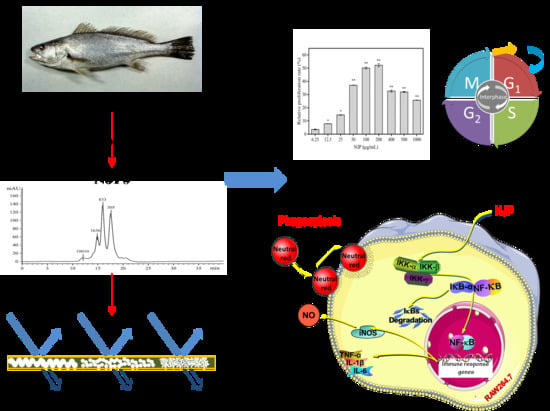
Immunomodulatory Activity of Low Molecular-Weight Peptides from Nibea japonica in RAW264.7 Cells via NF-κB Pathway
Abstract
:1. Introduction
2. Results and Discussion
2.1. Determination of Mw Distribution
2.2. Fractionation of the NJP from NJPs
2.3. Cytotoxicity of NJP on RAW264.7 Cells
2.4. Effect of NJP on RAW264.7 Cells Viability
2.5. Effect of NJP on RAW264.7 Cells’ Morphology
2.6. Effect of NJP on Phagocytosis
2.7. Effect of NJP on NO Production and iNOS Expression
2.8. Effect of NJP on TNF-α, IL-6, and IL-1β
2.9. Effect of NJP Cell-Cycle Distribution of RAW264.7 Cells
2.10. Effect of NJP on NF-κB Signal Pathway in RAW264.7 Cells
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of NJPs
3.3. Mw Distribution
3.4. Fractionation of the NJP by Ultrafiltration
3.5. Analysis of the Immunomodulatory Activity of the Peptide on RAW264.7 Cells
3.5.1. Culture of Macrophage RAW264.7 Cells
3.5.2. Cytotoxic Test
3.5.3. Cell Viability Assay
3.5.4. Morphological Evaluation
3.5.5. Phagocytosis of Neutral Red
3.5.6. Measurement of NO Level and related Inflammatory Cytokines
3.5.7. Cell Cycle Analysis
3.5.8. Immunofluorescence Staining
3.5.9. Western Blot Analysis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Erdmann, K.; Cheung, B.W.; Schröder, H. The possible roles of food-derived bioactive peptides in reducing the risk of cardiovascular disease. J. Nutr. Biochem. 2008, 19, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Kitts, D.; Weiler, K. Bioactive Proteins and Peptides from Food Sources. Applications of Bioprocesses used in Isolation and Recovery. Curr. Pharm. Des. 2003, 9, 1309–1323. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.-J.; Byun, H.-G.; Kim, S.-K. Improvement of functional properties of cod frame protein hydrolysates using ultrafiltration membranes. Process. Biochem. 1999, 35, 471–478. [Google Scholar] [CrossRef]
- Segura-Campos, M.; Chel-Guerrero, L.; Betancur-Ancona, D.; Hernandez-Escalante, V.M. Bioavailability of Bioactive Peptides. Food Rev. Int. 2011, 27, 213–226. [Google Scholar] [CrossRef]
- Shen, W.; Matsui, T. Current knowledge of intestinal absorption of bioactive peptides. Food Funct. 2017, 8, 4306–4314. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; You, L.; Yin, X.; Liu, Y.; Leng, X.; Wang, W.; Sai, N.; Ni, J. Heterophyllin B Ameliorates Lipopolysaccharide-Induced Inflammation and Oxidative Stress in RAW 264.7 Macrophages by Suppressing the PI3K/Akt Pathways. Molecules 2018, 23, 717. [Google Scholar] [CrossRef]
- Li, L.-L.; Li, B.; Ji, H.-F.; Ma, Q.; Wang, L.-Z. Immunomodulatory activity of small molecular (≤3 kDa) Coix glutelin enzymatic hydrolysate. CyTA J. Food 2017, 15, 41–48. [Google Scholar]
- Zhang, Y.; Dai, B.; Deng, Y.; Zhao, Y. In vitro anti-inflammatory and antioxidant activities and protein quality of high hydrostatic pressure treated squids (Todarodes pacificus). Food Chem. 2016, 203, 258–266. [Google Scholar] [CrossRef]
- Fernando, I.S.; Nah, J.-W.; Jeon, Y.-J. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016, 48, 22–30. [Google Scholar] [CrossRef]
- Ahn, C.-B.; Je, J.-Y.; Cho, Y.-S. Antioxidant and anti-inflammatory peptide fraction from salmon byproduct protein hydrolysates by peptic hydrolysis. Food Res. Int. 2012, 49, 92–98. [Google Scholar] [CrossRef]
- Sripokar, P.; Benjakul, S.; Klomklao, S. Antioxidant and functional properties of protein hydrolysates obtained from starry triggerfish muscle using trypsin from albacore tuna liver. Biocatal. Agric. Biotechnol. 2019, 17, 447–454. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Yu, W.; Wu, J. Immunomodulatory and anticancer protein hydrolysates (peptides) from food proteins: A review. Food Chem. 2018, 245, 205–222. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.-F.; Hu, F.-Y.; Wang, B.; Li, T.; Ding, G.-F. Antioxidant and anticancer peptides from the protein hydrolysate of blood clam (Tegillarca granosa) muscle. J. Funct. Foods 2015, 15, 301–313. [Google Scholar] [CrossRef]
- Beaulieu, L.; Thibodeau, J.; Bonnet, C.; Bryl, P.; Carbonneau, M. Élise Detection of antibacterial activity in an enzymatic hydrolysate fraction obtained from processing of Atlantic rock crab (Cancer irroratus) by-products. PharmaNutrition 2013, 1, 149–157. [Google Scholar] [CrossRef]
- Beaulieu, L.; Thibodeau, J.; Desbiens, M.; Saint-Louis, R.; Zatylny-Gaudin, C.; Thibault, S. Evidence of Antibacterial Activities in Peptide Fractions Originating from Snow Crab (Chionoecetes opilio) By-Products. Probiotics Antimicrob. Proteins 2010, 2, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Di Bernardini, R.; Harnedy, P.; Bolton, D.; Kerry, J.; O’Neill, E.; Mullen, A.M.; Hayes, M. Antioxidant and antimicrobial peptidic hydrolysates from muscle protein sources and by-products. Food Chem. 2011, 124, 1296–1307. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine Peptides: Bioactivities and Applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef]
- Batatinha, H.A.; Biondo, L.A.; Lira, F.S.; Castell, L.M.; Rosa-Neto, J.C. Nutrients, immune system, and exercise: Where will it take us? Nutrition 2019, 61, 151–156. [Google Scholar] [CrossRef]
- Ketha, K.; Gudipati, M. Purification, structural characterization of an arabinogalactan from green gram (Vigna radiata) and its role in macrophage activation. J. Funct. Foods 2018, 50, 127–136. [Google Scholar] [CrossRef]
- Chalamaiah, M.; Hemalatha, R.; Jyothirmayi, T.; Diwan, P.V.; Kumar, P.U.; Nimgulkar, C.; Kumar, B.D. Immunomodulatory effects of protein hydrolysates from rohu (Labeo rohita) egg (roe) in BALB/c mice. Food Res. Int. 2014, 62, 1054–1061. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, Z.; Luo, L.; Zhu, J.; Huang, F.; Yang, Z.; Tang, Y.; Ding, G. Identification and Molecular Docking Study of a Novel Angiotensin-I Converting Enzyme Inhibitory Peptide Derived from Enzymatic Hydrolysates of Cyclina sinensis. Mar. Drugs 2018, 16, 411. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zhang, Z.; Pei, X.; Han, X.; Wang, J.; Wang, L.; Long, Z.; Shen, X.; Li, Y. Immunomodulatory effects of marine oligopeptide preparation from Chum Salmon (Oncorhynchus keta) in mice. Food Chem. 2009, 113, 464–470. [Google Scholar] [CrossRef]
- Morris, H.J.; Carrillo, O.; Almarales, A.; Bermúdez, R.C.; Lebeque, Y.; Fontaine, R.; Llauradó, G.; Beltrán, Y. Immunostimulant activity of an enzymatic protein hydrolysate from green microalga Chlorella vulgaris on undernourished mice. Enzyme Microb. Technol. 2007, 40, 456–460. [Google Scholar] [CrossRef]
- Duarte, J.; Vinderola, G.; Ritz, B.; Perdigon, G.; Matar, C. Immunomodulating capacity of commercial fish protein hydrolysate for diet supplementation. Immunobiology 2006, 211, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Chai, X.; Ji, W.; Han, H.; Dai, Y.; Wang, Y. Growth, feed utilization, body composition and swimming performance of giant croaker, Nibea japonica Temminck and Schlegel, fed at different dietary protein and lipid levels. Aquac. Nutr. 2013, 19, 928–935. [Google Scholar] [CrossRef]
- Tang, Y.; Jin, S.; Li, X.; Li, X.; Hu, X.; Chen, Y.; Huang, F.; Yang, Z.; Yu, F.; Ding, G. Physicochemical Properties and Biocompatibility Evaluation of Collagen from the Skin of Giant Croaker (Nibea japonica). Mar. Drugs 2018, 16, 222. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Zong, C.; Jin, S.; Zheng, J.; Chen, N.; Huang, J.; Chen, Y.; Huang, F.; Yang, Z.; Tang, Y.; et al. Optimization of Extraction Conditions and Characterization of Pepsin-Solubilised Collagen from Skin of Giant Croaker (Nibea japonica). Mar. Drugs 2018, 16, 29. [Google Scholar] [CrossRef]
- Halim, N.; Yusof, H.; Sarbon, N.M. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Granath, K.A.; Kvist, B.E. Molecular weight distribution analysis by gel chromatography on sephadex. J. Chromatogr. A 1967, 28, 69–81. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Chi, C.; Gong, Y.; Luo, H.; Ding, G. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res. Int. 2013, 51, 283–293. [Google Scholar] [CrossRef]
- Park, S.Y.; Ahn, C.-B.; Je, J.-Y. Antioxidant and Anti-Inflammatory Activities of Protein Hydrolysates from Mytilus Edulis and Ultrafiltration Membrane Fractions. J. Food Biochem. 2014, 38, 460–468. [Google Scholar] [CrossRef]
- Halim, N.R.A.; Azlan, A.; Yusof, H.M.; Sarbon, N.M. Antioxidant and anticancer activities of enzymatic eel (Monopterus sp.) protein hydrolysate as influenced by different molecular weight. Biocatal. Agric. Biotechnol. 2018, 16, 10–16. [Google Scholar] [CrossRef]
- Razali, A.N.; Amin, A.M.; Sarbon, N.M. Antioxidant activity and functional properties of fractionated cobia skin gelatin hydrolysate at different molecular weight. Int. Food Res. J. 2015, 22, 651–660. [Google Scholar]
- Ghassem, M.; Arihara, K.; Babji, A.S.; Said, M.; Ibrahim, S. Purification and identification of ACE inhibitory peptides from Haruan (Channa striatus) myofibrillar protein hydrolysate using HPLC–ESI-TOF MS/MS. Food Chem. 2011, 129, 1770–1777. [Google Scholar] [CrossRef]
- Lassoued, I.; Mora, L.; Barkia, A.; Aristoy, M.C.; Nasri, M.; Toldrá, F. Bioactive peptides identified in thornback ray skin’s gelatin hydrolysates by proteases from Bacillus subtilis and Bacillus amyloliquefaciens. J. Proteomics 2015, 128, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Fan, Y.; Li, B.; Xue, C.; Yu, G. Preparation of immunomodulatory hydrolysates from Alaska pollock frame. J. Sci. Food Agric. 2012, 92, 3029–3038. [Google Scholar] [CrossRef]
- He, X.Q.; Cao, W.H.; Pan, G.K.; Yang, L.; Zhang, C.H. Enzymatic hydrolysis optimization of Paphia undulata and lymphocyte proliferation activity of the isolated peptide fractions. J. Sci. Food Agric. 2015, 95, 1544–1553. [Google Scholar] [CrossRef]
- Xu, Z.; Fang, Y.; Chen, Y.; Yang, W.; Ma, N.; Pei, F.; Kimatu, B.M.; Hu, Q.; Qiu, W. Protective effects of Se-containing protein hydrolysates from Se-enriched rice against Pb2+-induced cytotoxicity in PC12 and RAW264.7 cells. Food Chem. 2016, 202, 396–403. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, F.; Li, Q.; Chen, H.; Zhang, W.; Yu, P.; Zhao, T.; Mao, G.; Feng, W.; Yang, L.; et al. Structure characterization of one polysaccharide from Lepidium meyenii Walp., and its antioxidant activity and protective effect against H2O2 -induced injury RAW264.7 cells. Int. J. Boil. Macromol. 2018, 118, 816–833. [Google Scholar] [CrossRef]
- Ren, D.; Wang, P.; Liu, C.; Wang, J.; Liu, X.; Liu, J.; Min, W. Hazelnut protein-derived peptide LDAPGHR shows anti-inflammatory activity on LPS-induced RAW264.7 macrophage. J. Funct. Foods 2018, 46, 449–455. [Google Scholar] [CrossRef]
- Li, W.; Ye, S.; Zhang, Z.; Tang, J.; Jin, H.; Huang, F.; Yang, Z.; Tang, Y.; Chen, Y.; Ding, G.; et al. Purification and Characterization of a Novel Pentadecapeptide from Protein Hydrolysates of Cyclina sinensis and Its Immunomodulatory Effects on RAW264.7 Cells. Mar. Drugs 2019, 17, 30. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Qian, W.; Xu, Y.; Chen, G.; Wang, G.; Nie, S.; Shen, B.; Zhao, Z.; Liu, C.; Chen, K. Activation of RAW264.7 cells by a polysaccharide isolated from Antarctic bacterium Pseudoaltermonas sp. S-5. Carbohydr. Polym. 2015, 130, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Jinkai, Y.; Pengyan, S.; Xiao, W.; Wanyu, S. Effects of Salvia miltiorrhiza Polysaccharides on Lipopolysaccharide-Induced Inflammatory Factor Release in RAW264.7 Cells. J. Interferon Cytokine Res. 2018, 38, 29–37. [Google Scholar]
- Qi, B.; Wang, S.; Wang, Q.; Zhang, H.; Bai, X.-Y.; He, H.-N.; Sun, W.-J.; Liu, L.; Zhao, D.-Q. Characterization and immunostimulating effects on murine peritoneal macrophages of a novel protein isolated from Panax quinquefolius L. J. Ethnopharmacol. 2016, 193, 700–705. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Zhang, H.; Liu, Z.; Ma, C.; Kang, W. Immunomodulation of ADPs-1a and ADPs-3a on RAW264.7 cells through NF-κB/MAPK signaling pathway. Int. J. Boil. Macromol. 2019, 132, 1024–1030. [Google Scholar] [CrossRef] [PubMed]
- Lorsbach, R.B.; Murphy, W.J.; Lowenstein, C.J.; Snyder, S.H.; Russell, S.W. Expression of the nitric oxide synthase gene in mouse macrophages activated for tumor cell killing. Molecular basis for the synergy between interferon-gamma and lipopolysaccharide. J. Boil. Chem. 1993, 268, 1908–1913. [Google Scholar]
- Surayot, U.; Wang, J.; Lee, J.H.; Kanongnuch, C.; Peerapornpisal, Y.; You, S. Characterization and immunomodulatory activities of polysaccharides from Spirogyra neglecta (Hassall) Kützing. Biosci. Biotechnol. Biochem. 2015, 79, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, H.; Nie, C.; Ge, W.; Wang, Y.; Zhang, W. Oligopeptide derived from solid-state fermented cottonseed meal significantly affect the immunomodulatory in BALB/c mice treated with cyclophosphamide. Food Sci. Biotechnol. 2018, 27, 1791–1799. [Google Scholar] [CrossRef] [Green Version]
- Meram, C.; Wu, J. Anti-inflammatory effects of egg yolk livetins (α, β, and γ-livetin) fraction and its enzymatic hydrolysates in lipopolysaccharide-induced RAW 264.7 macrophages. Food Res. Int. 2017, 100, 449–459. [Google Scholar] [CrossRef]
- Jiang, S.; Jia, Y.; Tang, Y.; Zheng, D.; Han, X.; Yu, F.; Chen, Y.; Huang, F.; Yang, Z.; Ding, G. Anti-Proliferation Activity of a Decapeptide from Perinereies aibuhitensis toward Human Lung Cancer H1299 Cells. Mar. Drugs 2019, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.-J.; Wu, C.-H.; Tsai, G.-J. Chitooligosaccharides from the shrimp chitosan hydrolysate induces differentiation of murine RAW264.7 macrophages into dendritic-like cells. J. Funct. Foods 2015, 12, 70–79. [Google Scholar] [CrossRef]
- DiDonato, J.A.; Mercurio, F.; Karin, M. NF-κB and the link between inflammation and cancer. Immunol. Rev. 2012, 246, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-S.; Tang, Z.; Gu, L.-X.; Xiang, X.-W.; Qu, Y.-L. Immunomodulatory effect of low molecular-weight seleno-aminopolysaccharide on immunosuppressive mice. Int. J. Boil. Macromol. 2019, 123, 1278–1288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Nie, S.; Huang, D.; Li, W.; Xie, M. Immunomodulatory effect of Ganoderma atrum polysaccharide on CT26 tumor-bearing mice. Food Chem. 2013, 136, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, J.; Lei, L.; Li, F.; Tang, Y.; Yuan, Y.; Zhang, Y.; Wu, S.; Yin, R.; Ming, J. Acetylation of polysaccharide from Morchella angusticeps peck enhances its immune activation and anti-inflammatory activities in macrophage RAW264.7 cells. Food Chem. Toxicol. 2019, 125, 38–45. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Li, W.; Kong, X.-D.; Han, H.B.; Tang, Y.-P.; Yu, F.-M.; Yang, Z.-S.; Ding, G.-F. Optimization of Extraction Technology of Immunologically Active Peptides from Nibea Japonica by Response Surface Methodology. Sci. Technol. Food Ind. 2019. Available online: http://kns.cnki.net/kcms/detail/11.1759.ts.20190430.1115.016.html (accessed on 8 July 2019).
- Qin, L.; Zhu, B.-W.; Zhou, D.-Y.; Wu, H.-T.; Tan, H.; Yang, J.-F.; Li, D.-M.; Dong, X.-P.; Murata, Y. Preparation and antioxidant activity of enzymatic hydrolysates from purple sea urchin (Strongylocentrotus nudus) gonad. LWT 2011, 44, 1113–1118. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, S.; Liu, J.; Zou, Y.; Liao, S. Antioxidant Activity and Stability Study of Peptides from Enzymatically Hydrolyzed Male Silkmoth. J. Food Process. Preserv. 2017, 41, e13081. [Google Scholar] [CrossRef]
- Cheng, H.-W.; Lee, K.-C.; Cheah, K.-P.; Chang, M.-L.; Lin, C.-W.; Li, J.-S.; Yu, W.-Y.; Liu, E.-T.; Hu, C.-M. Polygonum viviparum L. inhibits the lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages through haem oxygenase-1 induction and activation of the Nrf2 pathway. J. Sci. Food Agric. 2013, 93, 491–497. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N. Antioxidant, immunomodulatory and antiproliferative effects of gelatin hydrolysate from unicorn leatherjacket skin. J. Sci. Food Agric. 2016, 96, 3220–3226. [Google Scholar] [CrossRef]
- Bølling, A.K.; Ovrevik, J.; Samuelsen, J.T.; Holme, J.A.; Rakkestad, K.E.; Mathisen, G.H.; Paulsen, R.E.; Korsnes, M.S.; Becher, R. Mono-2-ethylhexylphthalate (MEHP) induces TNF-α release and macrophage differentiation through different signalling pathways in RAW264.7 cells. Toxicol. Lett. 2012, 209, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Pei, F.; Kimatu, B.M.; Liu, K.; Du, M.; Qiu, W.; Hu, Q.; Fang, Y.; Chen, X. The Correlation Between In Vitro Antioxidant Activity and Immunomodulatory Activity of Enzymatic Hydrolysates from Selenium-Enriched Rice Protein. J. Food Sci. 2017, 82, 517–522. [Google Scholar]
- Yu, Q.; Nie, S.-P.; Li, W.-J.; Zheng, W.-Y.; Yin, P.-F.; Gong, D.-M.; Xie, M.-Y. Macrophage Immunomodulatory Activity of a Purified Polysaccharide Isolated from Ganoderma atrum. Phytother. Res. 2013, 27, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Sae-Leaw, T.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N.M. Antioxidant, immunomodulatory and antiproliferative effects of gelatin hydrolysates from seabass (Lates calcarifer) skins. Int. J. Food Sci. Technol. 2016, 51, 1545–1551. [Google Scholar] [CrossRef]
- Li, W.; Song, K.; Wang, S.; Zhang, C.; Zhuang, M.; Wang, Y.; Liu, T. Anti-tumor potential of astragalus polysaccharides on breast cancer cell line mediated by macrophage activation. Mater. Sci. Eng. C 2019, 98, 685–695. [Google Scholar] [CrossRef] [PubMed]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Hu, X.; Lin, L.; Ding, G.; Yu, F. Immunomodulatory Activity of Low Molecular-Weight Peptides from Nibea japonica in RAW264.7 Cells via NF-κB Pathway. Mar. Drugs 2019, 17, 404. https://doi.org/10.3390/md17070404
Zhang Z, Hu X, Lin L, Ding G, Yu F. Immunomodulatory Activity of Low Molecular-Weight Peptides from Nibea japonica in RAW264.7 Cells via NF-κB Pathway. Marine Drugs. 2019; 17(7):404. https://doi.org/10.3390/md17070404
Chicago/Turabian StyleZhang, Zhuangwei, Xuyang Hu, Lin Lin, Guofang Ding, and Fangmiao Yu. 2019. "Immunomodulatory Activity of Low Molecular-Weight Peptides from Nibea japonica in RAW264.7 Cells via NF-κB Pathway" Marine Drugs 17, no. 7: 404. https://doi.org/10.3390/md17070404
APA StyleZhang, Z., Hu, X., Lin, L., Ding, G., & Yu, F. (2019). Immunomodulatory Activity of Low Molecular-Weight Peptides from Nibea japonica in RAW264.7 Cells via NF-κB Pathway. Marine Drugs, 17(7), 404. https://doi.org/10.3390/md17070404