Abstract
Two new antimicrobial bisabolane-type sesquiterpenoid derivatives, ent-aspergoterpenin C (compound 1) and 7-O-methylhydroxysydonic acid (2), and two new butyrolactone-type monoterpenoids, pestalotiolactones C (3) and D (4), along with a known monoterpenoid pestalotiolactone A (5) and four known bisabolane sesquiterpenoids (6−9), were isolated and identified from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. The structures of these compounds were elucidated on the basis of spectroscopic analysis, and the absolute configurations of the new compounds 1−4 were determined by the combination of NOESY and TDDFT-ECD calculations and X-ray crystallographic analysis. Additionally, we first determined and reported the absolute configuration of the known monoterpenoid pestalotiolactone A (5) through the X-ray crystallographic experiment. All of these isolated compounds were evaluated for antimicrobial activities against human and aquatic pathogenic bacteria. Compounds 1, 2, 6 and 9 exhibited selective inhibitory activities against zoonotic pathogenic bacteria such as Escherichia coli, Edwardsiella tarda, Vibrio anguillarum and V. harveyi, with MIC values ranging from 1.0 to 8.0 μg/mL.
1. Introduction
Aspergillus versicolor is a chemically brilliant fungal species with immense potential to produce a wide range of unique secondary metabolites including alkaloids [1,2,3], polyketides [4,5,6], and terpenoids [7]. Some of these metabolites exhibit intriguing biological properties, including antimicrobial properties [3,5] and cytotoxicity [6] as well as enzyme-inhibiting activities [1,4].
The bisabolane-type derivatives are a family of sesquiterpenoids which have been isolated from several biological sources, such as herb plants [8,9], marine sponges [10,11,12], and marine micro-organisms [13,14,15,16,17]. Among the attractive examples, peniciaculins A and B were isolated in 2015 from the culture of a deep sea-derived Penicillium aculeatum strain, SD-321, with significant antimicrobial activities [16]. In addition, aspergiterpenoid A and three related derivatives were isolated from a strain of sponge-derived fungus Aspergillus sp. in 2012 [17].
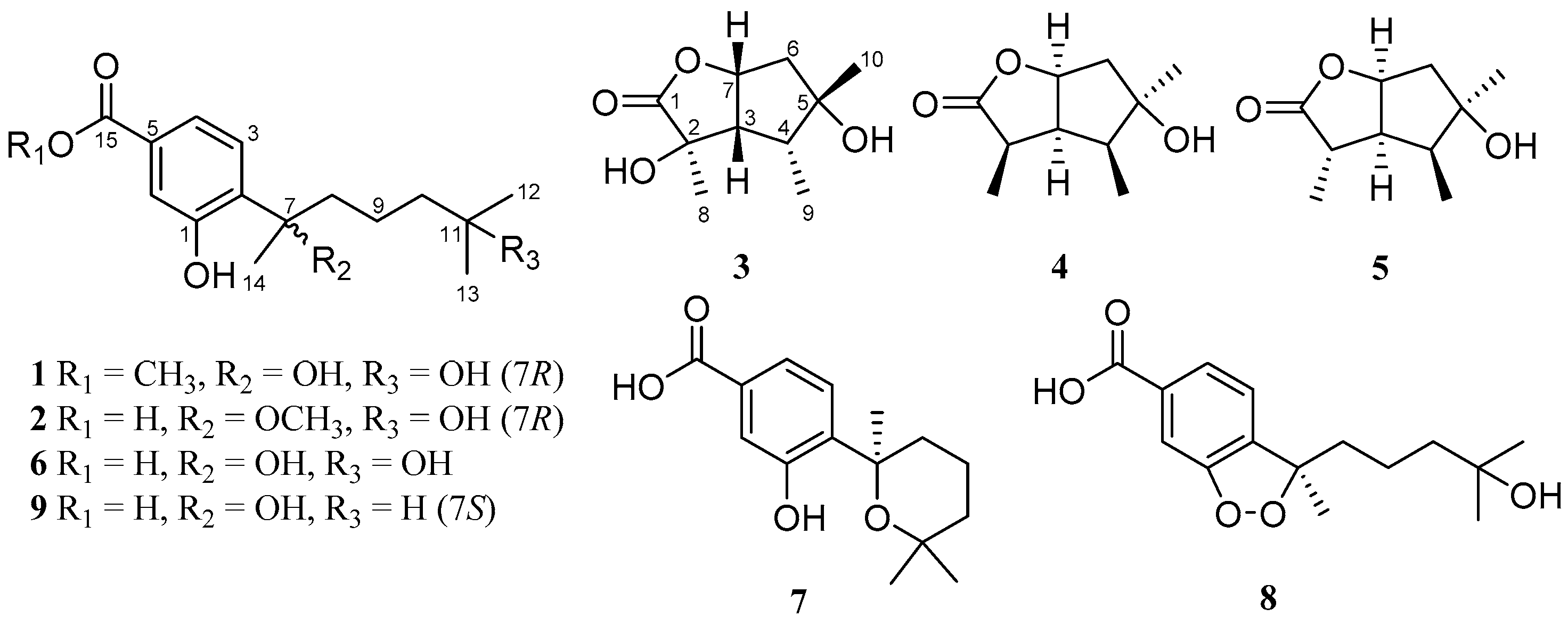
With the purpose of searching for new antibacterial metabolites from marine-derived micro-organisms, we carried out our studies to discover aromatic bisabolene-type derivatives. As a result, two new phenolic bisabolane sesquiterpenoids, ent-aspergoterpenin C (compound 1) and 7-O-methylhydroxysydonic acid (2) (Figure 1), together with four known bisabolane sesquiterpenoids (6−9), as well as two new butyrolactone-type monoterpenoids, pestalotiolactones C (3) and D (4), along with a known monoterpenoid, pestalotiolactone A (5), were isolated and identified from the culture extract of Aspergillus versicolor SD-330—a deep-sea sediment-derived fungus. The structures of the isolated compounds were established by the detailed interpretation of the nuclear magnetic resonance (NMR) and mass spectrometric data, and the absolute configurations of the new compounds 1–4 were determined by the combination of NOESY, quantum chemical ECD calculations, and X-ray crystallographic analysis. Additionally, the absolute configuration of the known monoterpenoid compound pestalotiolactone A (5) was first determined and reported through the X-ray crystallographic experiment. All of these compounds were examined for antimicrobial activities against human and aquatic pathogenic bacteria. Herein, details of the isolation, structure elucidation, and biological activities of compounds 1–9 are described.
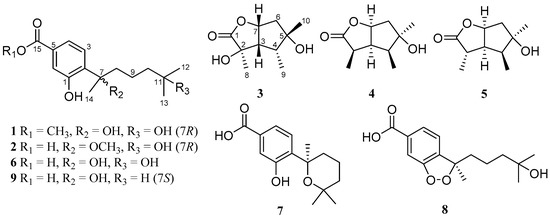
Figure 1.
Structures of compounds 1–9.
2. Results and Discussion
2.1. Structure Elucidation of the Compounds 1–5
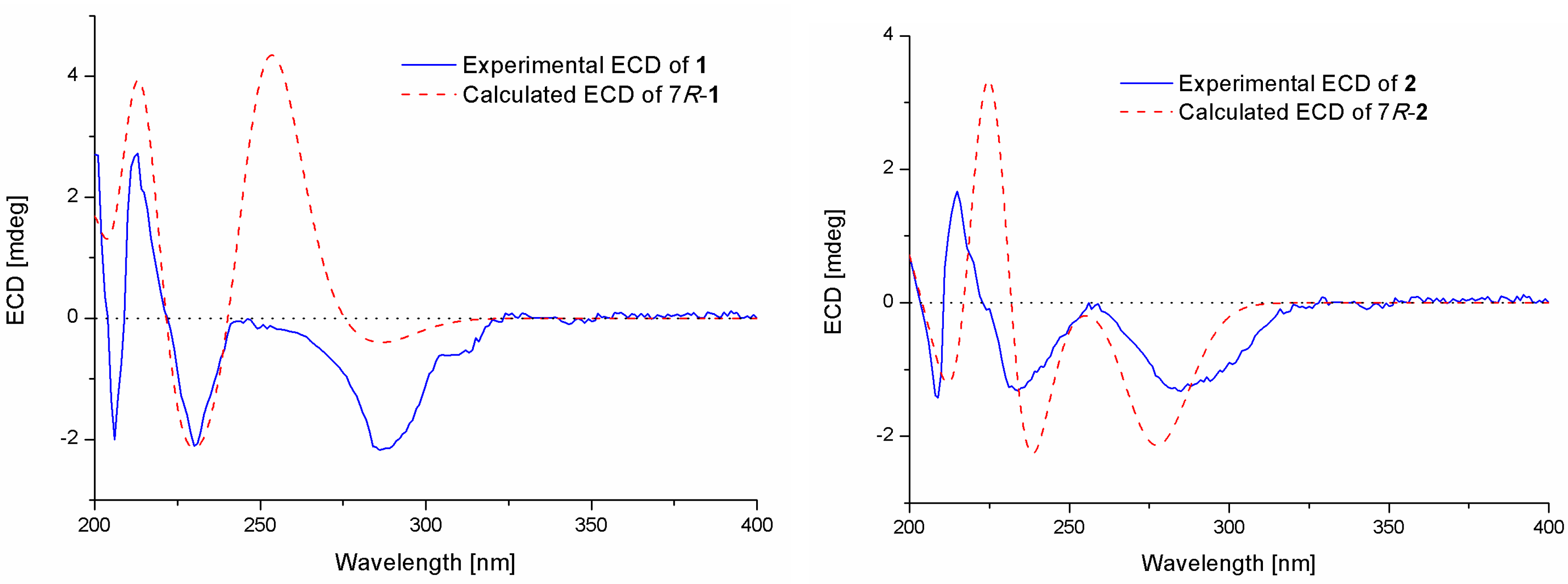
Compound 1 was isolated as a colorless oil. The molecular formula was determined to be C16H24O5 by HRESIMS, indicating five degrees of unsaturation. The 13C NMR along with DEPT spectroscopic data (Table 1) revealed the presence of 16 carbon atoms, which were clarified into six non-protonated carbons, three aromatic methines, three methylenes, and four methyls (with one oxygenated). Detailed analysis of the 1D and 2D NMR spectra displayed signals similar to those of hydroxysydonic acid (compound 6), a phenolic bisabolane sesquiterpenoid originally isolated from Aspergillus sydowi [18], revealing that compound 1 also belongs to the family of phenolic bisabolanes. The main differences between compounds 1 and 6 were the extra signals of a methoxy group at δH/C 3.80/51.8 observed in the 1H and 13C NMR spectrum of 1. The HMBC correlation (Figure 2) from the methoxy to C-15 indicated that compound 1 is a 15-COOH methyl esterificated derivative of 6 [18]. The absolute configuration of compound 1 was determined as 7R based on the TDDFT-ECD calculations. The experimental ECD spectrum of compound 1 matched well with the ECD calculated for (7R)-1 (Figure 3) and was opposite to the experimental ECD spectrum of aspergoterpenin C (7S), a bisabolane sesquiterpenoid derivative obtained from the endophytic fungus Aspergillus versicolor [19], revealing that compound 1 was the enantiomer of aspergoterpenin C. It is worth mentioning that aspergoterpenin C possessed a close ECD curve tp that of another related known analog, (+)-curcutetraol (7S) [20], suggesting that they have the same absolute configurations as 7S. However, the optical activities described for the two compounds were opposite ([α = +5.24 (c 0.74, MeOH) for (+)-curcutetraol and [α = −10.5 (c 0.07, MeOH) for aspergoterpenin C). To further confirm the absolute configuration of compound 1, the optical rotation values of aspergoterpenin C and ent-aspergoterpenin C (1) were calculated. Combining the calculated results of aspergoterpenin C ([α = +6.75 (c 0.10, MeOH)) and ent-aspergoterpenin C (1) ([α = −6.75 (c 0.10, MeOH)), as well as the experimental data measured for ent-aspergoterpenin C (1) ([α = −4.80 (c 0.40, MeOH)), we deduced that the absolute configuration of compound 1 should be assigned as 7R.

Table 1.
1H and 13C data of compounds 1 and 2 (measured in DMSO-d6).
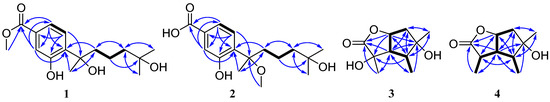
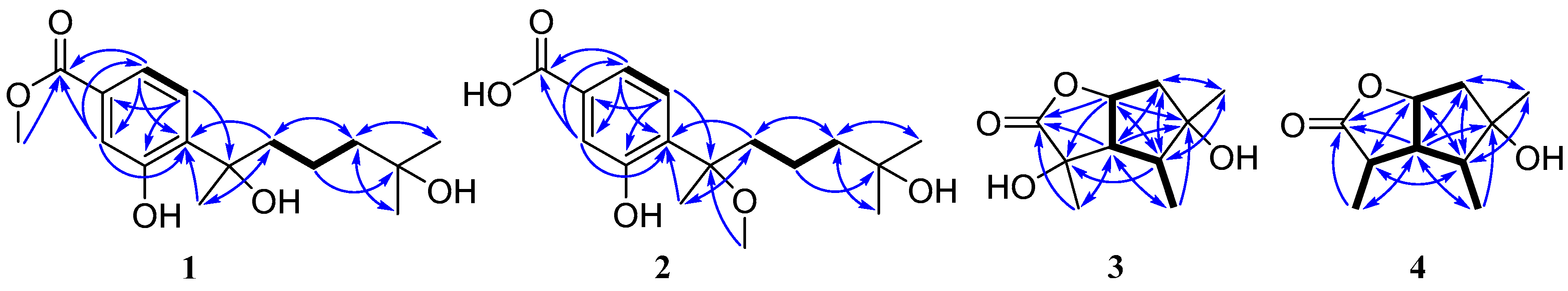
Figure 2.
Key COSY (bold lines) and HMBC (arrows) correlations for compounds 1–4.
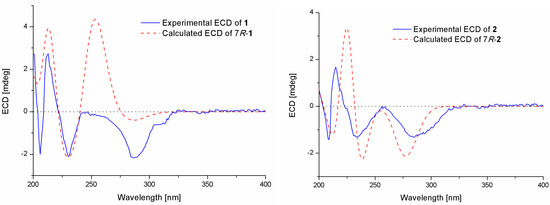
Figure 3.
Experimental and calculated ECD spectra of compounds 1 and 2.
Compound 2 was obtained as a white amorphous powder. The molecular formula was also determined as C16H24O5, the same as that of compound 1, based on HRESIMS data. The 1H, 13C, and DEPT NMR data (Table 1) of compound 2 showed identical patterns to those of compound 1, but with some variations for the chemical shifts of C-2, C-5, C-7, C-8, C-14, and the methoxy group. The upfielded shifts of C-2 (δC 134.2), C-8 (δC 38.4), C-14 (δC 22.2), and oxygenated methyl (δC 49.4), and the downfielded shifts of C-5 (δC 132.2) and C-7 (δC 79.5) indicated that compound 2 was a 7-methoxylated, 15-COOCH3 demethylated derivative of compound 1. Further evidence was observed by the key HMBC correlation from the proton of methoxyl to C-7 (Figure 2). Thus, the structure of compound 2 was determined to be 7-O-methylhydroxysydonic acid. The absolute configuration of compound 2 was established by the TDDFT-ECD calculation. The experimental ECD spectra of compound 2 matched well with that of calculated for (7R)-2 (Figure 3), leading to the absolute configuration of compound 2 being determined as 7R.
To verify if compounds 1 and 2 were artifacts produced during the process of chemical isolation by the esterification or methylation of hydroxysydonic acid (compound 6) with the methanol solvent in acidic condition, a confirmatory experiment was carried out. Hydroxysydonic acid was dissolved in MeOH with a small amount of silica gel after stirring at room temperature for 24 h. The result shows that compound 6 displayed good stability and did not react with MeOH. This was further proved by the evidence that the peaks of 1 and 2 were both detected from the high-performance liquid chromatography (HPLC) profile of crude fermented extract (Figure S15). Based on the above proofs, we tentatively presume that compounds 1 and 2 were natural products rather than artifacts.
The molecular formula of pestalotiolactone C (compound 3) was determined to be C10H16O4 on the basis of HRESIMS. The spectroscopic data (Table 2) revealed the presence of 10 carbon atoms, which were clarified into three non-protonated carbons, three methines, one methylene, and three methyls. Detailed analysis of NMR data showed that the signals in 1H and 13C NMR spectra of compound 3 were similar to those of pestalotiolactone A (compound 5), a butyrolactone-type monoterpenoid originally isolated from the axenic culture of the endophytic fungus Pestalotiopsis sp. [21], suggesting that compound 3 also possessed a butyrolactone-type monoterpenoid scaffold. The difference between compounds 3 and 5 was that signals of the methine (CH-2) in the 1D NMR spectra of compound 5 was absent in those of compound 3. Instead, the presence of one oxygenated non-protonated carbon at δC 75.6 was observed in the 13C NMR spectrum of compound 3. The above observation suggested that compound 3 was a 2-hydroxylated derivative of compound 5. This deduction was further verified by the COSY correlations from H-6 to H-7, from H-7 to H-3, from H-3 to H-4, and from H-4 to H3-9 along with key HMBC correlations from H-7 and Me-8 to C-1, C-2 and C-3, from H3-10 to C-4, C-5 and C-6 (Figure 2). Thus, the structure of compound 3 was determined and named pestalotiolactone C.

Table 2.
1H and 13C data of compounds 3 and 4 (measured in DMSO-d6).
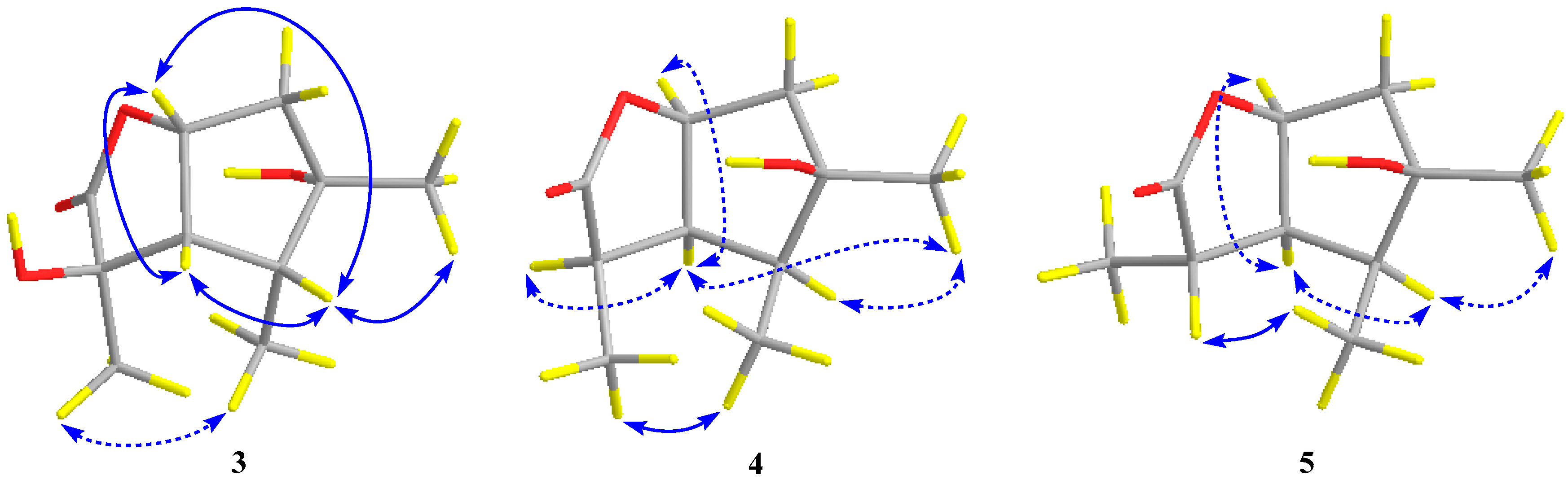
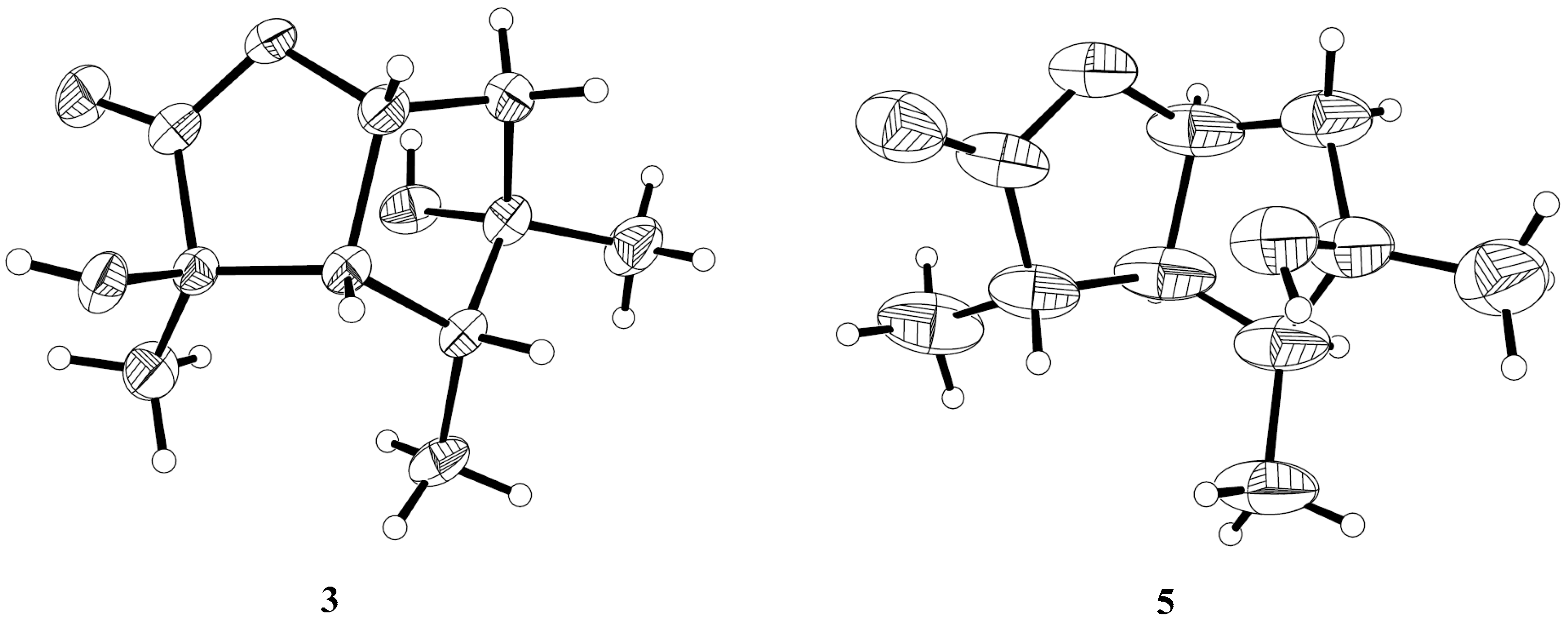
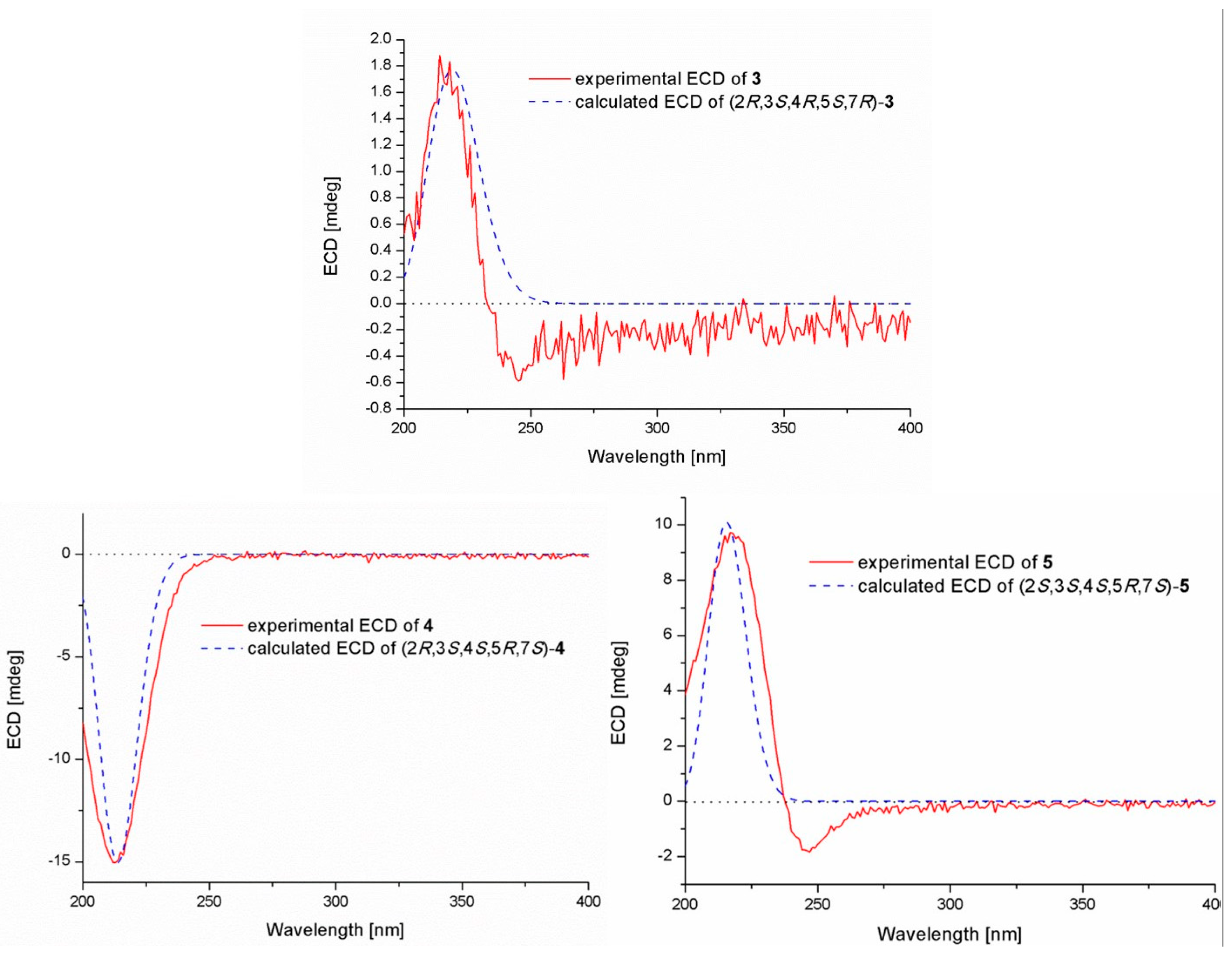
The relative configuration of compound 3 was assigned by the analysis of NOESY data (Figure 4). The key NOE correlations between H-3/H-4, H-3/H-7, H-4/H-7, and H-4/Me-10 indicated that these groups were on the same side of the molecule, while the NOEs between Me-8/Me-9 suggested that they were on the other face. On the basis of the above evidence, the relative configuration of compound 3 was determined. To confirm the absolute configuration of compound 3, we crystallized it for an X-ray single crystallographic analysis. After many attempts, single crystals that were suitable for X-ray analysis were obtained by the slow evaporation of a solution of compound 3 in CHCl3/MeOH = 1/9. Once the X-ray crystallographic experiment was conducted, the absolute configuration of compound 3 was unambiguously assigned as (2R, 3S, 4R, 5S, 7R)-3 (Figure 5).
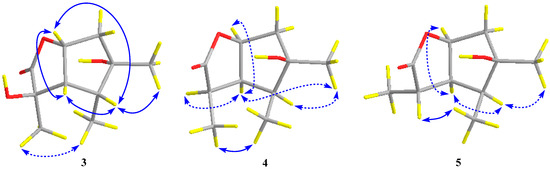
Figure 4.
Key NOESY correlations (blue lines: β-orientation; blue dotted lines: α-orientation) for compounds 3–5.
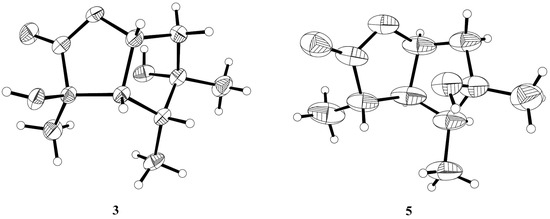
Figure 5.
X-ray structures of compounds 3 and 5.
Pestalotiolactone D (compound 4) was isolated as a white amorphous powder. HRESIMS data gave the molecular formula C10H16O3, the same as that of pestalotiolactone A (compound 5) [21]. The general features of the 1D NMR data (Table 2) resembled compound 5, and a minor difference was found for the chemical shifts of H-2, H-3 and H-8 in 1H NMR, and C-2, C-3, C-12, and C-8 in 13C NMR, suggesting that compound 4 is a diastereomer of pestalotiolactone A (compound 5). The relative configuration of 4 was assigned by analysis of NOESY data (Figure 4). The key NOE correlations between H-2/H-3, H-3/H-7, H-3/Me-10, and H-4/Me-10 indicated that these groups were on the same side of the molecule, while the NOEs between Me-8/Me-9 suggested them on the other face. On the basis of the above evidence, the relative configuration of compound 4 was determined. The absolute configuration of compound 4 was studied by the TDDFT-ECD calculation. The ECD spectrum of compound 4 exhibited negative CE at 213 nm, which matched well with that calculated for (2R, 3S, 4S, 5R, 7S)-4 (Figure 6).
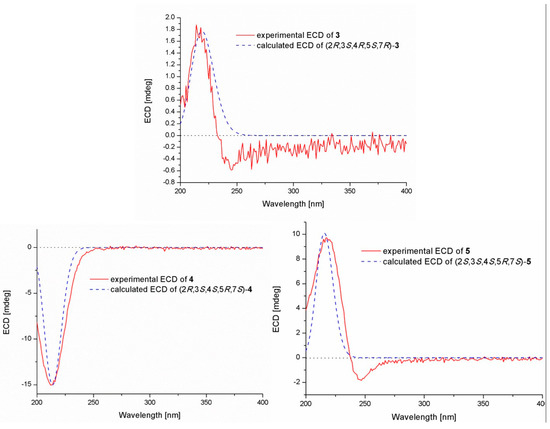
Figure 6.
Experimental and calculated ECD spectra of compounds 3–5.
The structure of compound 5 was identified through the detailed analysis of 1H, 13C, and DEPT NMR data and was compared with those of pestalotiolactone A reported in the literature [21]. The relative configuration of compound 5 was determined by the key NOE correlations between H-3/H-4, H-3/H-7, H-4/Me-10 and H-7/Me-8, and the correlations between H-2/Me-9 (Figure 4), which were also same as that of pestalotiolactone A. Combined with the close optical rotation values between compound 5 and pestalotiolactone A, we finally identified the compound which was the same as pestalotiolactone A. Considering that the absolute configuration of this compound has not been reported in the literature, we crystallized it for an X-ray single crystallographic analysis. Once the X-ray crystallographic experiment was conducted, the absolute configuration of compound 5 was finally determined as (2S, 3S, 4S, 5R, 7S) (Figure 5). Additionally, the experimental ECD spectrum for compound 5 was also in accordance with that calculated for (2S, 3S, 4S, 5R, 7S)-5 (Figure 6). This was the first report on the absolute configuration of the compound.
In addition to compounds 1–5, four known bisabolane-type sesquiterpenoids (compounds 6–9) were also isolated. By detailed spectroscopic analysis as well as comparisons with reported data, the structures of compounds 6–9 were identified as hydroxysydonic acid (compound 6) [22], sydowic acid (compound 7) [22], (−)-(R)-cyclo-hydroxysydonic acid (compound 8) [23], and sydonic acid (compound 9) [24].
2.2. Biological Activities of the Isolated Compounds
The obtained compounds 1–9 were tested for antimicrobial activities against seven zoonotic pathogenic bacteria (Table 3). The new compounds 1 and 2 exhibited obviously inhibitory activities against E. coli, E. tarda, V. harveyi, and V. parahaemolyticus, each with MIC values less than or equal to 8.0 μg/mL, while compound 6 exhibited significant inhibitory activity on E. coli, with an MIC value of 1.0 μg/mL, which is more potent than that of the positive control chloramphenicol (MIC 2.0 μg/mL). Moreover, compound 6 also exhibited potent activities against Aeromonas hydrophilia, E. tarda, V. anguillarum and V. harveyi each with an MIC value of 4.0 μg/mL, which is comparable to that of chloramphenicol. Compound 6 was more active toward bacteria than compounds 1 and 2, suggesting that the C-15 carboxy group methyl ester or the methylated C-7 hydroxy group are decreasing its antimicrobial activity. The fact that compound 6 was also more active than compounds 7 and 8 indicated that the formation of cyclohexanol ether or a peroxide ring in the molecule would also decrease its activity. In addition to the bisabolane-type sesquiterpenoids, the butyrolactone-type monoterpenoids—compounds 3–5—also exhibited some selective activities against the zoonotic pathogenic bacteria.

Table 3.
Antimicrobial activities of compounds 1–9 (MIC, μg/mL) a.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were acquired on an Optical Activity AA-55 polarimeter (Optical Activity Ltd., Cambridgeshire, UK). UV spectra were measured on a PuXi TU-1810 UV−visible spectrophotometer (Shanghai Lengguang Technology Co., Ltd., Shanghai, China). ECD spectra were measured on a Chirascan spectropolarimeter (Applied Photophysics Ltd., Surrey, UK). One-dimensional and 2D NMR spectra were obtained at 500 and 125 MHz for 1H and 13C, respectively, on a Bruker Avance 500 MHz spectrometer (Bruker Biospin Group, Karlsruhe, Germany) with TMS as the internal standard. Mass spectra were generated on a VG Autospec 3000 (VG Instruments, London, UK) or an API QSTAR Pulsar 1 mass spectrometer (Applied Biosystems, Foster, Waltham, MA, USA). Analytical and semi-preparative HPLC procedures were performed using a Dionex HPLC system (Dionex, Sunnyvale, CA, USA) equipped with a P680 pump, an ASI-100 automated sample injector, and a UVD340U multiple wavelength detector controlled by Chromeleon software (version 6.80). Commercially available Si gel (200−300 mesh, Qingdao Haiyang Chemical Co., Qingdao, China), Lobar LiChroprep RP-18 (40−63 μm, Merck, Darmstadt, Germany), and Sephadex LH-20 (Pharmacia, Pittsburgh, PA, USA) were used for open column chromatography. All solvents were distilled prior to use.
3.2. Fungal Material
The fungus Aspergillus versicolor SD-330 was isolated from a marine sediment sample collected in May 2012 from the South China Sea at a depth of 1487 m. The fungus was identified using a molecular biological protocol by DNA amplification and the sequencing of the intrnal transcribed spacer (ITS) region, as described in our previous report [25]. The sequenced data derived from the fungal strain have been deposited in GenBank (accession no. MN176407). A BLAST search result showed that the sequence was most similar (99%) to the sequence of Aspergillus versicolor (compared to accession no. MH911415.1). The strain is preserved at the Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences, with accession number SD-330.
3.3. Fermentation
For chemical investigations, the fungal strain was statically fermented for 35 days at room temperature in a liquid medium containing 20% potato juice, 2% glucose, 0.5% peptone, and 0.3% yeast extract.
3.4. Extraction and Isolation
The entire fermented cultures were filtered to separate the broth from the mycelia. The broth was extracted three times with EtOAc, while the mycelia was extracted three times with a mixture of ethyl alcohol and H2O (95:5, v/v). The ethyl alcohol solution was evaporated under reduced pressure to afford an aqueous solution, which was then extracted with EtOAc three times. Because the TLC and HPLC profiles of the two EtOAc solutions from the broth and mycelia were almost identical, they were combined and concentrated under reduced pressure to give an extract (25.1 g) for further separation.
The organic extract was fractionated by vacuum liquid chromatography (VLC) on silica gel eluting with different solvents of increasing polarity from petroleum ether (PE), to MeOH to yield 9 fractions (Frs. 1–9) that were pooled based on TLC analysis. Fr. 4 (4.5 g), eluted with PE–EtOAc (2:1), was further purified by column chromatography (CC) on Sephadex LH-20 (MeOH) to afford compound 5 (15.8 mg). Fr. 5 (3.7 g), eluted with CHCl3–MeOH (20:1), was further purified by CC on silica gel, eluting with a PE–EtOAc gradient (from 5:1 to 2:1), to afford two subfractions (Fr. 5-1 and Fr. 5-2). Fr. 5-1 was further purified by CC on Sephadex LH-20 (MeOH) and then purified by semi-preparative HPLC (75% MeOH–H2O, 5 mL/min) to afford compounds 3 (15.4 mg, tR 25.4 min) and 4 (10.7 mg, tR 28.2 min). Fr. 5-2 was further purified by CC over RP-18 eluting with a MeOH–H2O gradient (from 1:9 to 1:0) and by CC on Sephadex LH-20 (MeOH) to afford compounds 7 (8.7 mg, tR 19.2 min) and 8 (18.8 mg, tR 20.5 min). Fr. 6 (2.1 g), eluted with CHCl3–MeOH (10:1), was purified by CC on silica gel eluting with a CHCl3–MeOH gradient (40:1 to 10:1) to afford two subfrations (Fr. 6-1 and Fr. 6-2). Fr. 6-1 was further purified by CC over RP-18 eluting with a MeOH–H2O gradient (1:9 to 1:0) and by semi-preparative HPLC (MeOH/H2O, 80% to 85%, 5 mL/min) to obtain compound 6 (30.0 mg, tR 18.8 min). Fr. 6-2 was further purified by Sephadex LH-20 (MeOH) and by semi-preparative HPLC (80% MeOH/H2O, 5 mL/min) to yield compound 9 (51.5 mg, tR 18.2 min). Fr. 7 (3.7 g), eluted with CHCl3–MeOH (5:1), was further purified by CC on Sephadex LH-20 (MeOH) and then purified by semi-preparative HPLC (80% MeOH–H2O, 5 mL/min) to obtain compounds 1 (10.9 mg, tR 20.7 min) and 2 (18.8 mg, tR 22.3 min).
ent-Aspergoterpenin C (1): Colorless oily liquid; [α = −4.8 (c 0.40, MeOH); UV (MeOH) λmax (log ε) 215 (1.86), 248 (0.62), 303 (0.20) nm; ECD (0.26 mg/mL, MeOH) λmax (Δε) 206 (−0.68), 213 (+0.93), 231 (−0.71), 289 (−0.74) nm; 1H and 13C NMR data, Table 1 and Table 2; HRESIMS m/z 314.1960 [M + NH4]+ (calcd for C16H28O5N, 314.1962, Δ −0.5871 ppm), 319.1520 [M + Na]+ (calcd for C16H24O5Na, 319.1516, Δ 1.3681 ppm).
7-O-Methylhydroxysydonic acid (2): Amorphous powder; [α = −4.2 (c 0.35, MeOH); UV (MeOH) λmax (log ε) 212 (3.40), 245 (0.94), 301 (0.33) nm; ECD (0.24 mg/mL, MeOH) λmax (Δε) 209 (−0.53), 215 (+0.62), 235 (−0.47), 289 (−0.46) nm; 1H and 13C NMR data, Table 1 and Table 2; HRESIMS m/z 319.1514 [M + Na]+ (calcd for C16H24O5Na, 319.1516, Δ −0.4571 ppm).
Pestalotiolactones C (3): Colorless single crystal (MeOH); mp 103−106 °C; [α −17.5 (c 0.59, MeOH); ECD (1.1 mg/mL, MeOH) λmax (Δε) 214 (+0.10), 246 (−0.03) nm; 1H and 13C NMR data, Table 1 and Table 2; HRESIMS m/z 201.1121 [M + H]+ (calcd for C10H17O4, 201.1121, Δ −0.3112 ppm), 218.1388 [M + NH4]+ (calcd for C10H20O4N, 218.1387, Δ 0.3683 ppm), 223.0942 [M + Na]+ (calcd for C10H16O4Na, 223.0941, Δ 0.5540 ppm).
Pestalotiolactones D (4): Amorphous powder; [α −20.3 (c 0.58, MeOH); ECD (1.0 mg/mL, MeOH) λmax (Δε) 213 (−0.84) nm; 1H and 13C NMR data, Table 1 and Table 2; HRESIMS m/z 185.1169 [M + H]+ (calcd for C10H17O3, 185.1172, Δ −1.8331 ppm), 202.1435 [M + NH4]+ (calcd for C10H20O3N, 202.1438, Δ −1.3100 ppm).
Pestalotiolactones A (5): Colorless single crystal (MeOH); mp 104−107 °C; [α −30.3 (c 0.97, MeOH); ECD (1.40 mg/mL, MeOH) λmax (Δε) 220 (+0.55), 247 (−0.11) nm; HRESIMS m/z 185.1172 [M + H]+ (calcd for C10H17O3, 185.1172, Δ −0.1762 ppm), 202.1438 [M + NH4]+ (calcd for C10H20O3N, 202.1438, Δ −0.0112 ppm), 207.0991 [M + Na]+ (calcd for C10H16O3Na, 207.0992, Δ −0.4691 ppm).
3.5. Antimicrobial Assays
An antimicrobial evaluation against seven zoonotic pathogenic bacteria between humans and aquatic animals (E. coli QDIO-1, Aeromonas hydrophilia QDIO-3, E. tarda QDIO-4, Pseudomonas aeruginosa QDIO-6, V. anguillarum QDIO-8, V. harveyi QDIO-9, and V. parahaemolyticus QDIO-10) was carried out by the microplate assay with three repetitions [26]. For each organism, a loopful of glycerol stock was streaked on a Luria Broth (LB)-agar plate, which was incubated 24 h at 37 °C. A single bacterial colony was picked and suspended in Mueller–Hinton broth to approximately 5 × 105 cfu/mL. A two-fold serial dilution of each compound to be tested (2560 to 2.5 µg/mL in DMSO) was prepared and an aliquot of each dilution (5 μL) was added to a 96-well flat-bottom microtiter plate. An aliquot (95 μL) of bacterial suspension was then added to each well (to give final compound concentrations of 128 to 0.125 μg/mL in 2.0% DMSO) and the plate was incubated at 37 °C aerobically for 24 h. Finally, the optical density of each well at 600 nm was measured with an Tecan GENios multifunctional microplate reader (infinite M1000 PRO, Männedorf, Switzerland). MIC values were defined as the minimum concentration of compound that inhibited visible bacterial growth. The pathogenic bacteria and aquatic pathogen strains were provided by the Institute of Oceanology, Chinese Academy of Sciences. Chloramphenicol was used as a positive control, and DMSO as the negative control.
3.6. X-ray Crystallographic Analysis
Colorless crystals of compounds 3 and 5 were obtained by the slow evaporation of a solution in CHCl3/MeOH = 1/9, and from a solution of MeOH, respectively. Crystallographic data were collected on a Srigaku Mercury CCD/AFCR diffractometer equipped each with graphite-monochromatic Cu Kα radiation (λ = 1.54178 Å) at 293(2) K [27]. The data were corrected for absorption by using the program SADABS [28]. The structure was solved by direct methods and subsequent difference Fourier synthesis and refined by full-matrix least-squares techniques with the SHELXTL software package [29]. All non-hydrogen atoms were refined anisotropically. The H atoms belonging to C atoms were calculated theoretically, and those to O atoms were determined by difference Fourier maps [30].
Crystal data of compound 3: C10H16O4; fw = 200.23, Orthorhombic space group P2(1)2(1)2(1), unit cell dimensions a = 6.4948(6) Å, b = 6.6256(5) Å, c = 23.926(2) Å, V = 1029.57(16) Å3, α = 90.00, β = 90.00, γ = 90.00, Z = 4, dcalcd = 1.292 mg/m3, crystal dimensions 0.37 × 0.18 × 0.10 mm, μ = 0.826 mm−1, F(000) = 432. The 1452 measurements yielded 1067 independent reflections after equivalent data were averaged, and Lorentz and polarization corrections were applied. The final refinement gave R1 = 0.0283 and wR2 = 0.0585 [I > 2σ(I)]. The Flack parameter was 0.0(5) in the final refinement for all 1452 reflections with 1067 Friedel pairs.
Crystal data of compound 5: C10H16O3; fw = 184.23, Orthorhombic space group P2(1)2(1)2(1), unit cell dimensions a = 9.3551(8) Å, b = 9.6715(15) Å, c = 11.2278(8) Å, V = 1015.87(19) Å3, α = 90.00, β = 90.00, γ = 90.00, Z = 4, dcalcd = 1.205 mg/m3, crystal dimensions 0.23 × 0.14 × 0.08 mm, μ = 0.717 mm−1, F(000) = 400. The 1544 measurements yielded 786 independent reflections after equivalent data were averaged, and Lorentz and polarization corrections were applied. The final refinement gave R1 = 0.0337 and wR2 = 0.0708 [I > 2σ(I)]. The Flack parameter was 0.0(9) in the final refinement for all 1544 reflections with 786 Friedel pairs.
3.7. Computational Section
Conformational searches were performed via molecular mechanics using the MM+ method in HyperChem 8.0 software, and the geometries were further optimized at B3LYP/6-31G(d) PCM/MeCN level via Gaussian 09 software [31] to give the energy-minimized conformers. ECD and optical rotation (OR) calculations were performed with Gaussian 09. After this, the optimized conformers were subjected to the calculations of ECD spectra using TDDFT at PBE0/TZVP; solvent effects of the MeCN solution were evaluated at the same DFT level using the SCRF/Polarizable Continuum Model (PCM) method. OR values were computed using functional PBE0 and the TZVP basis set.
4. Conclusions
In summary, we have isolated and characterized two new antimicrobial bisabolane sesquiterpenoid derivatives, ent-aspergoterpenin C (compound 1) and 7-O-methylhydroxysydonic acid (2), and two new butyrolactone-type monoterpenoids, pestalotiolactones C (3) and D (4), along with a known monoterpenoid, pestalotiolactone A (5), and four known bisabolene sesquiterpenoids (6−9) from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. The new phenolic bisabolane derivatives, compounds 1 and 2, and the known phenolic bisabolane sesquiterpenoid, compound 6, may prove useful as antibacterial agents since they constitute potent antimicrobial activities against zoonotic pathogenic bacteria between humans and aquatic animals.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/10/563/s1, HRESIMS, 1D and 2D NMR spectra, UVs and ECDs of compounds 1–5.
Author Contributions
X.-D.L. performed the experiments for the isolation, structure elucidation, and antimicrobial evaluation and prepared the manuscript; X.-M.L. and X.-L.Y. performed the 1D and 2D NMR experiments; X.L. contributed to the structure determination, ECD and OR calculations; B.-G.W. supervised the research work and revised the manuscript.
Funding
This research work was financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences, Grant No. XDA22050401, the Aoshan Scientific and Technological Innovation Project of Qingdao National Laboratory for Marine Science and Technology (No. 2016ASKJ14), and the National Natural Science Foundation of China (31700043). X.-D. Li and X. Li appreciate the China Postdoctoral Science Foundation (2016LH00033, 2017M612358, and 2017M612360) for project funding. B.-G. Wang acknowledges the support of the Research Vessel KEXUE of the National Major Science and Technology Infrastructure from the Chinese Academy of Sciences (KEXUE2018G28) and the Taishan Scholar Project from Shandong Province. X. Li appreciate the Shandong Provincial Natural Science Foundation (ZR2017BB073).
Conflicts of Interest
The authors declare no conflict of interest.
References and Note
- Cheng, Z.; Lou, L.; Liu, D.; Li, X.; Proksch, P.; Yin, S.; Lin, W. Versiquinazolines A−K, fumiquinazoline-type alkaloids from the gorgonian-derived fungus Aspergillus versicolor LZD-14-1. J. Nat. Prod. 2016, 79, 2941–2952. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.-Y.; Liu, X.-H.; Miao, F.-P.; Qiao, M.-F. Aspeverin, a new alkaloid from an algicolous strain of Aspergillus versicolor. Org. Lett. 2013, 15, 2327–2329. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Miao, M.-M.; Du, G.; Li, X.-N.; Shang, S.-Z.; Zhao, W.; Liu, Z.-H.; Yang, G.-Y.; Che, C.-T.; Hu, Q.-F.; et al. Aspergillines A−E, highly oxygenated hexacyclic indole−tetrahydrofuran−tetramic acid derivatives from Aspergillus versicolor. Org. Lett. 2014, 16, 5016–5019. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, M.; Hao, H.; Lu, C. Avertoxins A−D, prenyl asteltoxin derivatives from Aspergillus versicolor Y10, an endophytic fungus of Huperzia serrata. J. Nat. Prod. 2015, 78, 3067–3070. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; He, W.; Huang, X.; Tian, X.; Liao, S.; Yang, B.; Wang, F.; Zhou, X.; Liu, Y. Antifungal new oxepine-containing alkaloids and xanthones from the deep-sea-derived fungus Aspergillus versicolor SCSIO 05879. J. Agric. Food Chem. 2016, 64, 2910–2916. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wu, G.; Sun, Z.; Zhang, X.; Che, Q.; Gu, Q.; Zhu, T.; Li, D.; Zhang, G. Cytotoxic tetrahydroxanthone dimers from the mangrove-associated fungus Aspergillus versicolor HDN1009. Mar. Drugs 2018, 16, 335. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Liu, Y.; Li, T.; Zhang, Z.; Ding, M.; Long, Y.; She, Z. 3-Arylisoindolinone and sesquiterpene derivatives from the mangrove endophytic fungi Aspergillus versicolor SYSU-SKS025. Fitoterapia 2018, 124, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Morinaga, H.; Masuoka, C.; Ikeda, T.; Okawa, M.; Kinjo, J.; Nohara, T. New bisabolane-type sesquiterpenes from the aerial parts of Lippia dulcis. Chem. Pharm. Bull. 2005, 53, 1175–1177. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, T.-H.; Bastow, K.F.; Lee, K.-H.; Chen, D.-F. Altaicalarins A-D, cytotoxic bisabolane sesquiterpenes from Ligularia altaica. J. Nat. Prod. 2010, 73, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Ogasawara, K. A new stereocontrolled route to (+)-curcuphenol, a phenolic sesquiterpene from the marine sponge Didiscus flavus. Tetrahedron: Asymmetry 1998, 9, 2215–2217. [Google Scholar] [CrossRef]
- Takamatsu, S.; Hodges, T.W.; Rajbhandari, I.; Gerwick, W.H.; Hamann, M.T.; Nagle, D.G. Marine natural products as novel antioxidant prototypes. J. Nat. Prod. 2003, 66, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Franzblau, S.G.; Zhang, F.; Hamann, M.T. Novel sesquiterpenes and a lactone from the Jamaican sponge Myrmekioderma styx. Tetrahedron Lett. 2002, 43, 9699–9702. [Google Scholar] [CrossRef]
- Almedia, C.; Elsaedi, S.; Kehraus, S.; Koenig, G.M. Novel bisabolene sequiterpenes from the marine-derived fungus Verticillium tenerum. Nat. Prod. Commun. 2010, 5, 507–510. [Google Scholar]
- Wei, M.-Y.; Wang, C.-Y.; Liu, Q.-A.; Shao, C.-L.; She, Z.-G.; Lin, Y.-C. Five sesquiterpenoids from a marine-derived fungus Aspergillus sp. isolated from a gorgonian Dichotella gemmacea. Mar. Drugs 2010, 8, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Trisuwan, K.; Rukachaisirikul, V.; Kaewpet, M.; Phongpaichit, S.; Hutadilok-Towatana, N.; Preedanon, S.; Sakayaroj, J. Sesquiterpene and xanthone derivatives from the sea fan-derived fungus Aspergillus sydowii PSU-F154. J. Nat. Prod. 2011, 74, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-D.; Li, X.-M.; Xu, G.-M.; Zhang, P.; Wang, B.-G. Antimicrobial phenolic bisabolanes and related derivatives from Penicillium aculeatum SD-321, a deep sea sediment-derived fungus. J. Nat. Prod. 2015, 78, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xu, Y.; Shao, C.-L.; Yang, R.-Y.; Zheng, C.-J.; Chen, Y.-Y.; Fu, X.-M.; Qian, P.-Y.; She, Z.-G.; Voogd, N.J.; et al. Antibacterial bisabolane-type sesquiterpenoids from the sponge-serived fungus Aspergillus sp. Mar. Drugs 2012, 10, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Hamasaki, T.; Nagayama, K.; Hatsuda, Y. Two new metabolites, sydonic acid and hydroxysydonic acid, from Aspergillus sydowi. Agric. Biol. Chem. 1978, 42, 37–40. [Google Scholar] [CrossRef]
- Guo, Z.-Y.; Tan, M.-H.; Liu, C.-X.; Lv, M.-M.; Deng, Z.-S.; Cao, F.; Zou, K.; Proksch, P. Aspergoterpenins A–D: Four new antimicrobial bisabolane sesquiterpenoid derivatives from an endophytic fungus Aspergillus versicolor. Molecules 2018, 23, 1291. [Google Scholar] [CrossRef] [PubMed]
- Mülhaupt, T.; Kaspar, H.; Otto, S.; Reichert, M.; Bringmann, G.; Lindel, T. Isolation, structural elucidation, and synthesis of curcutetraol. Eur. J. Org. Chem. 2005, 2, 334–341. [Google Scholar] [CrossRef]
- Liu, S.; Dai, H.-F.; Heering, C.; Janiak, C.; Lin, W.-H.; Liu, Z.; Proksch, P. Inducing new secondary metabolites through co-cultivation of the fungus Pestalotiopsis sp. with the bacterium Bacillus subtilis. Tetrahedron Lett. 2017, 58, 257–261. [Google Scholar] [CrossRef]
- Hamasaki, T.; Sato, Y.; Hatsuda, Y.; Tanabe, M.; Cary, L.-W. Sydowic acid, a new metabolite from Aspergillus sydowi. Tetrahedron Lett. 1975, 16, 659–660. [Google Scholar] [CrossRef]
- Li, X.-B.; Zhou, Y.-H.; Zhu, R.-X.; Chang, W.-Q.; Yuan, H.-Q.; Gao, W.; Zhang, L.-L.; Zhao, Z.-T.; Lou, H.-X. Identification and biological evaluation of secondary metabolites from the endolichenic fungus Aspergillus versicolor. Chem. Biodivers. 2015, 12, 575–592. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.; Murakami, T.; Miyanishi, J.; Tanaka, K.; Takada, N.; Hashimoto, M. Isolation and absolute stereochemistry of optically active sydonic acid from Glonium sp. (Hysterales, Aascomycota). Biosci. Biotechnol. Biochem. 2009, 73, 203–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, X.-M.; Teuscher, F.; Li, D.-L.; Diesel, A.; Ebel, R.; Proksch, P.; Wang, B.-G. Chaetopyranin, a benzaldehyde derivative, and other related metabolites from Chaetomium globosum, an endophytic fungus derived from the marine red alga Polysiphonia urceolata. J. Nat. Prod. 2006, 69, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Liu, X.; Guo, H.; Ren, B.; Chen, C.; Piggott, A.M.; Yu, K.; Gao, H.; Wang, Q.; Liu, M.; et al. Brevianamides with antitubercular potential from a marine-derived isolate of Aspergillus versicolor. Org. Lett. 2012, 14, 4770–4773. [Google Scholar] [CrossRef] [PubMed]
- Crystallographic data of compounds 3 and 5 have been deposited in the Cambridge Crystallographic Data Centre as CCDC 1940573 and 1940570, respectively. Those data can be obtained free of charge via http://www.ccdc.cam.ac.uk/data request/cif (or from the CCDC, 12 Union Road, Cambridge CB21EZ, U.K.; fax: +44-1223-336-033; e-mail: deposit@ccdc.cam.ac.uk).
- Sheldrick, G.M. SADABS, Software for Empirical Absorption Correction; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXTL, Structure Determination Software Programs; Bruker Analytical X-ray System Inc.: Madison, WI, USA, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXL-97 and SHELXS-97, Program for X-ray Crystal Structure Solution and Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).