Express Method for Isolation of Ready-to-Use 3D Chitin Scaffolds from Aplysina archeri (Aplysineidae: Verongiida) Demosponge
Abstract
:1. Introduction
2. Results
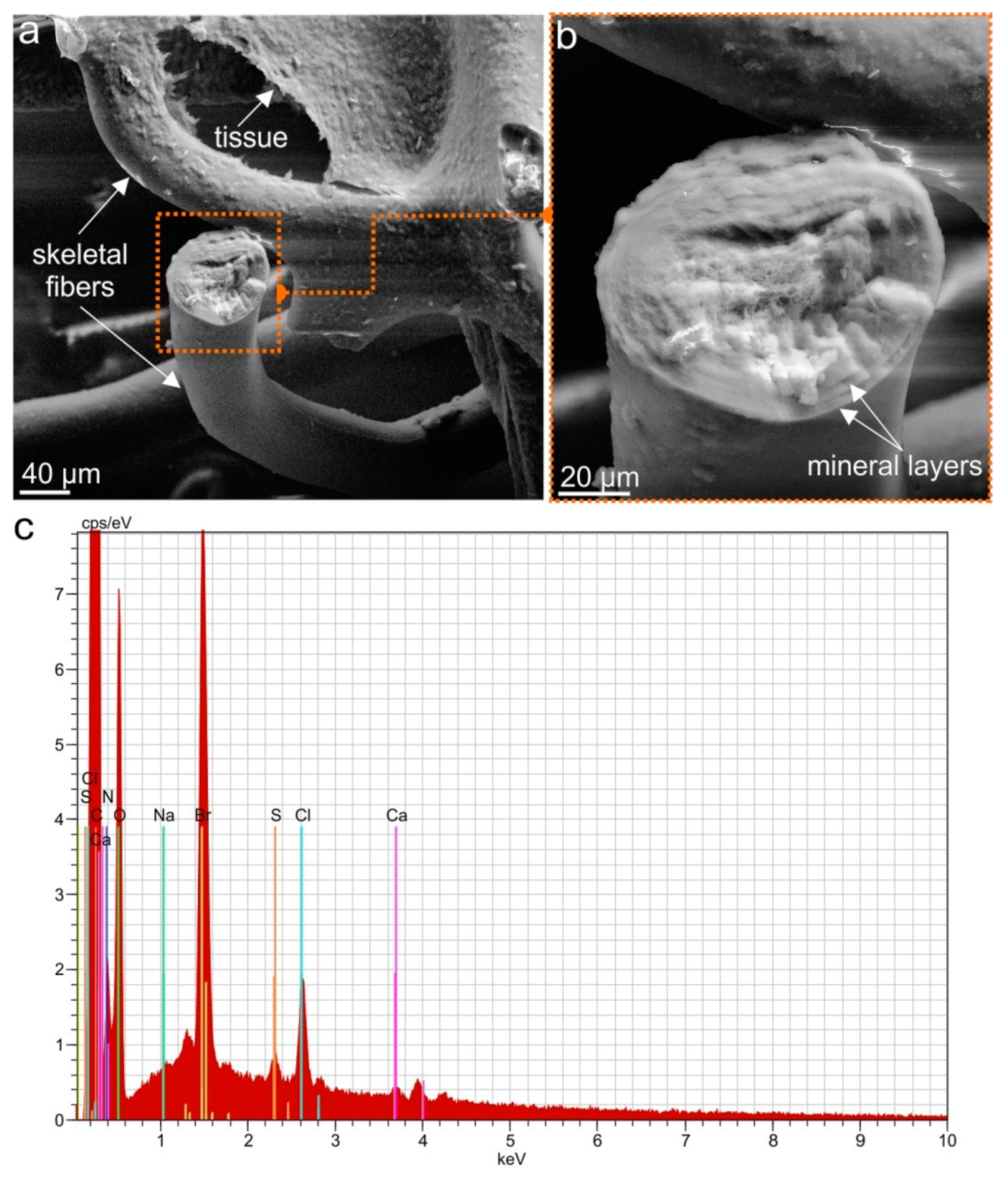
2.1. Isolation and Identification of Chitin
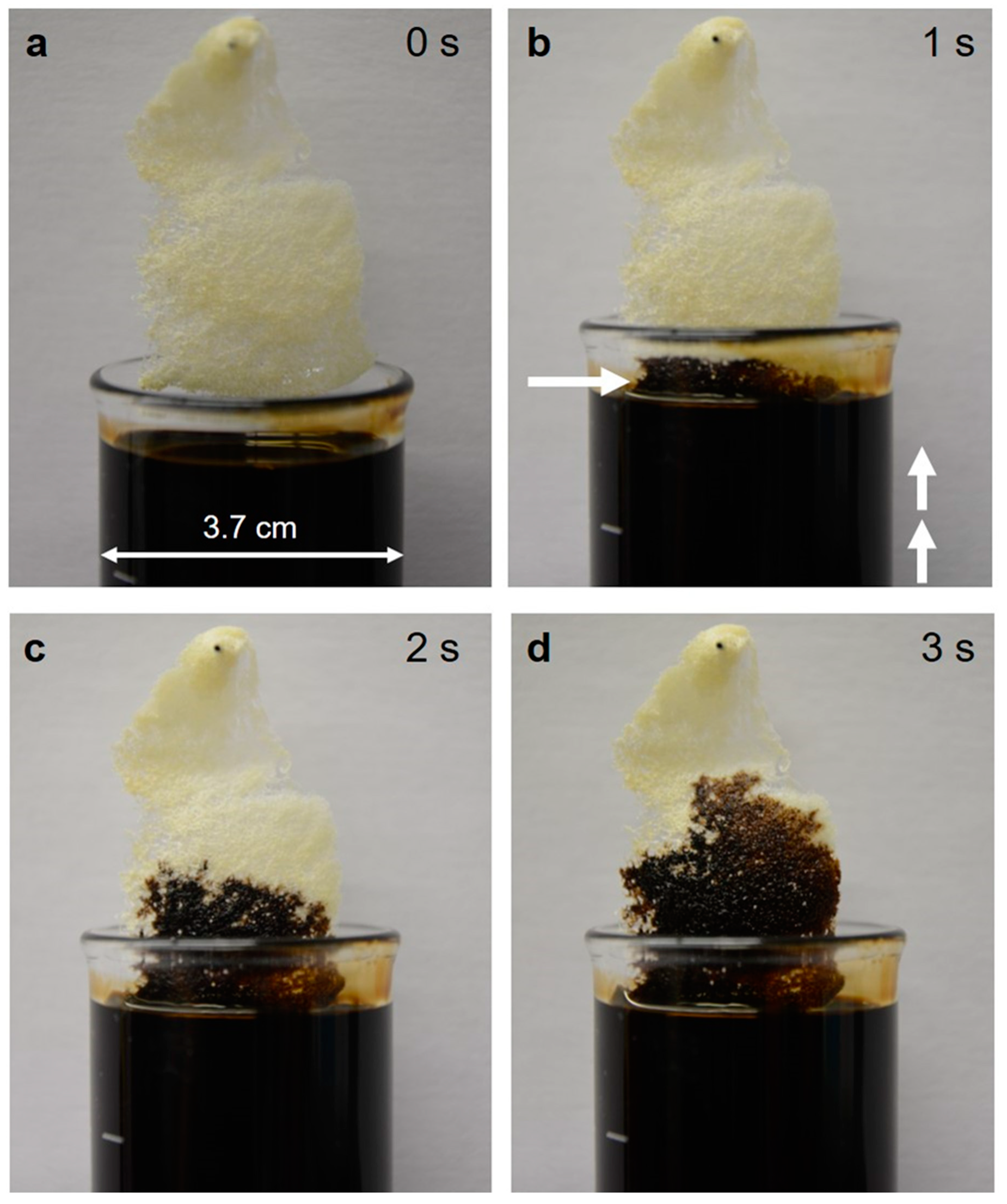
2.2. Capillary Effect of 3D Chitin Scaffolds
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Isolation of Chitin
4.2.1. Modified Standard Method
4.2.2. Microwave-Assisted Approach for Chitin Isolation
4.2.3. Fluorescent Microscopy Analysis
4.2.4. Calcofluor White Staining
4.2.5. Chitinase Digestion Test
4.2.6. ATR-FTIR Spectroscopy
4.2.7. Raman Spectroscopy
4.2.8. Scanning Electron Microscopy
4.2.9. EDX
4.2.10. Electrospray Ionisation Mass Spectrometry (ESI-MS)
4.2.11. Sorption Experiments
4.2.12. Swelling Capacity Measurements
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wysokowski, M.; Petrenko, I.; Stelling, A.L.; Stawski, D.; Jesionowski, T.; Ehrlich, H. Chitin as a versatile template for extreme biomimetics. Polymers 2015, 7, 235–265. [Google Scholar] [CrossRef]
- Ehrlich, H. Chitin and collagen as universal and alternative templates in biomineralization. Int. Geo. Rev. 2010, 52, 661–699. [Google Scholar] [CrossRef]
- Agbaje, O.B.A.; Ben, S.I.; Zax, D.B.; Schmidt, A.; Jacob, D.E. Biomacromolecules within bivalve shells: Is chitin abundant? Acta Biomater. 2018, 80, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Maldonado, M.; Spindler, K.D.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (demospongia: Porifera). J. Exp. Zool. B Mol. Dev. Evol. 2007, 308B, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Maldonado, M.; Parker, A.R.; Kulchin, Y.N.; Schilling, J.; Köhler, B.; Skrzypczak, U.; Simon, P.; Reiswig, H.M.; Tsurkan, M.V.; et al. Supercontinuum generation in naturally occurring glass sponges spicules. Adv. Opt. Mater. 2016, 4, 1608–1613. [Google Scholar] [CrossRef]
- Nikolov, S.; Petrov, M.; Lymperakis, L.; Friak, M.; Sachs, C.; Fabritius, H.O.; Raabe, D.; Neugebauer, J. Revealing the design principles of high-performance biological composites using Ab initio and multiscale simulations: The example of lobster cuticle. Adv. Mater. 2010, 22, 519–526. [Google Scholar] [CrossRef]
- Brunner, E.; Ehrlich, H.; Schupp, P.; Hedrich, R.; Hunoldt, S.; Kammer, M.; Machill, S.; Paasch, S.; Bazhenov, V.V.; Kurek, D.V.; et al. Chitin-based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. J. Struct. Biol. 2009, 168, 539–547. [Google Scholar] [CrossRef]
- Ehrlich, H.; Krautter, M.; Hanke, T.; Simon, P.; Knieb, C.; Heinemann, S.; Worch, H. First evidence of the presence of chitin in skeletons of marine sponges. Part II. Glass sponges (Hexactinellida: Porifera). J. Exp. Zool. 2007, 308B, 473–483. [Google Scholar] [CrossRef]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef]
- Ehrlich, H.; Steck, E.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part II: Biomimetic potential and applications. Int. J. Biol. Macromol. 2010, 47, 141–145. [Google Scholar] [CrossRef]
- Ehrlich, H.; Simon, P.; Carrillo-Cabrera, W.; Bazhenov, V.V.; Botting, J.P.; Ilan, M.; Ereskovsky, A.V.; Muricy, G.; Worch, H.; Mensch, A.; et al. Insights into chemistry of biological materials: Newly discovered silica-aragonite-chitin biocomposites in demosponges. Chem. Mater. 2010, 22, 1462–1471. [Google Scholar] [CrossRef]
- Roberts, G.A.F. Chitin Chemistry, 1st ed.; MacMillian: London, UK, 1992. [Google Scholar]
- Cahú, T.B.; Santos, S.D.; Mendes, A.; Córdula, C.R.; Chavante, S.F.; Carvalho, L.B.; Nader, H.B.; Bezerra, R.S. Recovery of protein, chitin, carotenoids and glycosaminoglycans from Pacific white shrimp (Litopenaeus vannamei) processing waste. Process Biochem. 2012, 47, 570–577. [Google Scholar] [CrossRef]
- Hackman, R.H. Studies on chitin V. The action of mineral acids on chitin. Aust. J. Biol. Sci. 1962, 15, 526–537. [Google Scholar] [CrossRef]
- Brine, C.J.; Austin, P.R. Chitin variability with species and method of preparation. Comp. Biochem. Physiol. 1981, 69B, 283–286. [Google Scholar] [CrossRef]
- Hayes, M.; Carney, B.; Slater, J.; Brück, W. Mining marine shellfish wastes for bioactive molecules: Chitin and chitosan – Part B: Applications. Biotechnol. J. 2008, 3, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Rasti, H.; Parivar, K.; Baharara, J.; Iranshahi, M.; Namvar, F. Chitin from the Mollusc chiton: extraction, characterization and chitosan preparation. Iran J. Pharm. Res. 2017, 16, 366–379. [Google Scholar] [PubMed]
- Bulut, E.; Sargin, I.; Arslan, O.; Odabasi, M.; Akyuz, B.; Kaya, M. In situ chitin isolation from body parts of a centipede and lysozyme adsorption studies. Mater. Sci. Eng. C 2017, 70, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Ibitoye, E.B.; Lokman, I.H.; Hezmee, M.N.M.; Goh, Y.M.; Zuki, A.B.Z.; Jimoh, A.A. Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed. Mater. 2018, 13, 025009. [Google Scholar] [CrossRef] [PubMed]
- Soon, C.Y.; Tee, Y.B.; Tan, C.H.; Rosnita, A.T.; Khalina, A. Extraction and physicochemical characterization of chitin and chitosan from Zophobas morio larvae in varying sodium hydroxide concentration. Int. J. Biol. Macromol. 2018, 108, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.B.; Hackman, R.H. Application of ethylenediaminetetraacetic acid in the isolation of crustacean chitin. Nature 1957, 180, 40–41. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin extraction from crustacean shells using biological methods - A review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Kaya, M.; Karaarslan, M.; Baran, T.; Can, E.; Ekemen, G.; Bitim, B.; Duman, F. The quick extraction of chitin from an epizoic crustacean species (Chelonibia patula). Nat. Prod. Res. 2014, 28, 2186–2190. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Synowiecki, J. Isolation and characterization of nutrients and value-added products from snow crab (Chionoecetes opilio) and shrimp (Pandalus borealis) processing discards. J. Agric. Food Chem. 1991, 39, 1527–1532. [Google Scholar] [CrossRef]
- Martin, R.E.; Flick, G.J.; Hebard, C.E.; Ward, D.R. Chemistry & Biochemistry of Marine Food Products; AVI Publishing Co.: Westport, CT, USA, 1982. [Google Scholar]
- Marquis-Duval, F.O. Isolation et valorisation des constituants de la carapace de la crevette nordique. Ph.D. Thesis, Laval University, Quebec, QC, Canada, 2008. [Google Scholar]
- Kaur, S.; Dhillon, G.S. Recent trends in biological extraction of chitin from marine shell wastes: A review. Crit. Rev. Biotechnol. 2015, 35, 44–61. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.O.; Hausler, R.; Monette, F.; Niquette, P. Valorisation des residus industriels de peches pour la transformation de chitosane par technique hydrothermo-chimique. Rev. Sci. Eau. 2007, 20, 253–262. [Google Scholar]
- Okafor, V. Isolation of chitin from the shell of the cuttlefish, Sepia officinalis L. BBA 1965, 101, 193–200. [Google Scholar] [CrossRef]
- Khanafari, A.; Marandi, R.; Sanatei, S. Recovery of chitin and chitosan from shrimp waste by chemical and microbial methods. Iran J. Env. Heal. Sci. Eng. 2008, 5, 1–24. [Google Scholar]
- Berton, P.; Shamshina, J.L.; Ostadjoo, S.; King, C.A.; Rogers, R.D. Enzymatic hydrolysis of ionic liquid-extracted chitin. Carbohydr. Polym. 2018, 199, 228–235. [Google Scholar] [CrossRef]
- Knidri, H.E.; Belaabed, R.; Addaou, A.; Laajeb, A.; Lahsini, A. Extraction, chemical modification and characterization of chitin and chitosan. Int. J. Biol. Macromol. 2018, 120, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Tokatli, K.; Demirdöven, A. Optimization of chitin and chitosan production from shrimp wastes and characterization. J. Food. Process. Preserv. 2017, 42, e13494. [Google Scholar] [CrossRef]
- Dun, Y.; Li, Y.; Xu, J.; Hu, Y.; Zhang, C.; Liang, Y.; Zhao, S. Simultaneous fermentation and hydrolysis to extract chitin from crayfish shell waste. Int. J. Biol. Macromol. 2018, 123, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Ho, T.C.; Chae, S.J.; Cho, Y.J.; Park, J.S.; Lee, H.J.; Chun, B.S. Deep eutectic solvent-based extraction and fabrication of chitin films from crustacean waste. Carbohydr. Polym. 2018, 195, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.; Guerrero-Legarreta, I.; Bórquez, R. Chitin extraction from Allopetrolisthes punctatus crab using lactic fermentation. Biotechnol. Rep. 2018, 20, e00287. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Zhao, D.; Guo, N.; Xue, C.; Mao, X. Green and facile production of chitin from crustacean shells using a natural deep eutectic solvent. J. Agric. Food Chem. 2018, 66, 11897–11901. [Google Scholar] [CrossRef]
- Hong, S.; Yang, Q.; Yuan, Y.; Chen, L.; Song, Y.; Lian, H. Sustainable co-solvent induced one step extraction of low molecular weight chitin with high purity from raw lobster shell. Carbohydr. Polym. 2019, 205, 236–243. [Google Scholar] [CrossRef]
- Jesionowski, T.; Norman, M.; Żółtowska-Aksamitowska, S.; Petrenko, I.; Joseph, Y.; Ehrlich, H. Marine spongin: naturally prefabricated 3D scaffold-based biomaterial. Mar. Drugs 2018, 16, 88. [Google Scholar] [CrossRef]
- Żółtowska-Aksamitowska, S.; Shaala, L.A.; Youssef, D.T.A.; Elhady, S.S.; Tsurkan, M.V.; Petrenko, I.; Wysokowski, M.; Tabachnick, K.; Meissner, H.; Ivanenko, V.N.; et al. First report on chitin in a non-Verongiid marine Demosponge: the Mycale euplectellioides case. Mar. Drugs 2018, 16, 68. [Google Scholar] [CrossRef]
- Żółtowska-Aksamitowska, S.; Tsurkan, M.V.; Lim, S.C.; Meissner, H.; Tabachnick, K.; Shaala, L.A.; Youssef, D.T.A.; Ivanenko, V.N.; Petrenko, I.; Wysokowski, M.; et al. The demosponge Pseudoceratina purpurea as a new source of fibrous chitin. Int. J. Biol. Macromol. 2018, 112, 1021–1028. [Google Scholar] [CrossRef]
- Van Soest, R.W.M.; Boury-Esnault, N.; Hooper, J.N.A.; Rützler, K.; de Voogd, N.J.; Alvarez, B.; Hajdu, E.; Pisera, A.B.; Manconi, R.; Schönberg, C.; et al. World Porifera database. Aplysina Archeri 1875. Available online: http://www.marinespecies.org/porifera/porifera.php?p=taxdetails&id=169636 (accessed on 22 February 2019).
- Bechmann, N.; Ehrlich, H.; Eisenhofer, G.; Ehrlich, A.; Meschke, S.; Ziegler, C.G.; Bornstein, S.R. Anti-tumorigenic and anti-metastatic activity of the sponge-derived marine drugs Aeroplysinin-1 and Isofistularin-3 against Pheochromocytoma in vitro. Mar. Drugs 2018, 16, 172. [Google Scholar] [CrossRef] [PubMed]
- Wysokowski, M.; Bazhenov, V.V.; Tsurkan, M.V.; Galli, R.; Stelling, A.L.; Stöcker, H.; Kaiser, S.; Niederschlag, E.; Gärtner, G.; Behm, T.; et al. Isolation and identification of chitin in three-dimensional skeleton of Aplysina fistularis marine sponge. Int. J. Biol. Macromol. 2013, 62, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Nickerl, J.; Tsurkan, M.; Hensel, R.; Neinhuis, C.; Werner, C. The multi-layered protective cuticle of Collembola: a chemical analysis. J. R. Soc. Interface 2014, 11, 6–19. [Google Scholar] [CrossRef]
- Ehrlich, H.; Rigby, J.K.; Botting, J.P.; Tsurkan, M.; Werner, C.; Schwille, P.; Petrasek, Z.; Pisera, A.; Simon, P.; Sivkov, V.; et al. Discovery of 505-million-year old chitin in the basal demosponge Vauxia gracilenta. Sci. Rep. 2013, 3, 3497. [Google Scholar] [CrossRef] [PubMed]
- Focher, B.; Naggi, T.; Cosani, A.; Terbojevich, M. Structural differences between chitin polymorphs and their precipitates from solutions—evidence from CP-MAS 13C-NMR, FT-IR and FT-Raman spectroscopy. Carbohydr. Polym. 1992, 17, 97–102. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef]
- Wysokowski, M.; Motylenko, M.; Bazhenov, V.V.; Stawski, D.; Petrenko, I.; Ehrlich, A.; Behm, T.; Kljajic, Z.; Stelling, A.L.; Jesionowski, T.; et al. Poriferan chitin as template for hydrotermal zirconia deposition. Front. Mater. Sci. 2013, 7, 248–260. [Google Scholar]
- Duan, B.; Gao, H.; He, M.; Zhang, L. Hydrophobic Modification on surface of chitin sponges for highly effective separation of oil. Appl. Mater. Interfaces 2014, 6, 19933–19942. [Google Scholar] [CrossRef]
- Setti, L.; Mazzieri, S.; Pifferi, P.G. Enhanced degradation of heavy oil in an aqueous system by a Pseudomonas sp. in the presence of natural and synthetic sorbents. Biores. Technology 1999, 67, 191–199. [Google Scholar] [CrossRef]
- De Freitas Barros, F.C.; Grombone Vasconcellos, L.C.; Vieira Carvalho, T.; Ferreira do Nascimento, R. Removal of petroleum spill in water by chitin and chitosan. Electron. J. Chem. 2014, 6, 70–74. [Google Scholar]
- Lv, L.; Tang, F.; Lan, G. Preparation and characterization of a chitin/platelet-poor plasma composite as a hemostatic material. RSC Advances 2016, 6, 95358–95368. [Google Scholar] [CrossRef]
- Drozd, N.N.; Torlopov, M.A.; Udoratina, E.V.; Logvinova, Y.S. Effect of nanocrystalline particles of chitin on blood components in humans and experimental animals. Bull Exp. Biol. Med. 2018, 164, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Dotto, G.L. Adsorption of Methylene Blue by ultrasonic surface modified chitin. J. Colloid Interface Sci. 2015, 446, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, S.; Karthikeyan, E. Microwave synthesis-a potential tool for green chemistry. Int. J. Chem. Tech. Res. 2011, 3, 466–470. [Google Scholar]
- Nüchter, M.; Ondruschka, B.; Bonrath, W.; Gum, A. Microwave assisted synthesis–a critical technology overview. Green Chem. 2004, 6, 128–141. [Google Scholar] [CrossRef]
- Duarte, K.; Justino, C.I.L.; Gomes, A.M.; Rocha-Santos, T.; Duarte, A.C. Green analytical methodologies for preparation of extracts and analysis of bioactive compounds. Compr. Anal. Chem. 2014, 65, 59–78. [Google Scholar]
- Soria, A.C.; Ruiz-Aceituno, L.; Ramos, L.; Sanz, L.M. Microwave-assisted extraction of polysaccharides. In Polysaccharides; Ramawat, K., Mérillon, J.M., Eds.; Springer: Cham, Germany, 2014; pp. 1–18. [Google Scholar]
- Apriyanti, D.T.; Susanto, H.; Rokhati, N. Influence of microwave irradiation on extraction of chitosan from shrimp shell waste. Reaktor 2018, 18, 45–50. [Google Scholar] [CrossRef]
- Al Sagheer, F.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Cabohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Sahu, A.; Goswami, P.; Bora, U. Microwave mediated rapid synthesis of chitosan. J. Mater Sci. Mater Med. 2009, 20, 171–175. [Google Scholar] [CrossRef]
- Lertwattanaseri, T.; Ichikawa, N.; Mizoguchi, T.; Tanaka, Y.; Chirachanchai, S. Microwave technique for efficient deacetylation of chitin nanowhiskers to a chitosan nanoscaffold. Carbohydr. Res. 2009, 344, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Peniston, Q.P.; Johnson, E.L. Process for activating chitin by microwave treatment and improved activated chitin product. US4159932, 7 March 1979. [Google Scholar]
- Wongpanit, P.; Sanchavanakit, N.; Pavasant, P.; Supaphol, P.; Tokura, S.; Rujiravanit, R. Preparation and characterization of microwave-treated carboxymethyl chitin and carboxymethyl chitosan films for potential use in wound care application. Macromol Biosci. 2005, 5, 1001–1012. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; Cross, S.S. Fistularin 3 and 11-ketofistularin 3. Feline leukemia virus active bromotyrosine metabolites from the marine sponge Aplysina archeri. J. Nat. Prod. 1992, 55, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Magno, S. Archerine, A novel anti-histaminic bromotyrosine-derived compound from the caribbean marine sponge Aplysina archeri. Eur. J. Org. Chem. 2001, 1, 55–60. [Google Scholar] [CrossRef]
- Cimino, G.; De Rosa, S.; De Stefano, S.; Sodano, G. The zoochrome of the sponge Verongia aerophoba (“Uranidine”). Tetrahedron Lett. 1984, 25, 2925–2928. [Google Scholar] [CrossRef]
- Schleuter, D.; Günther, A.; Paasch, S.; Ehrlich, H.; Kljajic, Z.; Hanke, T.; Bernhard, G.; Brunner, E. Chitin–based renewable materials from marine sponges for uranium adsorption. Carbohydr. Polym. 2013, 92, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Stepniak, I.; Galinski, M.; Nowacki, K.; Wysokowski, M.; Jakubowska, P.; Bazhenov, V.V.; Leisegang, T.; Ehrlich, H.; Jesionowski, T. A novel chitosan/sponge chitin origin material as a membrane for supercapacitors – preparation and characterization. RSC Advances 2016, 6, 4007–4013. [Google Scholar] [CrossRef]
- Wysokowski, M.; Behm, T.; Born, R.; Bazhenov, V.V.; Meissner, H.; Richter, G.; Szwarc–Rzepka, K.; Makarova, A.; Vyalikh, D.; Schupp, P.; et al. Preparation of chitin–silica composites by in vitro silicification of two–dimensional Ianthella basta demosponge chitinous scaffolds under modified Stöber conditions. Mater. Sci. Eng. 2013, 33, 3935–3941. [Google Scholar] [CrossRef]
- Ehrlich, H. Biomimetic potential of chitin–based composite biomaterials of poriferan origin. In Biomimetic Biomaterials: Structure and Applications; Ruys, A.J., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 47–67. [Google Scholar]
- Wysokowski, M.; Motylenko, M.; Beyer, J.; Makarova, A.; Stöcker, H.; Walter, J.; Galli, R.; Kaiser, S.; Vyalikh, D.; Bazhenov, V.V.; et al. Extreme biomimetic approach for development of novel chitin–GeO2 nanocomposites with photoluminescent properties. Nano Res. 2015, 8, 2288–2301. [Google Scholar] [CrossRef]
- Petrenko, I.; Bazhenov, V.V.; Galli, R.; Wysokowski, M.; Fromont, J.; Schupp, P.J.; Stelling, A.L.; Niederschlag, E.; Stöcker, H.; Kutsova, V.Z.; et al. Chitin of poriferan origin and the bioelectrometallurgy of copper/copper oxide. Int. J. Biol. Macromol. 2017, 104, 1626–1632. [Google Scholar] [CrossRef]
- Steck, E.; Burkhardt, M.; Ehrlich, H.; Richter, W. Discrimination between cells of murine and human origin in xenotransplants by species specific genomic in situ hybridization. Xenotransplantation 2010, 17, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.V.; Bazhenov, V.V.; Rogulska, O.; Tarusin, D.N.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; Meissner, H.; Lode, A.; et al. 3D chitinous scaffolds derived from cultivated marine demosponge Aplysina aerophoba for tissue engineering approaches based on human mesenchymal stromal cells. Int. J. Biol. Macromol. 2017, 104, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel chitin scaffolds derived from marine sponge Ianthella basta for tissue engineering approaches based on human mesenchymal stromal cells: biocompatibility and cryopreservation. Int. J. Biol. Macromol. 2017, 104, 1955–1965. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; John, B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog. Polym. Sci. 2013, 38, 1487–1503. [Google Scholar] [CrossRef]
- Prasadh, S.; Wong, R.C.W. Unraveling the mechanical strength of biomaterials used as a bone scaffold in oral and maxillofacial defects. Oral Sci. Int. 2018, 15, 48–55. [Google Scholar] [CrossRef]
- Chang, C.; Peng, N.; He, M.; Teramoto, Y.; Nishio, Y.; Zhang, L. Fabrication and properties of chitin/hydroxyapatite hybrid hydrogels as scaffold nano-materials. Carbohydr.Polym. 2013, 91, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.N.; Jiao, K.; Qi, Y.; Yiu, C.K.Y.; Ryou, H.; Arola, D.D.; Chen, J.; Breschi, L.; Pashley, D.H.; Tay, F.R. Infiltration of Silica Inside Fibrillar Collagen. Angew. Chem. Int. Ed. 2011, 50, 11688–11691. [Google Scholar] [CrossRef] [PubMed]
- Smolyakov, G.; Pruvost, S.; Cardoso, L.; Alonso, B.; Belamie, E.; Duchet-Rumeau, J. AFM PeakForce QNM mode: Evidencing nanometre-scale mechanical properties of chitin-silica hybrid nanocomposites. Carbohydr. Polym. 2016, 151, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, D.; Yano, H.; Abe, K. Bioinspired hydrogels: Quinone crosslinking reaction for chitin nanofibers with enhanced mechanical strength via surface deacetylation. Carbohydr. Polym. 2019, 207, 411–417. [Google Scholar] [CrossRef]
- Shaala, L.A.; Asfour, H.Z.; Youssef, D.T.A.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.; Galli, R.; Meissner, H.; Petrenko, I.; Tabachnick, K.; et al. New source of 3D chitin scaffolds: the Red Sea demosponge Pseudoceratina arabica (Pseudoceratinidae, Verongiida). Mar. Drugs 2019, 17, 92. [Google Scholar] [CrossRef]
- Rohde, S.; Schupp, P.J. Growth and regeneration of the elephant ear sponge Ianthella basta (Porifera). Hydrobiologia 2012, 687, 219–226. [Google Scholar] [CrossRef]
- Wojtasz-Pająk, A.; Szumilewicz, J. Degradation of chitin with hydrogen peroxide in microwave fields. In Progress on Chemistry of Chitin and Its Derivatives; Jaworska, M., Ed.; Polish Chitin Society: Łódź, Poland, 2007; Volume 12, pp. 13–24. [Google Scholar]
- Felinto, M.C.F.C.; Parra, D.F.; da Silva, C.C.; Angerami, J.; Oliveira, M.J.A.; Lugao, A.B. The swelling behavior of chitosan hydrogels membranes obtained by UV- and γ-radiation. Nucl. Instrum. Methods Phys. Res. B 2007, 265, 418–424. [Google Scholar] [CrossRef]












© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klinger, C.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Petrenko, I.; Machałowski, T.; Ereskovsky, A.; Martinović, R.; Muzychka, L.; et al. Express Method for Isolation of Ready-to-Use 3D Chitin Scaffolds from Aplysina archeri (Aplysineidae: Verongiida) Demosponge. Mar. Drugs 2019, 17, 131. https://doi.org/10.3390/md17020131
Klinger C, Żółtowska-Aksamitowska S, Wysokowski M, Tsurkan MV, Galli R, Petrenko I, Machałowski T, Ereskovsky A, Martinović R, Muzychka L, et al. Express Method for Isolation of Ready-to-Use 3D Chitin Scaffolds from Aplysina archeri (Aplysineidae: Verongiida) Demosponge. Marine Drugs. 2019; 17(2):131. https://doi.org/10.3390/md17020131
Chicago/Turabian StyleKlinger, Christine, Sonia Żółtowska-Aksamitowska, Marcin Wysokowski, Mikhail V. Tsurkan, Roberta Galli, Iaroslav Petrenko, Tomasz Machałowski, Alexander Ereskovsky, Rajko Martinović, Lyubov Muzychka, and et al. 2019. "Express Method for Isolation of Ready-to-Use 3D Chitin Scaffolds from Aplysina archeri (Aplysineidae: Verongiida) Demosponge" Marine Drugs 17, no. 2: 131. https://doi.org/10.3390/md17020131








