Spinochrome D Attenuates Doxorubicin-Induced Cardiomyocyte Death via Improving Glutathione Metabolism and Attenuating Oxidative Stress
Abstract
:1. Introduction
2. Results
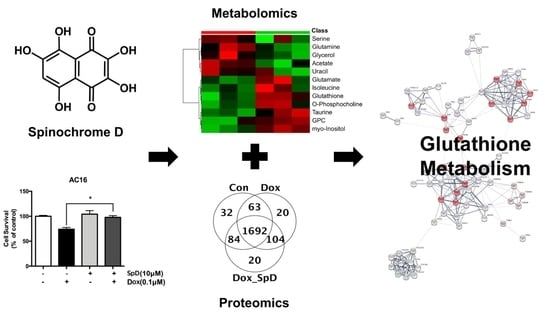
2.1. SpD Protected AC16 Cells against Doxorubicin Cytotoxicity
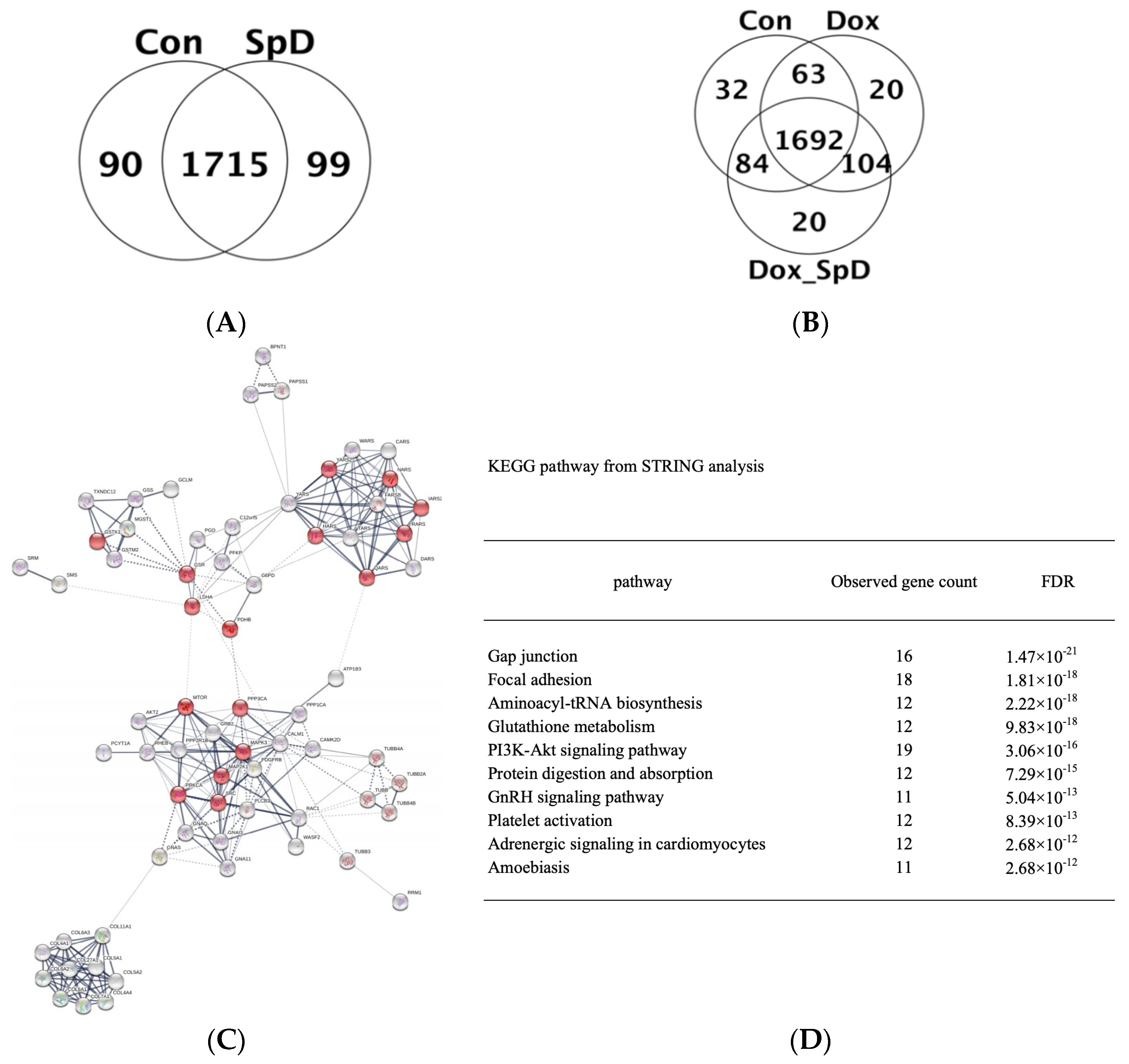
2.2. Liquid Chromatography–Mass Spectrometry-MS (LC-MS/MS)-Based Proteomics Analyses of SpD/Doxorubicin-Treated AC16 Cells
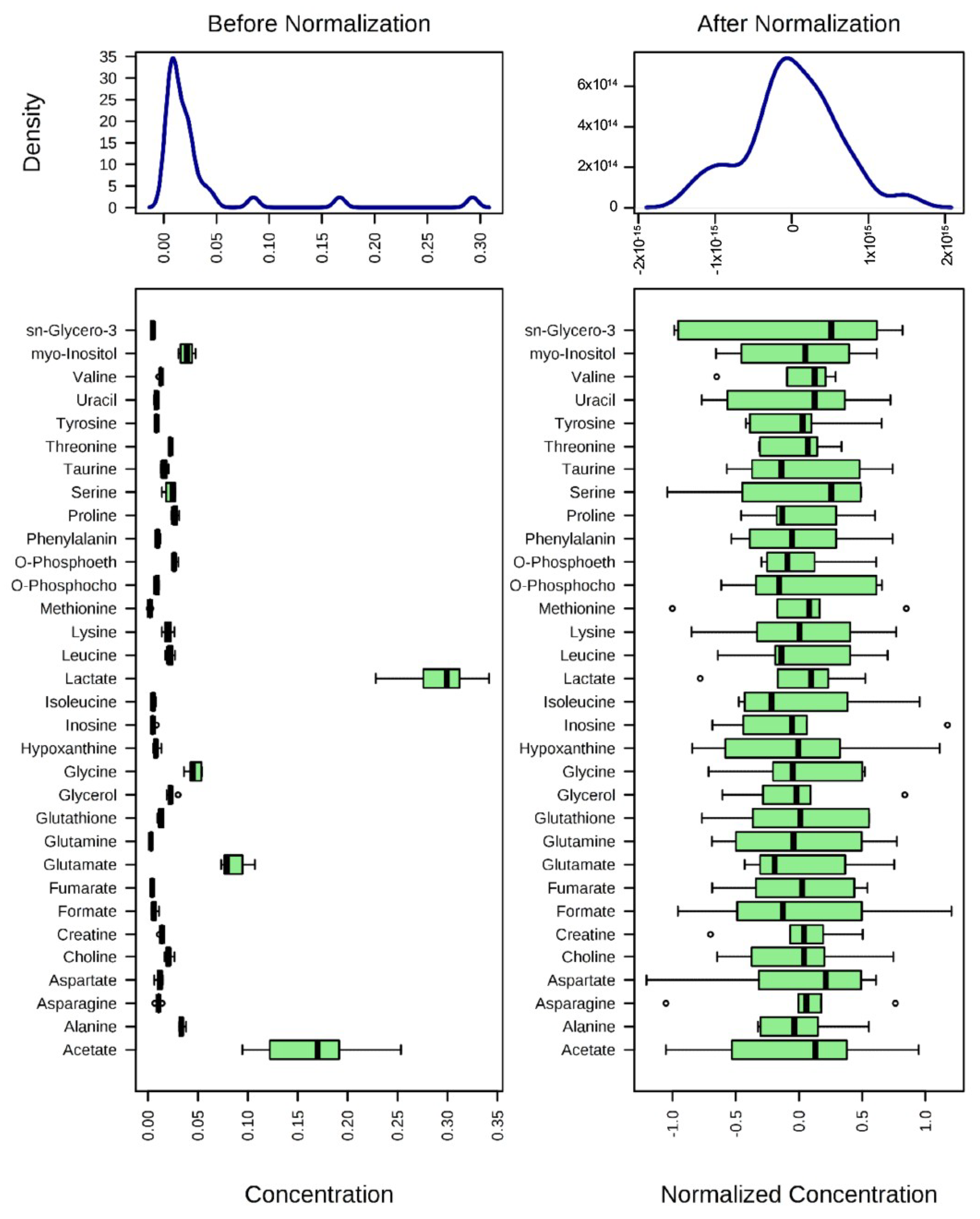
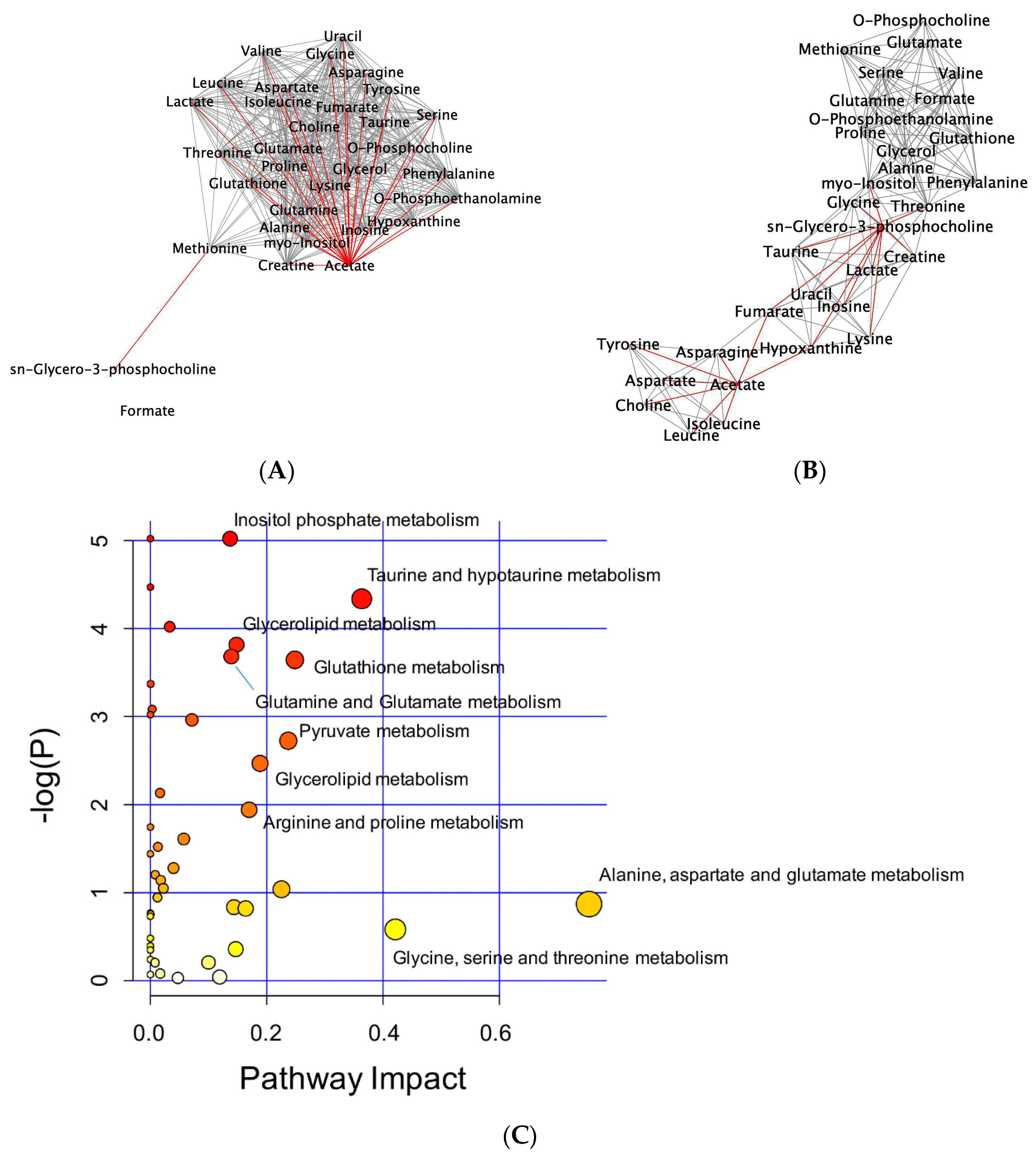
2.3. 1H-NMR Mediated Metabolomics Analysis of SpD-Treated AC16 Cells
2.4. Glutathione Metabolism in AC16 Cells Was Significantly Influenced by SpD Treatment
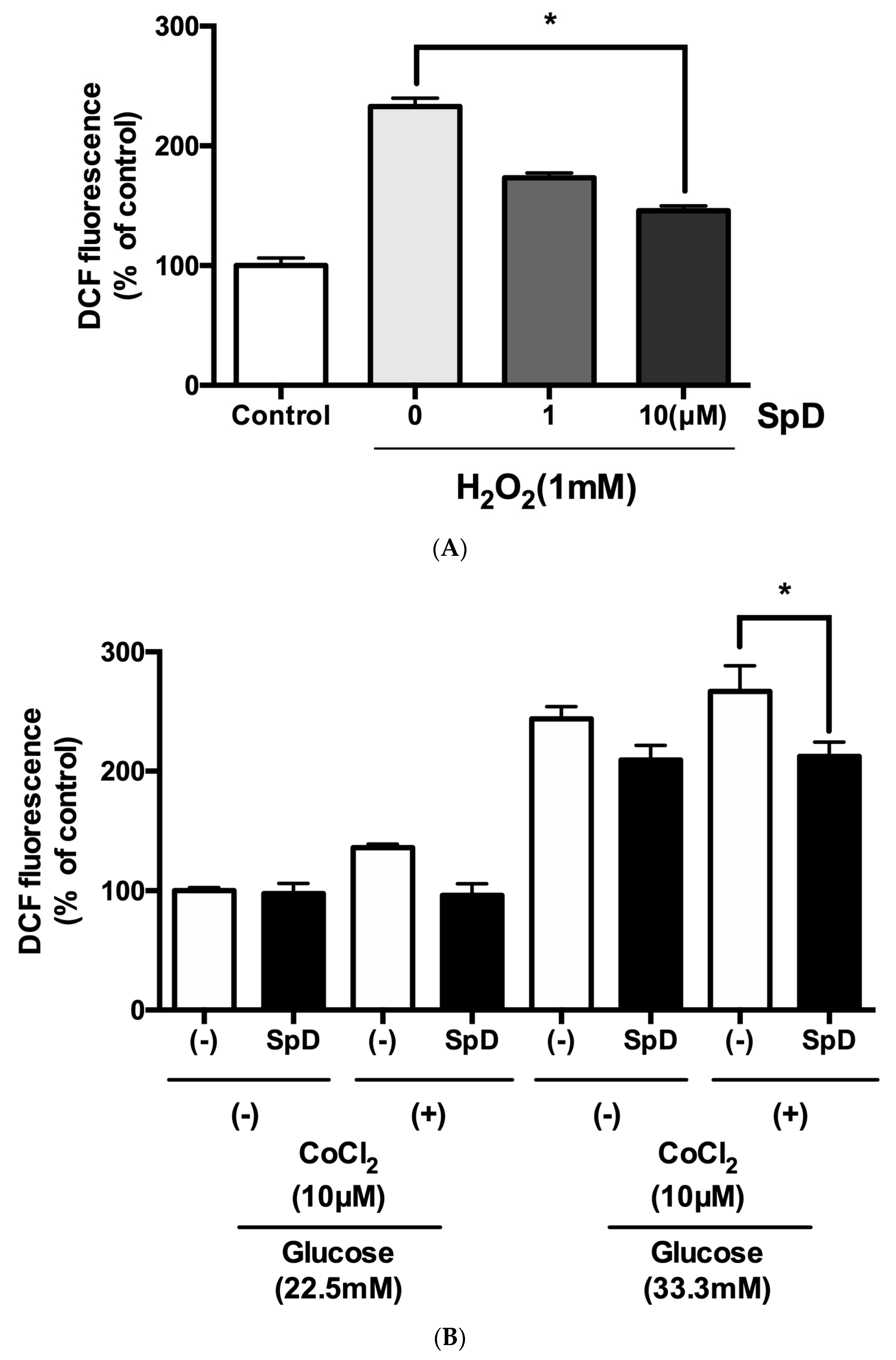
2.5. SpD Functioned as an Antioxidant in AC16 Cells
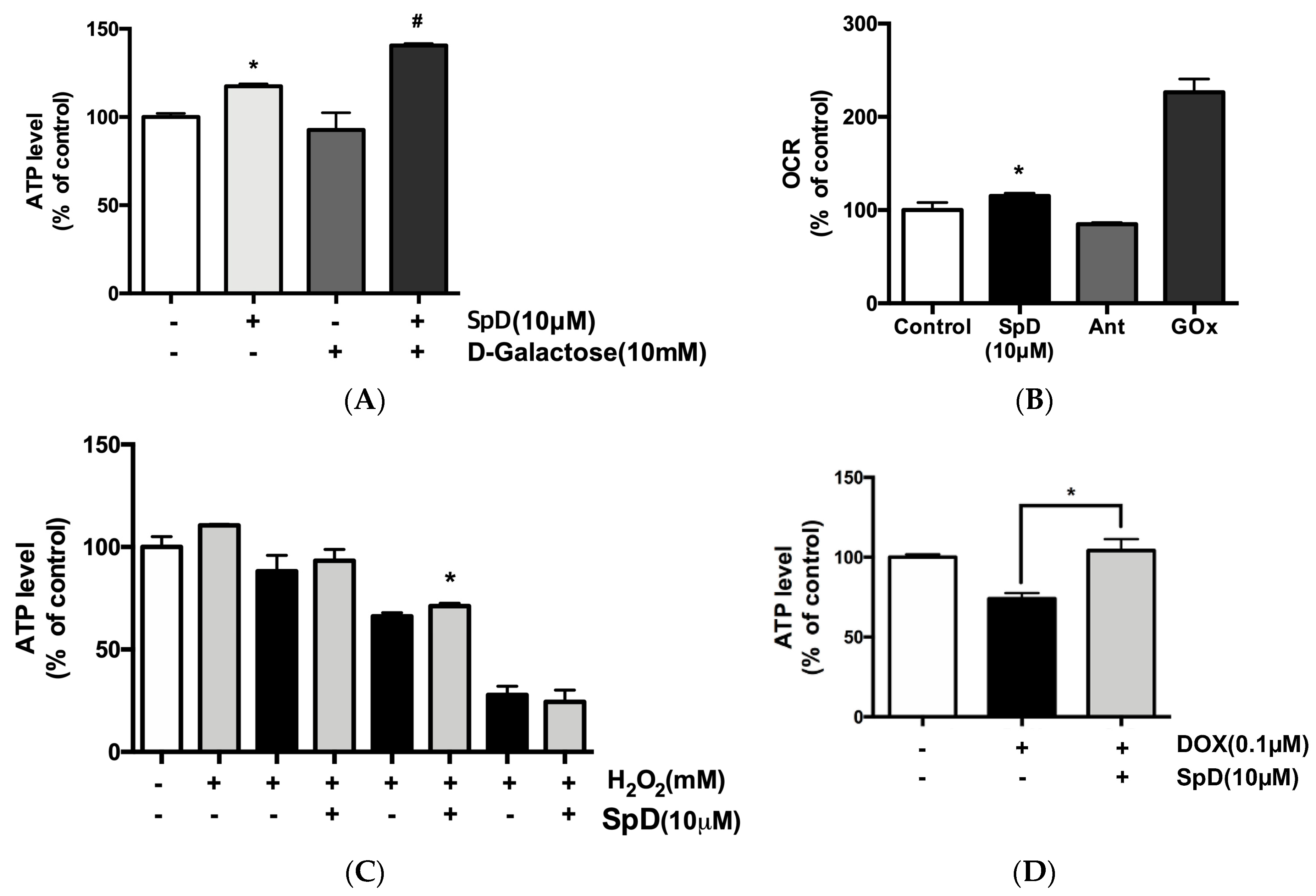
2.6. SpD Increased Mitochondrial ATP Production and Oxygen Consumption
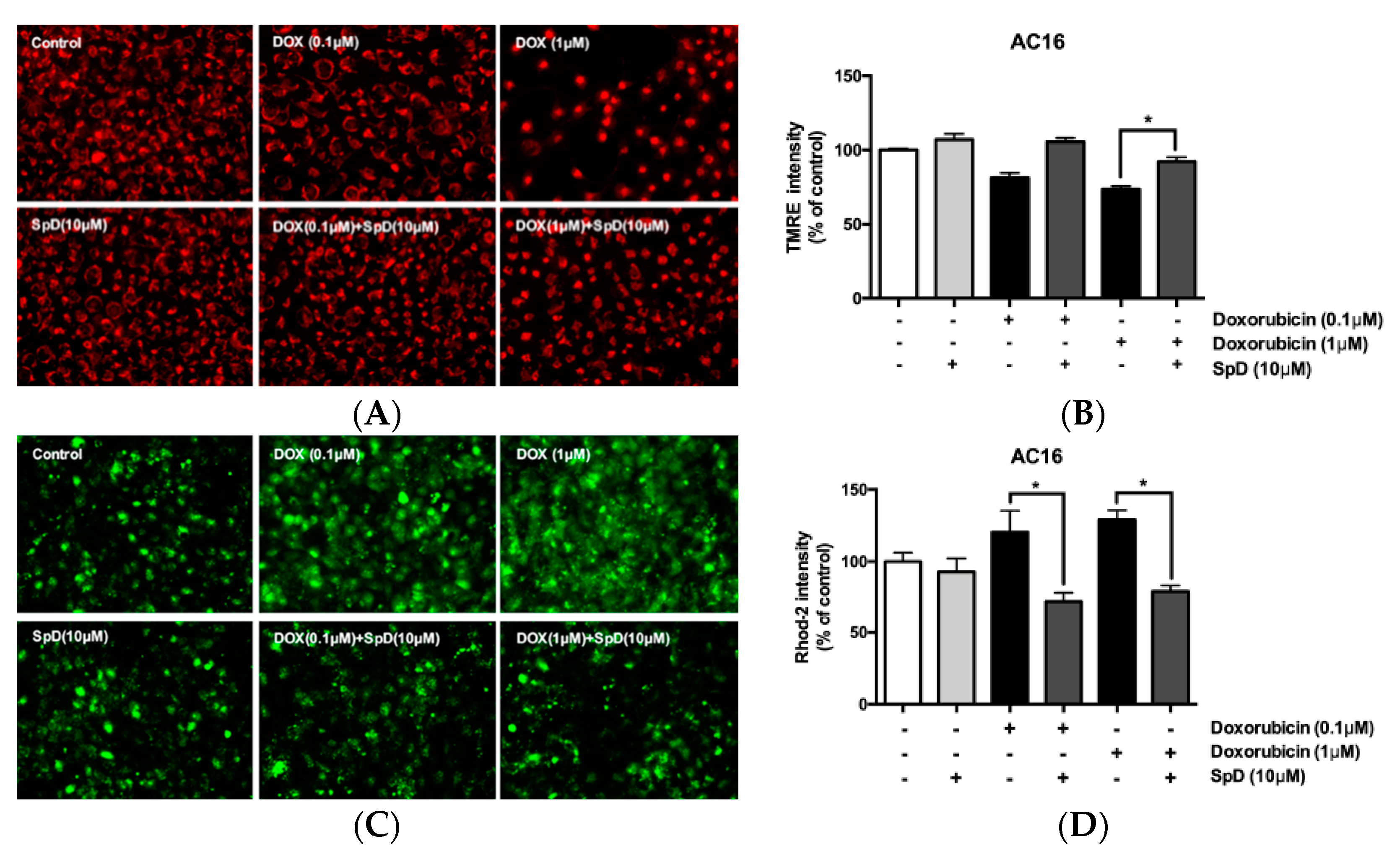
2.7. SpD Protected against Doxorubicin-Induced Mitochondrial Damage in AC16 Cells
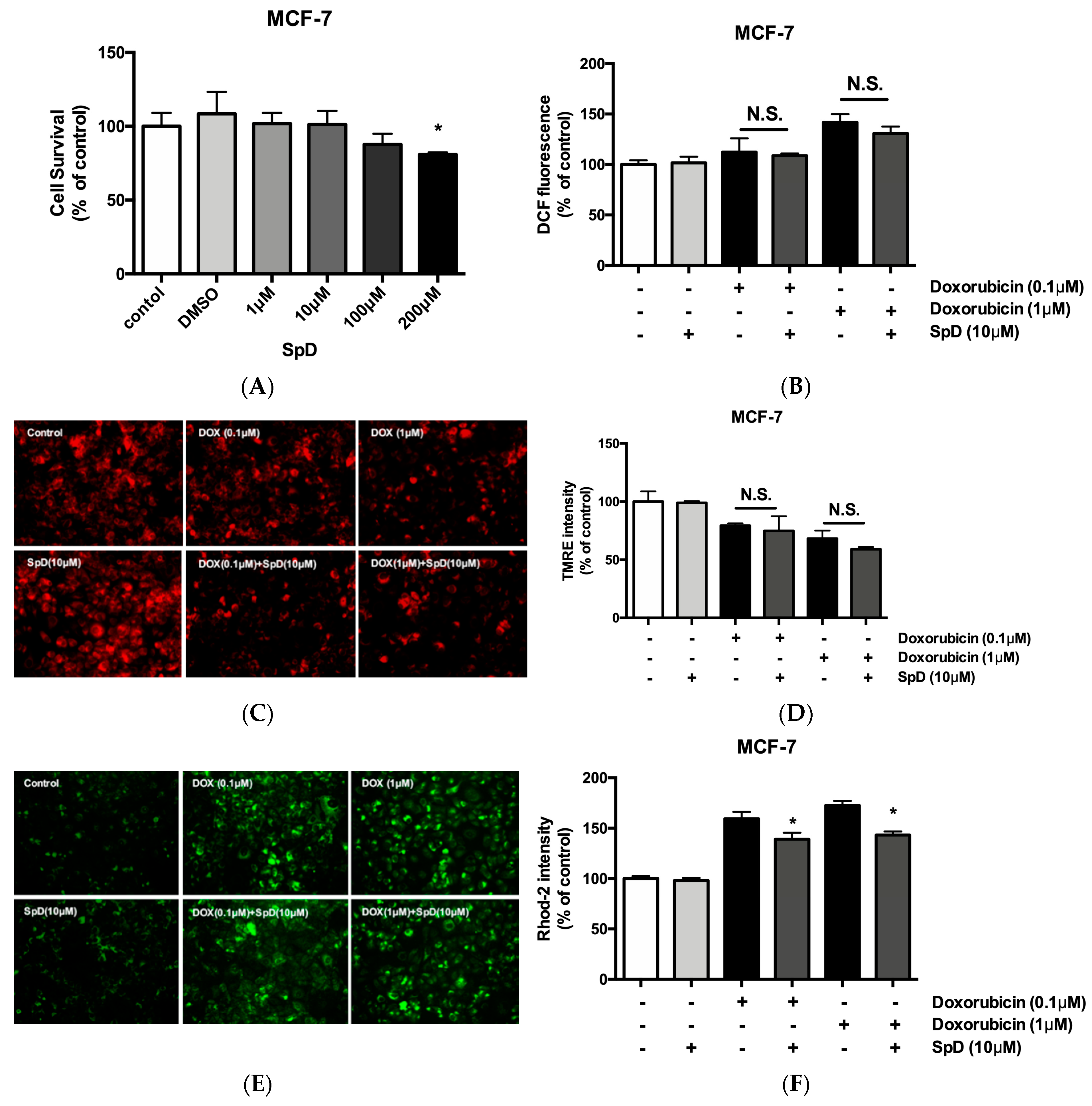
2.8. SpD Did Not Interfere with the Anticancer Effects of Doxorubicin in MCF-7 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.1.1. Cell Viability Assay
4.1.2. Intracellular ATP Measurement
4.1.3. Oxygen Consumption Ratio (OCR) Measurement
4.1.4. Intracellular ROS Levels
4.1.5. Measurement of Mitochondrial Membrane Potential
4.1.6. Measurement of Mitochondrial Calcium
4.1.7. Cell Migration Assay
4.2. LC-MS/MS Analysis and Database Searching
4.3. 1H-NMR Metabolomics
4.4. Pathway Enrichment Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lebedev, A.V.; Levitskaya, E.L.; Tikhonova, E.V.; Ivanova, M.V. Antioxidant properties, autooxidation, and mutagenic activity of echinochrome a compared with its etherified derivative. Biochemistry 2001, 66, 885–893. [Google Scholar] [PubMed]
- Lebedev, A.V.; Ivanova, M.V.; Levitsky, D.O. Echinochrome, a naturally occurring iron chelator and free radical scavenger in artificial and natural membrane systems. Life Sci. 2005, 76, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, H.K.; Song, I.S.; Lee, S.J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoryev, S.A.; Stonik, V.A.; et al. Echinochrome A protects mitochondrial function in cardiomyocytes against cardiotoxic drugs. Mar. Drugs 2014, 12, 2922–2936. [Google Scholar] [CrossRef]
- Pozharitskaya, O.N.; Shikov, A.N.; Makarova, M.N.; Ivanova, S.A.; Kosman, V.M.; Makarov, V.G.; Bazgier, V.; Berka, K.; Otyepka, M.; Ulrichova, J. Antiallergic effects of pigments isolated from green sea urchin (Strongylocentrotus droebachiensis) shells. Planta Med. 2013, 79, 1698–1704. [Google Scholar] [CrossRef]
- Anderson, H.A.; Mathieson, J.W.; Thomson, R.H. Distribution of spinochrome pigments in echinoids. Comp. Biochem. Physiol. 1969, 28, 333–345. [Google Scholar] [CrossRef]
- Nagaoka, S.; Shiraishi, J.; Utsuyama, M.; Seki, S.; Takemura, T.; Kitagawa, M.; Sawabe, M.; Takubo, K.; Hirokawa, K. Poor prognosis of colorectal cancer in patients over 80 years old is associated with down-regulation of tumor suppressor genes. J. Clin. Gastroenterol. 2003, 37, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Balaneva, N.N.; Shestak, O.P.; Anufriev, V.F.; Novikov, V.L. Synthesis of Spinochrome D, A Metabolite of Various Sea-Urchin Species. Chem. Nat. Compd. 2016, 52, 213–217. [Google Scholar] [CrossRef]
- Arcamone, F.; Cassinelli, G.; Fantini, G.; Grein, A.; Orezzi, P.; Pol, C.; Spalla, C. Adriamycin, 14-hydroxydaunomycin, a new antitumor antibiotic from S. peucetius var. caesius. Biotechnol. Bioeng. 1969, 11, 1101–1110. [Google Scholar] [CrossRef]
- Cortes-Funes, H.; Coronado, C. Role of anthracyclines in the era of targeted therapy. Cardiovasc. Toxicol. 2007, 7, 56–60. [Google Scholar] [CrossRef]
- Takemura, G.; Fujiwara, H. Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog. Cardiovasc. Dis. 2007, 49, 330–352. [Google Scholar] [CrossRef]
- Bachur, N.R.; Gee, M.V.; Friedman, R.D. Nuclear catalyzed antibiotic free radical formation. Cancer Res. 1982, 42, 1078–1081. [Google Scholar] [PubMed]
- Sinha, B.K.; Katki, A.G.; Batist, G.; Cowan, K.H.; Myers, C.E. Adriamycin-stimulated hydroxyl radical formation in human breast tumor cells. Biochem. Pharmacol. 1987, 36, 793–796. [Google Scholar] [CrossRef]
- Lefrak, E.A.; Pitha, J.; Rosenheim, S.; Gottlieb, J.A. A clinicopathologic analysis of adriamycin cardiotoxicity. Cancer 1973, 32, 302–314. [Google Scholar] [CrossRef] [Green Version]
- Franco, Y.L.; Vaidya, T.R.; Ait-Oudhia, S. Anticancer and cardio-protective effects of liposomal doxorubicin in the treatment of breast cancer. Breast Cancer 2018, 10, 131–141. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Siveski-Iliskovic, N.; Hill, M.; Chow, D.A.; Singal, P.K. Probucol protects against adriamycin cardiomyopathy without interfering with its antitumor effect. Circulation 1995, 91, 10–15. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Kassem, H.H. Protective effect of nebivolol on doxorubicin-induced cardiotoxicity in rats. Arch. Med. Sci. 2018, 14, 1450–1458. [Google Scholar] [CrossRef]
- Studneva, I.; Palkeeva, M.; Veselova, O.; Molokoedov, A.; Ovchinnikov, M.; Sidorova, M.; Pisarenko, O. Protective Effects of a Novel Agonist of Galanin Receptors Against Doxorubicin-Induced Cardiotoxicity in Rats. Cardiovasc. Toxicol. 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pilco-Ferreto, N.; Calaf, G.M. Influence of doxorubicin on apoptosis and oxidative stress in breast cancer cell lines. Int. J. Oncol. 2016, 49, 753–762. [Google Scholar] [CrossRef]
- Agafonova, I.G.; Kotel’nikov, V.N.; Mischenko, N.P.; Kolosova, N.G. Evaluation of effects of histochrome and mexidol on structural and functional characteristics of the brain in senescence-accelerated OXYS rats by magnetic resonance imaging. Bull. Exp. Biol. Med. 2011, 150, 739–743. [Google Scholar] [CrossRef]
- Al-Ghamdi, S.S. Time and dose dependent study of doxorubicin induced DU-145 cytotoxicity. Drug Metab. Lett. 2008, 2, 47–50. [Google Scholar] [CrossRef]
- Wang, S.; Konorev, E.A.; Kotamraju, S.; Joseph, J.; Kalivendi, S.; Kalyanaraman, B. Doxorubicin induces apoptosis in normal and tumor cells via distinctly different mechanisms. intermediacy of H(2)O(2)- and p53-dependent pathways. J. Biol. Chem. 2004, 279, 25535–25543. [Google Scholar] [CrossRef]
- Magnelli, L.; Cinelli, M.; Chiarugi, V. Phorbol esters attenuate the expression of p53 in cells treated with doxorubicin and protect TS-P53/K562 from apoptosis. Biochem. Biophys. Res. Commun. 1995, 215, 641–645. [Google Scholar] [CrossRef] [PubMed]
- McCurrach, M.E.; Connor, T.M.; Knudson, C.M.; Korsmeyer, S.J.; Lowe, S.W. bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA 1997, 94, 2345–2349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worley, B.; Powers, R. Multivariate Analysis in Metabolomics. Curr. Metab. 2013, 1, 92–107. [Google Scholar]
- Kotze, H.L.; Armitage, E.G.; Sharkey, K.J.; Allwood, J.W.; Dunn, W.B.; Williams, K.J.; Goodacre, R. A novel untargeted metabolomics correlation-based network analysis incorporating human metabolic reconstructions. BMC Syst. Biol. 2013, 7, 107. [Google Scholar] [CrossRef]
- Hosios, A.M.; Vander Heiden, M.G. Acetate metabolism in cancer cells. Cancer Metab. 2014, 2, 27. [Google Scholar] [CrossRef]
- Knowles, S.E.; Jarrett, I.G.; Filsell, O.H.; Ballard, F.J. Production and utilization of acetate in mammals. Biochem. J. 1974, 142, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Rajaram, R.D.; Dissard, R.; Jaquet, V.; de Seigneux, S. Potential benefits and harms of NADPH oxidase type 4 in the kidneys and cardiovascular system. Nephrol. Dial. Transpl. 2018. [Google Scholar] [CrossRef]
- Wu, H.; Huang, S.; Chen, Z.; Liu, W.; Zhou, X.; Zhang, D. Hypoxia-induced autophagy contributes to the invasion of salivary adenoid cystic carcinoma through the HIF-1alpha/BNIP3 signaling pathway. Mol. Med. Rep. 2015, 12, 6467–6474. [Google Scholar] [CrossRef]
- Kraskiewicz, H.; FitzGerald, U. Partial XBP1 knockdown does not affect viability of oligodendrocyte precursor cells exposed to new models of hypoxia and ischemia in vitro. J. Neurosci. Res. 2011, 89, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Chhunchha, B.; Fatma, N.; Kubo, E.; Rai, P.; Singh, S.P.; Singh, D.P. Curcumin abates hypoxia-induced oxidative stress based-ER stress-mediated cell death in mouse hippocampal cells (HT22) by controlling Prdx6 and NF-kappaB regulation. Am. J. Physiol. Cell Physiol. 2013, 304, C636–C655. [Google Scholar] [CrossRef] [PubMed]
- Fakhruddin, S.; Alanazi, W.; Jackson, K.E. Diabetes-Induced Reactive Oxygen Species: Mechanism of Their Generation and Role in Renal Injury. J. Diabetes Res. 2017, 2017, 8379327. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.; Hunton, D.L.; Bounelis, P.; Marchase, R.B. Hyperglycemia inhibits capacitative calcium entry and hypertrophy in neonatal cardiomyocytes. Diabetes 2002, 51, 3461–3467. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.; Anderson, M.E. CaMKII is a nodal signal for multiple programmed cell death pathways in heart. J. Mol. Cell Cardiol. 2017, 103, 102–109. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.M.; Waqar, T.; Howarth, F.C.; Adeghate, E.; Bidasee, K.; Singh, J. Hyperglycemia-induced cardiac contractile dysfunction in the diabetic heart. Heart Fail. Rev. 2018, 23, 37–54. [Google Scholar] [CrossRef]
- Adams, J.W.; Pagel, A.L.; Means, C.K.; Oksenberg, D.; Armstrong, R.C.; Brown, J.H. Cardiomyocyte apoptosis induced by Galphaq signaling is mediated by permeability transition pore formation and activation of the mitochondrial death pathway. Circ. Res. 2000, 87, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Diogo, C.V.; Suski, J.M.; Lebiedzinska, M.; Karkucinska-Wieckowska, A.; Wojtala, A.; Pronicki, M.; Duszynski, J.; Pinton, P.; Portincasa, P.; Oliveira, P.J.; et al. Cardiac mitochondrial dysfunction during hyperglycemia--the role of oxidative stress and p66Shc signaling. Int. J. Biochem. Cell Biol. 2013, 45, 114–122. [Google Scholar] [CrossRef]
- Lane, R.S.; Fu, Y.; Matsuzaki, S.; Kinter, M.; Humphries, K.M.; Griffin, T.M. Mitochondrial respiration and redox coupling in articular chondrocytes. Arthritis Res. Ther. 2015, 17, 54. [Google Scholar] [CrossRef]
- Aguer, C.; Gambarotta, D.; Mailloux, R.J.; Moffat, C.; Dent, R.; McPherson, R.; Harper, M.E. Galactose enhances oxidative metabolism and reveals mitochondrial dysfunction in human primary muscle cells. PLoS ONE 2011, 6, e28536. [Google Scholar] [CrossRef] [PubMed]
- Kase, E.T.; Nikolic, N.; Bakke, S.S.; Bogen, K.K.; Aas, V.; Thoresen, G.H.; Rustan, A.C. Remodeling of oxidative energy metabolism by galactose improves glucose handling and metabolic switching in human skeletal muscle cells. PLoS ONE 2013, 8, e59972. [Google Scholar] [CrossRef]
- Kuznetsov, A.V.; Margreiter, R.; Amberger, A.; Saks, V.; Grimm, M. Changes in mitochondrial redox state, membrane potential and calcium precede mitochondrial dysfunction in doxorubicin-induced cell death. Biochim. Biophys. Acta 2011, 1813, 1144–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishchenko, N.P.; Vasileva, E.A.; Fedoreev, S.A. Mirabiquinone, a new unsymmetrical binaphthoquinone from the sea urchin Scaphechinus mirabilis. Tetrahedron Lett. 2014, 55, 5967–5969. [Google Scholar] [CrossRef]
- Vasileva, E.A.; Mishchenko, N.P.; Vo, H.M.; Bui, L.M.; Denisenko, V.A.; Fedoreyev, S.A. Quinoid pigments from the sea urchin Astropyga radiata. Chem. Nat. Compd. 2017, 53, 356–358. [Google Scholar] [CrossRef]

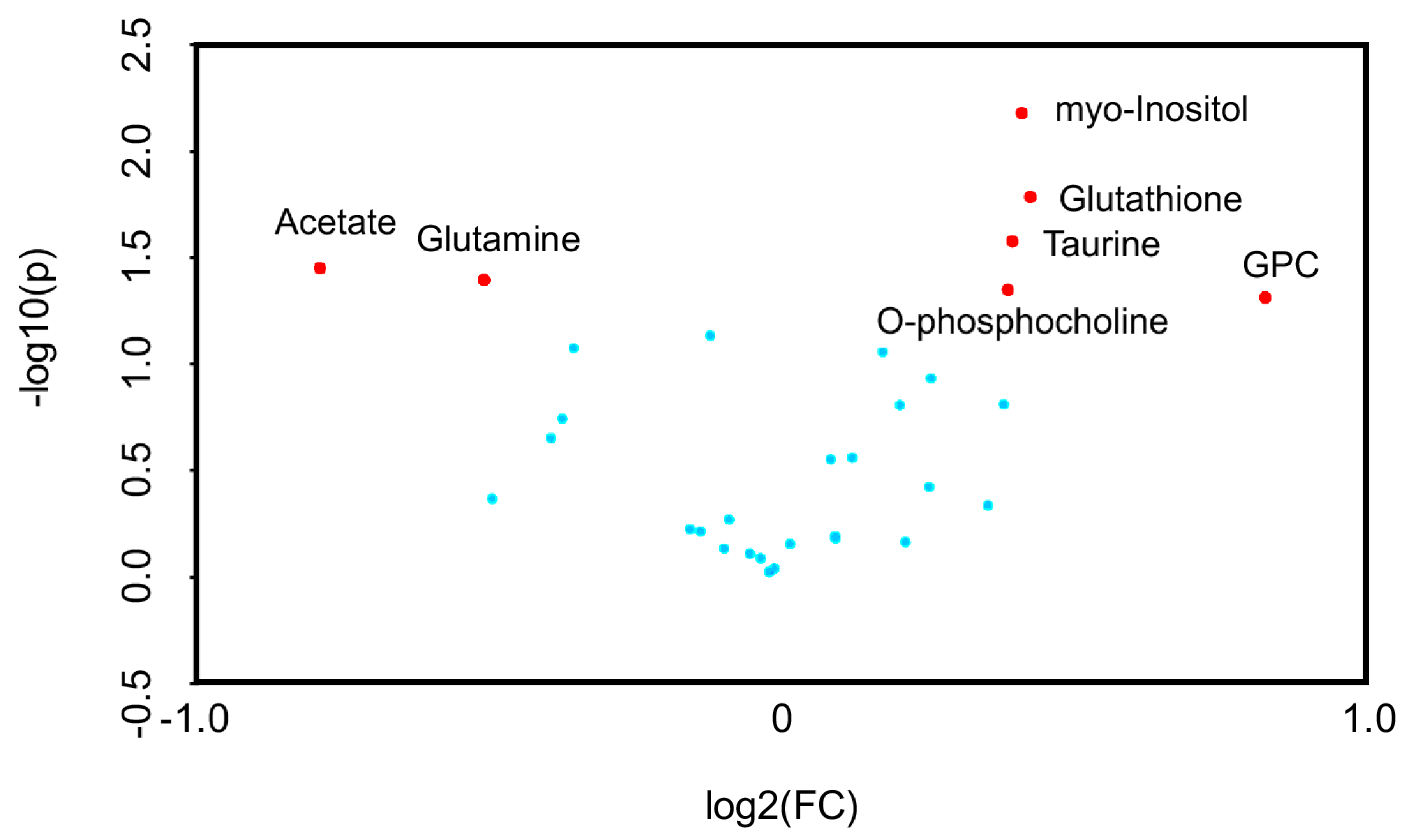


| Metabolite | Control | SpD | ||
|---|---|---|---|---|
| Mean (mM) | S.D. | Mean (mM) | S.D. | |
| Acetate | 8.218 | 5.815 | 4.820 | 1.541 |
| Alanine | 1.236 | 0.665 | 1.341 | 0.315 |
| Asparagine | 0.388 | 0.194 | 0.397 | 0.095 |
| Aspartate | 0.412 | 0.139 | 0.428 | 0.138 |
| Choline | 0.751 | 0.437 | 0.831 | 0.111 |
| Creatine | 0.463 | 0.190 | 0.557 | 0.051 |
| Formate | 0.230 | 0.034 | 0.197 | 0.048 |
| Fumarate | 0.143 | 0.047 | 0.164 | 0.043 |
| Glutamate | 2.877 | 1.640 | 3.622 | 0.791 |
| Glutamine | 0.129 | 0.048 | 0.101 | 0.006 |
| Glutathione | 0.383 | 0.158 | 0.572 | 0.081 |
| Glycerol | 0.912 | 0.405 | 0.788 | 0.088 |
| Glycine | 1.650 | 0.952 | 1.900 | 0.656 |
| Hypoxanthine | 0.285 | 0.222 | 0.349 | 0.089 |
| Inosine | 0.169 | 0.068 | 0.209 | 0.056 |
| Isoleucine | 0.169 | 0.080 | 0.235 | 0.031 |
| Lactate | 9.551 | 3.793 | 12.374 | 2.715 |
| Leucine | 0.829 | 0.536 | 0.824 | 0.073 |
| Lysine | 0.783 | 0.489 | 0.719 | 0.124 |
| Methionine | 0.064 | 0.017 | 0.090 | 0.029 |
| O-Phosphocholine | 0.274 | 0.124 | 0.392 | 0.066 |
| O-Phosphoethanolamine | 0.939 | 0.468 | 1.068 | 0.091 |
| Phenylalanine | 0.376 | 0.200 | 0.364 | 0.018 |
| Proline | 0.907 | 0.435 | 1.114 | 0.202 |
| Serine | 0.948 | 0.536 | 0.790 | 0.352 |
| Taurine | 0.505 | 0.270 | 0.723 | 0.198 |
| Threonine | 0.853 | 0.434 | 0.846 | 0.152 |
| Tyrosine | 0.323 | 0.168 | 0.329 | 0.004 |
| Uracil | 0.363 | 0.243 | 0.279 | 0.021 |
| Valine | 0.479 | 0.272 | 0.474 | 0.073 |
| myo-Inositol | 1.198 | 0.554 | 1.750 | 0.352 |
| sn-Glycero-3-phosphocholine (GPC) | 0.123 | 0.058 | 0.245 | 0.068 |
| Pathway | No. of Overlapping Genes | No. of Genes in Pathway | No. of Overlapping Metabolites | No. of Metabolites in Pathway | p-Value | q-Value |
|---|---|---|---|---|---|---|
| Glutathione metabolism | 12 | 54 (54) | 2 | 38 (38) | 8 × 10−6 | 4 × 10−4 |
| Protein digestion and absorption | 12 | 90 (90) | 3 | 47 (47) | 3 × 10−5 | 9 × 10−4 |
| Gap junction | 16 | 88 (88) | 1 | 11 (11) | 7 × 10−5 | 0.002 |
| Sulfur metabolism | 4 | 10 (10) | 2 | 33 (33) | 8 × 10−5 | 0.002 |
| Aminoacyl-tRNA biosynthesis | 12 | 66 (66) | 2 | 52 (52) | 9 × 10−5 | 0.002 |
| Choline metabolism in cancer | 12 | 99 (99) | 2 | 11 (11) | 1 × 10−4 | 0.002 |
| Central carbon metabolism in cancer | 11 | 65 (65) | 2 | 37 (37) | 1 × 10−4 | 0.003 |
| Long-term depression | 9 | 60 (60) | 1 | 9 (9) | 0.003 | 0.033 |
| Long-term potentiation | 9 | 67 (67) | 1 | 7 (7) | 0.004 | 0.043 |
| Pathway | Gene | Direction of Log(FC) vs. Control | Protein | ||
|---|---|---|---|---|---|
| SpD | Dox | Dox and SpD | |||
| Glutathione metabolism | TXNDC12 | DOWN | DOWN | UP | Thioredoxin domain-containing protein 12 |
| Protein digestion and absorption | COL4A1 | DOWN | DOWN | UP | Collagen alpha-1(IV) chain |
| COL5A2 | DOWN | DOWN | UP | Collagen alpha-2(V) chain | |
| Gap junction | MAPK3 | DOWN | UP | DOWN | Mitogen-activated protein kinase |
| MAP2K1 | UP | DOWN | UP | Dual specificity mitogen-activated protein kinase kinase 1 | |
| Sulfur metabolism | BPNT1 | DOWN | DOWN | UP | 3′(2′),5′-bisphosphate nucleotidase 1 |
| Aminoacyl-tRNA biosynthesis | FARSB | DOWN | DOWN | UP | Phenylalanine--tRNA ligase beta subunit |
| TARS | UP | UP | DOWN | Threonine--tRNA ligase, cytoplasmic | |
| Choline metabolism in cancer | MAP2K1 | UP | DOWN | UP | Dual specificity mitogen-activated protein kinase kinase 1 |
| AKT2 | DOWN | UP | UP | RAC-beta serine/threonine-protein kinase | |
| RAC1 | DOWN | DOWN | UP | Isoform B of Ras-related C3 botulinum toxin substrate 1 | |
| RHEB | DOWN | UP | UP | GTP-binding protein Rheb | |
| MAPK3 | DOWN | UP | DOWN | Mitogen-activated protein kinase | |
| Central carbon metabolism in cancer | MAP2K1 | UP | DOWN | UP | Dual specificity mitogen-activated protein kinase kinase 1 |
| PDHB | UP | DOWN | DOWN | Isoform 2 of Pyruvate dehydrogenase E1 component subunit beta, mitochondrial | |
| MTOR | UP | DOWN | UP | Serine/threonine-protein kinase mTOR | |
| AKT2 | DOWN | UP | UP | RAC-beta serine/threonine-protein kinase | |
| MAPK3 | DOWN | UP | DOWN | Mitogen-activated protein kinase | |
| Long-term depression | MAP2K1 | UP | DOWN | UP | Dual specificity mitogen-activated protein kinase kinase 1 |
| MAPK3 | DOWN | UP | DOWN | Mitogen-activated protein kinase | |
| Long-term potentiation | MAP2K1 | UP | DOWN | UP | Dual specificity mitogen-activated protein kinase kinase 1 |
| CAMK2D | DOWN | UP | UP | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, C.S.; Kim, H.K.; Mishchenko, N.P.; Vasileva, E.A.; Fedoreyev, S.A.; Stonik, V.A.; Han, J. Spinochrome D Attenuates Doxorubicin-Induced Cardiomyocyte Death via Improving Glutathione Metabolism and Attenuating Oxidative Stress. Mar. Drugs 2019, 17, 2. https://doi.org/10.3390/md17010002
Yoon CS, Kim HK, Mishchenko NP, Vasileva EA, Fedoreyev SA, Stonik VA, Han J. Spinochrome D Attenuates Doxorubicin-Induced Cardiomyocyte Death via Improving Glutathione Metabolism and Attenuating Oxidative Stress. Marine Drugs. 2019; 17(1):2. https://doi.org/10.3390/md17010002
Chicago/Turabian StyleYoon, Chang Shin, Hyoung Kyu Kim, Natalia P. Mishchenko, Elena A. Vasileva, Sergey A. Fedoreyev, Valentin A. Stonik, and Jin Han. 2019. "Spinochrome D Attenuates Doxorubicin-Induced Cardiomyocyte Death via Improving Glutathione Metabolism and Attenuating Oxidative Stress" Marine Drugs 17, no. 1: 2. https://doi.org/10.3390/md17010002
APA StyleYoon, C. S., Kim, H. K., Mishchenko, N. P., Vasileva, E. A., Fedoreyev, S. A., Stonik, V. A., & Han, J. (2019). Spinochrome D Attenuates Doxorubicin-Induced Cardiomyocyte Death via Improving Glutathione Metabolism and Attenuating Oxidative Stress. Marine Drugs, 17(1), 2. https://doi.org/10.3390/md17010002