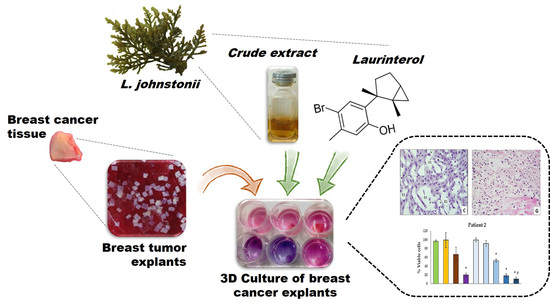
Antitumoral Effect of Laurinterol on 3D Culture of Breast Cancer Explants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction and Isolation
2.2. Cytotoxicity Assays
2.3. Antitumoral Effect on Breast Cancer Explants
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Material
3.3. Extraction and Isolation
3.4. Cell Culture
3.5. Cytotoxicity Activity
3.6. In Situ H&E Staining of MCF-7 Cells
3.7. Tumor Samples
3.8. Preparation of Slices and Explants from Breast Cancer
3.9. Treatment of Tumor Explants
3.10. Alamar Blue™ Viability Assay
3.11. Histopatological Analysis
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alves, C.; Silva, J.; Pinteus, S.; Gaspar, H.; Alpoim, M.C.; Botana, L.M.; Pedrosa, R. From marine origin to therapeutics: The antitumor potential of marine algae-derived compounds. Front. Pharmacol. 2018, 9, 777. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D.; Guiry, M.D. AlgaBase. World-wide electronic publication, National University of Irland, Galway. Available online: www.algaebase.org (accessed on 12 May 2018).
- Vázquez-Borja, R. Análisis Comparativo de la Ficoflora de Baja California Sur; Maestría en Ciencias, Instituto Politécnico Nacional: Mexico City, Mexico, 1999. [Google Scholar]
- Harizani, M.; Ioannou, E.; Roussis, V. The Laurencia paradox: An endless source of chemodiversity. Prog. Chem. Org. Nat. Prod. 2016, 102, 91–252. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Suzuki, M.; Abe, T.; Masuda, M. Halogenated metabolites with antibacterial activity from the Okinawan Laurencia species. Phytochemistry 2001, 58, 517–523. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Kawamoto, T.; Miwa, H.; Suzuki, M. Potent antibacterial activity of halogenated compounds against antibiotic-resistant bacteria. Planta Med. 2004, 70, 1087–1090. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Yamashita, Y.; Ohta, T. New cytotoxic and antibacterial compounds isolated from sea hare, Aplysia kurodai. Mar Drugs 2005, 3, 22–28. [Google Scholar] [CrossRef]
- Kladi, M.; Xenaki, H.; Vagias, C.; Papazafiri, P.; Roussis, V. New cytotoxic sesquiterpenes from the red algae Laurencia obtusa and Laurencia microcladia. Tetrahedron 2006, 62, 182–189. [Google Scholar] [CrossRef]
- Oguri, Y.; Watanabe, M.; Ishikawa, T.; Kamada, T.; Vairappan, C.; Matsuura, H.; Kaneko, K.; Ishii, T.; Suzuki, M.; Yoshimura, E.; Nogata, Y. New marine antifouling compounds from the red alga Laurencia sp. Mar. Drugs 2017, 15, 267. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Nitanda, N.; Ojika, M.; Sakagami, Y. Aplysiallene, a new bromoallene as an Na, K-ATPase inhibitor from the sea hare, Aplysia kurodai. Biosci. Biotechnol. Biochem. 2001, 65, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Nagamine, T.; Nguyen, B.C.; Tawata, S. Insecticidal and repellent activities of laurinterol from the Okinawan red alga Laurencia nidifica. Rec. Nat. Prod. 2017, 11, 63–68. [Google Scholar]
- García-Davis, S.; Sifaoui, I.; Reyes-Batlle, M.; Viveros-Valdez, E.; Piñero, J.E.; Lorenzo-Morales, J.; Fernández, J.J.; Díaz-Marrero, A.R. Anti-Acanthamoeba activity of brominated sesquiterpenes from Laurencia johnstonii. Mar. Drugs 2018, 16, 443. [Google Scholar] [CrossRef] [PubMed]
- WHO. Cancer Fact Sheet. Available online: www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/en/ (accessed on 22 January 2019).
- Perez, E.A. Impact, mechanisms, and novel chemotherapy strategies for overcoming resistance to anthracyclines and taxanes in metastatic breast cancer. Breast. Cancer Res. Treat. 2009, 114, 195–201. [Google Scholar] [CrossRef]
- Ambudkar, S.V.; Dey, S.; Hrycyna, C.A.; Ramachandra, M.; Pastan, I.; Gottesman, M.M. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol Toxicol. 1999, 39, 361–398. [Google Scholar] [CrossRef]
- Maass, N.; Schütz, F.; Fasching, P.A.; Fehm, T.; Janni, W.; Kümmel, S.; Lüfner, D.; Wallwiener, M.; Lux, M.P. Breast cancer update 2014—Focus on the patient and the tumor. Geburtshile Frauenheilkd. 2015, 75, 170–182. [Google Scholar] [CrossRef]
- Taran, F.A.; Schneeweiss, A.; Lux, M.P.; Janni, W.; Hartkopf, A.D.; Nabieva, N.; Overkamp, F.; Kolberg, H.C.; Hadji, P.; Tesch, H.; Wöckel, A. Update breast cancer 2018 (Part 1)—Primary breast cancer and biomarkers. Geburtshile Frauenheilkd. 2018, 78, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Carranza-Rosales, P.; Guzmán-Delgado, N.E.; Carranza-Torres, I.E.; Viveros-Valdez, E.; Morán-Martínez, J. Breast organotypic cancer models. Curr. Top. Microbiol. Immunol. 2018, 1–25. [Google Scholar] [CrossRef]
- Sun, J.; Shi, D.; Ma, M.; Wang, S.; Han, L.; Yang, Y.; Fan, X.; Shi, J.; He, L. Sesquiterpenes from the red alga Laurencia tristicha. J. Nat. Prod. 2005, 68, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Zaleta-Pinet, D.A.; Holland, I.P.; Muñoz-Ochoa, M.; Murillo-Alvarez, J.I.; Sakoff, J.A.; van Altena, I.A.; McCluskey, A. Cytotoxic compounds from Laurencia pacifica. Org. Med. Chem. Lett. 2014, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhuang, T.; Liang, Z.; Li, L.; Xue, M.; Liu, J.; Liang, H. Breast cancer suppression by aplysin is associated with inhibition of PI3K/AK/FOXO3a pathway. Oncotarget 2017, 8, 63923–63934. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.M.; Mendis, E.; Kim, S.K. Laurencia okamurai extract containing laurinterol induces apoptosis in melanoma cells. J. Med. Food 2008, 11, 260–266. [Google Scholar] [CrossRef] [PubMed]
- García-Davis, S.; Murillo-Alvarez, I.; Muñoz-Ochoa, M.; Carranza-Torres, E.; Garza-Padrón, R.; Morales-Rubio, E.; Viveros-Valdez, E. Bactericide, antioxidant and cytotoxic activities from marine algae of genus Laurencia collected in Baja California Sur. Int. J. Pharmacol. 2018, 14, 391–396. [Google Scholar] [CrossRef]
- Irie, T.; Suzuki, M.; Masamune, T. Laurinterol and debromolaurinterol costituents from Laurencia intermedia. Tetraheron Lett. 1966, 7, 1837–1840. [Google Scholar] [CrossRef]
- Jesus, A.; Correia-da-Silva, M.; Alfonso, C.; Pinto, M.; Cidade, H. Isolation and potential biological applications of haloaryl secondary metabolites from macroalgae. Mar. Drugs 2019, 17, 73. [Google Scholar] [CrossRef]
- Fernández Freire, P.; Peropadre, A.; Pérez Martín, J.M.; Herrero, O.; Hazen, M.J. An integrated cellular model to evaluate cytotoxic effects in mammalian cell lines. Toxicol. In Vitro 2009, 23, 1553–1558. [Google Scholar] [CrossRef] [PubMed]
- Sombatsri, A.; Thummanant, Y.; Sribuhom, T.; Wongphakham, P.; Senawong, T.; Yenjai, C. Atalantums H-K from the peels of Atalantia monophylla and their cytotoxicity. Nat. Prod Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kumarihamy, M.; Ferreira, D.; Croom, E.M., Jr.; Sahu, R.; Tekwani, B.L.; Duke, S.O.; Khan, S.; Techen, N.; Nanayakkara, N.P.D. Antiplasmodial and cytotoxic cytochalasins from an endophytic fungus, Nemania sp. UM10M, isolated from a diseased Torreya taxifolia Leaf. Molecules 2019, 24, 777. [Google Scholar] [CrossRef] [PubMed]
- Mashjoor, S.; Yousefzadi, M.; Esmaeili, M.A.; Rafiee, R. Cytotoxicity and antimicrobial activity of marine macro algae (Dictyotaceae and Ulvaceae) from the Persian Gulf. Cytotechnology 2016, 68, 1717–1726. [Google Scholar] [CrossRef]
- Sit, N.W.; Chan, Y.S.; Lai, S.C.; Lim, L.N.; Looi, G.T.; Tay, P.L.; Tee, Y.T.; Woon, Y.Y.; Khoo, K.S.; Ong, H.C. In vitro antidermatophytic activity and cytotoxicity of extracts derived from medicinal plants and marine algae. J. Mycol. Med. 2018, 28, 561–567. [Google Scholar] [CrossRef]
- Siddiqui, S.; Ahmad, R.; Khan, M.A.; Upadhyay, S.; Husain, I.; Srivastava, A.N. Cytostatic and anti-tumor potential of Ajwa date pulp against human hepatocellular carcinoma HepG2 cells. Sci. Rep. 2019, 9, 245. [Google Scholar] [CrossRef]
- Stein, E.M.; Andreguetti, D.X.; Rocha, C.S.; Fujii, M.T.; Baptista, M.S.; Colepicolo, P.; Indig, G.L. Search for cytotoxic agents in multiple Laurencia complex seaweed species (Ceramiales, Rhodophyta) harvested from the Atlantic Ocean with emphasis on the Brazilian State of Espírito Santo. Rev. Bras. Farmacogn. 2011, 21, 239–243. [Google Scholar] [CrossRef]
- Esselin, H.; Sutour, S.; Liberal, J.; Cruz, M.T.; Salgueiro, L.; Siegler, B.; Freuze, I.; Castola, V.; Paoli, M.; Bighelli, A.; et al. Chemical composition of Laurencia obtuse extract and isolation of a New C15 acetogenin. Molecules 2017, 22. [Google Scholar] [CrossRef]
- Garzon, C.D.; Flores, F.J. Hormesis: Biphasic dose-responses to fungicides in plant pathogens and their potential threat to agriculture. In Fungicides—Showcases of Integrated Plant Disease Management from Around the World; Mizuho, N., Ed.; InTech: Rijeka, Croatia, 2013; pp. 311–328. [Google Scholar] [CrossRef]
- Afford, S.; Randhawa, S. Apoptosis. Mol. Pathol. 2000, 53, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Mgbonyebi, O.P.; Russo, J.; Russo, I.H. Roscovitine induces cell death and morphological changes indicative of apoptosis in MDA-MB-231 breast cancer cells. Cancer Res. 1999, 59, 1903–1910. [Google Scholar] [PubMed]
- Mahassni, S.H.; Al-Reemi, R.M. Apoptosis and necrosis of human breast cancer cells by an aqueous extract of garden cress (Lepidium sativum) seeds. Saudi J. Biol. Sci. 2013, 20, 131–139. [Google Scholar] [CrossRef]
- Lv, Z.D.; Liu, X.P.; Zhao, W.J.; Dong, Q.; Li, F.N.; Wang, H.B.; Kong, B. Curcumin induces apoptosis in breast cancer cells and inhibits tumor growth in vitro and in vivo. Int. J. Clin. Exp. Pathol. 2014, 7, 2818. [Google Scholar]
- Gong, A.J.; Gong, L.L.; Yao, W.C.; Ge, N.; Lu, L.X.; Liang, H. Aplysin induces apoptosis in glioma cells through HSP90/AKT pathway. Exp. Biol. Med. 2015, 240, 639–644. [Google Scholar] [CrossRef]
- Shakeel, E.; Akhtar, S.; Khan, M.K.A.; Lohani, M.; Arif, J.M.; Siddiqui, M.H. Molecular docking analysis of aplysin analogs targeting survivin protein. Bioinformation 2017, 13, 293–300. [Google Scholar] [CrossRef]
- Shakeel, E.; Sharma, N.; Akhtar, S.; Khan, M.K.A.; Lohani, M.; Siddiqui, M.H. Decoding the antineoplastic efficacy of aplysin targeting Bcl-2: A de novo perspective. Comput. Biol. Chem. 2018, 77, 390–401. [Google Scholar] [CrossRef]
- Sims, J.J.; Fenical, W.; Wing, R.M.; Radlick, P. Marine natural products III. Johnstonnol, an unusual halogenated epoxide from the red alga Laurencia johnstonii. Tetrahedron Lett. 1972, 13, 195–198. [Google Scholar] [CrossRef]
- Faulkner, J.D.; Stallard, M.O.; Ireland, C. Prepacifenol epoxide, a halogenated sesquiterpene diepoxide. Tetrahedron Lett. 1974, 15, 3571–3574. [Google Scholar] [CrossRef]
- Liu, J.; Ma, N.; Liu, G.; Zheng, L.; Lin, X. Aplysin sensitizes cancer cells to TRAIL by suppressing P38 MAPK/survivin pathway. Mar. Drugs 2014, 12, 5072–5088. [Google Scholar] [CrossRef]
- Rocha, D.H.A.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed secondary metabolites in vitro and in vivo anticancer activity. Mar. Drugs 2018, 16, 410. [Google Scholar] [CrossRef]
- Hait, W.N. Anticancer drug development: The grand challenges. Nat. Rev. Drug Discov. 2010, 9, 253–254. [Google Scholar] [CrossRef]
- De Graaf, I.A.; Olinga, P.; de Jager, M.H.; Merema, M.T.; de Kanter, R.; Van de Kerkhof, E.G.; Groothuis, G.M. Preparation and incubation of precisión-cut liver and intestinal slice for application in drug metabolism and toxicity studies. Nat. Protoc. 2010, 5, 1540–1551. [Google Scholar] [CrossRef]
- Van der Kuip, H.; Mürdter, T.E.; Sonnenberg, M.; McClenllan, M.; Gutzeit, S.; Gerteis, A.; Simon, W.; Fritz, P.; Aulitzky, W.E. Short term culture of breast cancer tissues to study the activity of the anticancer drug taxol in an intact tumor environment. BMC Cancer 2006, 6, 86. [Google Scholar] [CrossRef] [PubMed]
- Mestres, P.; Morguet, A.; Schmidt, W.; Kob, A.; Thedinga, E. A new method to assess drug sensitivity on breast tumor acute slices preparation. Ann. N. Y. Acad. Sci. 2006, 1091, 460–469. [Google Scholar] [CrossRef]
- Holliday, D.L.; Moss, M.A.; Pollock, S.; Lane, S.; Shaaban, A.M.; Millican-Slater, R.; Nash, C.; Hanby, A.M.; Speirs, V. The practicalities of using tissue slices as preclinical organotypics breast cancer models. J. Clin. Pathol. 2013, 66, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Naipal, K.A.T.; Verkaik, N.S.; Sánchez, H.; van Deurzen, C.H.M.; den Bakker, M.A.; Hoeijmakers, J.H.J.; Kanaar, R.; Vreeswijk, M.P.G.; Jager, A.; van Gent, D.C. Tumor slice culture system to assess drug response of primary breast cancer. BMC Cancer 2016, 16, 78. [Google Scholar] [CrossRef]
- Garcia-Chagollan, M.; Carranza-Torres, I.E.; Carranza-Rosales, P.; Guzmán-Delgado, N.E.; Ramírez-Montoya, H.; Martínez-Silva, M.G.; Mariscal-Ramirez, I.; Barrón-Gallardo, C.A.; Pereira-Suárez, A.L.; Aguilar-Lemarroy, A. Expression of NK cell Surface receptors in breast cancer tissue as predictors of resistance to antineoplastic treatment. Technol. Cancer Res. Treat. 2018, 17. [Google Scholar] [CrossRef] [PubMed]
- Kern, M.A.; Haugg, A.M.; Eiteneuer, E.; Konze, E.; Drebber, U.; Dienes, H.P.; Breuhahn, K.; Schirmacher, P.; Kasper, H.U. Ex vivo analysis of antineoplastic agents in precision-cut tissue slices of human origin: Effects of cyclooxygenase-2 inhibition in hepatocellular carcinoma. Liver Int. 2006, 26, 604–612. [Google Scholar] [CrossRef]
- Gerlach, M.M.; Merz, F.; Kubick, C.; Wittekind, C.; Lordick, F.; Dietz, A.; Bechmann, I. Slice cultures from head and neck squamous cell carcinoma: A novel test system for drug susceptibility and mechanisms of resistance. Br. J. Cancer 2014, 110, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Peria, M.; Donnadieu, J.; Racz, C.; Ikoli, J.F.; Galmiche, A.; Chauffert, B.; Page, C. Evaluation of individual sensitivity of head and neck squamous cell carcinoma to cetuximab by short-term culture of tumor slices. Head Neck 2016, 38 (Suppl. 1), E911–E915. [Google Scholar] [CrossRef] [PubMed]
- Unger, F.T.; Bentz, S.; Krüger, J.; Rosenbrock, C.; Schaller, J.; Pursche, K.; Sprüssel, A.; Juhl, H.; David, K.A. Precision cut cancer tissue slices in anticancer drug testing. J. Mol. Pathophysiol. 2015, 4, 108–121. [Google Scholar] [CrossRef]
- Koefer, J.; Kallendrusch, S.; Merz, F.; Kubick, C.; Kassahun, W.T.; Schumacher, G.; Moebius, C.; Gaßler, N.; Schopow, N.; Geister, D.; Wiechmann, V.; et al. Organotypic slice cultures of human gastric and esophagogastric junction cancer. Cancer Med. 2016, 5, 1444–1453. [Google Scholar] [CrossRef] [Green Version]
- Peranzoni, E.; Bougherara, H.; Barrin, S.; Mansuet-Lupo, A.; Alifano, M.; Damotte, D.; Donnadieu, E. Ex vivo imaging of resident CD8 T lymphocytes in human lung tumor slices using confocal microcopy. J. Vis. Exp. 2017, 130. [Google Scholar] [CrossRef]
- Dayot, S.; Speisky, D.; Couvelard, A.; Bourgoin, P.; Gratio, V.; Cros, J.; Rebours, V.; Sauvanet, A.; Bedossa, P.; Paradis, V.; et al. In vitro, in vivo and ex vivo demonstration of the antitumoral role of hypocretin-1/orexin-A and almorexant in pancreatic ductal adenocarcinoma. Oncotarget 2018, 9, 6952–6967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, C.Y.; Chang, J.H.; Lee, K.M.; Yoon, Y.C.; Kim, J.; Park, I.Y. Organotypic slice cultures of pancreatic ductal adenocarcinoma preserve the tumor microenvironment and provide a plataform for drugs response. Pancreatology 2018, 18, 913–927. [Google Scholar] [CrossRef] [PubMed]
- Van de Merbel, A.F.; van der Horst, G.; van der Mark, M.H.; van Uhm, J.I.M.; van Gennep, E.J.; Kloen, P.; Beimers, L.; Pelger, R.C.M.; van der Plujim, G. An ex vivo tissue culture model for the assessment of individualized drug responses in prostate and bladder cancer. Front. Oncol. 2018, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Carranza-Torres, I.E.; Guzmán-Delgado, N.E.; Coronado-Martínez, C.; Bañuelos-García, J.I.; Viveros-Valdez, E.; Morán-Martínez, J.; Carranza-Rosales, P. Organotypic culture of breast tumor explants as a multicellular system for the screening of natural compounds with antineoplastic potential. Biomed. Res. Int. 2015, 2015, 618021. [Google Scholar] [CrossRef]
- Ross, J.S.; Linette, G.P.; Stec, J.; Clark, E.; Ayers, M.; Leschly, N.; Symmans, W.F.; Hortobagyi, G.N.; Pusztai, L. Breast cancer biomarkers an molecular medicine. Expert Rev. Mol. Diagn. 2003, 3, 573–585. [Google Scholar] [CrossRef]
- Brenton, J.D.; Carey, L.A.; Ahmed, A.A.; Caldas, C. Molecular classification and molecular forecasting of breast cancer: Ready for clinical application? J. Clin. Oncol. 2005, 23, 7350–7360. [Google Scholar] [CrossRef]
- Yeo, S.K.; Guan, J.L. Breast cancer: Multiples subtypes within a tumor? Trends Cancer 2017, 3, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Hoarau-Véchot, J.; Rafii, A.; Touboul, C.; Pasquier, J. Halfway between 2D and animal models: Are 3D cultures the ideal tool to study cancer-microenvironment interactions? Int. J. Mol. Sci. 2018, 19, 181. [Google Scholar] [CrossRef] [PubMed]







| Patient | Age | 1 CS (grading) | 2 HT | 3 TS | 4 ER | 5 PR | 6 HER2 | 7 MC |
|---|---|---|---|---|---|---|---|---|
| 1 | 38 | T2N1MO (IIIA) | ID | 3 | (+) | (+) | (-) | LA |
| 2 | 54 | T4dN1M0 (IIIB) | ID | 5 | (+) | (+) | (-) | LA |
| 3 | 46 | T3N1M0 (IIIA) | ID | 4 | (-) | (-) | (+) | HER2 |
| 4 | 63 | T2N0M0 (IIA) | ID | 3 | (-) | (-) | (+) | HER2 |
| 5 | 61 | T2N0M0 (IIA) | ID | 4 | (-) | (-) | (+) | HER2 |
| 6 | 42 | T4bN0M0 (IIIB) | ID | 4 | (-) | (-) | (-) | BL |
| 7 | 74 | T4bN0M0 (IIIB) | IL | 4 | (+) | (+) | (-) | LA |
| 8 | 39 | T2N2M0 (IIIB) | ID | 5 | (-) | (-) | (-) | BL |
| 9 | 57 | T3N1M0 (IIIA) | IL | 5.5 | (+) | (+) | (-) | LA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Davis, S.; Viveros-Valdez, E.; Díaz-Marrero, A.R.; Fernández, J.J.; Valencia-Mercado, D.; Esquivel-Hernández, O.; Carranza-Rosales, P.; Carranza-Torres, I.E.; Guzmán-Delgado, N.E. Antitumoral Effect of Laurinterol on 3D Culture of Breast Cancer Explants. Mar. Drugs 2019, 17, 201. https://doi.org/10.3390/md17040201
García-Davis S, Viveros-Valdez E, Díaz-Marrero AR, Fernández JJ, Valencia-Mercado D, Esquivel-Hernández O, Carranza-Rosales P, Carranza-Torres IE, Guzmán-Delgado NE. Antitumoral Effect of Laurinterol on 3D Culture of Breast Cancer Explants. Marine Drugs. 2019; 17(4):201. https://doi.org/10.3390/md17040201
Chicago/Turabian StyleGarcía-Davis, Sara, Ezequiel Viveros-Valdez, Ana R. Díaz-Marrero, José J. Fernández, Daniel Valencia-Mercado, Olga Esquivel-Hernández, Pilar Carranza-Rosales, Irma Edith Carranza-Torres, and Nancy Elena Guzmán-Delgado. 2019. "Antitumoral Effect of Laurinterol on 3D Culture of Breast Cancer Explants" Marine Drugs 17, no. 4: 201. https://doi.org/10.3390/md17040201
APA StyleGarcía-Davis, S., Viveros-Valdez, E., Díaz-Marrero, A. R., Fernández, J. J., Valencia-Mercado, D., Esquivel-Hernández, O., Carranza-Rosales, P., Carranza-Torres, I. E., & Guzmán-Delgado, N. E. (2019). Antitumoral Effect of Laurinterol on 3D Culture of Breast Cancer Explants. Marine Drugs, 17(4), 201. https://doi.org/10.3390/md17040201