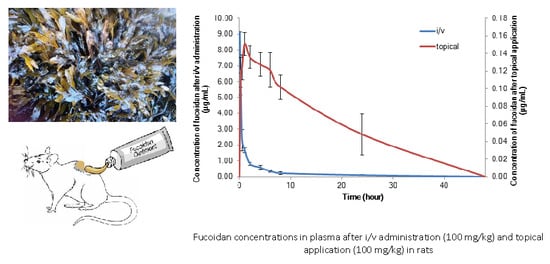
The Pharmacokinetics of Fucoidan after Topical Application to Rats
Abstract
Share and Cite
Pozharitskaya, O.N.; Shikov, A.N.; Obluchinskaya, E.D.; Vuorela, H. The Pharmacokinetics of Fucoidan after Topical Application to Rats. Mar. Drugs 2019, 17, 687. https://doi.org/10.3390/md17120687
Pozharitskaya ON, Shikov AN, Obluchinskaya ED, Vuorela H. The Pharmacokinetics of Fucoidan after Topical Application to Rats. Marine Drugs. 2019; 17(12):687. https://doi.org/10.3390/md17120687
Chicago/Turabian StylePozharitskaya, Olga N., Alexander N. Shikov, Ekaterina D. Obluchinskaya, and Heikki Vuorela. 2019. "The Pharmacokinetics of Fucoidan after Topical Application to Rats" Marine Drugs 17, no. 12: 687. https://doi.org/10.3390/md17120687
APA StylePozharitskaya, O. N., Shikov, A. N., Obluchinskaya, E. D., & Vuorela, H. (2019). The Pharmacokinetics of Fucoidan after Topical Application to Rats. Marine Drugs, 17(12), 687. https://doi.org/10.3390/md17120687