Degradation of Fucoxanthin to Elucidate the Relationship between the Fucoxanthin Molecular Structure and Its Antiproliferative Effect on Caco-2 Cells
Abstract
:1. Introduction
2. Results and Discussion
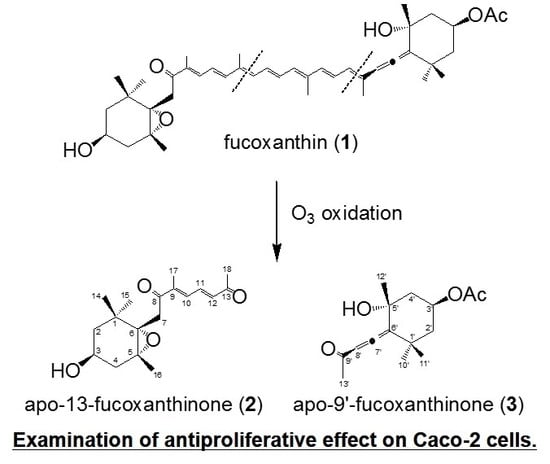
2.1. Chemistry
2.2. Biology
3. Materials and Methods
3.1. Reagents and Conditions
3.2. Synthesis of Two Six-Membered Ring Derivatives {apo-13-Fucoxanthinone (2) and apo-9′-Fucoxanthinone (3)}
3.3. Antiproliferation Activity of Caco-2 Cells by apo-13-Fucoxanthinone (2) and apo-9′-Fucoxanthinone (3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, S.M.; Jung, Y.J.; Kwon, O.N.; Cha, K.H.; Um, B.H.; Chung, D.; Pan, C.H. A potential commercial source of fucoxanthin extracted from the microalga Phaeodactylum tricornutum. Appl. Biochem. Biotechnol. 2012, 166, 1843–1855. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.; Ozaki, Y.; Hashimoto, T.; Das, S.K.; Matsushita, S.; Hirano, M.; Okada, T.; Komoto, A.; Mori, N.; Nakatsuka, M. Commercial-scale preparation of biofunctional fucoxanthin from waste parts of brown sea algae Laminalia japonica. Food Sci. Technol. Res. 2008, 14, 573–582. [Google Scholar] [CrossRef]
- Xiao, X.H.; Si, X.X.; Yuan, Z.Q.; Xu, X.F.; Li, G.K. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Ooi, T.; Hiraoka, M.; Oka, N.; Hamada, H.; Tamura, M.; Kusumi, T. Fucoxanthin and its metabolites in edible brown algae cultivated in deep seawater. Mar. Drugs 2004, 2, 63–72. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Miyashita, K. Fucoxanthin extractions of brown seaweeds and analysis of their lipid fraction in methanol. Food Sci. Technol. Res. 2012, 18, 251–257. [Google Scholar] [CrossRef]
- Jaswir, I.; Noviendri, D.; Salleh, H.M.; Taher, M.; Miyashita, K.; Ramli, N. Analysis of fucoxanthin content and purification of all-trans-fucoxanthin from Turbinaria turbinata and Sargassum plagyophyllum by SiO2 open column chromatography and reversed phase-HPLC. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 1340–1354. [Google Scholar]
- Kim, S.M.; Kang, S.W.; Kwon, O.N.; Chung, D.; Pan, C.H. Fucoxanthin as a major carotenoid in isochrysis aff. galbana: Characterization of extraction for commercial application. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 477–483. [Google Scholar] [CrossRef]
- Pasquet, V.; Cherouvrier, J.R.; Farhat, F.; Thiery, V.; Piot, J.M.; Berard, J.B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.P.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef]
- Xia, S.; Wang, K.; Wan, L.L.; Li, A.F.; Hu, Q.; Zhang, C.W. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef] [PubMed]
- Okuzumi, J.; Takahashi, T.; Yamane, T.; Kitao, Y.; Inagake, M.; Ohya, K.; Nishino, H.; Tanaka, Y. Inhibitory effects of fucoxanthin, a natural carotenoid, on N-ethyl-N′-nitro-N-nitrosoguanidine-induced mouse duodenal carcinogenesis. Cancer Lett. 1993, 68, 159–168. [Google Scholar] [CrossRef]
- Das, S.K.; Hashimoto, T.; Baba, M.; Nishino, H.; Komoto, A.; Kanazawa, K. Japanese kelp (kombu) extract suppressed the formation of aberrant crypt foci in azoxymethane challenged mouse colon. J. Clin. Biochem. Nutr. 2006, 38, 119–125. [Google Scholar] [CrossRef]
- Kotake-Nara, E.; Kushiro, M.; Zhang, H.; Sugawara, T.; Miyashita, K.; Nagao, A. Carotenoids affect proliferation of human prostate cancer cells. J. Nutr. 2001, 131, 3303–3306. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, M.; Kudo, M.; Maeda, H.; Kohno, H.; Tanaka, T.; Miyashita, K. Fucoxanthin induces apoptosis and enhances the antiproliferative effect of the PPARγ ligand, troglitazone, on colon cancer cells. Biochim. Biophys. Acta 2004, 1675, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Hashimoto, T.; Shimizu, K.; Yoshida, T.; Sakai, T.; Sowa, Y.; Komoto, A.; Kanazawa, K. Fucoxanthin induces cell cycle arrest at G0/G1 phase in human colon carcinoma cells through up-regulation of p21WAF1/Cip1. Biochim. Biophys. Acta 2005, 1726, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, H.; Imai, T.; Mase, T.; Sekiya, M.; Yokoi, K.; Hayashi, H.; Shibata, A.; Hayashi, T.; Nishikawa, M.; Suda, N.; et al. Seaweed prevents breast cancer? Jpn. J. Cancer Res. 2001, 92, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, H.; Imai, T.; Tanaka, Y.; Tsukamura, K.; Hayakawa, Y.; Kikumori, T.; Mase, T.; Itoh, T.; Nishikawa, M.; Hayashi, H.; et al. Wakame seaweed suppresses the proliferation of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. Jpn. J. Cancer Res. 1999, 90, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Okai, Y.; Higashiokai, K.; Nakamura, S. Identification of heterogenous antimutagenic activities in the extract of edible brown seaweeds, Laminaria-japonica (Makonbu) and Undaria-pinnatifida (Wakame) by the umu gene expression system in Salmonella typhimurium (TA1535/pSK1002). Mutat. Res. 1993, 303, 63–70. [Google Scholar] [CrossRef]
- Teas, J.; Vena, S.; Cone, D.L.; Irhimeh, M. The consumption of seaweed as a protective factor in the etiology of breast cancer: Proof of principle. J. Appl. Phycol. 2013, 25, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Karimian, H.; Khanabdali, R.; Razavi, M.; Firoozinia, M.; Zandi, K.; Kadir, H.A. Anticancer and antitumor potential of fucoidan and fucoxanthin, two main metabolites isolated from brown algae. Sci. World J. 2014, 2014, 768323. [Google Scholar] [CrossRef]
- Kumar, S.R.; Hosokawa, M.; Miyashita, K. Fucoxanthin: A marine carotenoid exerting anti-cancer effects by affecting multiple mechanisms. Mar. Drugs 2013, 11, 5130–5147. [Google Scholar] [CrossRef] [PubMed]
- Satomi, Y. Antitumor and cancer-preventative function of fucoxanthin: A marine carotenoid. Anticancer Res. 2017, 37, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Takahashi, N.; Kawada, T.; Miyashita, K. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int. J. Mol. Med. 2006, 18, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Nishikawa, S.; Beppu, F.; Tsukui, T.; Abe, M.; Hosokawa, A. The allenic carotenoid fucoxanthin, a novel marine nutraceutical from brown seaweeds. J. Sci. Food Agric. 2011, 91, 1166–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heo, S.J.; Yoon, W.J.; Kim, K.N.; Oh, C.; Choi, Y.U.; Yoon, K.T.; Kang, D.H.; Qian, Z.J.; Choi, I.W.; Jung, W.K. Anti-inflammatory effect of fucoxanthin derivatives isolated from Sargassum siliquastrum in lipopolysaccharide-stimulated RAW 264.7 macrophage. Food Chem. Toxicol. 2012, 50, 3336–3342. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Matsubara, K.; Akagi, R.; Mori, M.; Hirata, T. Antiangiogenic activity of brown algae fucoxanthin and its deacetylated product, fucoxanthinol. J. Agric. Food. Chem. 2006, 54, 9805–9810. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Sugawara, T.; Nagao, A. Antiproliferative effect of neoxanthin and fucoxanthin on cultured cells. Fish. Sci. 2005, 71, 459–461. [Google Scholar] [CrossRef]
- Komba, S.; Kotake-Nara, E.; Machida, S. Fucoxanthin derivatives: Synthesis and their chemical properties. J. Oleo Sci. 2015, 64, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Kim, E.A.; Kim, S.Y.; Ye, B.R.; Kim, J.; Ko, S.C.; Lee, W.W.; Kim, K.N.; Choi, I.W.; Jung, W.K.; Heo, S.J. Anti-inflammatory effect of Apo-9′-fucoxanthinone via inhibition of MAPKs and NF-κB signaling pathway in LPS-stimulated RAW 264.7 macrophages and zebrafish model. Int. Immunopharmacol. 2018, 59, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Chae, D.; Manzoor, Z.; Kim, S.C.; Kim, S.; Oh, T.H.; Yoo, E.S.; Kang, H.K.; Hyun, J.W.; Lee, N.H.; Ko, M.H.; et al. Apo-9′-fucoxanthinone, isolated from Sargassum muticum, inhibits CpG-induced inflammatory response by attenuating the mitogen-activated protein kinase pathway. Mar. Drugs 2013, 11, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Ham, Y.M.; Lee, W.J.; Lee, N.H.; Hyun, C.G. Anti-inflammatory effects of apo-9′-fucoxanthinone from the brown alga, Sargassum muticum. DARU J. Pharm. Sci. 2013, 21, 62. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.I.; Yoo, E.S.; Hyun, J.W.; Koh, Y.S.; Lee, N.H.; Ko, M.H.; Ko, C.S.; Kang, H.K. Promotion effect of apo-9′-fucoxanthinone from Sargassum muticum on hair growth via the activation of Wnt/β-catenin and VEGF-R2. Biol. Pharm. Bull. 2016, 39, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Kushiro, M.; Zhang, H.; Nara, E.; Ono, H.; Nagao, A. Lysophosphatidylcholine enhances carotenoid uptake from mixed micelles by Caco-2 human intestinal cells. J. Nutr. 2001, 131, 2921–2927. [Google Scholar] [CrossRef] [PubMed]
- Kotake-Nara, E.; Hase, M.; Kobayashi, M.; Nagao, A. 3′-Hydroxy-ε,ε-caroten-3-one inhibits the differentiation of 3T3-L1 cells to adipocytes. Biosci. Biotechnol. Biochem. 2016, 80, 518–523. [Google Scholar] [CrossRef] [PubMed]



| Comp. 2 | 1H | 13C |
|---|---|---|
| C-1 | - | 35.1 |
| CH2-2 | 1.34, 1.50 | 47.2 |
| CH-3 | 3.81 | 64.2 |
| CH2-4 | 1.79, 2.31 | 41.6 |
| C-5 | - | 66.1 |
| C-6 | - | 66.7 |
| CH2-7 | 2.61, 3.63 | 41.4 |
| C-8 | - | 198.1 |
| C-9 | - | 143.1 |
| CH-10 | 7.05 | 134.5 |
| CH-11 | 7.47 | 136.7 |
| CH-12 | 6.46 | 135.6 |
| C-13 | - | 197.7 |
| CH3-14 | 1.03 | 24.9 |
| CH3-15 | 0.94 | 28.1 |
| CH3-16 | 1.21 | 21.0 |
| CH3-17 | 2.03 | 12.5 |
| CH3-18 | 2.35 | 28.2 |
| Comp. 3 | 1H | 13C |
|---|---|---|
| C-1′ | - | 36.0 |
| CH2-2′ | 1.44, 2.02 | 45.00 |
| CH-3′ | 5.39 | 67.4 |
| CH2-4′ | 1.53, 2.33 | 45.04 |
| C-5′ | - | 72.0 |
| C-6′ | - | 118.5 |
| C-7′ | - | 209.5 |
| CH-8′ | 5.87 | 100.9 |
| C-9′ | - | 198.0 |
| CH3-10′ | 1.16 | 31.6 |
| CH3-11′ | 1.43 | 28.9 |
| CH3-12′ | 1.43 | 30.8 |
| CH3-13′ | 2.19 | 26.4 |
| CH3-Ac | 2.05 | 21.3 |
| C-Ac | - | 170.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komba, S.; Kotake-Nara, E.; Tsuzuki, W. Degradation of Fucoxanthin to Elucidate the Relationship between the Fucoxanthin Molecular Structure and Its Antiproliferative Effect on Caco-2 Cells. Mar. Drugs 2018, 16, 275. https://doi.org/10.3390/md16080275
Komba S, Kotake-Nara E, Tsuzuki W. Degradation of Fucoxanthin to Elucidate the Relationship between the Fucoxanthin Molecular Structure and Its Antiproliferative Effect on Caco-2 Cells. Marine Drugs. 2018; 16(8):275. https://doi.org/10.3390/md16080275
Chicago/Turabian StyleKomba, Shiro, Eiichi Kotake-Nara, and Wakako Tsuzuki. 2018. "Degradation of Fucoxanthin to Elucidate the Relationship between the Fucoxanthin Molecular Structure and Its Antiproliferative Effect on Caco-2 Cells" Marine Drugs 16, no. 8: 275. https://doi.org/10.3390/md16080275
APA StyleKomba, S., Kotake-Nara, E., & Tsuzuki, W. (2018). Degradation of Fucoxanthin to Elucidate the Relationship between the Fucoxanthin Molecular Structure and Its Antiproliferative Effect on Caco-2 Cells. Marine Drugs, 16(8), 275. https://doi.org/10.3390/md16080275