Antioxidative, Anti-Inflammatory, and Anti-Aging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging
Abstract
:1. Introduction
2. Mechanisms of the UV-Induced Skin Aging
2.1. Cellular DNA Damage
2.2. Reactive Oxygen Species Generation
2.3. Inflammatory Responses
2.4. Induction of Matrix Metalloproteinases
2.5. Induction of Protein Glycation
3. Natural Compounds that Prevent UV radiation (UVR)-Induced Photo-Damage
3.1. Plant Extracts
3.2. Mycosporine-Like Amino Acids
4. Molecular Properties of MAAs
4.1. General Description
4.2. Chemical Structure
4.3. Biosynthetic Pathways
5. Potential of Anti-Photoaging and Photo-Protective Activity of MAAs
5.1. DNA Damage-Protecting Activity
5.2. Antioxidant Activity
5.3. Anti-Inflammatory Activity
5.4. Anti-Protein-Glycation Activity
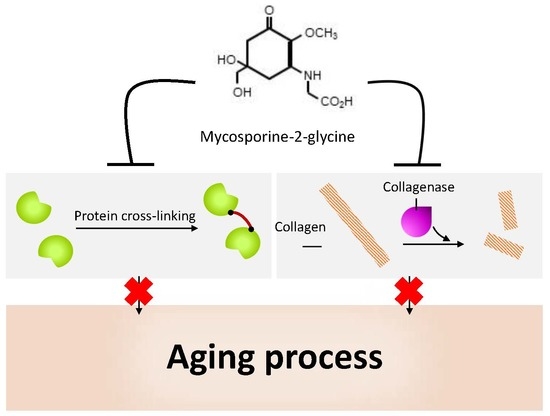
5.5. Bacterial Collagenase Inhibitory Activity
5.6. Other Activity
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Jallad, K.N. Chemical characterization of sunscreens composition and its related potential adverse health effects. J. Cosmet. Dermatol. 2017, 16, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Garcia-Pichel, F. Microbial ultraviolet sunscreens. Nature reviews. Microbiology 2011, 9, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Gruber, F.; Peharda, V.; Kastelan, M.; Brajac, I. Occupational skin diseases caused by UV radiation. Acta Dermatovenerol. Croat. ADC 2007, 15, 191–198. [Google Scholar] [PubMed]
- Ikehata, H. Mechanistic considerations on the wavelength-dependent variations of UVR genotoxicity and mutagenesis in skin: The discrimination of UVA-signature from UV-signature mutation. Photochem. Photobiol. Sci. 2018, 17, 1861–1871. [Google Scholar] [CrossRef]
- Browne, N.; Donovan, F.; Murray, P.; Saha, S. Cyanobacteria as bio-factories for production of UV-screening compounds. OA Biotechnol. 2014, 3, 6. [Google Scholar]
- Oyamada, C.; Kaneniwa, M.; Ebitani, K.; Murata, M.; Ishihara, K. Mycosporine-like amino acids extracted from scallop (Patinopecten yessoensis) ovaries: UV protection and growth stimulation activities on human cells. Mar. Biotechnol. 2008, 10, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, M.; Ando, H. The maximal cumulative solar UVB dose allowed to maintain healthy and young skin and prevent premature photoaging. Exp. Dermatol. 2014, 23, 43–46. [Google Scholar] [CrossRef] [Green Version]
- Rastogi, R.P.; Incharoensakdi, A. Analysis of UV-absorbing photoprotectant mycosporine-like amino acid (MAA) in the cyanobacterium Arthrospira sp. CU2556. Photochem. Photobiol. Sci. 2014, 13, 1016–1024. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Chollet-Krugler, M.; Gouault, N.; Tomasi, S. UV-protectant metabolites from lichens and their symbiotic partners. Natl. Prod. Rep. 2013, 30, 1490–1508. [Google Scholar] [CrossRef]
- Rosic, N.N.; Dove, S. Mycosporine-like amino acids from coral dinoflagellates. Appl. Environ. Microbiol. 2011, 77, 8478–8486. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, H.; Waditee-Sirisattha, R. Cyanobacterial UV sunscreen: Biosynthesis, regulation, and application. In Sunscreens: Source, Formulations, Efficacy and Recommendations; Rastogi, R.P., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2018; pp. 1–28. [Google Scholar]
- Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-like amino acids as multifunctional secondary metabolites in cyanobacteria: From biochemical to application aspects. In Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 59, pp. 153–194. [Google Scholar]
- Hu, C.; Voller, G.; Sussmuth, R.; Dittmann, E.; Kehr, J.C. Functional assessment of mycosporine-like amino acids in Microcystis aeruginosa strain PCC 7806. Environ. Microbiol. 2015, 17, 1548–1559. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 2015, 4, 603. [Google Scholar] [CrossRef]
- Chrapusta, E.; Kaminski, A.; Duchnik, K.; Bober, B.; Adamski, M.; Bialczyk, J. Mycosporine-like amino acids: potential health and beauty ingredients. Mar. Drugs 2017, 15, 326. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef]
- Brunt, E.G.; Burgess, J.G. The promise of marine molecules as cosmetic active ingredients. Int. J. Cosmet. Sci. 2018, 40, 1–15. [Google Scholar] [CrossRef]
- Nair, R.; Maseeh, A. Vitamin D: The “sunshine” vitamin. J. Pharmacol. Pharm. 2012, 3, 118–126. [Google Scholar] [CrossRef]
- Bernstein, E.F.; Chen, Y.Q.; Kopp, J.B.; Fisher, L.; Brown, D.B.; Hahn, P.J.; Robey, F.A.; Lakkakorpi, J.; Uitto, J. Long-term sun exposure alters the collagen of the papillary dermis. Comparison of sun-protected and photoaged skin by northern analysis, immunohistochemical staining, and confocal laser scanning microscopy. J. Am. Acad. Dermatol. 1996, 34, 209–218. [Google Scholar] [CrossRef]
- Panich, U.; Sittithumcharee, G.; Rathviboon, N.; Jirawatnotai, S. Ultraviolet radiation-induced skin aging: the role of DNA Damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int. 2016, 2016, 7370642. [Google Scholar] [CrossRef]
- Cadet, J.; Sage, E.; Douki, T. Ultraviolet radiation-mediated damage to cellular DNA. Mutat. Res. 2005, 571, 3–17. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, T.H.; Wahedi, H.; Baek, S.-H.; Kim, S. Resveratrol-Enriched Rice Attenuates UVB-ROS-Induced Skin Aging via Downregulation of Inflammatory Cascades. Oxid. Med. Cell. Longev. 2017, 1–15. [Google Scholar] [CrossRef]
- Valencia, A.; Kochevar, I.E. Nox1-based NADPH oxidase is the major source of UVA-induced reactive oxygen species in human keratinocytes. J. Investig. Dermatol. 2008, 128, 214–222. [Google Scholar] [CrossRef]
- Masaki, H.; Okano, Y.; Sakurai, H. Generation of active oxygen species from advanced glycation end-products (AGEs) during ultraviolet light A (UVA) irradiation and a possible mechanism for cell damaging. Biochim. Biophys. Acta 1999, 1428, 45–56. [Google Scholar] [CrossRef]
- Sakurai, H.; Yasui, H.; Yamada, Y.; Nishimura, H.; Shigemoto, M. Detection of reactive oxygen species in the skin of live mice and rats exposed to UVA light: a research review on chemiluminescence and trials for UVA protection. Photochem. Photobiol. Sci. 2005, 4, 715–720. [Google Scholar] [CrossRef]
- Glady, A.; Tanaka, M.; Moniaga, C.S.; Yasui, M.; Hara-Chikuma, M. Involvement of NADPH oxidase 1 in UVB-induced cell signaling and cytotoxicity in human keratinocytes. Biochem. Biophys. Rep. 2018, 14, 7–15. [Google Scholar] [CrossRef]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and non-enzymic antioxidants in epidermis and dermis of human skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef]
- Hruza, L.L.; Pentland, A.P. Mechanisms of UV-induced inflammation. J. Investig. Dermatol. 1993, 100, 35s–41s. [Google Scholar] [CrossRef]
- Radhiga, T.; Agilan, B.; Muzaffer, U.; Karthikeyan, R.; Kanimozhi, G.; Paul, V.I.; Prasad, N. Phytochemicals as modulators of ultraviolet-B radiation induced cellular and molecular events: A Review. J. Radiat. Cancer Res. 2016, 7, 2–12. [Google Scholar]
- Bowden, G.T. Prevention of non-melanoma skin cancer by targeting ultraviolet-B-light signalling. Nat. Rev. Cancer 2004, 4, 23–35. [Google Scholar] [CrossRef]
- Onodera, Y.; Teramura, T.; Takehara, T.; Shigi, K.; Fukuda, K. Reactive oxygen species induce Cox-2 expression via TAK1 activation in synovial fibroblast cells. FEBS Open Bio 2015, 5, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef]
- Brennan, M.; Bhatti, H.; Nerusu, K.C.; Bhagavathula, N.; Kang, S.; Fisher, G.J.; Varani, J.; Voorhees, J.J. Matrix metalloproteinase-1 is the major collagenolytic enzyme responsible for collagen damage in UV-irradiated human skin. Photochem. Photobiol. 2003, 78, 43–48. [Google Scholar] [CrossRef]
- Pillai, S.; Oresajo, C.; Hayward, J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation—A review. Int. J. Cosmet. Sci. 2005, 27, 17–34. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Bohm, M. Advanced glycation end products: Key players in skin aging? Dermato Endocrinol. 2012, 4, 259–270. [Google Scholar] [CrossRef]
- Ghosh, S.; Pandey, N.K.; Singha Roy, A.; Tripathy, D.R.; Dinda, A.K.; Dasgupta, S. Prolonged glycation of hen egg white lysozyme generates non amyloidal structures. PLoS ONE 2013, 8, e74336. [Google Scholar] [CrossRef]
- Hori, M.; Yagi, M.; Nomoto, K.; Shimode, A.; Ogura, M.; Yonei, Y. Inhibition of advanced glycation end product formation by herbal teas and its relation to anti-skin aging. J. Anti-Aging Med. 2012, 9, 135–148. [Google Scholar]
- Mohania, D.; Chandel, S.; Kumar, P.; Verma, V.; Digvijay, K.; Tripathi, D.; Choudhury, K.; Mitten, S.K.; Shah, D. Ultraviolet radiations: Skin defense-damage mechanism. Adv. Exp. Med. Biol. 2017, 996, 71–87. [Google Scholar] [CrossRef]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of photoaging and cutaneous photocarcinogenesis, and photoprotective strategies with phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef]
- Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Afaq, F.; Mukhtar, H. Photochemopreventive effect of pomegranate fruit extract on UVA-mediated activation of cellular pathways in normal human epidermal keratinocytes. Photochem. Photobiol. 2006, 82, 398–405. [Google Scholar] [CrossRef]
- Sharma, S.D.; Meeran, S.M.; Katiyar, S.K. Dietary grape seed proanthocyanidins inhibit UVB-induced oxidative stress and activation of mitogen-activated protein kinases and nuclear factor-kappaB signaling in in vivo SKH-1 hairless mice. Mol. Cancer Ther. 2007, 6, 995–1005. [Google Scholar] [CrossRef]
- Adhami, V.M.; Afaq, F.; Ahmad, N. Suppression of ultraviolet B exposure-mediated activation of NF-κB in normal human keratinocytes by resveratrol. Neoplasia 2003, 5, 74–82. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.K.; Bode, A.M.; Heo, Y.S.; Lee, H.J.; Dong, Z. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 2009, 30, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Lee, K.W.; Jung, S.K.; Lee, E.J.; Heo, Y.S.; Bode, A.M.; Lubet, R.A.; Lee, H.J.; Dong, Z. Kaempferol inhibits UVB-induced COX-2 expression by suppressing Src kinase activity. Biochem. Pharmacol. 2010, 80, 2042–2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.; Park, K.; Ryang Kweon, G.; Jang, B.-C.; Baek, W.; Suh, M.; Kim, C.-W.; Lee, K.-S.; Suh, S.-I. Curcumin inhibits the expression of COX-2 in UVB-irradiated human keratinocytes (HaCaT) by inhibiting activation of AP-1: p38 MAP kinase and JNK as potential upstream targets. Exp. Mol. Med. 2005, 37, 186–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afaq, F. Natural agents: Cellular and molecular mechanisms of photoprotection. Arch. Biochem. Biophys. 2011, 508, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Durr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef] [PubMed]
- Cefali, L.C.; Ataide, J.A.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Plant-based active photoprotectants for sunscreens. Int. J. Cosmet. Sci. 2016, 38, 346–353. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Castenholz, R.W. Occurrence of UV-absorbing, Mycosporine-like compounds among cyanobacterial isolates and an estimate of their screening capacity. Appl. Environ. Microbiol. 1993, 59, 163–169. [Google Scholar] [PubMed]
- Lawrence, K.P.; Long, P.F.; Young, A.R. Mycosporine-like amino acids for skin photoprotection. Curr. Med. Chem. 2018, 25, 5512–5527. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Garg, A.; Sharma, K.; Kumar, S.; Sharma, A.; Purohit, A.P. Mycosporine and mycosporine-like amino acids: A paramount tool against ultra violet irradiation. Pharm. Rev. 2011, 5, 138–146. [Google Scholar] [CrossRef]
- De la Coba, F.; Aguilera, J.; Figueroa, F.L.; de Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Daniel, S.; Cornelia, S.; Fred, Z. UV-A sunscreen from red algae for protection against premature skin aging. Cosmet. Toilet. Manuf. Worldw 2004, 139–143. [Google Scholar]
- Cheewinthamrongrod, V.; Kageyama, H.; Palaga, T.; Takabe, T.; Waditee-Sirisattha, R. DNA damage protecting and free radical scavenging properties of mycosporine-2-glycine from the Dead Sea cyanobacterium in A375 human melanoma cell lines. J. Photochem. Photobiol. B Biol. 2016, 164, 289–295. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.J.; Kim, I.H.; Choi, Y.H.; Nam, T.J. Protective effect of porphyra-334 on UVA-induced photoaging in human skin fibroblasts. Int. J. Mol. Med. 2014, 34, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Suh, S.S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.S.; Lee, J.H.; Moh, S.H.; Lee, T.K. Anti-inflammation activities of mycosporine-like amino acids (MAAs) in response to UV radiation suggest potential anti-skin aging activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef]
- Oren, A. Mycosporine-like amino acids as osmotic solutes in a community of halophilic cyanobacteria. Geomicrobiol. J. 1997, 14, 231–240. [Google Scholar] [CrossRef]
- Patipong, T.; Hibino, T.; Waditee-Sirisattha, R.; Kageyama, H. Efficient bioproduction of mycosporine-2-glycine, which functions as potential osmoprotectant, using Escherichia coli cells. Natl. Prod. Commun. 2017, 12, 1593–1594. [Google Scholar] [CrossRef]
- Suh, H.J.; Lee, H.W.; Jung, J. Mycosporine glycine protects biological systems against photodynamic damage by quenching singlet oxygen with a high efficiency. Photochem. Photobiol. 2003, 78, 109–113. [Google Scholar] [CrossRef]
- Ngoennet, S.; Nishikawa, Y.; Hibino, T.; Waditee-Sirisattha, R.; Kageyama, H. A method for the isolation and characterization of mycosporine-like amino acids from cyanobacteria. Methods Protocols 2018, 1, 46. [Google Scholar] [CrossRef]
- Balskus, E.P.; Walsh, C.T. The genetic and molecular basis for sunscreen biosynthesis in cyanobacteria. Science 2010, 329, 1653–1656. [Google Scholar] [CrossRef] [Green Version]
- Waditee-Sirisattha, R.; Kageyama, H.; Sopun, W.; Tanaka, Y.; Takabe, T. Identification and upregulation of biosynthetic genes required for accumulation of Mycosporine-2-glycine under salt stress conditions in the halotolerant cyanobacterium Aphanothece halophytica. Appl. Environ. Microbiol. 2014, 80, 1763–1769. [Google Scholar] [CrossRef]
- Shinzato, C.; Shoguchi, E.; Kawashima, T.; Hamada, M.; Hisata, K.; Tanaka, M.; Fujie, M.; Fujiwara, M.; Koyanagi, R.; Ikuta, T.; et al. Using the Acropora digitifera genome to understand coral responses to environmental change. Nature 2011, 476, 320. [Google Scholar] [CrossRef]
- Micallef, M.L.; D’Agostino, P.M.; Sharma, D.; Viswanathan, R.; Moffitt, M.C. Genome mining for natural product biosynthetic gene clusters in the Subsection V cyanobacteria. BMC Genom. 2015, 16, 669. [Google Scholar] [CrossRef]
- Waditee-Sirisattha, R.; Kageyama, H.; Fukaya, M.; Rai, V.; Takabe, T. Nitrate and amino acid availability affects glycine betaine and mycosporine-2-glycine in response to changes of salinity in a halotolerant cyanobacterium Aphanothece halophytica. FEMS Microbiol. Lett. 2015, 362, fnv198. [Google Scholar] [CrossRef]
- Schmid, D.; Schürch, C.; Zülli, F. Mycosporine-like amino acids from red algae protect against premature skin-aging. Euro Cosmet. 2006, 9, 1–4. [Google Scholar]
- Lawrence, K.P.; Gacesa, R.; Long, P.F.; Young, A.R. Molecular photoprotection of human keratinocytes in vitro by the naturally occurring mycosporine-like amino acid palythine. Br. J. Dermatol. 2018, 178, 1353–1363. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Ishihara, K.; Watanabe, R.; Uchida, H.; Suzuki, T.; Yamashita, M.; Takenaka, H.; Nazifi, E.; Matsugo, S.; Yamaba, M.; Sakamoto, T. Novel glycosylated mycosporine-like amino acid, 13-O-(β-galactosyl)-porphyra-334, from the edible cyanobacterium Nostoc sphaericum-protective activity on human keratinocytes from UV light. J. Photochem. Photobiol. B 2017, 172, 102–108. [Google Scholar] [CrossRef]
- Gacesa, R.; Lawrence, K.P.; Georgakopoulos, N.D.; Yabe, K.; Dunlap, W.C.; Barlow, D.J.; Wells, G.; Young, A.R.; Long, P.F. The mycosporine-like amino acids porphyra-334 and shinorine are antioxidants and direct antagonists of Keap1-Nrf2 binding. Biochimie 2018, 154, 35–44. [Google Scholar] [CrossRef]
- Tarasuntisuk, S.; Palaga, T.; Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-2-glycine exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. Arch. Biochem. Biophys. 2019, 662, 33–39. [Google Scholar] [CrossRef]
- Nazifi, E.; Wada, N.; Yamaba, M.; Asano, T.; Nishiuchi, T.; Matsugo, S.; Sakamoto, T. Glycosylated porphyra-334 and palythine-threonine from the terrestrial cyanobacterium Nostoc commune. Mar. Drugs 2013, 11, 3124–3154. [Google Scholar] [CrossRef]
- Matsui, K.; Nazifi, E.; Kunita, S.; Wada, N.; Matsugo, S.; Sakamoto, T. Novel glycosylated mycosporine-like amino acids with radical scavenging activity from the cyanobacterium Nostoc commune. J. Photochem. Photobiol. B Biol. 2011, 105, 81–89. [Google Scholar] [CrossRef]
- Biswal, S. Oxidative stress and astaxanthin: the novel supernutrient carotenoid. Int. J. Health Allied Sci. 2014, 3, 147–153. [Google Scholar] [CrossRef]
- Dose, J.; Matsugo, S.; Yokokawa, H.; Koshida, Y.; Okazaki, S.; Seidel, U.; Eggersdorfer, M.; Rimbach, G.; Esatbeyoglu, T. Free radical scavenging and cellular antioxidant properties of astaxanthin. Int. J. Mol. Sci. 2016, 17, 103. [Google Scholar] [CrossRef]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.M.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochimica et biophysica acta. Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Krajka-Kuzniak, V.; Paluszczak, J.; Baer-Dubowska, W. The Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatment. Pharmacol. Rep. PR 2017, 69, 393–402. [Google Scholar] [CrossRef]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4. [Google Scholar] [CrossRef]
- Siomek, A. NF-kappaB signaling pathway and free radical impact. Acta Biochim. Polonica 2012, 59, 323–331. [Google Scholar] [CrossRef]
- Mitchell, S.; Vargas, J.; Hoffmann, A. Signaling via the NFkappaB system. Wiley interdisciplinary reviews. Syst. Biol. Med. 2016, 8, 227–241. [Google Scholar] [CrossRef]
- Chun, K.S.; Cha, H.H.; Shin, J.W.; Na, H.K.; Park, K.K.; Chung, W.Y.; Surh, Y.J. Nitric oxide induces expression of cyclooxygenase-2 in mouse skin through activation of NF-kappaB. Carcinogenesis 2004, 25, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef]
- Tarasuntisuk, S.; Patipong, T.; Hibino, T.; Waditee-Sirisattha, R.; Kageyama, H. Inhibitory effects of mycosporine-2-glycine isolated from a halotolerant cyanobacterium on protein glycation and collagenase activity. Lett. Appl. Microbiol. 2018, 67, 314–320. [Google Scholar] [CrossRef]
- Peyroux, J.; Sternberg, M. Advanced glycation endproducts (AGEs): Pharmacological inhibition in diabetes. Pathol. Biol. 2006, 54, 405–419. [Google Scholar] [CrossRef]
- Duarte, A.S.; Correia, A.; Esteves, A.C. Bacterial collagenases—A review. Crit. Rev. Microbiol. 2016, 42, 106–126. [Google Scholar] [CrossRef]
- Hartmann, A.; Gostner, J.; Fuchs, J.E.; Chaita, E.; Aligiannis, N.; Skaltsounis, L.; Ganzera, M. Inhibition of collagenase by mycosporine-like amino acids from marine sources. Planta Med. 2015, 81, 813–820. [Google Scholar] [CrossRef]
- Volkmann, M.; Gorbushina, A.A.; Kedar, L.; Oren, A. Structure of euhalothece-362, a novel red-shifted mycosporine-like amino acid, from a halophilic cyanobacterium (Euhalothece sp.). FEMS Microbiol. Lett. 2006, 258, 50–54. [Google Scholar] [CrossRef]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef]
- Di Petrillo, A.; González-Paramás, A.M.; Era, B.; Medda, R.; Pintus, F.; Santos-Buelga, C.; Fais, A. Tyrosinase inhibition and antioxidant properties of Asphodelus microcarpus extracts. BMC Complement. Altern. Med. 2016, 16, 453. [Google Scholar] [CrossRef]



| Mycosporine-like Amino Acids (MAAs) | IC50 | References |
|---|---|---|
| Mono-Substituted MAAs | ||
| Mycosporine-glycine | 3 µM a at pH 8.5 | [14,52] |
| 43 µM b | [54] | |
| Mycosporine-γ-aminobutyric acid | 0.6 mM a | [14] |
| Di-Substituted MAAs | ||
| Mycosporine-2-glycine | 45 µM a | [54] |
| 22 µM b | [71] | |
| Palythine | 21.3 µM b | [67] |
| 714 µM c | [67] | |
| Porphyra-334 | 133 µM a | [60] |
| 185.2 µM b | [70] | |
| Shinorine | 94 µM a | [60] |
| 399 µM b | [70] | |
| Glycosylated MAAs | ||
| Hexose-bound-P334 | 58 mM a | [69] |
| 7-O-(β-arabinopyranosyl)-P334 | 9.5 mM a | [69] |
| 13-O-β-galactosyl-porphyra-334 | 17 mM a | [69] |
| Standard antioxidants | ||
| Trolox | 10 µM b | [60] |
| Ascorbic acid | 21.3 µM b | [67] |
| α-Tocopherol | 11.1 µM b | [67] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kageyama, H.; Waditee-Sirisattha, R. Antioxidative, Anti-Inflammatory, and Anti-Aging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging. Mar. Drugs 2019, 17, 222. https://doi.org/10.3390/md17040222
Kageyama H, Waditee-Sirisattha R. Antioxidative, Anti-Inflammatory, and Anti-Aging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging. Marine Drugs. 2019; 17(4):222. https://doi.org/10.3390/md17040222
Chicago/Turabian StyleKageyama, Hakuto, and Rungaroon Waditee-Sirisattha. 2019. "Antioxidative, Anti-Inflammatory, and Anti-Aging Properties of Mycosporine-Like Amino Acids: Molecular and Cellular Mechanisms in the Protection of Skin-Aging" Marine Drugs 17, no. 4: 222. https://doi.org/10.3390/md17040222