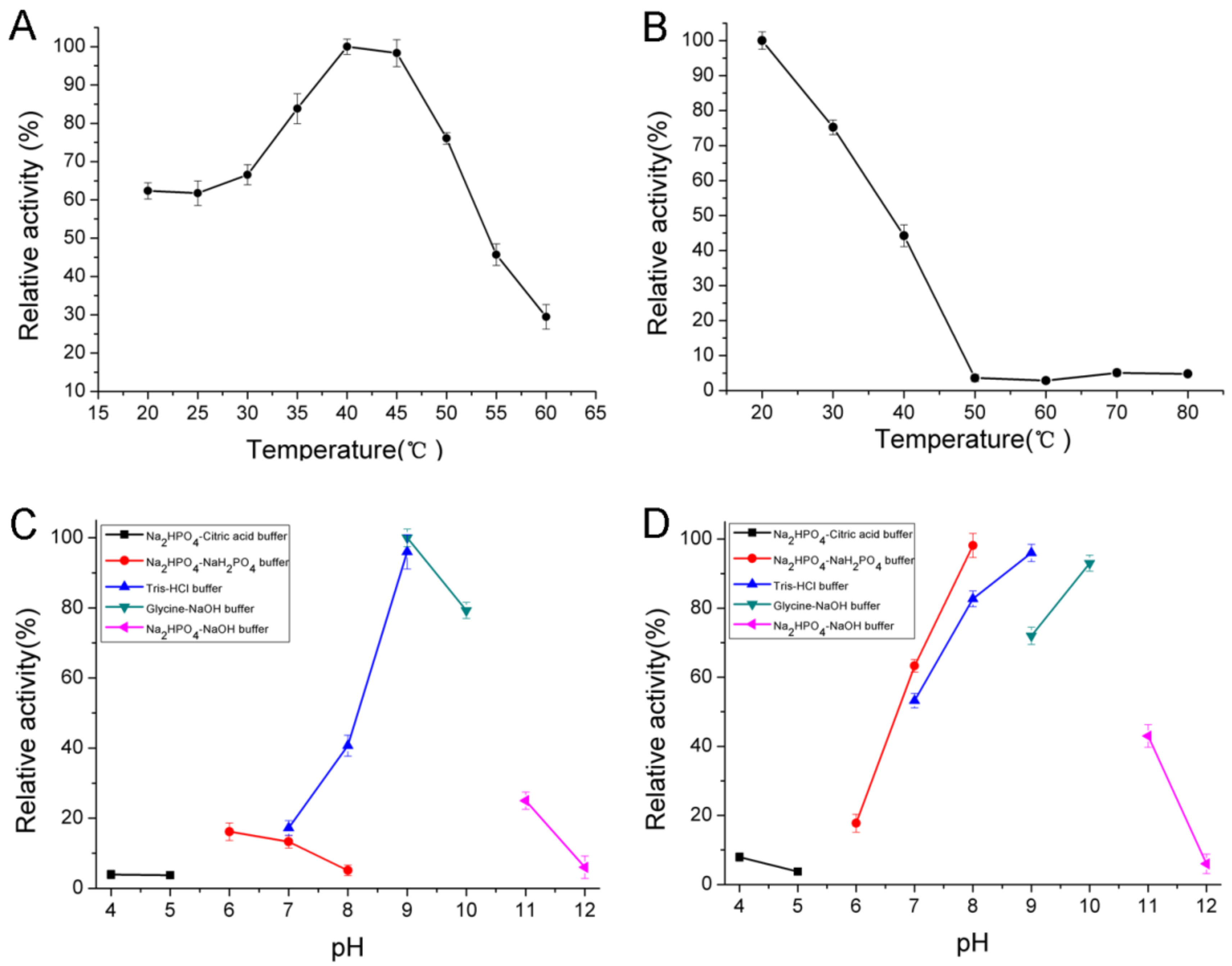
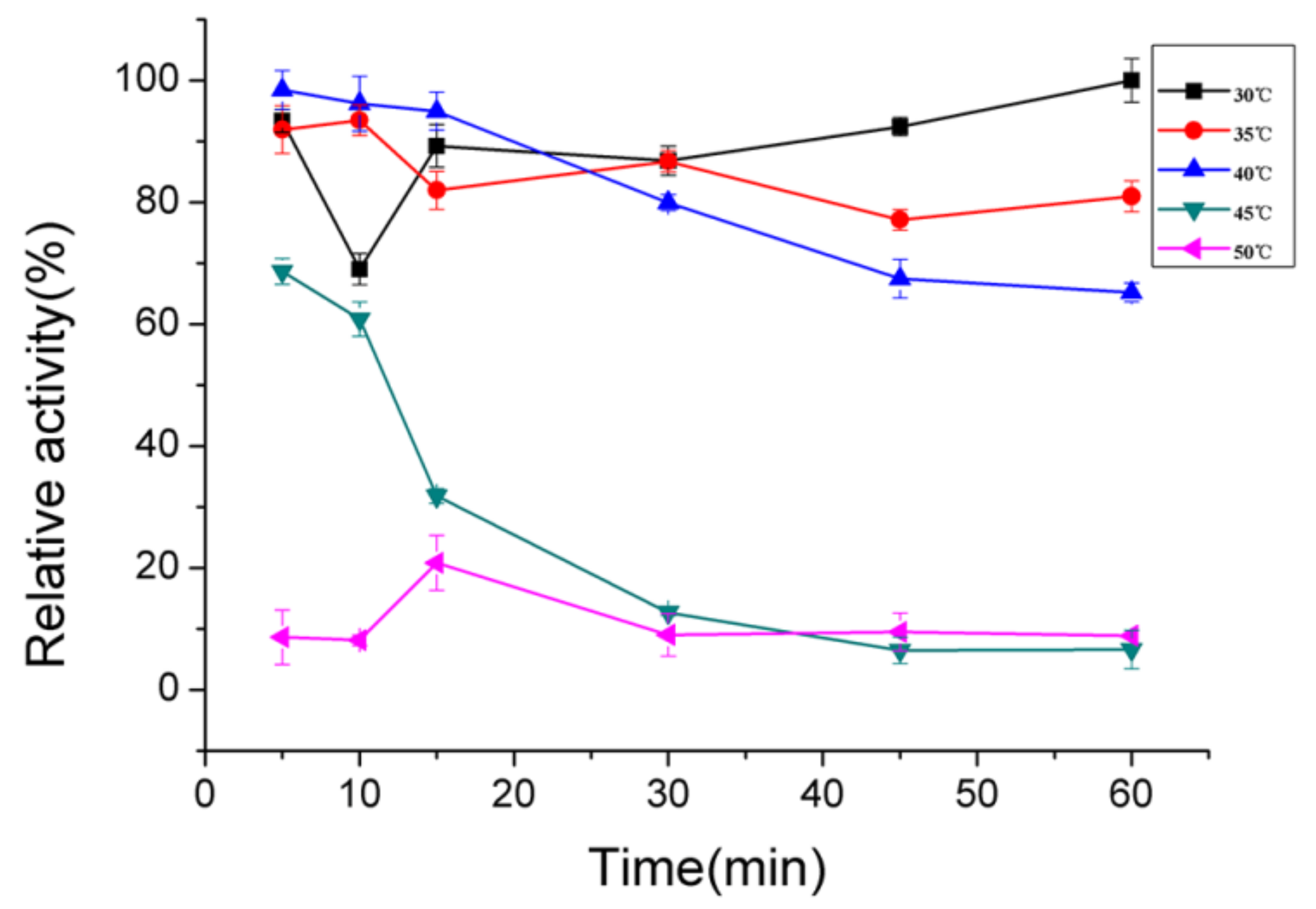
2.4. Biochemical Characterization of AlgNJ-07
3. Materials and Methods
3.1. Materials
3.2. Screening and Identification of Strain NJ-07
3.3. Production and Purification of the Alginate Lyase
3.4. Enzyme Activity Assay
3.5. Substrate Specificity and Kinetic Measurement of Alginate Lyase
3.6. Biochemical Characterization of AlgNJ-07
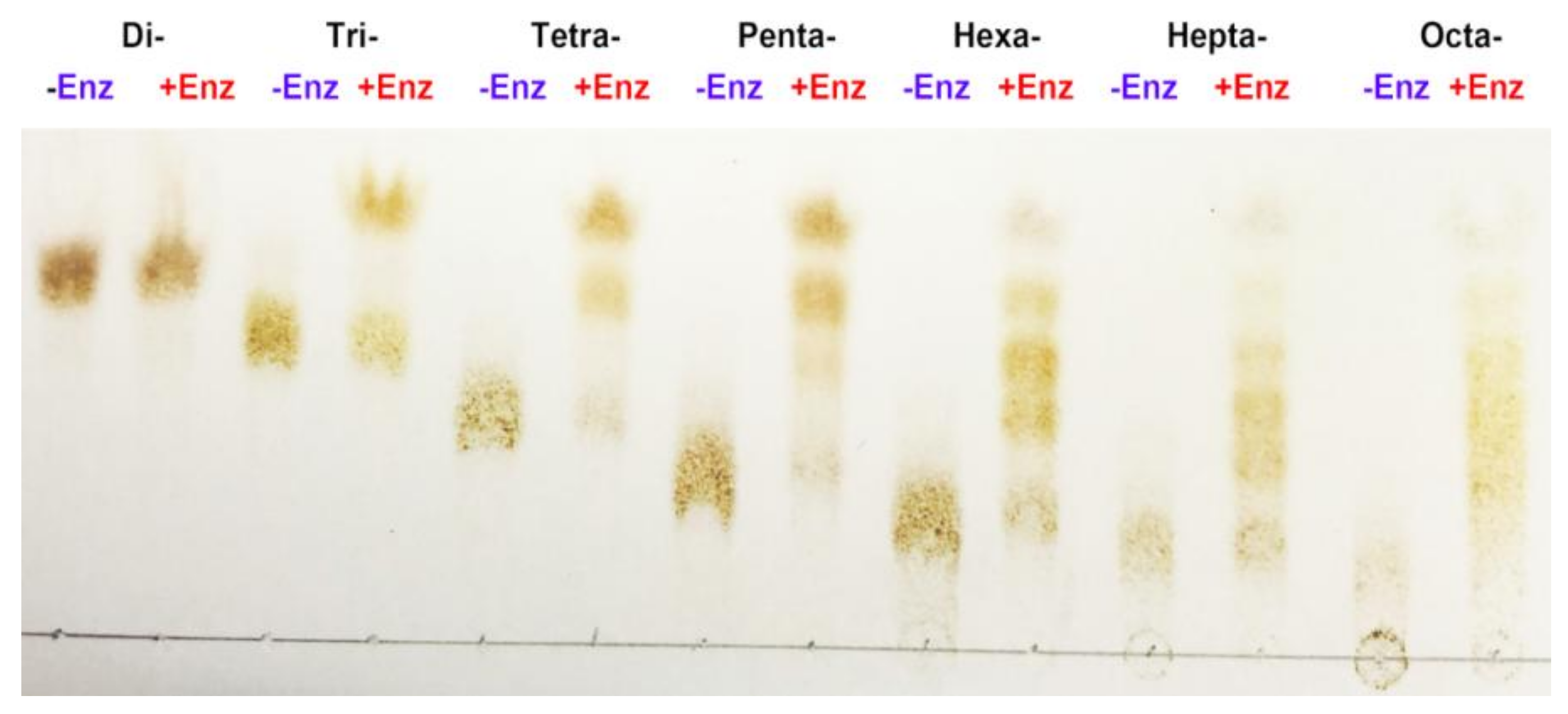
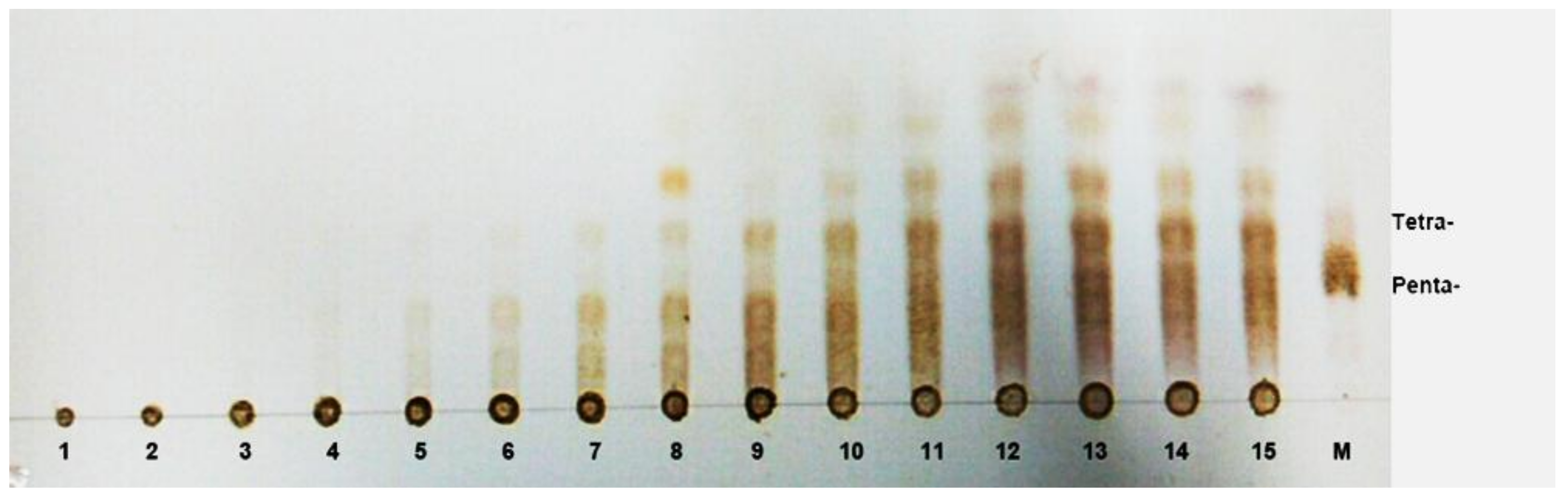
3.7. Substrate Binding Subsites of AlgNJ-07
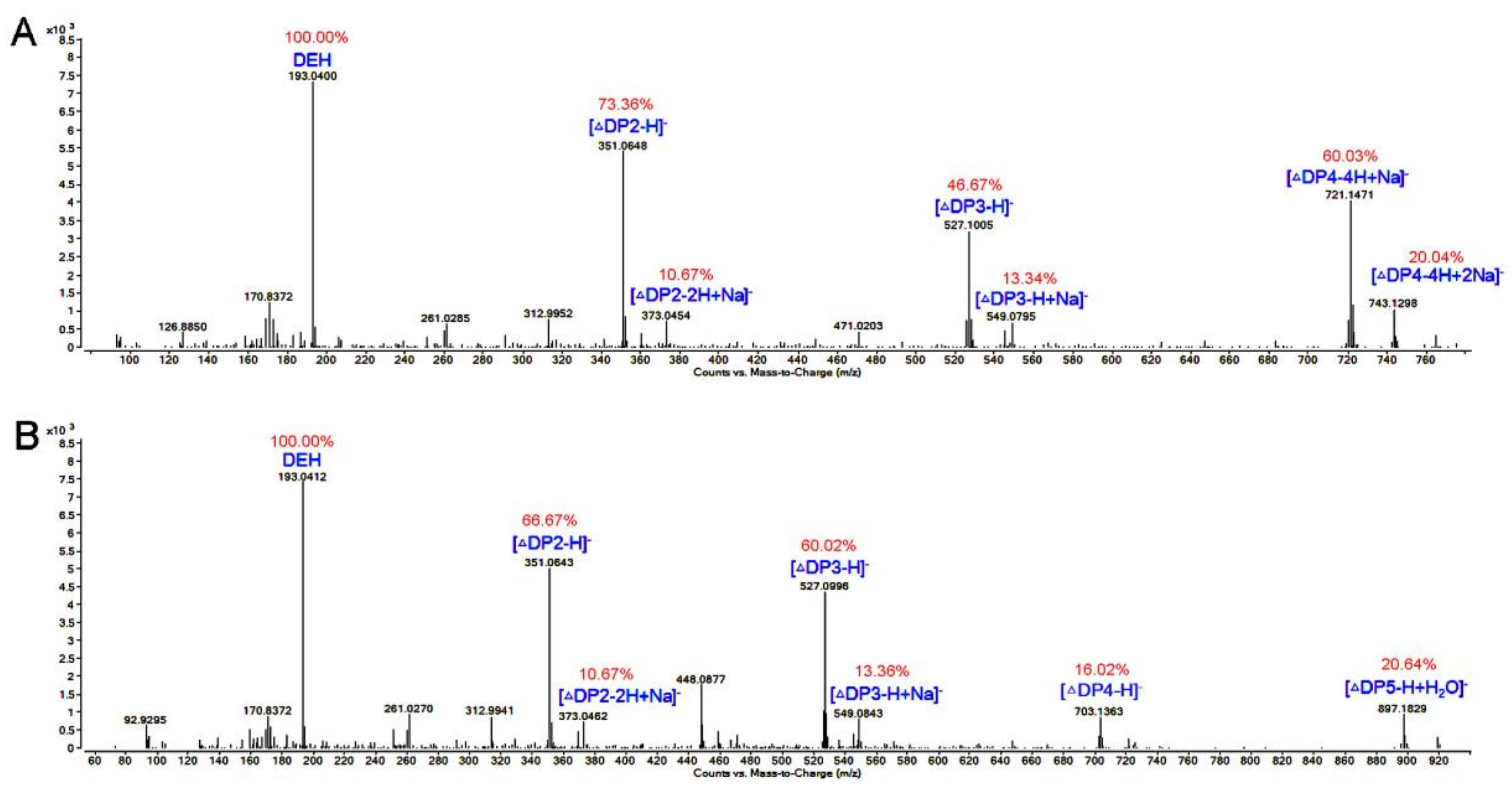
3.8. TLC and ESI-MS Analysis of the Degradation Products of AlgNJ-07
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gacesa, P. Enzymic degradation of alginates. Int. J. Biochem. 1992, 24, 545–552. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Mabeau, S.; Kloareg, B. Isolation and analysis of the cell walls of brown algae: Fucus spiralis, F. Ceranoides, F. Vesiculosus, F. Serratus, bifurcaria bifurcata and laminaria digitata. J. Exp. Bot. 1987, 38, 1573–1580. [Google Scholar] [CrossRef]
- Fujihara, M.; Nagumo, T. An influence of the structure of alginate on the chemotactic activity of macrophages and the antitumor activity. Carbohydr. Res. 1993, 243, 211–216. [Google Scholar] [CrossRef]
- Otterlei, M.; Ostgaard, K.; Skjak-Braek, G.; Smidsrod, O.; Soon-Shiong, P.; Espevik, T. Induction of cytokine production from human monocytes stimulated with alginate. J. Immunother. 1991, 10, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Bergero, M.F.; Liffourrena, A.S.; Opizzo, B.A.; Fochesatto, A.S.; Lucchesi, G.I. Immobilization of a microbial consortium on ca-alginate enhances degradation of cationic surfactants in flasks and bioreactor. Int. Biodeterior. Biodegrad. 2017, 117, 39–44. [Google Scholar] [CrossRef]
- Yang, J.S.; Xie, Y.J.; He, W. Research progress on chemical modification of alginate: A review. Carbohydr. Polym. 2011, 84, 33–39. [Google Scholar] [CrossRef]
- Tai, H.B.; Tang, L.W.; Chen, D.D.; Irbis, C.; Bioconvertion, L.O. Progresses on preparation of alginate oligosaccharide. Life Sci. Res. 2015, 19, 75–79. [Google Scholar]
- Do, J.R.; Back, S.Y.; Kim, H.K.; Lim, S.D.; Jung, S.K. Effects of aginate oligosaccharide on lipid metabolism in mouse fed a high cholesterol diet. J. Korean Soc. Food Sci. Nutr. 2014, 43, 491–497. [Google Scholar]
- Iwamoto, M.; Kurachi, M.; Nakashima, T.; Kim, D.; Yamaguchi, K.; Oda, T.; Iwamoto, Y.; Muramatsu, T. Structure-activity relationship of alginate oligosaccharides in the induction of cytokine production from raw264.7 cells. FEBS Lett. 2005, 579, 4423–4429. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kurachi, M.; Yamaguchi, K.; Oda, T. Induction of multiple cytokine secretion from raw264.7 cells by alginate oligosaccharides. J. Agric. Chem. Soc. Jpn. 2007, 71, 238–241. [Google Scholar] [CrossRef]
- Wong, T.Y.; And, L.A.P.; Schiller, N.L. Alginate lyase: Review of major sources and enzyme characteristics, structure-function analysis, biological roles, and applications. Annu. Rev. Microbiol. 2000, 54, 289–340. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, W.; Miyake, O.; Momma, K.; Kawai, S.; Murata, K. Molecular identification of oligoalginate lyase of sphingomonas sp. Strain a1 as one of the enzymes required for complete depolymerization of alginate. J. Bacteriol. 2000, 182, 4572–4577. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Tan, H.; Qin, Y.; Xu, Q.; Du, Y.; Yin, H. Characterization of a new endo-type alginate lyase from Vibrio sp. W13. Int. J. Biol. Macromol. 2015, 75, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Inoue, A.; Tanaka, H.; Ojima, T. Isolation and characterization of two alginate lyase isozymes, akaly28 and akaly33, from the common sea hare aplysia kurodai. Comp. Biochem. Phys. 2010, 157, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Dong, S.; Song, J.; Li, C.B.; Chen, X.L.; Xie, B.B.; Zhang, Y.Z. Purification and characterization of a bifunctional alginate lyase from Pseudoalteromonas sp. Sm0524. Mar. Drugs 2011, 9, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, X.; Shi, H.; Zhou, J.; Tan, Z.; Yuan, M.; Yao, P.; Liu, X. Characterization of a novel alginate lyase from marine Bacteriumvibrio furnissii H1. Mar. Drugs 2018, 16, 30. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Zhu, Y.; Men, Y.; Zeng, Y.; Sun, Y. Purification and characterization of a novel alginate lyase from the marine Bacterium bacillus sp. Alg07. Mar. Drugs 2018, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Yin, H. Alginate lyase: Review of major sources and classification, properties, structure-function analysis and applications. Bioengineered 2015, 6, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1991, 280 Pt 2, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, C.G.; Lee, E.Y. Alginate lyase: Structure, property, and application. Biotechnol. Bioprocess Eng. 2011, 16, 843. [Google Scholar] [CrossRef]
- Kim, H.T.; Ko, H.J.; Kim, N.; Kim, D.; Lee, D.; Choi, I.G.; Woo, H.C.; Kim, M.D.; Kim, K.H. Characterization of a recombinant endo-type alginate lyase (alg7d) from Saccharophagus degradans. Biotechnol. Lett. 2012, 34, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Heetaek, K.; Jaehyuk, C.; Wang, D.M.; Jieun, L.; Heechul, W.; Ingeol, C.; Kyoungheon, K. Depolymerization of alginate into a monomeric sugar acid using alg17c, an exo-oligoalginate lyase cloned from Saccharophagus degradans 2–40. Appl. Microbiol. Biotechnol. 2012, 93, 2233–2239. [Google Scholar]
- Zhang, Z.; Yu, G.; Guan, H.; Zhao, X.; Du, Y.; Jiang, X. Preparation and structure elucidation of alginate oligosaccharides degraded by alginate lyase from vibro sp. 510. Carbohydr. Res. 2004, 339, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Chen, M.; Yin, H.; Du, Y.; Ning, L. Enzymatic hydrolysis of alginate to produce oligosaccharides by a new purified endo-type alginate lyase. Mar. Drugs 2016, 14, 108. [Google Scholar] [CrossRef] [PubMed]
- Min, K.H.; Sasaki, S.F.; Kashiwabara, Y.; Umekawa, M.; Nisizawa, K. Fine structure of smg alginate fragment in the light of its degradation by alginate lyases of pseudomonas sp. J. Biochem. 1977, 81, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Boyen, C.; Kloareg, B.; Polne-Fuller, M.; Gibor, A. Preparation of alginate lyases from marine molluscs for protoplast isolation in brown algae. Phycologia. 1990, 29, 173–181. [Google Scholar] [CrossRef]
- Inoue, A.; Kagaya, M.; Ojima, T. Preparation of protoplasts from laminaria japonica using native and recombinant abalone alginate lyases. J. Appl. Phycol. 2008, 20, 633–640. [Google Scholar] [CrossRef]
- Alkawash, M.A.; Soothill, J.S.; Schiller, N.L. Alginate lyase enhances antibiotic killing of mucoid Pseudomonas aeruginosa in biofilms. APMIS 2006, 114, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Farrell, E.K.; Tipton, P.A. Functional characterization of algl, an alginate lyase from Pseudomonas aeruginosa. Biochemistry 2012, 51, 10259–10266. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, K.; Kodama, T. Purification and propertes of poly(β-d-mannuronate) lyase from azotobacter chroococcum. Appl. Microbiol. Biotechnol. 1996, 44, 576–581. [Google Scholar] [CrossRef]
- Kraiwattanapong, J.; Motomura, K.; Ooi, T.; Kinoshita, S. Characterization of alginate lyase (alyii) from Pseudomonas sp. Os-alg-9 expressed in recombinant escherichia coli. World J. Microb. Biotechnol. 1999, 15, 105–109. [Google Scholar] [CrossRef]
- Kam, N.; Park, Y.J.; Lee, E.Y.; Kim, H.S. Molecular identification of a polym-specific alginate lyase from Pseudomonas sp. Strain ks-408 for degradation of glycosidic linkages between two mannuronates or mannuronate and guluronate in alginate. Can. J. Microbiol. 2011, 57, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.W.; Huang, L.S.X.; Tan, H.D.; Qin, Y.Q.; Du, Y.G.; Yin, H. Characterization of a new endo-type polym-specific alginate lyase from Pseudomonas sp. Biotechnol. Lett. 2015, 37, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Romeo, T.; Iii, J.F.P. Purification and structural properties of an extracellular (1–4)-.beta.-d-mannuronan-specific alginate lyase from a marine bacterium. Biochemistry 1986, 25, 8385–8391. [Google Scholar] [CrossRef]
- Eftekhar, F.; Schiller, N.L. Partial purification and characterization of a mannuronan-specific alginate lyase from Pseudomonas aeruginosa. Curr. Microbiol. 1994, 29, 37–42. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Biochem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Kruger, N.J. The bradford method for protein quantitation. Methods Mol. Biol. 1988, 32, 9–15. [Google Scholar]
- Zhu, B.; Ni, F.; Sun, Y.; Yao, Z. Expression and characterization of a new heat-stable endo-type alginate lyase from deep-sea bacterium Flammeovirga sp. Nj-04. Extremophiles 2017, 21, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Swift, S.M.; Hudgens, J.W.; Heselpoth, R.D.; Bales, P.M.; Nelson, D.C. Characterization of algmsp, an alginate lyase from Microbulbifer sp. 6532a. PLoS ONE 2014, 9, e112939. [Google Scholar] [CrossRef] [PubMed]
| Substrate | Activity (U/mg) |
|---|---|
| Sodium alginate | 2742.5 |
| PolyM | 3842.3 |
| PolyG | N.D. * |
| Pullulan | N.D. |
| Pectin | N.D. |
| Xylan | N.D. |
| Heparin | N.D. |
| Substrate | Sodium Alginate | polyM |
|---|---|---|
| Km (mM) | 0.53 | 0.27 |
| Vmax (nmol/s) | 74 | 67 |
| kcat (s−1) | 34 | 31 |
| kcat/Km (s−1/mM) | 64 | 115 |
| Reagent | Relative Activity (%) |
|---|---|
| Control | 100 ± 0.5 |
| K+ (100 mM) | 87 ± 0.5 |
| K+ (300 mM) | 94 ± 0.3 |
| K+ (500 mM) | 92 ± 2.2 |
| Na+ (100 mM) | 106 ± 0.6 |
| Na+ (300 mM) | 120 ± 1.1 |
| Na+ (500 mM) | 103 ± 2.6 |
| Zn2+ | 1 ± 0.3 |
| Cu2+ | 5 ± 0.5 |
| Mn2+ | 4 ± 0.1 |
| Co2+ | 25 ± 0.3 |
| Ca2+ | 90 ± 0.3 |
| Fe3+ | 18 ± 0.1 |
| Mg2+ | 98 ± 0.5 |
| Ni2+ | 72 ± 1.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, B.; Hu, F.; Yuan, H.; Sun, Y.; Yao, Z. Biochemical Characterization and Degradation Pattern of a Unique pH-Stable PolyM-Specific Alginate Lyase from Newly Isolated Serratia marcescens NJ-07. Mar. Drugs 2018, 16, 129. https://doi.org/10.3390/md16040129
Zhu B, Hu F, Yuan H, Sun Y, Yao Z. Biochemical Characterization and Degradation Pattern of a Unique pH-Stable PolyM-Specific Alginate Lyase from Newly Isolated Serratia marcescens NJ-07. Marine Drugs. 2018; 16(4):129. https://doi.org/10.3390/md16040129
Chicago/Turabian StyleZhu, Benwei, Fu Hu, Heng Yuan, Yun Sun, and Zhong Yao. 2018. "Biochemical Characterization and Degradation Pattern of a Unique pH-Stable PolyM-Specific Alginate Lyase from Newly Isolated Serratia marcescens NJ-07" Marine Drugs 16, no. 4: 129. https://doi.org/10.3390/md16040129
APA StyleZhu, B., Hu, F., Yuan, H., Sun, Y., & Yao, Z. (2018). Biochemical Characterization and Degradation Pattern of a Unique pH-Stable PolyM-Specific Alginate Lyase from Newly Isolated Serratia marcescens NJ-07. Marine Drugs, 16(4), 129. https://doi.org/10.3390/md16040129






