Detecting Neurodevelopmental Toxicity of Domoic Acid and Ochratoxin A Using Rat Fetal Neural Stem Cells
Abstract
1. Introduction
2. Results
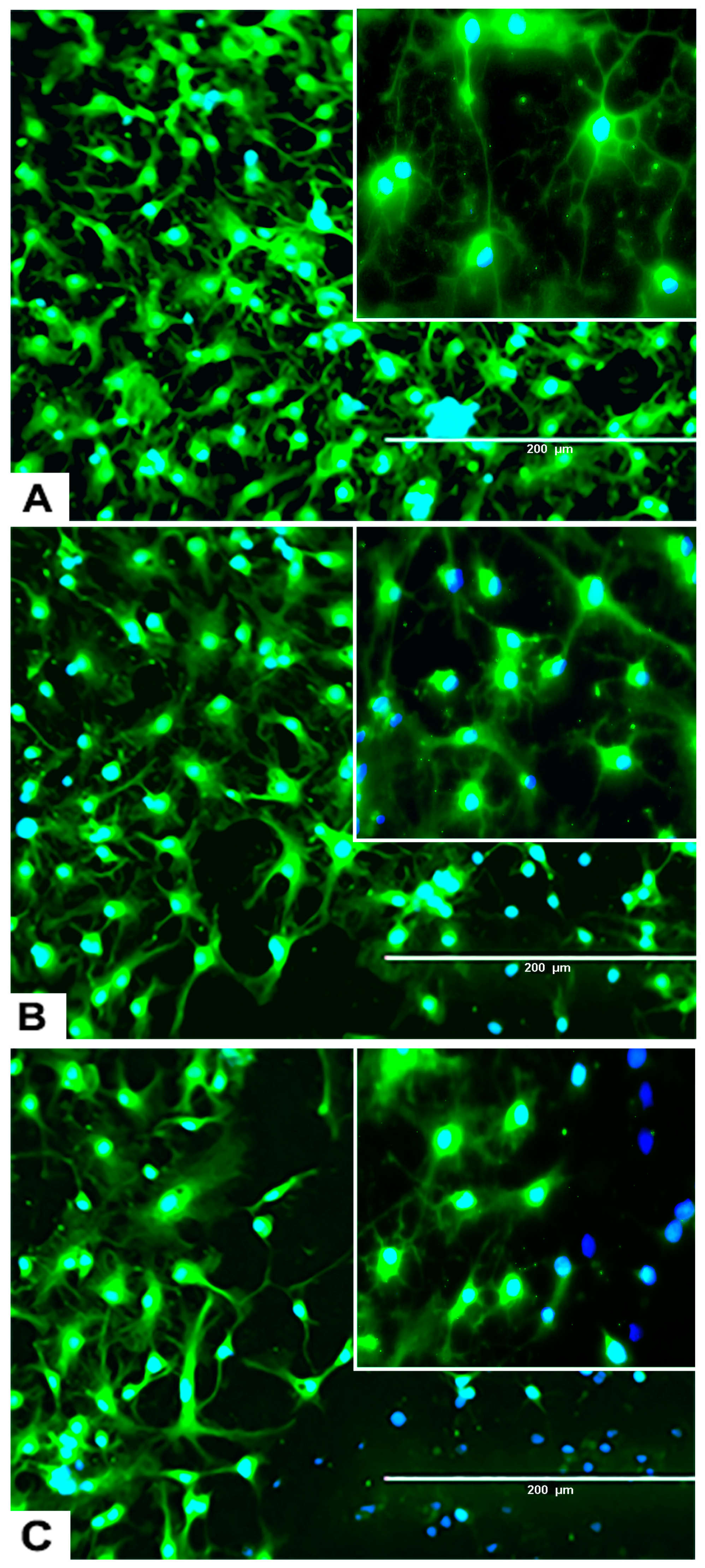
2.1. rNSC Proliferation Without Differentiation
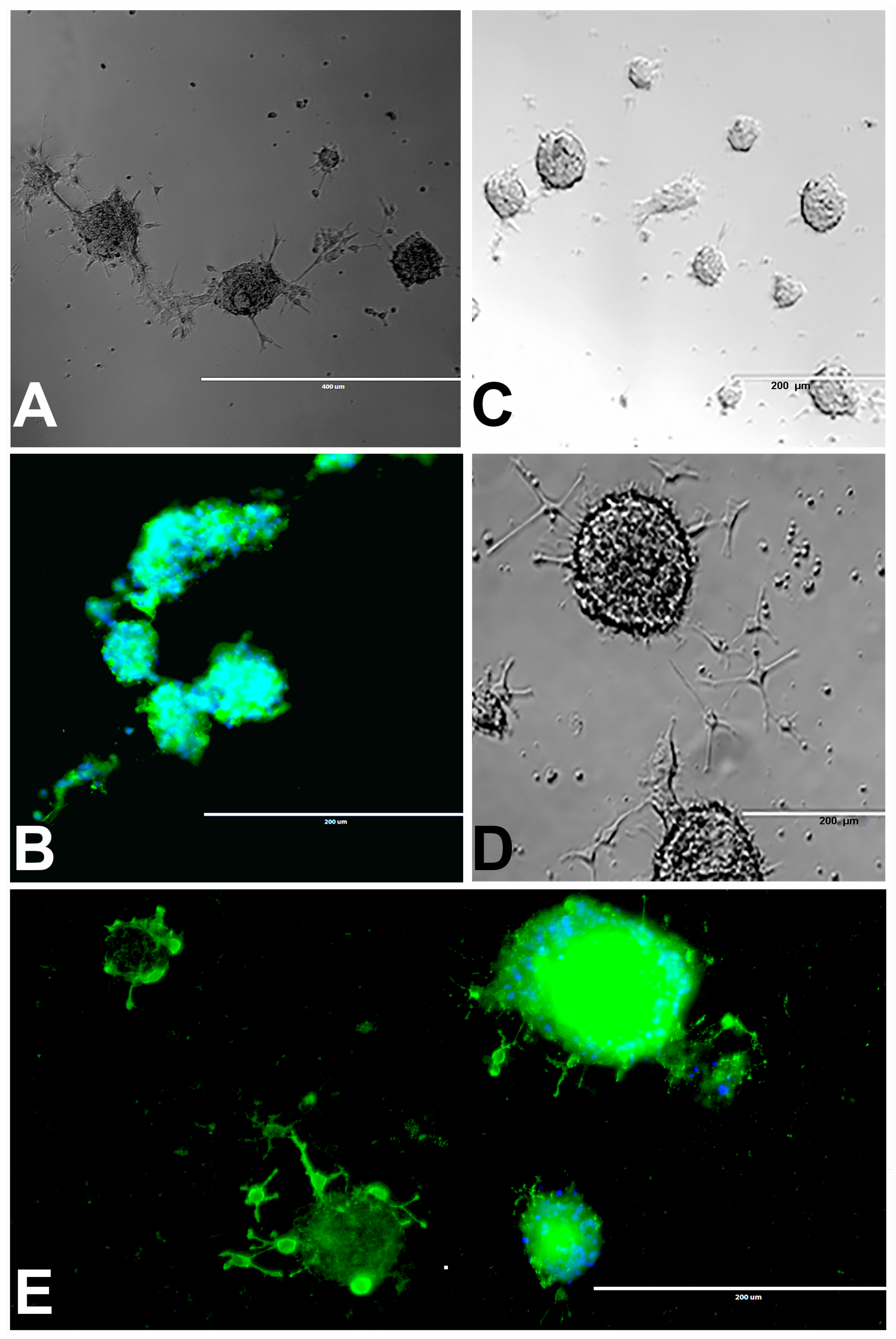
2.2. Neurosphere Assay
2.2.1. Neurosphere Proliferation Without Differentiation
2.2.2. Neurosphere Differentiation into Oligodendrocytes
2.3. rNSC Monolayer-Based Models for Three Types of Differentiation Processes
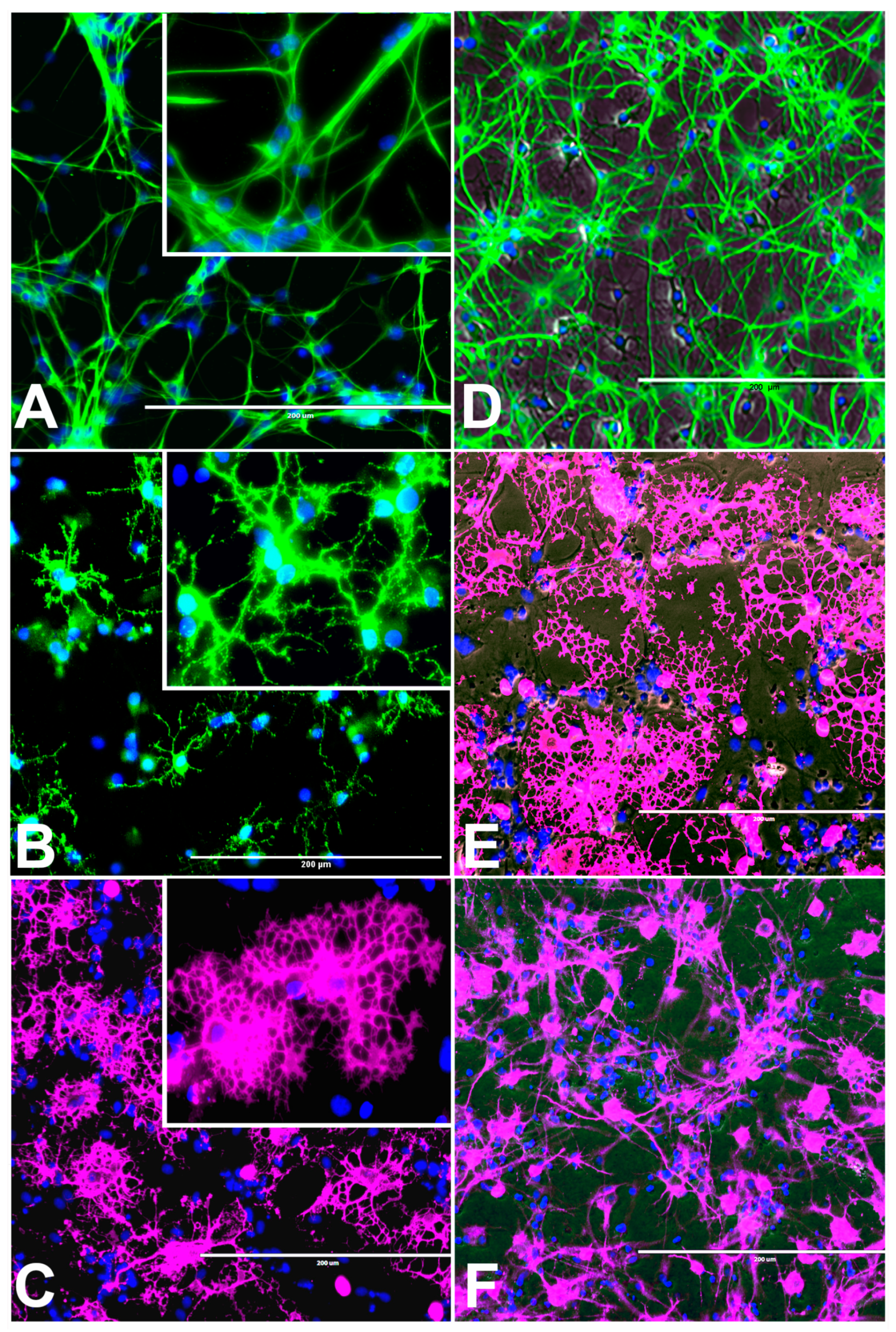
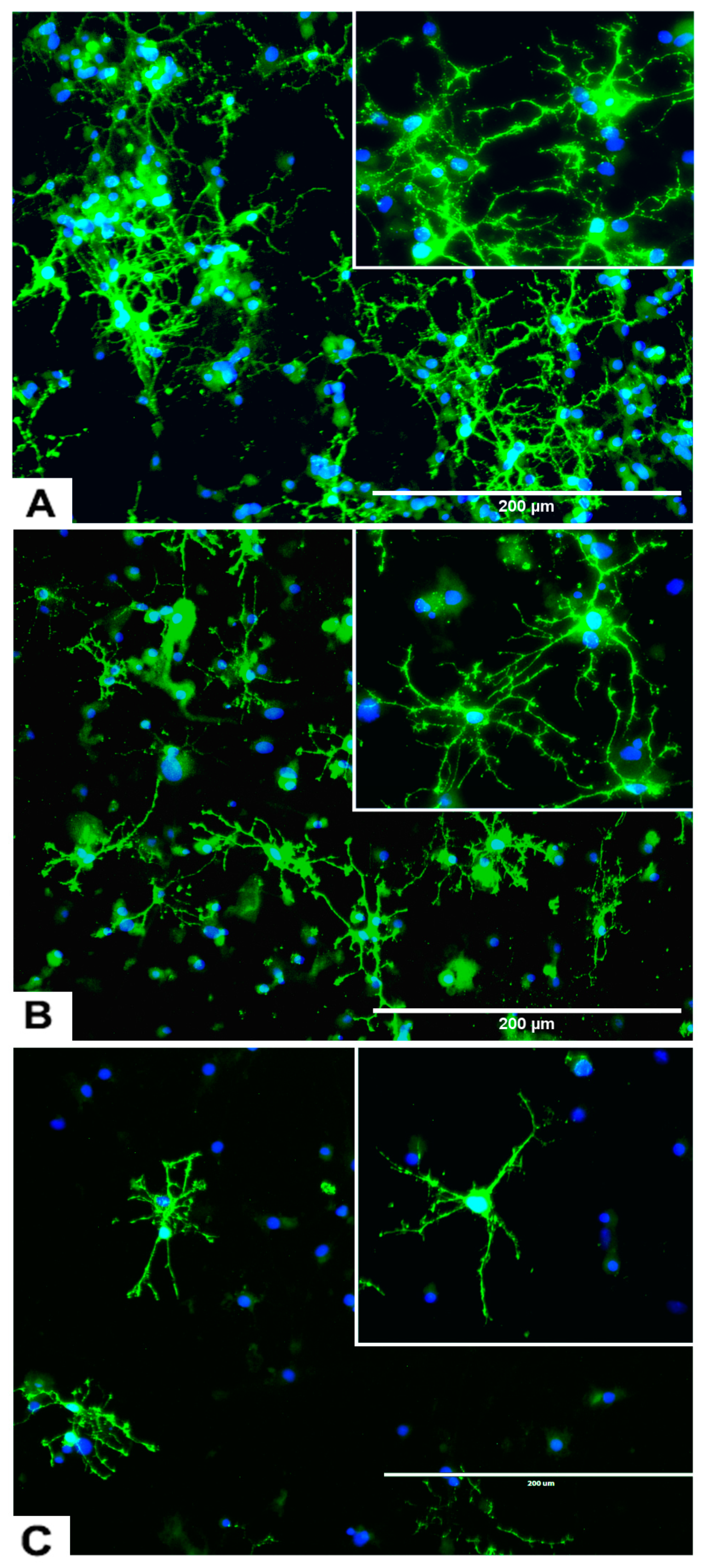
2.3.1. rNSC Differentiation Directed into Oligodendrocytes
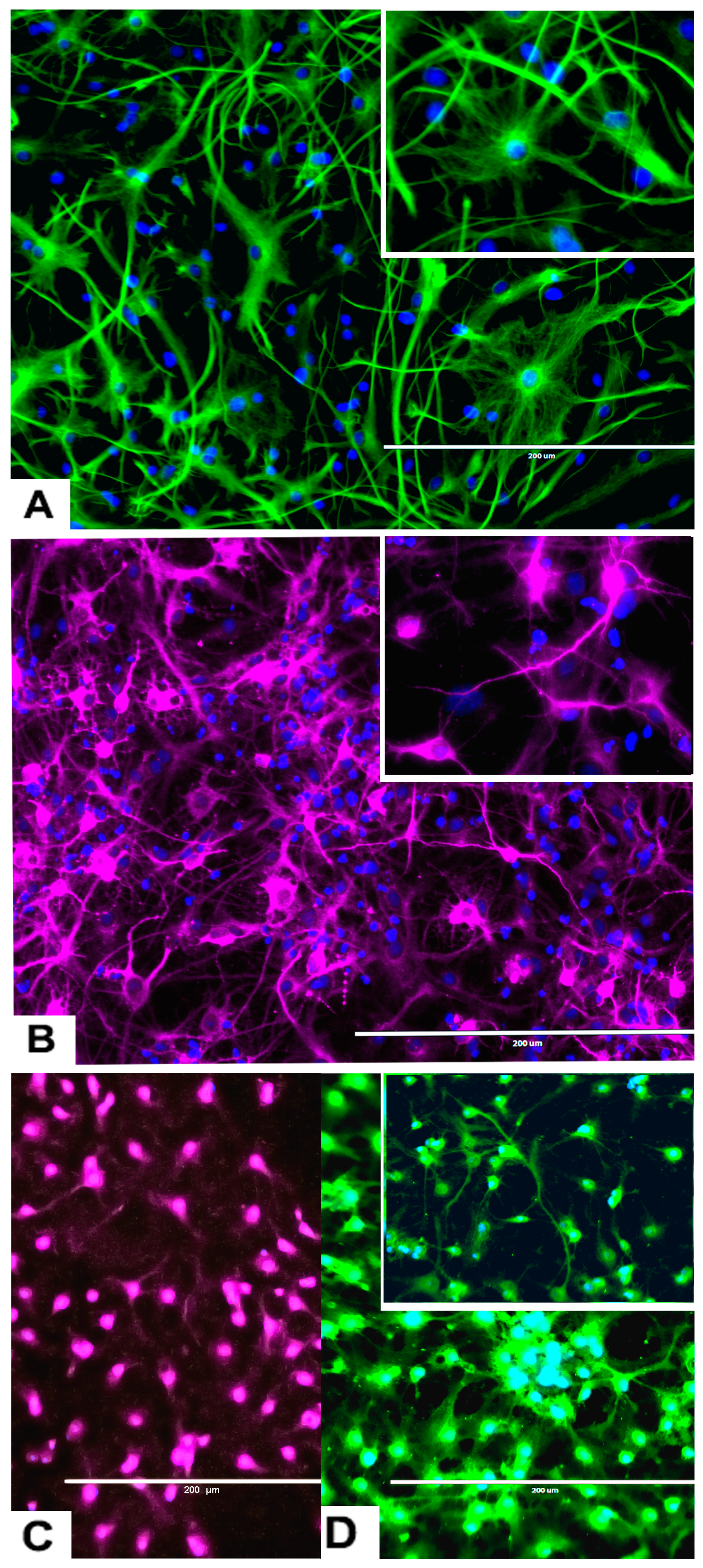
2.3.2. rNSC Differentiation Directed into Astrocytes
2.3.3. rNSC Differentiation Directed into Neurons
2.3.4. Effects of Domoic Acid on rNSC Differentiation Directed into Astrocytes, Neurons, and Oligodendrocytes
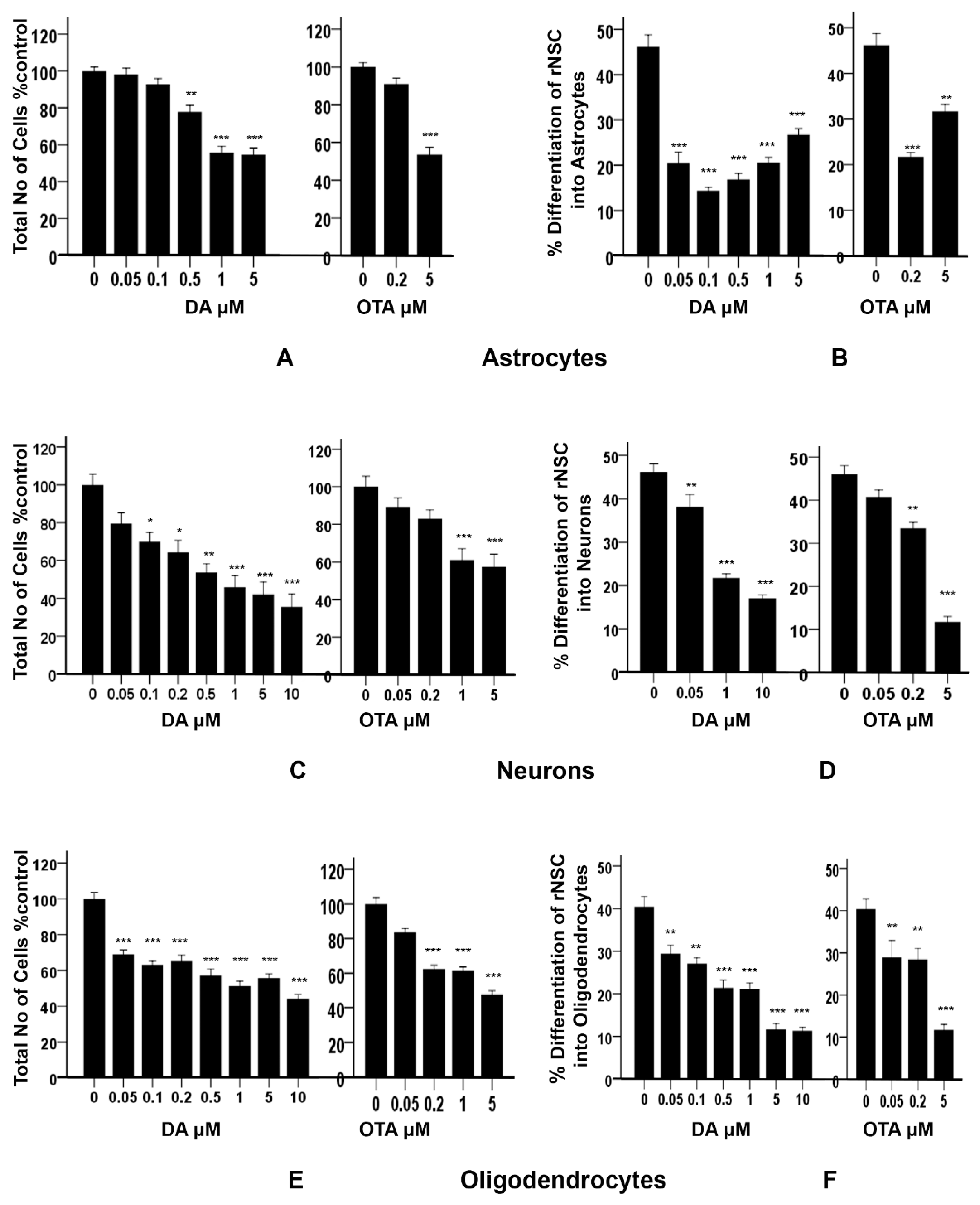
2.4. Effects of DA and OTA on Cytotoxicity and on the Differentiation of rNSC Directed into Astrocytes
2.5. Effects of DA and OTA on Cytotoxicity and on the Differentiation of rNSC Directed into Neurons
2.6. Effects of DA and OTA on Cytotoxicity and on the Differentiation of rNSC Directed into Oligodendrocytes
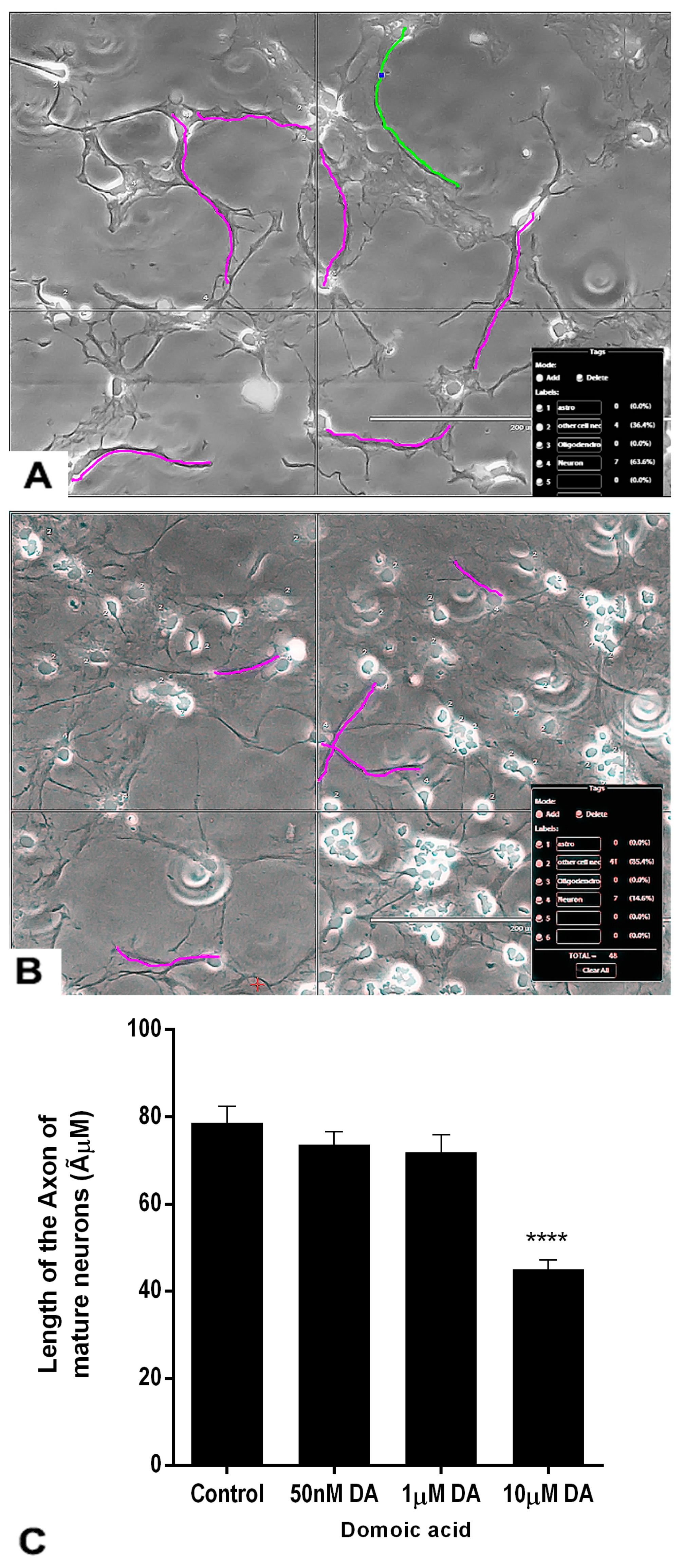
2.7. Effects of DA and OTA on the Axonal Length of the Mature Neurons
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.2.1. Propagation of rNSC
4.2.2. rNSC Neurosphere-Based Model for Differentiation
4.2.3. rNSC Monolayer-Based Model for Differentiation
A. Coating Chamber Slides for Differentiation into Various Cell Types
B. Differentiating Media
C. rNSC Culturing for Differentiation
4.3. Cytotoxicity Assays (Relative Cell Count)
4.4. Effects of DA and OTA on Directed Differentiation on the Different Cell Types
4.5. Immunochemistry
4.6. Image Analysis
4.7. Tracing and Measurement of the Axonal Length of the Mature Neurons
4.8. Statistical Analysis
5. Conclusion and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood Brain Barrier |
| CNS | Central Nervous System |
| DA | Domoic Acid |
| DNT | Developmental Neurotoxicology |
| EGF | Epidermal Growth Factor |
| FGFb | Fibroblast Growth Factor basic |
| GFAP | Glial Fibrillary Acidic Protein |
| MAP2 | Microtubule-Associated Protein 2 |
| OTA | Ochratoxin A |
| PSC | Pluripotent Stem Cells |
| rNSC | rat fetal Neural Stem Cells |
| RCC | Relative Cell Count |
| RT | Room Temperature |
| T3 | Thyroxin hormone |
References
- Rock, K.D.; Patisaul, H.B. Environmental Mechanisms of Neurodevelopmental Toxicity. Curr. Environ. Health Rep. 2018, 5, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Rice, D.; Barone, S. Critical periods of vulnerability for the developing nervous system: Evidence from humans and animal models. Environ. Health Perspect. 2000, 108 (Suppl. 3), 511–533. [Google Scholar] [PubMed]
- Tohyama, C. Developmental neurotoxicity test guidelines: Problems and perspectives. J. Toxicol. Sci. 2016, 41, SP69–SP79. [Google Scholar] [CrossRef] [PubMed]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Pei, Y.; Peng, J.; Behl, M.; Sipes, N.S.; Shockley, K.R.; Rao, M.S.; Tice, R.R.; Zeng, X. Comparative neurotoxicity screening in human iPSC-derived neural stem cells, neurons and astrocytes. Brain Res. 2016, 1638, 57–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Choi, E.; Monaco, M.C.G.; Campanac, E.; Medynets, M.; Do, T.; Rao, P.; Johnson, K.R.; Elkahloun, A.G.; Von Geldern, G.; et al. Derivation of neural stem cells from human adult peripheral CD34+ cells for an autologous model of neuroinflammation. PLoS ONE 2013, 8, e81720. [Google Scholar] [CrossRef]
- Bal-Price, A.; Pistollato, F.; Sachana, M.; Bopp, S.K.; Munn, S.; Worth, A. Strategies to improve the regulatory assessment of developmental neurotoxicity (DNT) using in vitro methods. Toxicol. Appl. Pharmacol. 2018, 354, 7–18. [Google Scholar] [CrossRef]
- Fritsche, E.; Grandjean, P.; Crofton, K.M.; Aschner, M.; Goldberg, A.; Heinonen, T.; Hessel, E.V.S.; Hogberg, H.T.; Bennekou, S.H.; Lein, P.J.; et al. Consensus statement on the need for innovation, transition and implementation of developmental neurotoxicity (DNT) testing for regulatory purposes. Toxicol. Appl. Pharmacol. 2018, 354, 3–6. [Google Scholar] [CrossRef]
- Sava, V.; Velasquez, A.; Song, S.; Sanchez-Ramos, J. Adult hippocampal neural stem/progenitor cells in vitro are vulnerable to the mycotoxin ochratoxin-A. Toxicol. Sci. 2007, 98, 187–197. [Google Scholar] [CrossRef]
- Buzanska, L.; Sypecka, J.; Nerini-Molteni, S.; Compagnoni, A.; Hogberg, H.T.; del Torchio, R.; Domanska-Janik, K.; Zimmer, J.; Coecke, S. A human stem cell-based model for identifying adverse effects of organic and inorganic chemicals on the developing nervous system. Stem Cells 2009, 27, 2591–2601. [Google Scholar] [CrossRef]
- Zurich, M.-G.; Honegger, P. Ochratoxin A at nanomolar concentration perturbs the homeostasis of neural stem cells in highly differentiated but not in immature three-dimensional brain cell cultures. Toxicol. Lett. 2011, 205, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, T.A.; Skavicus, S.; Card, J.; Levin, E.D.; Seidler, F.J. Diverse neurotoxicants target the differentiation of embryonic neural stem cells into neuronal and glial phenotypes. Toxicology 2016, 372, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Malik, N.; Efthymiou, A.G.; Mather, K.; Chester, N.; Wang, X.; Nath, A.; Rao, M.S.; Steiner, J.P. Compounds with species and cell type specific toxicity identified in a 2000 compound drug screen of neural stem cells and rat mixed cortical neurons. Neurotoxicology 2014, 45, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Kirkley, K.S.; Madl, J.E.; Duncan, C.; Gulland, F.M.; Tjalkens, R.B. Domoic acid-induced seizures in California sea lions (Zalophus californianus) are associated with neuroinflammatory brain injury. Aquat. Toxicol. 2014, 156, 259–268. [Google Scholar] [CrossRef]
- Pulido, O.M. Domoic acid toxicologic pathology: A review. Mar. Drugs 2008, 6, 180–219. [Google Scholar] [CrossRef]
- Ramsdell, J.S.; Zabka, T.S. In utero domoic acid toxicity: A fetal basis to adult disease in the California sea lion (Zalophus californianus). Mar. Drugs 2008, 6, 262–290. [Google Scholar] [CrossRef]
- Sharon, M. Gwaltney-Brant. Reproductive and Developmental Toxicology. In Zootoxins, 2nd ed.; Elsevier Inc, Academic Press: London UK, 2017; pp. 963–972. [Google Scholar]
- Dos Santos, J.A.; Vieira, J.M.F.; Videira, A.; Meirelles, L.A.; Rodrigues, A.; Taniwaki, M.H.; Sette, L.D. Marine-derived fungus Aspergillus cf. tubingensis LAMAI 31: A new genetic resource for xylanase production. AMB Express 2016, 6, 25. [Google Scholar] [CrossRef]
- Xu, X.; He, F.; Zhang, X.; Bao, J.; Qi, S. New mycotoxins from marine-derived fungus Aspergillus sp. SCSGAF0093. Food Chem. Toxicol. 2013, 53, 46–51. [Google Scholar] [CrossRef]
- Bondy, G.S.; Coady, L.; Ross, N.; Caldwell, D.; Gannon, A.M.; Kwong, K.; Hayward, S.; Lefebvre, D.E.; Liston, V.; Raju, J.; et al. A reproductive and developmental screening study of the fungal toxin ochratoxin A in Fischer rats. Mycotoxin Res. 2018, 34, 241–255. [Google Scholar] [CrossRef]
- Kuiper-Goodman, T.; Hilts, C.; Billiard, S.M.; Kiparissis, Y.; Richard, I.D.K.; Hayward, S. Health risk assessment of ochratoxin A for all age-sex strata in a market economy. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2010, 27, 212–240. [Google Scholar] [CrossRef]
- Paradells, S.; Rocamonde, B.; Llinares, C.; Herranz-Pérez, V.; Jimenez, M.; Garcia-Verdugo, J.M.; Zipancic, I.; Soria, J.M.; Garcia-Esparza, M.A. Neurotoxic effects of ochratoxin A on the subventricular zone of adult mouse brain. J. Appl. Toxicol. 2015, 35, 737–751. [Google Scholar] [CrossRef] [PubMed]
- Cahoy, J.D.; Emery, B.; Kaushal, A.; Foo, L.C.; Zamanian, J.L.; Christopherson, K.S.; Xing, Y.; Lubischer, J.L.; Krieg, P.A.; Krupenko, S.A.; et al. A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci. 2008, 28, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Louis, S.A.; Rietze, R.L.; Deleyrolle, L.; Wagey, R.E.; Thomas, T.E.; Eaves, A.C.; Reynolds, B.A. Enumeration of neural stem and progenitor cells in the neural colony-forming cell assay. Stem Cells 2008, 26, 988–996. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.B.; Parmar, M. Strengths and limitations of the neurosphere culture system. Mol. Neurobiol. 2006, 34, 153–161. [Google Scholar] [CrossRef]
- Reynolds, B.A.; Rietze, R.L. Neural stem cells and neurospheres--re-evaluating the relationship. Nat. Methods 2005, 2, 333–336. [Google Scholar] [CrossRef]
- Chandrasekaran, A.; Avci, H.X.; Leist, M.; Kobolák, J.; Dinnyés, A. Astrocyte Differentiation of Human Pluripotent Stem Cells: New Tools for Neurological Disorder Research. Front Cell Neurosci. 2016, 10, 215. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.C.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Goldman, S.A.; Nedergaard, M. Heterogeneity of astrocytic form and function. Methods Mol. Biol. 2012, 814, 23–45. [Google Scholar]
- Baumann, J. Application of the Neurosphere Assay for DNT Hazard Assessment: Challenges and Limitations. In Methods in Pharmacology and Toxicology; Humana Press: Totowa, NJ, USA, 2015. [Google Scholar]
- Molofsky, A.V.; Krencik, R.; Krenick, R.; Ullian, E.M.; Ullian, E.; Tsai, H.; Deneen, B.; Richardson, W.D.; Barres, B.A.; Rowitch, D.H. Astrocytes and disease: A neurodevelopmental perspective. Genes Dev. 2012, 26, 891–907. [Google Scholar] [CrossRef]
- Mirella, D.; Alice, P.; Martin, F.P. Protocols for Neural Cell Culture, 4th ed.; Springer Protocols Handbooks; Doering, L.C., Ed.; Humana Press: Totowa, NJ, USA, 2010; ISBN 978-1-60761-291-9. [Google Scholar]
- Mirella, D.; Martin, F.P. Neural Stem Cells; Methods in Molecular BiologyTM; Weiner, L.P., Ed.; Humana Press: Totowa, NJ, USA, 2008; Volume 438, ISBN 978-1-58829-846-1. [Google Scholar]
- Dolci, S.; Pino, A.; Berton, V.; Gonzalez, P.; Braga, A.; Fumagalli, M.; Bonfanti, E.; Malpeli, G.; Pari, F.; Zorzin, S.; et al. High Yield of Adult Oligodendrocyte Lineage Cells Obtained from Meningeal Biopsy. Front. Pharmacol. 2017, 8, 703. [Google Scholar] [CrossRef]
- Chetty, S.; Friedman, A.R.; Taravosh-Lahn, K.; Kirby, E.D.; Mirescu, C.; Guo, F.; Krupik, D.; Nicholas, A.; Geraghty, A.; Krishnamurthy, A.; et al. Stress and glucocorticoids promote oligodendrogenesis in the adult hippocampus. Mol. Psychiatry 2014, 19, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Gimsa, U.; ØRen, A.; Pandiyan, P.; Teichmann, D.; Bechmann, I.; Nitsch, R.; Brunner-Weinzierl, M.C. Astrocytes protect the CNS: Antigen-specific T helper cell responses are inhibited by astrocyte-induced upregulation of CTLA-4 (CD152). J. Mol. Med. 2004, 82, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Quaegebeur, A.; Lange, C.; Carmeliet, P. The neurovascular link in health and disease: Molecular mechanisms and therapeutic implications. Neuron 2011, 71, 406–424. [Google Scholar] [CrossRef] [PubMed]
- Windrem, M.S.; Schanz, S.J.; Morrow, C.; Munir, J.; Chandler-Militello, D.; Wang, S.; Goldman, S.A. A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J. Neurosci. 2014, 34, 16153–16161. [Google Scholar] [CrossRef] [PubMed]
- Blasko, I.; Humpel, C.; Grubeck-Loebenstein, B. Glial Cells: Astrocytes and Oligodendrocytes during Normal Brain Aging. In Encyclopedia of Neuroscience; Academic Press: Cambridge, MA, USA, 2009; pp. 743–747. ISBN 978-0-08-045046-9. [Google Scholar]
- Nave, K.-A.; Werner, H.B. Myelination of the nervous system: Mechanisms and functions. Annu. Rev. Cell Dev. Biol. 2014, 30, 503–533. [Google Scholar] [CrossRef] [PubMed]
- Zawadzka, M.; Rivers, L.E.; Fancy, S.P.J.; Zhao, C.; Tripathi, R.; Jamen, F.; Young, K.; Goncharevich, A.; Pohl, H.; Rizzi, M.; et al. CNS-resident glial progenitor/stem cells produce Schwann cells as well as oligodendrocytes during repair of CNS demyelination. Cell Stem Cell 2010, 6, 578–590. [Google Scholar] [CrossRef] [PubMed]
- Nantel, J. Albert Domoic Acid, International Programme on Chemical Safety Poisons Information. In Monograph; 670; Centre de Toxicologie du Quebec: Quebec, QC, Canada, 1996. [Google Scholar]
- Coleman, M. Axon degeneration mechanisms: Commonality amid diversity. Nat. Rev. Neurosci. 2005, 6, 889–898. [Google Scholar] [CrossRef]
- Pérez-Gómez, A.; Tasker, R.A. Enhanced mossy fiber sprouting and synapse formation in organotypic hippocampal cultures following transient domoic acid excitotoxicity. Neurotox. Res. 2014, 25, 402–410. [Google Scholar] [CrossRef]
- Ramsdell, J.S.; Gulland, F.M. Domoic acid epileptic disease. Mar. Drugs 2014, 12, 1185–1207. [Google Scholar] [CrossRef]
- Tiedeken, J.A.; Ramsdell, J.S. Persistent neurological damage associated with spontaneous recurrent seizures and atypical aggressive behavior of domoic acid epileptic disease. Toxicol. Sci. 2013, 133, 133–143. [Google Scholar] [CrossRef]
- Doucette, T.A.; Strain, S.M.; Allen, G.V.; Ryan, C.L.; Tasker, R.A. Comparative behavioural toxicity of domoic acid and kainic acid in neonatal rats. Neurotoxicol. Teratol. 2000, 22, 863–869. [Google Scholar] [CrossRef]
- Lefebvre, K.A.; Kendrick, P.S.; Ladiges, W.; Hiolski, E.M.; Ferriss, B.E.; Smith, D.R.; Marcinek, D.J. Chronic low-level exposure to the common seafood toxin domoic acid causes cognitive deficits in mice. Harmful Algae 2017, 64, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, K.A.; Hendrix, A.; Halaska, B.; Duignan, P.; Shum, S.; Isoherranen, N.; Marcinek, D.J.; Gulland, F.M.D. Domoic acid in California sea lion fetal fluids indicates continuous exposure to a neuroteratogen poses risks to mammals. Harmful Algae 2018, 79, 53–57. [Google Scholar] [CrossRef]
- Grant, K.S.; Crouthamel, B.; Kenney, C.; McKain, N.; Petroff, R.; Shum, S.; Jing, J.; Isoherranen, N.; Burbacher, T.M. Preclinical modeling of exposure to a global marine bio-contaminant: Effects of in utero Domoic acid exposure on neonatal behavior and infant memory. Neurotoxicol. Teratol. 2019, 73, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Burbacher, T.M.; Grant, K.S.; Petroff, R.; Shum, S.; Crouthamel, B.; Stanley, C.; McKain, N.; Jing, J.; Isoherranen, N. Effects of oral domoic acid exposure on maternal reproduction and infant birth characteristics in a preclinical nonhuman primate model. Neurotoxicol. Teratol. 2019, 72, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Petroff, R.; Richards, T.; Crouthamel, B.; McKain, N.; Stanley, C.; Grant, K.S.; Shum, S.; Jing, J.; Isoherranen, N.; Burbacher, T.M. Chronic, low-level oral exposure to marine toxin, domoic acid, alters whole brain morphometry in nonhuman primates. Neurotoxicology 2019, 72, 114–124. [Google Scholar] [CrossRef]
- Tong, L.M.; Fong, H.; Huang, Y. Stem cell therapy for Alzheimer’s disease and related disorders: Current status and future perspectives. Exp. Mol. Med. 2015, 47, e151. [Google Scholar] [CrossRef]
- Yamasaki, T.R.; Blurton-Jones, M.; Morrissette, D.A.; Kitazawa, M.; Oddo, S.; LaFerla, F.M. Neural stem cells improve memory in an inducible mouse model of neuronal loss. J. Neurosci. 2007, 27, 11925–11933. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, K.S.; Kim, E.J.; Choi, H.B.; Lee, K.H.; Park, I.H.; Ko, Y.; Jeong, S.W.; Kim, S.U. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells 2007, 25, 1204–1212. [Google Scholar] [CrossRef]
- Berman, F.W.; LePage, K.T.; Murray, T.F. Domoic acid neurotoxicity in cultured cerebellar granule neurons is controlled preferentially by the NMDA receptor Ca(2+) influx pathway. Brain Res. 2002, 924, 20–29. [Google Scholar] [CrossRef]
- Berman, F.W.; Murray, T.F. Domoic acid neurotoxicity in cultured cerebellar granule neurons is mediated predominantly by NMDA receptors that are activated as a consequence of excitatory amino acid release. J. Neurochem. 1997, 69, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Wilk-Zasadna, I.; Minta, M. Developmental toxicity of Ochratoxin A in rat embryo midbrain micromass cultures. Int. J. Mol. Sci. 2009, 10, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Kashem, M.A.; Sultana, N.; Balcar, V.J. Exposure of Rat Neural Stem Cells to Ethanol Affects Cell Numbers and Alters Expression of 28 Proteins. Neurochem. Res. 2018, 43, 1841–1854. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Zhao, X.; Wang, F.; Tan, R.; Wang, J.; Li, X.; Chen, C.; An, J.; Lu, H. Short term exposure to oxycodone alters the survival, proliferation and differentiation of rat embryonic neural stem cell in vitro. Brain Res. Bull. 2018, 143, 66–72. [Google Scholar] [CrossRef]
- Brewer, G.J.; Torricelli, J.R.; Evege, E.K.; Price, P.J. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 1993, 35, 567–576. [Google Scholar] [CrossRef]
- O’Donovan, M. A critique of methods to measure cytotoxicity in mammalian cell genotoxicity assays. Mutagenesis 2012, 27, 615–621. [Google Scholar] [CrossRef][Green Version]
- Shi, J.; Springer, S.; Escobar, P. Coupling cytotoxicity biomarkers with DNA damage assessment in TK6 human lymphoblast cells. Mutat. Res. 2010, 696, 167–178. [Google Scholar] [CrossRef]
- Shaltouki, A.; Peng, J.; Liu, Q.; Rao, M.S.; Zeng, X. Efficient generation of astrocytes from human pluripotent stem cells in defined conditions. Stem Cells 2013, 31, 941–952. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, S.; Kumara, V.M.R. Detecting Neurodevelopmental Toxicity of Domoic Acid and Ochratoxin A Using Rat Fetal Neural Stem Cells. Mar. Drugs 2019, 17, 566. https://doi.org/10.3390/md17100566
Gill S, Kumara VMR. Detecting Neurodevelopmental Toxicity of Domoic Acid and Ochratoxin A Using Rat Fetal Neural Stem Cells. Marine Drugs. 2019; 17(10):566. https://doi.org/10.3390/md17100566
Chicago/Turabian StyleGill, Santokh, and V. M. Ruvin Kumara. 2019. "Detecting Neurodevelopmental Toxicity of Domoic Acid and Ochratoxin A Using Rat Fetal Neural Stem Cells" Marine Drugs 17, no. 10: 566. https://doi.org/10.3390/md17100566
APA StyleGill, S., & Kumara, V. M. R. (2019). Detecting Neurodevelopmental Toxicity of Domoic Acid and Ochratoxin A Using Rat Fetal Neural Stem Cells. Marine Drugs, 17(10), 566. https://doi.org/10.3390/md17100566



