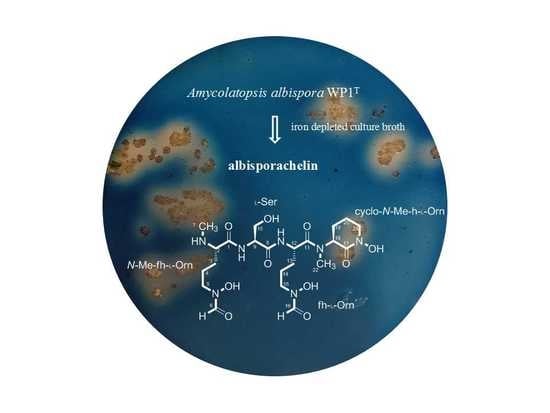
Albisporachelin, a New Hydroxamate Type Siderophore from the Deep Ocean Sediment-Derived Actinomycete Amycolatopsis albispora WP1T
Abstract
:1. Introduction
2. Results
2.1. Structural Identification of Albisporachelin from Amycolatopsis albispora WP1T
2.2. Iron-Chelating Ability of Albisporachelin (1)
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Bacteria Strain
4.3. Siderophore Screening
4.4. Fermentation, Extraction and Isolation
4.5. Acid Hydrolysis of Compound 1 and Assignment of the Absolute Configuration by Modified Marfey’s Method
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Milero, F.J. The solubility of iron in seawater. Mar. Chem. 2002, 77, 43–54. [Google Scholar] [CrossRef]
- Butler, A.; Theisen, R.M. Iron (III)-siderophore coordination chemistry: Reactivity of marine sideophores. Coord. Chem. Rev. 2010, 254, 288–296. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.; Fernandes, P. Production of metabolites as bacterial responses to the marine environment. Mar. Drugs 2010, 8, 705–727. [Google Scholar] [CrossRef] [PubMed]
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657. [Google Scholar] [CrossRef] [PubMed]
- Takehana, Y.; Umekita, M.; Hatano, M.; Kato, C.; Sawa, R.; Igarashi, M. Fradiamine A, a new siderophore from the deep-sea actinomycete Streptomyces fradiae MM456M-mF7. J. Antibiot. 2017, 70, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Shang, F.; Xi, L.; Huang, Y. Tetroazolemycins A and B, two new oxazole-thiazole siderophores from deep-sea Streptomyces olivaceus FXJ8.012. Mar. Drugs 2013, 11, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Zane, H.K.; Butler, A. Isolation, structure elucidation, and iron-binding properties of lystabactins, siderophores isolated from a marine Pseudoalteromonas sp. J. Nat. Prod. 2013, 76, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wu, Q.; Shen, Q.; Wang, H. Progress in understanding the genetic information and biosynthetic pathways behind Amycolatopsis antibiotics, with implications for the continue discovery of novel drugs. ChemBioChem 2016, 17, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Seyedsayamdost, M.R.; Traxler, M.F.; Zheng, S.; Kolter, R.; Clardy, J. Structure and biosynthesis of amychelin, an unusual mixed-ligand siderophore from Amycolatopsis sp. AA4. J. Am. Chem. Soc. 2011, 133, 11434–11437. [Google Scholar] [CrossRef] [PubMed]
- Kodani, S.; Komaki, H.; Suzuki, M.; Hemmi, H.; Ohnishi-Kameyama, M. Isolation and structure determination of new siderophore albachelin from Amycolatopsis alba. Biometals 2015, 28, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, L.; Li, J.; Zhou, Y. Amycolatopsis albispora sp. nov., isolated from deep-sea sediment. Int. J. Syst. Evol. Microbiol. 2016, 66, 3860–3864. [Google Scholar] [CrossRef] [PubMed]
- Milagres, A.M.F.; Machuca, A.; Napoleao, D. Detection of siderophore production from several fungi and bacteria by a modification of chrome azurol S (CAS) agar plate assay. J. Microbiol. Methods 1999, 37, 1–6. [Google Scholar] [CrossRef]
- Bhushan, R.; Bruckner, H. Marfey’s reagent for chiral amino acid analysis: A review. Amino Acids 2004, 27, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Sharman, G.J.; Williams, D.H.; Ewing, D.F.; Ratledge, C. Determination of the structure of exochelin MN, the extracellular siderophore from Mycobacterium neoaurum. Chem. Biol. 1995, 2, 553–561. [Google Scholar] [CrossRef]
- Zucchi, T.D.; Tan, G.Y.; Bonda, A.N.; Frank, S.; Kshetrimayum, J.D.; Goodfellow, M. Amycolatopsis granulosa sp. nov. Amycolatopsis ruanii sp. nov. and Amycolatopsis thermalba sp. nov. thermophilic actinomycetes isolated from arid soils. Int. J. Syst. Evol. Microbiol. 2012, 62, 1245–1251. [Google Scholar] [PubMed]
- Miao, Q.; Qin, S.; Bian, G.K.; Yuan, B.; Xing, K.; Zhang, Y.J.; Li, Q.; Tang, S.K.; Li, W.J.; Jiang, J.H. Amycolatopsis endophytica sp. nov. a novel endophytic actinomycete isolated from oil-seed plant Jatropha curcas L. Antonie Leeuwenhoek 2011, 100, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Labeda, D.P.; Donahue, J.M.; Williams, N.M.; Sells, S.F.; Henton, M.M. Amycolatopsis kentuckyensis sp. nov. Amycolatopsis lexingtonensis sp. nov. and Amycolatopsis pretoriensis sp. nov. isolated from equine placentas. Int. J. Syst. Evol. Microbiol. 2003, 53, 1601–1605. [Google Scholar] [PubMed]
- Kodani, S.; Bicz, J.; Song, L.; Deeth, R.J.; Ohnishi-Kameyama, M.; Yoshida, M.; Ochi, K.; Challis, G.L. Structure and biosynthesis of scabichelin, a novel tris-hydroxamate siderophore produced by the plant pathogen Streptomyces scabies 87.22. Org. Biomol. Chem. 2013, 11, 4686–4694. [Google Scholar] [CrossRef] [PubMed]
- Boyd, P.W.; Ellwood, M.J. The biogeochemical cycle of iron in the ocean. Nat. Geosci. 2010, 3, 675–682. [Google Scholar] [CrossRef] [Green Version]
- Boiteau, R.M.; Mende, D.R.; Hawco, N.J.; McIlvin, M.R.; Fitzsimmons, J.N.; Saito, M.A.; Sedwick, P.N.; DeLong, E.F.; Repeta, D.J. Siderophore-based microbial adaptations to iron scarcity across the eastern Pacific Ocean. Proc. Natl. Acad. Sci. USA 2016, 113, 14237–14242. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Toner, B.M.; Baker, B.J.; Breier, J.A.; Sheik, C.S.; Dick, G.J. Microbial iron uptake as a mechanism for dispersing iron from deep-sea hydrothermal vents. Nat. Commun. 2014, 5, 3192–3199. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, S.; Nishimura, S.; Hattori, A.; Tsujimoto, M.; Hatano, M.; Igarashi, M.; Kakeya, H. Chlorocatechelins A and B from Streptomyces sp.: New siderophores containing chlorinated catecholate groups and an acylguanidine structure. Org. Lett. 2014, 16, 6108–6111. [Google Scholar] [CrossRef] [PubMed]





| Residue | Position | δC, Type | δH (J in Hz) | 1H-1H COSY | HMBC |
|---|---|---|---|---|---|
| N-Me-hf-Orn | C-1 | 167.1, qC | - | - | - |
| C-2 | 60.2, CH | 3.86 m | NH-1, H-3 | C-1, C-3, C-4 | |
| C-3 | 32.8, CH2 | 1.54, 1.72 m | H-2, H-4 | C-1, C-2, C-4, C-5 | |
| C-4 | 22.6, CH2 | 1.62 m | H-3, H-5 | C-2, C-3, C-5 | |
| C-5 | 48.7, CH2 | 3.43 m | H-4 | C-3, C-4, C-6 | |
| C-6 | 157.2, CH | 7.90 d (8.5) | - | - | |
| C-7 | 31.3, CH3 | 2.48 s | - | C-2 | |
| NH-1 | - | 8.87 s | - | - | |
| Ser | C-8 | 168.7, qC | - | - | - |
| C-9 | 52.8, CH | 5.04 m | H-10 | C-1, C-8, C-10 | |
| C-10 | 60.6, CH2 | 3.56, 3.75 m | H-9 | C-8, C-9 | |
| NH-2 | - | 8.79 d (7.6) | H-9 | C-1 | |
| Hf-Orn | C-11 | 166.0, qC | - | - | - |
| C-12 | 54.3, CH | 3.75 m | NH-3, H-13 | C-8, C-11 | |
| C-13 | 32.4, CH2 | 1.54, 1.72 m | H-12, H-14 | C-11, C-12, C-15 | |
| C-14 | 26.5, CH2 | 1.72 m | H-13, H-15 | C-12, C-13, C-15 | |
| C-15 | 45.5, CH2 | 3.43 m | H-14 | C-13, C-14, C-16 | |
| C-16 | 161.8, CH | 8.24 d (6.9) | - | C-15 | |
| NH-3 | - | 8.37 d (17.5) | - | - | |
| Cyclic N-Me-fh-Orn | C-17 | 166.6, qC | - | - | - |
| C-18 | 60.4, CH | 3.85 m | H-19 | C-17, C-19, C-20, C-22 | |
| C-19 | 28.8, CH2 | 1.63, 1.81 m | H-18, H-20 | C-17, C-18, C-20 | |
| C-20 | 21.9, CH2 | 1.63 m | H-19, H-21 | C-18, C-19, C-21 | |
| C-21 | 47.1, CH2 | 3.73, 3.37 m | H-20 | C-17, C-19, C-20 | |
| C-22 | 31.9, CH3 | 2.81 s | - | C-11, C-18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Deering, R.W.; Zhang, G.; Wang, B.; Li, X.; Sun, J.; Chen, J.; Zhang, H.; Rowley, D.C.; Wang, H. Albisporachelin, a New Hydroxamate Type Siderophore from the Deep Ocean Sediment-Derived Actinomycete Amycolatopsis albispora WP1T. Mar. Drugs 2018, 16, 199. https://doi.org/10.3390/md16060199
Wu Q, Deering RW, Zhang G, Wang B, Li X, Sun J, Chen J, Zhang H, Rowley DC, Wang H. Albisporachelin, a New Hydroxamate Type Siderophore from the Deep Ocean Sediment-Derived Actinomycete Amycolatopsis albispora WP1T. Marine Drugs. 2018; 16(6):199. https://doi.org/10.3390/md16060199
Chicago/Turabian StyleWu, Qihao, Robert W. Deering, Gaiyun Zhang, Bixia Wang, Xin Li, Jiadong Sun, Jianwei Chen, Huawei Zhang, David C. Rowley, and Hong Wang. 2018. "Albisporachelin, a New Hydroxamate Type Siderophore from the Deep Ocean Sediment-Derived Actinomycete Amycolatopsis albispora WP1T" Marine Drugs 16, no. 6: 199. https://doi.org/10.3390/md16060199
APA StyleWu, Q., Deering, R. W., Zhang, G., Wang, B., Li, X., Sun, J., Chen, J., Zhang, H., Rowley, D. C., & Wang, H. (2018). Albisporachelin, a New Hydroxamate Type Siderophore from the Deep Ocean Sediment-Derived Actinomycete Amycolatopsis albispora WP1T. Marine Drugs, 16(6), 199. https://doi.org/10.3390/md16060199