Mycousfurans A and B, Antibacterial Usnic Acid Congeners from the Fungus Mycosphaerella sp., Isolated from a Marine Sediment
Abstract
:1. Introduction
2. Results
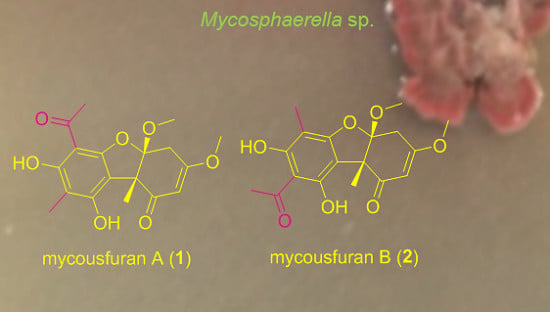
2.1. Isolation and Structure Elucidation
2.2. Bioactivity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Purifircation
3.4. Computer-Assisted Conformational Analyses and ECD Calculations
3.5. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Millot, M.; Dieua, A.; Tomasib, S. Dibenzofurans and derivatives from lichens and ascomycetes. Nat. Prod. Rep. 2016, 33, 801–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingólfsdóttir, K. Molecules of interest usnic acid. Phytochemistry 2002, 61, 729–736. [Google Scholar] [CrossRef]
- Luzina, O.A.; Salakhutdinov, N.F. Usnic Aacid and its derivatives for pharmaceutical use: A patent review (2000-2017). Expert Opin. Ther. Pat. 2018, 28, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Cocchietto, M.; Skert, N.; Nimis, P.L.; Sava, G. A review on usnic acid, an interesting natural compound. Naturwissenschaften 2002, 89, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Alahmadi, A.A. Usnic acid biological activity: History, evaluation and usage. Int. J. Basic Clin. Pharmacol. 2017, 6, 2752–2759. [Google Scholar] [CrossRef]
- Kim, K.-J.; Jeong, M.-H.; Lee, Y.; Hwang, S.-J.; Shin, H.-B.; Hur, J.-S.; Son, Y.-J. Effect of usnic acid on osteoclastogenic activity. J. Clin. Med. 2018, 7, 345. [Google Scholar] [CrossRef] [PubMed]
- Luzina, O.A.; Salakhutdinov, N.F. Biological activity of usnic acid and tts derivatives: Part 1. Activity against unicellular organisms. Russ. J. Bioorg. Chem. 2016, 42, 115–132. [Google Scholar] [CrossRef]
- Albinati, A.; Bruckner, S.; Camarda, L.; Nasini, G. Rosigenin, an unusual metabolite from Mycosphaerella rosigena. Tetrahedron 1980, 36, 117–121. [Google Scholar] [CrossRef]
- Arnone, A.; Camarda, L.; Nasini, G.; Assante, G. Secondary metabolites. Part 15. Structure elucidation of rubellins A and B, two novel anthraquinone metabolites from Mycosphaerella rubella. J. Chem. Soc. 1986, 1, 255–260. [Google Scholar] [CrossRef]
- Sassa, T.; Igarashi, M. Structures of (−)-mycousnine, (+)-isomycousnine and (+)-oxymycousnine. Agric. Biol. Chem. 1990, 54, 2231–2237. [Google Scholar] [CrossRef]
- De Oliveira, D.M.; Pereira, C.B.; Mendes, G.; Junker, J.; Kolloff, M.; Rosa, L.H.; Rosa, C.A.; Alves, T.M.A.; Zani, C.L.; Johann, S.; et al. Two new usnic acid derivatives from the endophytic fungus Mycosphaerella sp. Naturforsch C. 2018, 73, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Webber, F.C. Observations on the structure, life history and biology of Mycosphaerella ascophylli. Trans. Br. Mycol. Soc. 1967, 50, 583–601. [Google Scholar] [CrossRef]
- Kohlmeyer, J. Geography of marine fungi. Aust. J. Bot. Suppl. Ser. 1983, 10, 67–76. [Google Scholar] [CrossRef]
- Fries, N. Physiological characteristics of Mycosphaerella ascophylli, a fungal endophyte of the marine brown alga Ascophyllum nodosum. Physiol. Plant. 1979, 45, 117–121. [Google Scholar] [CrossRef]
- Lyons, J.I.; Alber, M.; Hollibaugh, J.T. Ascomycete fungal communities associated with early decaying leaves of Spartina spp. from central California estuaries. Oecologia 2010, 162, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Newell, S.Y.; Blum, L.K.; Crawford, R.E.; Dai, T.; Dionne, M. Autumnal biomass and potential productivity of salt marsh fungi from 29° to 43° north latitude along the United States Atlantic coast. Appl. Environ. Microbiol. 2000, 66, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Connolly, J.D.; Freer, A.A.; Huneck, S. Crystal Structure of (-)-placodiolic acid, a dibenzofuran derivative from the lichen Rhizoplaca chrysoleuca. Phytochem. 1984, 23, 702. [Google Scholar] [CrossRef]
- Seo, C.; Sohn, J.H.; Park, S.M.; Yim, J.H.; Lee, H.K.; Oh, H. Usimines A-C, bioactive usnic acid derivatives from the Antarctic lichen Stereocaulon alpinum. J. Nat. Prod. 2008, 71, 710–712. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]



| Position | 1 | HMBC | 2 | ||
|---|---|---|---|---|---|
| δC, Type | δH, mult. (J in Hz) | δC, Type | δH, mult. (J in Hz) | ||
| 1 | 200.5, C | 201.0, C | |||
| 2 | 100.6, CH | 5.55, s | 100.2, CH | 5.55, s | |
| 3 | 175.4, C | 175.9, C | |||
| 4α 4β | 34.3, CH2 | 3.15, d (17.5), 2.96, d (17.5) | 2, 4a 2, 4a | 34.2, CH2 | 3.20, d (17.5) 2.94, d (17.5) |
| 4a | 111.6, C | 110.6, C | |||
| 5a | 157.1, C | 160.7, C | |||
| 6 | 102.0, C | 100.4, C | |||
| 7 | 163.3, C | 165.5, C | |||
| 8 | 107.5, C | 107.1, C | |||
| 9 | 159.6, C | 156.4, C | |||
| 9a | 106.5, C | 106.1, C | |||
| 9b | 57.9, C | 58.9, C | |||
| 10 | 16.6, CH3 | 1.62, s | 1, 4a, 9a, 9b | 16.2, CH3 | 1.66, s |
| 11 | 7.4, CH3 | 2.04, s | 8 | 7.5, CH3 | 2.02, s |
| 12 | 201.2, C | 203.8, C | |||
| 13 | 31.3, CH3 | 2.61, s | 6, 12 | 32.9, CH3 | 2.73, s |
| 14 | 50.9, CH3 | 3.49, s | 4a | 50.3, CH3 | 3.47, s |
| 15 | 56.9, CH3 | 3.81, s | 2, 3 | 56.8, CH3 | 3.84, s |
| 7-OH | 13.34, s | 6, 7, 8 | 14.32, s | ||
| 9-OH | 9.34, s | 8, 9a | 9.61, s | ||
| Compound | Gram (+) Bacteria | Gram (−) Bacteria | ||||
|---|---|---|---|---|---|---|
| B. subtilis ATCC 6633 | K. rhizophila ATCC 9341 | S. aureus ATCC 6538 | E. coli ATCC 11775 | S. typhimurium ATCC 14208 | K. pneumonia ATCC 4352 | |
| 1 | >128 | 8 | 32 | >128 | >128 | >128 |
| 2 | >128 | 16 | 32 | >128 | >128 | >128 |
| 3 | 4 | 8 | 4 | >128 | >128 | >128 |
| 4 | 4 | 8 | 4 | >128 | >128 | >128 |
| 5 | 2 | 8 | 16 | >128 | >128 | >128 |
| Vancomycin | 0.25 | 0.25 | 0.5 | >128 | >128 | >128 |
| Ampicillin | 0.5 | 0.25 | 2 | 16 | 8 | >128 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Lee, J.; Kim, G.J.; Yang, I.; Wang, W.; Nam, J.-W.; Choi, H.; Nam, S.-J.; Kang, H. Mycousfurans A and B, Antibacterial Usnic Acid Congeners from the Fungus Mycosphaerella sp., Isolated from a Marine Sediment. Mar. Drugs 2019, 17, 422. https://doi.org/10.3390/md17070422
Lee J, Lee J, Kim GJ, Yang I, Wang W, Nam J-W, Choi H, Nam S-J, Kang H. Mycousfurans A and B, Antibacterial Usnic Acid Congeners from the Fungus Mycosphaerella sp., Isolated from a Marine Sediment. Marine Drugs. 2019; 17(7):422. https://doi.org/10.3390/md17070422
Chicago/Turabian StyleLee, Jihye, Jusung Lee, Geum Jin Kim, Inho Yang, Weihong Wang, Joo-Won Nam, Hyukjae Choi, Sang-Jip Nam, and Heonjoong Kang. 2019. "Mycousfurans A and B, Antibacterial Usnic Acid Congeners from the Fungus Mycosphaerella sp., Isolated from a Marine Sediment" Marine Drugs 17, no. 7: 422. https://doi.org/10.3390/md17070422
APA StyleLee, J., Lee, J., Kim, G. J., Yang, I., Wang, W., Nam, J.-W., Choi, H., Nam, S.-J., & Kang, H. (2019). Mycousfurans A and B, Antibacterial Usnic Acid Congeners from the Fungus Mycosphaerella sp., Isolated from a Marine Sediment. Marine Drugs, 17(7), 422. https://doi.org/10.3390/md17070422