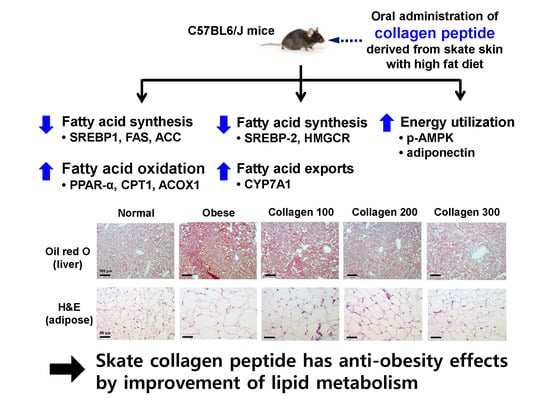
Anti-Obesity Effects of Collagen Peptide Derived from Skate (Raja kenojei) Skin Through Regulation of Lipid Metabolism
Abstract
:1. Introduction
2. Results
2.1. Effect of Skate Collagen Peptide on Body Weight Gain and Changes in Adipose Tissue Weight and Size
2.2. Effect of Skate Collagen Peptide on Lipid Levels in the Plasma and Hepatic Tissue
2.3. Effect of Skate Collagen Peptide on β-Oxidation in the Liver
2.4. Effect of Skate Collagen Peptide on Fatty Acid Synthesis in the Liver
2.5. Effect of Skate Collagen Peptide on Cholesterol Metabolism in the Liver
2.6. Effect of Skate Collagen Peptide on AMPK in the Liver
2.7. Effect of Skate Collagen Peptide on Adiponectin and Leptin Levels
3. Discussion
4. Materials and Methods
4.1. Animals and Diets
4.2. Plasma Lipid, Aminotransferase, and Adipokines Levels
4.3. Hepatic Lipid Concentration
4.4. Western Blot Analysis
4.5. Histological Analysis
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Savage, D.B.; Petersen, K.F.; Shulman, G.I. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol. Rev. 2007, 87, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Viljanen, A.P.; Iozzo, P.; Borra, R.; Kankaanpää, M.; Karmi, A.; Lautamäki, R.; Järvisalo, M.; Parkkola, R.; Ronnemaa, T.; Guiducci, L. Effect of weight loss on liver free fatty acid uptake and hepatic insulin resistance. J. Clin. Endocrinol. Metab. 2009, 94, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Jongjareonrak, A.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Isolation and characterisation of acid and pepsin-solubilised collagens from the skin of brownstripe red snapper (Lutjanus vitta). Food Chem. 2005, 93, 475–484. [Google Scholar] [CrossRef]
- Han, S.-H.; Uzawa, Y.; Moriyama, T.; Kawamura, Y. Effect of collagen and collagen peptides from bluefin tuna abdominal skin on cancer cells. Health 2011, 3, 129–134. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Ri, S.X.; Hideyuki, K.; Koretaro, T. Characterization of molecular species of collagen in scallop mantle. Food Chem. 2007, 102, 1187–1191. [Google Scholar] [Green Version]
- Park, S.-H.; Lee, J.-K.; Jeon, J.-K.; Byun, H.-G. Characterization of a collagenase-1 inhibitory peptide purified from skate dipturus chilensis skin. Korean J. Fish. Aquat. Sci. 2011, 44, 456–463. [Google Scholar] [CrossRef]
- Zhuang, Y.; Sun, L.; Zhao, X.; Wang, J.; Hou, H.; Li, B. Antioxidant and melanogenesis-inhibitory activities of collagen peptide from jellyfish (Rhopilema esculentum). J. Sci. Food Agric. 2009, 89, 1722–1727. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.-K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.; Wu, P.; Wu, C.; Shiau, C. Studies on antioxidative activities of hydrolysates from fish scales collagen of tilapia. J. Taiwan Fish. Res 2008, 15, 99–108. [Google Scholar]
- Tanaka, M.; Koyama, Y.-I.; Nomura, Y. Effects of collagen peptide ingestion on UV-B-induced skin damage. Biosci. Biotechnol. Biochem. 2009, 73, 930–932. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, A.; Morimura, S.; Guo, H.-C.; Shigematsu, T.; Kida, K.; Uemura, Y. Production of angiotensin I converting enzyme inhibitory peptides from sea bream scales. Process Biochem. 2004, 39, 1195–1200. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, Z.; Xu, S. Macroporous resin purification of grass carp fish (Ctenopharyngodon idella) scale peptides with in vitro angiotensin-I converting enzyme (ACE) inhibitory ability. Food Chem. 2009, 117, 387–392. [Google Scholar] [CrossRef]
- Zolotarev, Y.A.; Badmaeva, K.; Bakaeva, Z.; Samonina, G.; Kopylova, G.; Dadayan, A.; Zverkov, Y.B.; Garanin, S.; Vaskovsky, B.; Ashmarin, I. Short peptide fragments with antiulcer activity from a collagen hydrolysate. Russ. J. Bioorg. Chem. 2006, 32, 174–178. [Google Scholar] [CrossRef]
- Bello, A.E.; Oesser, S. Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: A review of the literature. Curr. Med. Res. Opin. 2006, 22, 2221–2232. [Google Scholar] [CrossRef] [PubMed]
- Berillis, P. Marine collagen: Extraction and applications. In Research Trends in Biochemistry, Molecular Biology and Microbiology; Madhukar, S., Ed.; SM Group: Dover, DE, USA, 2015; pp. 1–13. [Google Scholar]
- Liang, J.; Pei, X.-R.; Wang, N.; Zhang, Z.-F.; Wang, J.-B.; Li, Y. Marine collagen peptides prepared from chum salmon (Oncorhynchus keta) skin extend the life span and inhibit spontaneous tumor incidence in sprague-dawley rats. J. Med. Food 2010, 13, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Nagai, T.; Yamashita, E.; Taniguchi, K.; Kanamori, N.; Suzuki, N. Isolation and characterisation of collagen from the outer skin waste material of cuttlefish (Sepia lycidas). Food Chem. 2001, 72, 425–429. [Google Scholar] [CrossRef]
- Heu, M.S.; Lee, J.H.; Kim, H.J.; Jee, S.J.; Lee, J.S.; Jeon, Y.-J.; Shahidi, F.; Kim, J.-S. Characterization of acid-and pepsin-soluble collagens from flatfish skin. Food Sci. Biotechnol. 2010, 19, 27–33. [Google Scholar] [CrossRef]
- Nagai, T.; Araki, Y.; Suzuki, N. Collagen of the skin of ocellate puffer fish (Takifugu rubripes). Food Chem. 2002, 78, 173–177. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Kishimura, H.; Shahidi, F. Isolation and characterisation of collagen from the skin of brownbanded bamboo shark (Chiloscyllium punctatum). Food Chem. 2010, 119, 1519–1526. [Google Scholar] [CrossRef]
- Sadowska, M.; Kołodziejska, I.; Niecikowska, C. Isolation of collagen from the skins of Baltic cod (Gadus morhua). Food Chem. 2003, 81, 257–262. [Google Scholar] [CrossRef]
- Duan, R.; Zhang, J.; Du, X.; Yao, X.; Konno, K. Properties of collagen from skin, scale and bone of carp (Cyprinus carpio). Food Chem. 2009, 112, 702–706. [Google Scholar] [CrossRef]
- Singh, P.; Benjakul, S.; Maqsood, S.; Kishimura, H. Isolation and characterisation of collagen extracted from the skin of striped catfish (Pangasianodon hypophthalmus). Food Chem. 2011, 124, 97–105. [Google Scholar] [CrossRef]
- Nagai, T.; Suzuki, N. Preparation and partial characterization of collagen from paper nautilus (Argonauta argo, Linnaeus) outer skin. Food Chem. 2002, 76, 149–153. [Google Scholar] [CrossRef]
- Tziveleka, L.A.; Ioannou, E.; Tsiourvas, D.; Berillis, P.; Foufa, E.; Roussis, V. Collagen from the marine sponges Axinella cannabina and Suberites carnosus: Isolation and morphological, biochemical, and biophysical characterization. Mar. Drugs 2017, 15, 152. [Google Scholar] [CrossRef] [PubMed]
- Baek, J.M.; Kang, K.H.; Kim, S.H.; Noh, J.S.; Jeong, K.S. Development of high functional collagen peptide materials using skate skins. J. Environ. Sci. Int. 2016, 25, 579–588. [Google Scholar] [CrossRef]
- Lee, H.J.; Woo, M.; Song, Y.O.; Noh, J.S. Inhibitory effect of skate skin collagen on hepatic lipid accumulation through regulation of lipid metabolism. J. Korean Soc. Food Sci. Nutr. 2018, 47, 235–242. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, O.-K.; Yoon, H.-G.; Park, J.; You, Y.; Kim, K.; Lee, Y.-H.; Choi, K.-C.; Lee, J.; Jun, W. Anti-obesity effect of extract from fermented Curcuma longa L. through regulation of adipogenesis and lipolysis pathway in high-fat diet-induced obese rats. Food Nutr. Res. 2016, 60, 30428. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Popkin, B.M. Dietary fat intake does affect obesity! Am. J. Clin. Nutr. 1998, 68, 1157–1173. [Google Scholar] [CrossRef] [PubMed]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [PubMed]
- Hafidi, M.E.; Pérez, I.; Zamora, J.; Soto, V.; Carvajal-Sandoval, G.; Banos, G. Glycine intake decreases plasma free fatty acids, adipose cell size, and blood pressure in sucrose-fed rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R1387–R1393. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Perez, J.; Alarcon-Aguilar, F.; Blancas-Flores, G.; Campos-Sepulveda, A.; Roman-Ramos, R.; Garcia-Macedo, R.; Cruz, M. Glycine regulates inflammatory markers modifying the energetic balance through PPAR and UCP-2. Biomed. Pharmacother. 2010, 64, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xie, Y.; Pei, X.; Yang, R.; Zhang, Z.; Li, Y. The lipid-lowering and antioxidative effects of marine collagen peptides. Chin. J. Prev. Med. 2008, 42, 226–230. [Google Scholar]
- Saito, M.; Kiyose, C.; Higuchi, T.; Uchida, N.; Suzuki, H. Effect of collagen hydrolysates from salmon and trout skins on the lipid profile in rats. J. Agric. Food Chem. 2009, 57, 10477–10482. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Seong, J.H.; Lee, Y.G.; Xie, C.L.; Choi, W.S.; Kim, S.H.; Yoon, H.D. Effect of low-molecular-weight collagen peptide extract isolated from scales of the flathead mullet (Mugil cephalus) on lipid metabolism in hyperlipidemic rats. Korean J. Food Preserv. 2009, 16, 938–945. [Google Scholar]
- Zhu, C.-F.; Li, G.-Z.; Peng, H.-B.; Zhang, F.; Chen, Y.; Li, Y. Treatment with marine collagen peptides modulates glucose and lipid metabolism in Chinese patients with type 2 diabetes mellitus. Appl. Physiol. Nutr. Metab. 2010, 35, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.R.; Portugal, L.R.; Cara, D.C.; Vieira, E.C.; Alvarez-Leite, J.I. Gelatin intake increases the atheroma formation in apoE knock out mice. Atherosclerosis 2001, 154, 71–77. [Google Scholar] [CrossRef]
- Strable, M.S.; Ntambi, J.M. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.K.; Hashimoto, T. Peroxisomal β-oxidation and peroxisome proliferator–activated receptor α: An adaptive metabolic system. Annu. Rev. Nutr. 2001, 21, 193–230. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Kim, I.H.; Choi, J.W.; Lee, M.K.; Nam, T.J. The anti-obesity effects of a tuna peptide on 3T3-L1 adipocytes are mediated by the inhibition of the expression of lipogenic and adipogenic genes and by the activation of the Wnt/β-catenin signaling pathway. Int. J. Mol. Med. 2015, 36, 327–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lage, R.; Diéguez, C.; Vidal-Puig, A.; López, M. AMPK: A metabolic gauge regulating whole-body energy homeostasis. Trends Mol. Med. 2008, 14, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.-J. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols stimulate AMP-activated protein kinase, lower lipids, and inhibit accelerated atherosclerosis in diabetic LDL receptor–deficient mice. Diabetes 2006, 55, 2180–2191. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Guerra, J.F.; Maciel, P.S.; de Abreu, I.C.M.E.; Pereira, R.R.; Silva, M.; de Morais Cardoso, L.; Pinheiro-Sant’Ana, H.M.; de Lima, W.G.; Silva, M.E.; Pedrosa, M.L. Dietary açai attenuates hepatic steatosis via adiponectin-mediated effects on lipid metabolism in high-fat diet mice. J. Funct. Foods 2015, 14, 192–202. [Google Scholar] [CrossRef]
- Park, K.-G.; Park, K.S.; Kim, M.-J.; Kim, H.-S.; Suh, Y.-S.; Ahn, J.D.; Park, K.-K.; Chang, Y.-C.; Lee, I.-K. Relationship between serum adiponectin and leptin concentrations and body fat distribution. Diabetes Res. Clin. Pract. 2004, 63, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Macedo, R.; Sanchez-Muñoz, F.; Almanza-Perez, J.C.; Duran-Reyes, G.; Alarcon-Aguilar, F.; Cruz, M. Glycine increases mRNA adiponectin and diminishes pro-inflammatory adipokines expression in 3T3-L1 cells. Eur. J. Pharmacol. 2008, 587, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Sakai, H.; Ishii, Y.; Shirai, K. Preparation and some properties of type I collagen from fish scales. Biosci. Biotechnol. Biochem. 1996, 60, 2092–2094. [Google Scholar] [CrossRef] [PubMed]
- USFDA. Guidance for industry. In Estimating the Maximum Safe Starting Dose in Adult Healthy Volunteer; US Food and Drug Administration: Rockville, MD, USA, 2005. [Google Scholar]
- Hariri, N.; Thibault, L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010, 23, 270–299. [Google Scholar] [CrossRef] [PubMed]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [PubMed]
- Folch, J.; Lees, M.; Sloane-Stanley, G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Jung, K.; Hong, S.H.; Kim, M.; Han, J.-S.; Jang, M.-S.; Song, Y.O. Antiatherogenic effects of Korean cabbage kimchi with added short arm octopus. Food Sci. Biotechnol. 2015, 24, 249–255. [Google Scholar] [CrossRef]






| Group (1) | Leptin | Adiponectin |
|---|---|---|
| NOR | 54.6 ± 5.0 c | 198.6 ± 14.1 d |
| CON | 122.6 ± 34.9 a | 214.4 ± 46.3 c,d |
| CL100 | 98.0 ± 24.6 a,b | 236.3 ± 21.5 b,c |
| CL200 | 97.0 ± 22.9 a,b | 263.8 ± 35.3 a,b |
| CL300 | 94.0 ± 15.2 b | 281.1 ± 17.9 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, M.; Song, Y.O.; Kang, K.-H.; Noh, J.S. Anti-Obesity Effects of Collagen Peptide Derived from Skate (Raja kenojei) Skin Through Regulation of Lipid Metabolism. Mar. Drugs 2018, 16, 306. https://doi.org/10.3390/md16090306
Woo M, Song YO, Kang K-H, Noh JS. Anti-Obesity Effects of Collagen Peptide Derived from Skate (Raja kenojei) Skin Through Regulation of Lipid Metabolism. Marine Drugs. 2018; 16(9):306. https://doi.org/10.3390/md16090306
Chicago/Turabian StyleWoo, Minji, Yeong Ok Song, Keon-Hee Kang, and Jeong Sook Noh. 2018. "Anti-Obesity Effects of Collagen Peptide Derived from Skate (Raja kenojei) Skin Through Regulation of Lipid Metabolism" Marine Drugs 16, no. 9: 306. https://doi.org/10.3390/md16090306
APA StyleWoo, M., Song, Y. O., Kang, K. -H., & Noh, J. S. (2018). Anti-Obesity Effects of Collagen Peptide Derived from Skate (Raja kenojei) Skin Through Regulation of Lipid Metabolism. Marine Drugs, 16(9), 306. https://doi.org/10.3390/md16090306