Zoanthamine Alkaloids from the Zoantharian Zoanthus cf. pulchellus and Their Effects in Neuroinflammation
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Biological Material
4.3. Extraction and Isolation
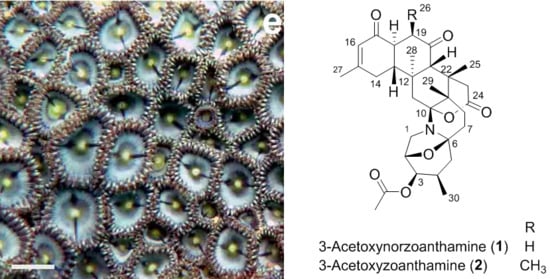
4.4. 3-Acetoxynorzoanthamine (1)
4.5. 3-Acetoxyzoanthamine (2)
4.6. Biological Assays
4.6.1. Cell Culture
4.6.2. Cell Viability
4.6.3. Measurement of Intracellular ROS Production
4.6.4. NO Determination
4.6.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, C.B.; Anjaneyula, A.S.R.; Sarma, S.S.; Venkatateswarlu, Y.; Chen, M.; Clardy, J.; Rosser, R.; Faulkner, J. Zoanthamine: A novel alkaloid from a marine zoanthid. J. Am. Chem. Soc. 1984, 106, 7984–7985. [Google Scholar] [CrossRef]
- Rao, C.B.; Anjaneyulu, A.S.R.; Sarma, N.S.; Venkateswarlu, Y.; Rosser, R.M.; Faulkner, J. Alkaloids from a marine zoanthid. J. Org. Chem. 1985, 50, 3757–3760. [Google Scholar] [CrossRef]
- Fukuzawa, S.; Hayashi, Y.; Uemura, D.; Nagatsu, A.; Yamada, K.; Ijuin, Y. The isolation and structures of five new alkaloids, norzoanthamine, oxyzoanthamine, norzoanthamine, cyclozoanthamine and epinorzoanthamine. Heterocycl. Commun. 1995, 1, 207–214. [Google Scholar] [CrossRef]
- Atta-ur-Rahman; Alvi, K.A.; Abbas, S.A.; Choudhary, M.I.; Clardy, J. Zoanthaminone, a new alkaloid from a marine zoanthid. Tetrahedron Lett. 1989, 30, 6825–6828. [Google Scholar] [CrossRef]
- Cen-Pacheco, F.; Norte, M.; Fernández, J.J.; Daranas, A.H. Zoaramine, a zoanthamine-like alkaloid with a new skeleton. Org. Lett. 2014, 16, 2880–2883. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-B.; Lo, I.-W.; Shyur, L.-F.; Yang, C.-C.; Hsu, Y.-M.; Su, J.-H.; Lu, M.-C.; Chiou, S.-F.; Lan, C.-C.; Wu, Y.-C.; et al. New alkaloids from Formosan zoanthid Zoanthus kuroshio. Tetrahedron 2015, 71, 8001–8006. [Google Scholar] [CrossRef]
- Daranas, A.H.; Fernández, J.J.; Gavin, J.A.; Norte, M. Epioxyzoanthamine, a new zoanthamine-type alkaloid and the unusual deuterium exchange in this series. Tetrahedron 1998, 54, 7891–7896. [Google Scholar] [CrossRef]
- Daranas, A.H.; Fernandez, J.J.; Gavin, J.A.; Norte, M. New alkaloids from a marine zoanthid. Tetrahedron 1999, 55, 5539–5546. [Google Scholar] [CrossRef]
- Behenna, D.C.; Stockdill, J.L.; Stoltz, B.M. The biology and chemistry of the zoanthamine alkaloids. Angew. Chem. Int. Ed. 2008, 47, 2365–2386. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-M.; Chang, F.-R.; Lo, I.W.; Lai, K.-H.; El-Shazly, M.; Wu, T.-Y.; Du, Y.-C.; Hwang, T.-L.; Cheng, Y.-B.; Wu, Y.-C. Zoanthamine-type alkaloids from the zoanthid Zoanthus kuroshio collected in Taiwan and their effects on inflammation. J. Nat. Prod. 2016, 79, 2674–2680. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, M.; Sasaki, M.; Hattori, I.; Sakai, M.; Tanino, K. Total synthesis of norzoanthamine. Science 2004, 305, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yoshimura, F.; Tanino, K.; Miyashita, M. Total synthesis of zoanthenol. Angew. Chem. Int. Ed. 2009, 48, 8905–8908. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, F.; Sasaki, M.; Hattori, I.; Komatsu, K.; Sakai, M.; Tanino, K.; Miyashita, M. Synthetic studies of the zoanthamine alkaloids: The total syntheses of norzoanthamine and zoanthamine. Chem. Eur. J. 2009, 15, 6626–6644. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, F.; Tanino, K.; Miyashita, M. Total synthesis of zoanthamine alkaloids. Acc. Chem. Res. 2012, 45, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Fischer, D.; Nguyen, T.X.; Trzoss, L.; Dakanali, M.; Theodorakis, E.A. Intramolecular cyclization strategies toward the synthesis of zoanthamine alkaloids. Tetrahedron Lett. 2011, 52, 4920–4923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, T.; Yamashita, D.; Suzuki, K.; Nakazaki, A.; Suzuki, T.; Kobayashi, S. Different modes of cyclization in zoanthamine alkaloid system, bisaminal versus spiroketal formation. Org. Lett. 2011, 13, 2980–2983. [Google Scholar] [CrossRef] [PubMed]
- Villar, R.M.; Gil-Longo, J.; Daranas, A.H.; Souto, M.L.; Fernández, J.J.; Peixinho, S.; Barral, M.A.; Santafé, G.; Rodríguez, J.; Jiménez, C. Evaluation of the effect of several zoanthamine-type alkaloids on the aggregation of human platelets. Bioorg. Med. Chem. 2003, 11, 2301–2306. [Google Scholar] [CrossRef]
- Genji, T.; Fukuzawa, S.; Tachibana, K. Distribution and possible function of the marine alkaloid, norzoanthamine, in the zoanthid Zoanthus sp. using MALDI imaging mass spectrometry. Mar. Biotechnol. 2010, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Guillen, P.O.; Calabro, K.; Jaramillo, K.B.; Dominguez, C.; Genta-Jouve, G.; Rodriguez, J.; Thomas, O.P. Ecdysonelactones, ecdysteroids from the Tropical Eastern Pacific zoantharian Antipathozoanthus hickmani. Mar. Drugs 2018, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Guillen, P.O.; Jaramillo, K.B.; Genta-Jouve, G.; Sinniger, F.; Rodriguez, J.; Thomas, O.P. Terrazoanthines, 2-aminoimidazole alkaloids from the Tropical Eastern Pacific zoantharian Terrazoanthus onoi. Org. Lett. 2017, 19, 1558–1561. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, K.B.; Reverter, M.; Guillen, P.O.; McCormack, G.; Rodriguez, J.; Sinniger, F.; Thomas, O.P. Assessing the zoantharian diversity of the Tropical Eastern Pacific through an integrative approach. Sci. Rep. 2018, 8, 7138. [Google Scholar] [CrossRef] [PubMed]
- Kuramoto, M.; Hayashi, K.; Fujitani, Y.; Yamaguchi, K.; Tsuji, T.; Yamada, K.; Ijuin, Y.; Uemura, D. Absolute configuration of norzoanthamine, a promising candidate for an osteoporotic drug. Tetrahedron Lett. 1997, 38, 5683–5686. [Google Scholar] [CrossRef]
- Dumont, M.; Beal, M.F. Neuroprotective strategies involving ROS in Alzheimer disease. Free Radic. Biol. Med. 2011, 51, 1014–1026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, J.A.; Alfonso, A.; Leiros, M.; Alonso, E.; Rateb, M.E.; Jaspars, M.; Houssen, W.E.; Ebel, R.; Tabudravu, J.; Botana, L.M. Identification of Spongionella compounds as cyclosporine A mimics. Pharmacol. Res. 2016, 107, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Leiros, M.; Alonso, E.; Rateb, M.E.; Houssen, W.E.; Ebel, R.; Jaspars, M.; Alfonso, A.; Botana, L.M. Gracilins: Spongionella-derived promising compounds for Alzheimer disease. Neuropharmacology 2015, 93, 285–293. [Google Scholar] [CrossRef] [PubMed]



| 1 | 2 | |||
|---|---|---|---|---|
| No. | δH, mult. (J in Hz) | δC | δH, mult. (J in Hz) | δC |
| 1 | 3.24, t (7.0) | 45.3 | 3.24, t (7.5) | 45.5 |
| 3.19, d (7.0) | 3.20, d (7.0) | |||
| 2 | 4.58, br d (6.5) | 75.6 | 4.59, d (7.0) | 75.7 |
| 3 | 4.62, br t (3.0) | 72.5 | 4.63, t (3.0) | 72.6 |
| 4 | 2.44, br sext (5.5) | 26.0 | 2.43, br sext (6.0) | 26.1 |
| 5 | 1.92, dd (12.0, 6.0) | 40.3 | 1.95, dd (12.5, 6.0) | 40.4 |
| 1.36, t (12.5) | 1.37, t (13.0) | |||
| 6 | - | 90.1 | - | 90.2 |
| 7 | 1.88, dd (12.5, 4.5) | 29.8 | 1.90, dd (12.5, 4.5) | 29.9 |
| 1.80, dt (12.5, 3.5) | 1.80, dt (12.5, 3.5) | |||
| 8 | 1.66, td (13.5, 3.5) | 23.7 | 1.67, td (14.0, 3.5) | 23.8 |
| 1.57, dt (13.5, 4.0) | 1.57, dt (14.0, 4.0) | |||
| 9 | - | 40.0 | - | 40.5 |
| 10 | - | 100.9 | - | 101.0 |
| 11 | 2.08, d (13.0) | 41.8 | 2.11, d (13.0) | 42.0 |
| 1.94, d (13.0) | 1.93, d (13.0) | |||
| 12 | - | 39.9 | - | 39.8 |
| 13 | 2.20, td (12.0, 4.5) | 53.1 | 2.41, td (12.0, 4.5) | 48.1 |
| 14 | 2.26, br s | 32.0 | 2.24, br s | 30.7 |
| 2.24, br s | 2.22, br s | |||
| 15 | - | 160.0 | - | 160.1 |
| 16 | 5.90, s | 125.6 | 5.92, s | 127.0 |
| 17 | - | 198.5 | - | 197.3 |
| 18 | 2.69, td (12.0, 6.5) | 46.4 | 2.66, dd (12.5, 6.5) | 48.2 |
| 19 | 2.62, dd (14.5, 6.5) | 42.4 | 3.02, dq (7.0, 6.5) | 45.9 |
| 2.50, dd (14.5, 12.0) | ||||
| 20 | - | 209.0 | - | 212.2 |
| 21 | 2.83, s | 59.1 | 3.23, s | 53.9 |
| 22 | - | 36.5 | - | 40.3 |
| 23 | 3.65, d (20.0) | 35.9 | 3.68, d (20.0) | 36.1 |
| 2.36, d (20.0) | 2.37, d (20.0) | |||
| 24 | - | 172.3 | - | 172.4 |
| 25 | 0.99, s | 21.1 | 0.98, s | 20.8 |
| 26 | - | - | 1.17, d (7.0) | 13.9 |
| 27 | 2.00, s | 24.4 | 2.01, s | 24.6 |
| 28 | 0.97, s | 18.5 | 0.99, s | 18.5 |
| 29 | 1.15, s | 18.4 | 1.21, s | 18.4 |
| 30 | 0.87, d (7.0) | 16.3 | 0.89, d (7.0) | 16.4 |
| Ac | - | 171.2 | - | 171.4 |
| 2.11, s | 21.1 | 2.14, s | 21.2 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillen, P.O.; Gegunde, S.; Jaramillo, K.B.; Alfonso, A.; Calabro, K.; Alonso, E.; Rodriguez, J.; Botana, L.M.; Thomas, O.P. Zoanthamine Alkaloids from the Zoantharian Zoanthus cf. pulchellus and Their Effects in Neuroinflammation. Mar. Drugs 2018, 16, 242. https://doi.org/10.3390/md16070242
Guillen PO, Gegunde S, Jaramillo KB, Alfonso A, Calabro K, Alonso E, Rodriguez J, Botana LM, Thomas OP. Zoanthamine Alkaloids from the Zoantharian Zoanthus cf. pulchellus and Their Effects in Neuroinflammation. Marine Drugs. 2018; 16(7):242. https://doi.org/10.3390/md16070242
Chicago/Turabian StyleGuillen, Paul O., Sandra Gegunde, Karla B. Jaramillo, Amparo Alfonso, Kevin Calabro, Eva Alonso, Jenny Rodriguez, Luis M. Botana, and Olivier P. Thomas. 2018. "Zoanthamine Alkaloids from the Zoantharian Zoanthus cf. pulchellus and Their Effects in Neuroinflammation" Marine Drugs 16, no. 7: 242. https://doi.org/10.3390/md16070242
APA StyleGuillen, P. O., Gegunde, S., Jaramillo, K. B., Alfonso, A., Calabro, K., Alonso, E., Rodriguez, J., Botana, L. M., & Thomas, O. P. (2018). Zoanthamine Alkaloids from the Zoantharian Zoanthus cf. pulchellus and Their Effects in Neuroinflammation. Marine Drugs, 16(7), 242. https://doi.org/10.3390/md16070242