Inhibitory Effect of Depolymerized Sulfated Galactans from Marine Red Algae on the Growth and Adhesion of Diarrheagenic Escherichia coli
Abstract
:1. Introduction
2. Results
2.1. Yield and Composition of Polysaccharides
2.2. Effect of Depolymerization on the Antibacterial Activity of Sulfated Galactans
2.3. Effect of Molecular Weight on Antibacterial Activity
2.4. Effect of Anionic Properties on Antibacterial Activity
2.5. Effect of Monosaccharide Composition on Antibacterial Activity
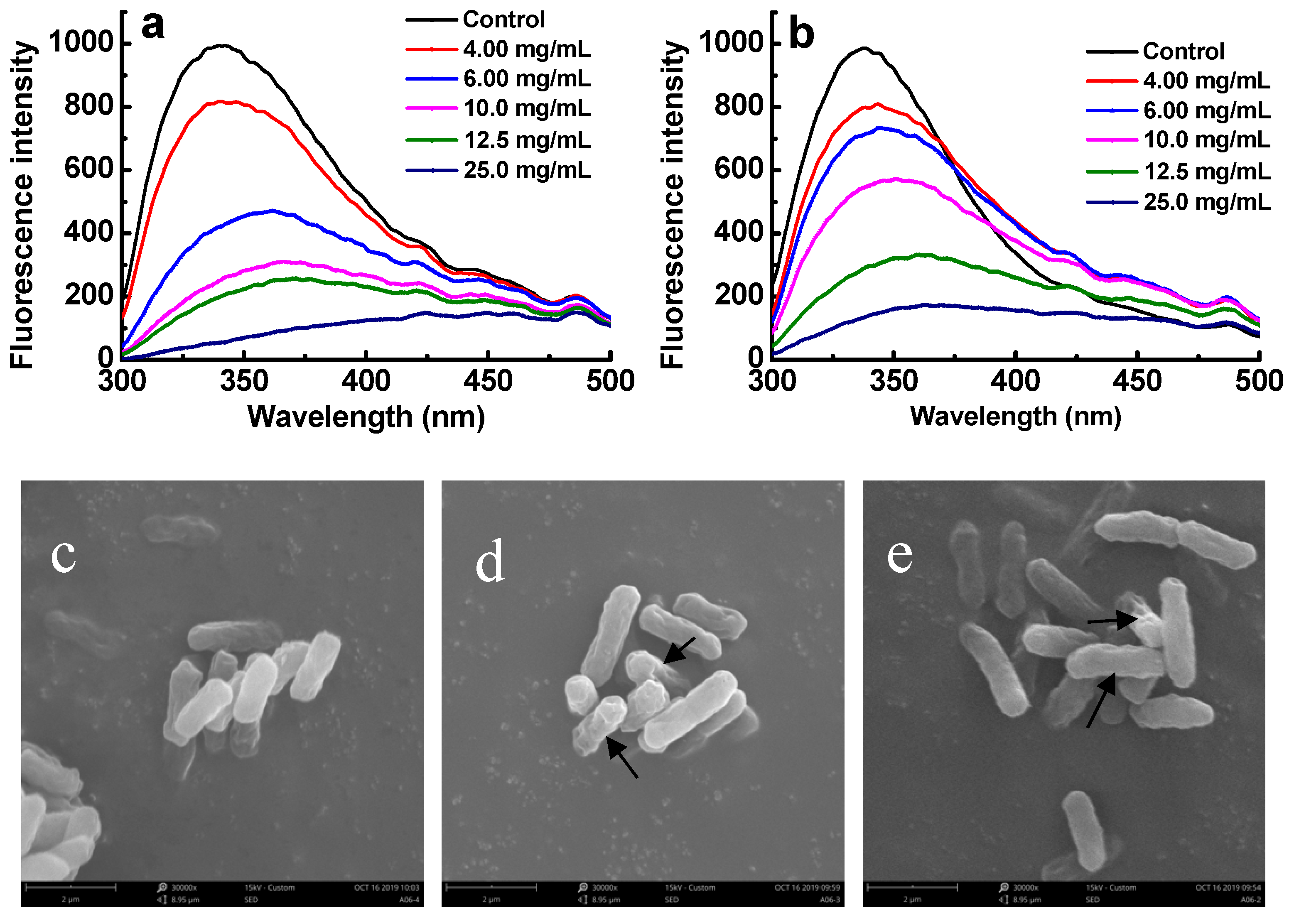
2.6. Effect of Sulfated Galactans on Cell Membrane Integrity
2.7. Effect of Sulfated Galactans on Cell Membrane Proteins
2.8. Effect of Sulfated Galactans on Bacterial Adhesion
3. Discussion
4. Materials and Methods
4.1. Chemicalsand Reagents
4.2. Extraction of Crude Sulfated Galactans
4.3. Purification of Sulfated Galactans
4.4. Analysis of Chemical Composition
4.5. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
4.6. Preparation of Depolymerized Sulfated Galactans and Physicochemical Property Analysis
4.6.1. Depolymerization and Molecular Weight Classification
4.6.2. Reducing Sugars and Viscosity
4.7. Fractionation by Ion-Exchange Chromatography
4.8. Microbial Strains and Culture
4.9. Antibacterial Assay
4.10. Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC)
4.11. Integrity of Cell Membrane
4.12. Effect on Cell Membrane Proteins
4.13. Inhibition of Bacterial Adhesion
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saeed, A.; Abd, H.; Sandstrom, G.E. Microbial aetiology of acute diarrhoea in children under five years of age in Khartoum, Sudan. J. Med. Microbiol. 2015, 64, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J. Infect. Dis. 1987, 155, 377–389. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qi, Y.; Chen, L.; Qu, D.; Li, Z.; Gao, K.; Chen, J.; Sun, Y. Effects of Panax ginseng polysaccharides on the gut microbiota in mice with antibiotic–associated diarrhea. Int. J. Biol. Macromol. 2019, 124, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Tziveleka, L.; Ioannou, E.; Roussis, V. Ulvan, a bioactive marine sulphated polysaccharide as a key constituent of hybrid biomaterials: A review. Carbohyd. Polym. 2019, 218, 355–370. [Google Scholar] [CrossRef] [PubMed]
- Marudhupandi, T.; Kumar, T.T.A. Antibacterial effect of fucoidan from Sargassum wightii against the chosen human bacterial pathogens. Int. Curr. Pharm. J. 2013, 2, 156–158. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Liu, Y.; Cao, M.; Liu, G.; Chen, Q.; Sun, L.; Chen, H. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohyd. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Pierre, G.; Sopena, V.; Juin, C.; Mastouri, A.; Graber, M.; Maugard, T. Antibacterial activity of a sulfated galactan extracted from the marine alga chaetomorphaaerea against staphylococcus aureus. Biotechnol. Bioproc. E 2011, 16, 937–945. [Google Scholar] [CrossRef]
- De Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci. 2014, 101, 56–63. [Google Scholar] [CrossRef]
- Sousa, N.A.; Barros, F.C.N.; Araújo, T.S.L.; Costa, D.S.; Souza, L.K.M.; Sousa, F.B.M.; Leódido, A.C.M.; Pacífico, D.M.; Araújo, S.D.; Bezerra, F.F.; et al. The efficacy of a sulphated polysaccharide fraction from Hypnea musciformis against diarrhea in rodents. Int. J. Biol. Macromol. 2016, 86, 865–875. [Google Scholar] [CrossRef]
- Bhadja, P.; Lunagariya, J.; Ouyang, J. Seaweed sulphated polysaccharide as an inhibitor of calcium oxalate renal stone formation. J. Funct. Foods 2016, 27, 685–694. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, W.; Song, W.; Chen, H.; Teng, A.; Liu, A. Partial characterization and anti–tumor activity of an acidic polysaccharide from Gracilaria verrucosa. Carbohyd. Polym. 2012, 88, 1313–1318. [Google Scholar] [CrossRef]
- Seedevi, P.; Moovendhan, M.; Viramani, S.; Shanmugam, A. Bioactive potential and structural chracterization of sulfated polysaccharide from seaweed (Gracilaria corticata). Carbohyd. Polym. 2017, 155, 516–524. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.; Mody, K.H. Heparinoid–active sulphated polysaccharides from marine algae as potential blood anticoagulant agents. J. Am. Oil Chem. Soc. 2000, 40, A6–A40. [Google Scholar]
- Amorim, R.D.N.D.S.; Rodrigues, J.A.G.; Holanda, M.L.; Quinderé, A.L.G.; Paula, R.C.M.D.; Melo, V.M.M.; Benevides, N.M.B. Antimicrobial effect of a crude sulfated polysaccharide from the red seaweed Gracilaria ornata. Braz. Arch. Biol. Technol. 2012, 55, 171–181. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Salah, H.B.; Jardak, N.; Chaaben, R.; Jribi, I.; Feki, A.E.; Rebai, T.; Jamoussi, K.; Allouche, N.; Blecker, C.; et al. Sulphated polysaccharide isolated from Sargassum vulgare: Characterization and hypolipidemic effects. Carbohyd. Polym. 2017, 170, 148–159. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Maleki, S.J.; Alcocer, M.; Xu, S.; Shi, C.; Cao, M.; Liu, G. Anti-food allergic activity of sulfated polysaccharide from Gracilaria verrucosa is dependent on immunosuppression and inhibition of p38 MAPK. J. Agric. Food Chem. 2016, 64, 4536–4544. [Google Scholar] [CrossRef]
- Abdelhedi, O.; Nasri, R.; Souissi, N.; Nasri, M.; Jridi, M. Sulfated polysaccharides from common smooth hound: Extraction and assessment of anti-ACE, antioxidant and antibacterial activities. Carbohyd. Polym. 2016, 152, 605–614. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Jiménez-Escrig, A.; Rupérez, P. Bioactivity of sulfated polysaccharides from the edible red seaweed Mastocarpus stellatus. Biol. Carbohyd. Diet. Fib. 2014, 3, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.I.; Kim, H.J. Preparation of low molecular weight fucoidan by gamma-irradiation and its anticancer activity. Carbohyd. Polym. 2013, 97, 358–362. [Google Scholar] [CrossRef]
- Hwang, P.A.; Hung, Y.L.; Phan, N.N.; Chang, P.M.; Li, K.L.; Lin, Y.C. The in vitro and in vivo effects of the low molecular weight fucoidan on the bone osteogenic differentiation properties. Cytotechnology 2016, 68, 1349–1359. [Google Scholar] [CrossRef]
- Palanisamy, S.; Vinosha, M.; Rajasekar, P.; Anjali, R.; Sathiyaraj, G.; Marudhupandi, T.; Narayanasamy, S.S.; Prabhu, M.; You, S. Antibacterial efficacy of a fucoidan fraction (Fu-F2) extracted from Sargassum polycystum. Int. J. Biol. Macromol. 2019, 125, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Shah, N.P. Antioxidant and antibacterial activities of sulphated polysaccharides from Pleurotus eryngii and Streptococcus thermophilus ASCC 1275. Food Chem. 2014, 165, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Rostand, K.S.; Esko, J.D. Microbial adherence to and invasion through proteoglycans. Infect. Immun. 1997, 65, 1–8. [Google Scholar] [PubMed]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Salmonella infection—Prevention and treatment by antibiotics and probiotic yeasts: A review. Microbiology 2018, 164, 1327–1344. [Google Scholar] [CrossRef] [PubMed]
- Lombard, Y.; Giaimis, J.; Makaya-Kumba, M.; Fonteneau, P.; Poindron, P. A new method for studying the binding and ingestion of zymosan particles by macrophages. J. Immunol. Methods 1994, 174, 155–165. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Heleno, S.A.; Reis, F.S.; Stojkovic, D.; Queiroz, M.J.R.; Vasconcelos, M.H.; Sokovic, M. Chemical features of Ganoderma polysaccharides with antioxidant, antitumor and antimicrobial activities. Phytochemistry 2015, 114, 38–55. [Google Scholar] [CrossRef] [Green Version]
- Tolstoguzov, V. Why were polysaccharides necessary? Origins Life Evol. B 2004, 34, 571–597. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.T.; Zheng, W.; Han, X.X.; Jiang, Y.H.; Hu, P.L.; Tang, Z.H.; Shi, L.E. The antibacterial activity and antibacterial mechanism of a polysaccharide from Cordyceps cicadae. J. Funct. Foods 2017, 38, 273–279. [Google Scholar] [CrossRef]
- Böttcher, T.; Szamosvári, D.; Clardy, J. A repeating sulfated galactan motif resuscitates dormant Micrococcus luteus bacteria. Appl. Environ. Microbiol. 2018, 84, e00745-18. [Google Scholar] [CrossRef] [Green Version]
- Balamayooran, G.; Batra, S.; Fessler, M.B.; Happel, K.I.; Jeyaseelan, S. Mechanisms of neutrophil accumulation in the lungs against bacteria. Am. J. Resp. Cell Mol. 2010, 43, 5–16. [Google Scholar] [CrossRef]
- Chen, C.Z.; Cooper, S.L. Interactions between dendrimer biocides and bacterial membranes. Biomaterials 2002, 23, 3359–3368. [Google Scholar] [CrossRef]
- Silva, F.R.F.; Dore, C.M.P.G.; Marques, C.T.; Nascimento, M.S.; Benevides, N.M.B.; Rocha, H.A.O.; Chavante, S.F.; Leite, E.L. Anticoagulant activity, paw edema and pleurisy induced carrageenan: Action of major types of commercial carrageenans. Carbohyd. Polym. 2010, 79, 26–33. [Google Scholar] [CrossRef]
- Gedek, B.R. Adherence of Escherichia coli serogroup O 157 and the Salmonella typhimurium mutant DT 104 to the surface of Saccharomyces boulardii. Mycoses 1999, 42, 261–264. [Google Scholar] [CrossRef]
- Tiago, F.C.P.; Martins, F.S.; Souza, E.L.S.; Pimenta, P.F.P.; Araujo, H.R.C.; Castro, I.M.; Brandao, R.L.; Nicoli, J.R. Adhesion to the yeast cell surface as a mechanism for trapping pathogenic bacteria by Saccharomyces probiotics. J. Med. Microbiol. 2012, 61, 1194–1207. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, X.; Ma, H.; Yan, J.; Guo, D. Purification, characterization and antitumor activity of polysaccharides extracted from Phellinus igniarius mycelia. Carbohyd. Polym. 2015, 133, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Leyva, A.; Quintana, A.; Sánchez, M.; Rodríguez, E.N.; Cremata, J.; Sánchez, J.C. Rapid and sensitive anthrone-sulfuric acid assay in microplate format to quantify carbohydrate in biopharmaceutical products: Method development and validation. Biologicals 2008, 36, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Kokoulin, M.S.; Kuzmich, A.S.; Kalinovsky, A.I.; Tomshich, S.V.; Romanenko, L.A.; Mikhailov, V.V.; Komandrova, N.A. Structure and anticancer activity of sulfated O-polysaccharide from marine bacterium Cobetia litoralis KMM 3880T. Carbohyd. Polym. 2016, 154, 55–61. [Google Scholar] [CrossRef]
- Theander, O.; Aman, P.; Westerlund, E.; Andersson, R.; Pettersson, D. Total dietary fiber determined as neutral sugar residues, uronic acid residues, and Klason lignin (the Uppsala method): Collaborative study. J. AOAC Int. 1995, 78, 1030–1044. [Google Scholar]
- Shi, C.; Pan, T.; Cao, M.; Liu, Q.; Zhang, L.; Liu, G. Suppression of Th2 immune responses by the sulfated polysaccharide from Porphyra haitanensis in tropomyosin–sensitized mice. Int. Immunopharmacol. 2015, 24, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Zhang, S.Z.; Yang, B.P.; Cheng, P.; Zhuan-Di, W.U.; Jun-Yu, H.U. Application of 3,5-dinitrosalicylic acid (DNS) method to test the reducing sugar and water-soluble total sugar content in sugarcane internodes. Sugarcane Canesugar 2008, 5, 45–49. [Google Scholar]
- Behrouzian, F.; Razavi, S.M.A.; Karazhiyan, H. Intrinsic viscosity of cress (Lepidium sativum) seed gum: Effect of salts and sugars. Food Hydrocolloid. 2014, 35, 100–105. [Google Scholar] [CrossRef]
- Li, R.; Feke, D.L. Rheological and kinetic study of the ultrasonic degradation of xanthan gum in aqueous solutions. Food Chem. 2015, 172, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Cheng, F.; Swing, C.J.; Xia, S.; Zhang, X. Modulation effect of core-wall ratio on the stability and antibacterial activity of cinnamaldehyde liposomes. Chem. Phys. Lipids 2019, 223, 104790. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Zhu, Y.; Li, C.; Liu, L.; Surendhiran, D.; Cui, H. Antibacterial activity of PEO nanofibers incorporating polysaccharide from dandelion and its derivative. Carbohyd. Polym. 2018, 198, 225–232. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yang, Y.; Yang, G.; Yu, L. Studies on antibacterial activity and antibacterial mechanism of a novel polysaccharide from Streptomyces virginia H03. Food Control 2010, 21, 1257–1262. [Google Scholar] [CrossRef]
- Gut, A.M.; Vasiljevic, T.; Yeager, T.; Donkor, O.N. Characterization of yeasts isolated from traditional kefir grains for potential probiotic properties. J. Funct. Foods 2019, 58, 56–66. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.; Zhu, J.; Huang, J.; Zhang, H. Inhibition of bacterial adhesion and biofilm formation of sulfonated chitosan against Pseudomonas aeruginosa. Carbohyd. Polym. 2019, 206, 412–419. [Google Scholar] [CrossRef]






| Items | ESP a | GSP b | |
|---|---|---|---|
| Yield (%, w/w) c | 19.5 ± 1.2 | 7.6 ± 0.5 | |
| Sulfate (%, w/w) | 28.2 ± 2.1 | 13.1 ± 1.9 | |
| Total carbohydrate (%, w/w) | 78.3 ± 4.5 | 83.8 ± 3.1 | |
| Uronic acid (%, w/w) | 2.2 ± 0.3 | 4.2 ± 0.4 | |
| 3,6-anhydrogalactose (%, w/w) | 9.8 ± 0.3 | 13.4 ± 0.5 | |
| Monosaccharide composition (%) d | Mannose | 3.2 | 2.7 |
| Glucuronic acid | 0.9 | 0.4 | |
| Galacturonic acid | 0.9 | 1.2 | |
| Glucose | 0.6 | 2.2 | |
| Galactose | 93.4 | 93.5 | |
| Xylose | 1.1 | 0 | |
| Fractions | D-ESP | D-GSP | ||||
|---|---|---|---|---|---|---|
| Yield (%) | Sulfate Group (%) | MIC a (mg/mL) | Yield (%) | Sulfate Group (%) | MIC (mg/mL) | |
| Mixure | 100.0 | 28.3 ± 1.9 | 25.0 | 100.0 | 13.3 ± 1.7 | 40.0 |
| ≤6 kDa | 43.2 | 27.2 ± 2.4 | 15.0 | 22.6 | 12.5 ± 1.2 | 25.0 |
| 6–20 kDa | 23.5 | 31.4 ± 3.4 | 20.0 | 33.1 | 12.1 ± 2.1 | 30.0 |
| 20–50 kDa | 16.9 | 29.7 ± 2.6 | - | 7.5 | 10.5 ± 2.3 | - |
| 50–80 kDa | 9.3 | 31.4 ± 3.2 | - | 9.3 | 12.4 ± 2.4 | - |
| >80 kDa | 7.1 | 24.9 ± 1.9 | - | 27.5 | 11.6 ± 1.1 | - |
| Test Items | D-ESP | D-GSP | |||
|---|---|---|---|---|---|
| E1 | E2 | E3 | G1 | G2 | |
| MIC (mg/mL) | 10.0 | 10.0 | 8.0 | 12.5 | 10.0 |
| MBC (mg/mL) | 25 | 25 | 12.5 | 25.0 | 25.0 |
| Sulfate group (%, w/w) | 19.4 ± 2.8 | 23.8 ± 3.1 | 29.2 ± 2.2 | 16.9 ± 2.1 | 23.1 ± 1.4 |
| Uronic acid (%, w/w) | 0.7 ± 0.1 | 1.2 ± 0.1 | 1.6 ± 0.1 | 2.2 ± 0.2 | 2.9 ± 0.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liu, W.; Wang, Y.; Ma, Y.; Huang, L.; Zou, C.; Li, D.; Cao, M.-J.; Liu, G.-M. Inhibitory Effect of Depolymerized Sulfated Galactans from Marine Red Algae on the Growth and Adhesion of Diarrheagenic Escherichia coli. Mar. Drugs 2019, 17, 694. https://doi.org/10.3390/md17120694
Liu Y, Liu W, Wang Y, Ma Y, Huang L, Zou C, Li D, Cao M-J, Liu G-M. Inhibitory Effect of Depolymerized Sulfated Galactans from Marine Red Algae on the Growth and Adhesion of Diarrheagenic Escherichia coli. Marine Drugs. 2019; 17(12):694. https://doi.org/10.3390/md17120694
Chicago/Turabian StyleLiu, Yixiang, Wenqiang Liu, Yanbo Wang, Yu Ma, Ling Huang, Chao Zou, Donghui Li, Min-Jie Cao, and Guang-Ming Liu. 2019. "Inhibitory Effect of Depolymerized Sulfated Galactans from Marine Red Algae on the Growth and Adhesion of Diarrheagenic Escherichia coli" Marine Drugs 17, no. 12: 694. https://doi.org/10.3390/md17120694





