Prostaglandins in Marine Organisms: A Review
Abstract
1. Introduction
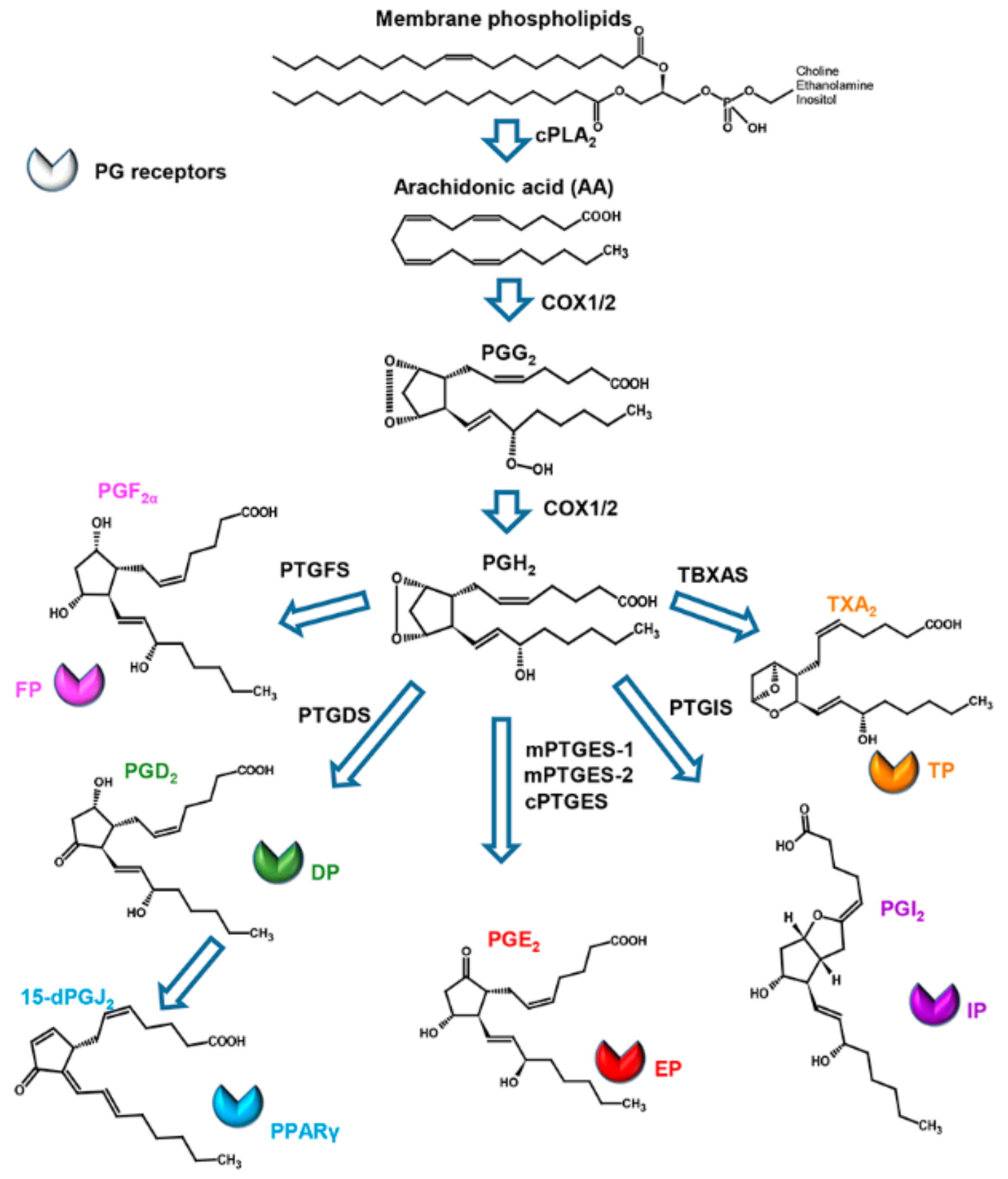
2. Structure, Biosynthesis, and Activity of Prostaglandins in Mammals
3. Prostaglandins and Derivative Molecules in Marine Organisms
3.1. Corals
3.2. Other Marine Invertebrates
3.3. Marine Vertebrates
3.4. Macroalgae
3.4.1. Red Macroalgae
3.4.2. Brown Macroalgae
3.5. Microalgae
4. Marine Cyclopentenone Prostaglandins
4.1. Clavulones and Related Molecules
4.2. Punaglandins
5. Marine Thromboxane
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chen, Y.; Chan, W.Y. Marine natural products with anti-inflammatory activity. Appl. Microbiol. Biotechnol. 2016, 100, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Tittensor, D.P.; Adl, S.; Simpson, A.G.B.; Worm, B. How many species are there on Earth and in the ocean? PLoS Biol. 2011, 9, e1001127. [Google Scholar] [CrossRef] [PubMed]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N.; Stockinger, B.; Tak, P.P. The resolution of inflammation. Nat. Rev. Immunol. 2013, 13, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, J.N.; Gilroy, D.W. Resolution of inflammation: A new therapeutic frontier. Nat. Rev. Drug Discov. 2016, 15, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, D.; De Maeyer, R. New insights into the resolution of inflammation. Semin. Immunol. 2015, 27, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Headland, S.E.; Norling, L.V. The resolution of inflammation: Principles and challenges. Semin. Immunol. 2015, 27, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Gómez, A.; Perretti, M.; Soehnlein, O. Resolution of inflammation: An integrated view. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harb. Perspect. Biol. 2014, 7, a016311. [Google Scholar] [CrossRef] [PubMed]
- Rajakariar, R.; Yaqoob, M.M.; Gilroy, D.W. COX-2 in inflammation and resolution. Mol. Interv. 2006, 6, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Maehara, T. Discovery of anti-inflammatory role of prostaglandin D2. J. Vet. Med. Sci. 2016, 78, 1643–1647. [Google Scholar] [CrossRef] [PubMed]
- Euler, U.S.v. Zur Kenntnis der pharmakologischen Wirkungen von Nativsekreten und Extrakten männlicher accessorischer Geschlechtsdrüsen. Arch. Exp. Pathol. Pharmakol. 1934, 175, 78–84. [Google Scholar] [CrossRef]
- Goldblatt, M.W. Properties of human seminal plasma. J. Physiol. 1935, 84, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, L.S. Eicosanoids: Prostaglandins, thromboxanes, leukotrienes, and other derivatives of carbon-20 unsaturated fatty acids. J. Neurochem. 1982, 38, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Piper, P.J. Introduction to the biosynthesis and metabolism of prostaglandins. Postgrad. Med. J. 1977, 53, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Bhakuni, D.S.; Rawat, D.S. Bioactive Marine Natural Products; Springer: New York, NY, USA; Anamaya: New Delhi, India, 2005; ISBN 978-1-4020-3472-5. [Google Scholar]
- Nomura, T.; Ogata, H. Distribution of prostaglandins in the animal kingdom. Biochim. Biophys. Acta 1976, 431, 127–131. [Google Scholar] [PubMed]
- Rowley, A.F.; Vogan, C.L.; Taylor, G.W.; Clare, A.S. Prostaglandins in non-insectan invertebrates: Recent insights and unsolved problems. J. Exp. Biol. 2005, 208, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Sajiki, J.; Kakimi, H. Identification of eicosanoids in the red algae, Gracilaria asiatica, using high-performance liquid chromatography and electrospray ionization mass spectrometry. J. Chromatogr. A 1998, 795, 227–237. [Google Scholar] [CrossRef]
- Ritter, A.; Goulitquer, S.; Salaün, J.-P.; Tonon, T.; Correa, J.A.; Potin, P. Copper stress induces biosynthesis of octadecanoid and eicosanoid oxygenated derivatives in the brown algal kelp Laminaria digitata. New Phytol. 2008, 180, 809–821. [Google Scholar] [CrossRef]
- Groenewald, E.G.; van der Westhuizen, A.J. Prostaglandins and related substances in plants. Bot. Rev. 1997, 63, 199–220. [Google Scholar] [CrossRef]
- Ruggeri, B.; Thoroughgood, C. Prostaglandins in aquatic fauna: A comprehensive review. Mar. Ecol. Prog. Ser. 1985, 23, 301–306. [Google Scholar] [CrossRef]
- Carté, B.K. Biomedical potential of marine natural products. Marine organisms are yielding novel molecules for use in basic research and medical applications. BioScience 1996, 46, 271–286. [Google Scholar]
- Di Dato, V.; Orefice, I.; Amato, A.; Fontanarosa, C.; Amoresano, A.; Cutignano, A.; Ianora, A.; Romano, G. Animal-like prostaglandins in marine microalgae. ISME J. 2017, 11, 1722–1726. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Rosenberg, D.W. Multifaceted roles of PGE2 in inflammation and cancer. Semin. Immunopathol. 2013, 35, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Lands, W.E.M. The biosynthesis and metabolism of prostaglandins. Annu. Rev. Physiol. 1979, 41, 633–652. [Google Scholar] [CrossRef] [PubMed]
- Bos, C.L.; Richel, D.J.; Ritsema, T.; Peppelenbosch, M.P.; Versteeg, H.H. Prostanoids and prostanoid receptors in signal transduction. Int. J. Biochem. Cell Biol. 2004, 36, 1187–1205. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D. Prostaglandins and leukotrienes: Advances in eicosanoid biology. Science 2001, 294, 1871–1875. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.S. 15-hydroxyprostaglandin dehydrogenase. A review. Prostaglandins 1976, 12, 647–679. [Google Scholar] [CrossRef]
- Wang, D.; DuBois, R.N. Prostaglandins and cancer. Gut 2006, 55, 115–122. [Google Scholar] [CrossRef]
- Jakobsson, P.J.; Thorén, S.; Morgenstern, R.; Samuelsson, B. Identification of human prostaglandin E synthase: A microsomal, glutathione-dependent, inducible enzyme, constituting a potential novel drug target. Proc. Natl. Acad. Sci. USA 1999, 96, 7220–7225. [Google Scholar] [CrossRef]
- Sykes, L.; MacIntyre, D.A.; Teoh, T.G.; Bennett, P.R. Anti-inflammatory prostaglandins for the prevention of preterm labour. Reproduction 2014, 148, R29–R40. [Google Scholar] [CrossRef]
- Basu, S. Novel cyclooxygenase-catalyzed bioactive prostaglandin F2α from physiology to new principles in inflammation. Med. Res. Rev. 2007, 27, 435–468. [Google Scholar] [CrossRef] [PubMed]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Nakao, A.; Emerling, D.; Hashimoto, Y.; Tsukamoto, K.; Horie, Y.; Kinoshita, M.; Kurokawa, K. Prostaglandin F2 alpha enhances tyrosine phosphorylation and DNA synthesis through phospholipase C-coupled receptor via Ca(2+)-dependent intracellular pathway in NIH-3T3 cells. J. Biol. Chem. 1994, 269, 17619–17625. [Google Scholar] [PubMed]
- Straus, D.S.; Glass, C.K. Cyclopentenone prostaglandins: New insights on biological activities and cellular targets. Med. Res. Rev. 2001, 21, 185–210. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.A.; Bucknall, R.M. Antidiuretic and cardiovascular actions of prostaglandin E2 in the rainbow trout Salmo gairdneri. Gen. Comp. Endocrinol. 1986, 61, 330–337. [Google Scholar] [CrossRef]
- Di Marzo, V.; Cimino, G.; Crispino, A.; Minardi, C.; Sodano, G.; Spinella, A. A novel multifunctional metabolic pathway in a marine mollusc leads to unprecedented prostaglandin derivatives (prostaglandin 1,15-lactones). Biochem. J. 1991, 273 (Pt 3), 593–600. [Google Scholar] [CrossRef]
- Weinheimer, A.J.; Spraggins, R.L. The occurrence of two new prostaglandin derivatives (15-epi-PGA2 and its acetate, methyl ester) in the gorgonian Plexaura homomalla chemistry of coelenterates. XV. Tetrahedron Lett. 1969, 59, 5185–5188. [Google Scholar] [CrossRef]
- Light, R.J.; Samuelsson, B. Identification of prostaglandins in the gorgonian, Plexaura homomalla. Eur. J. Biochem. 1972, 28, 232–240. [Google Scholar] [CrossRef]
- Schneider, W.P.; Hamilton, R.D.; Rhuland, L.E. Occurrence of esters of (15S)-prostaglandin A 2 and E 2 in coral. J. Am. Chem. Soc. 1972, 94, 2122–2123. [Google Scholar] [CrossRef]
- Bayer, F.; Weinheimer, A. Prostaglandins from Plexaura homomalla: Ecology, Utilization and Conservation of a Major Medical Marine Resource. A Symposium; Studies in Tropical Oceanography; University of Miami Press on Behalf of the Upjohn Co.: Coral Gables, FL, USA, 1974. [Google Scholar]
- Gerhart, D.J. Prostaglandin A2: An agent of chemical defense in the Caribbean gorgonian Plexaura homomalla. Mar. Ecol. Prog. Ser. 1984, 19, 181–187. [Google Scholar] [CrossRef]
- Reina, E.; Ramos, F.A.; Castellanos, L.; Aragón, M.; Ospina, L.F. Anti-inflammatory R-prostaglandins from Caribbean Colombian Soft Coral Plexaura homomalla. J. Pharm. Pharmacol. 2013, 65, 1643–1652. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Tsukitani, Y.; Iguchi, K.; Yamada, Y. Absolute stereochemistry of new prostanoids clavulone I, II and III, from Clavularia viridis Quoy and Gaimard. Tetrahedron Lett. 1983, 24, 1549–1552. [Google Scholar] [CrossRef]
- Honda, A.; Yamamoto, Y.; Mori, Y.; Yamada, Y.; Kikuchi, H. Antileukemic effect of coral-prostanoids clavulones from the stolonifer Clavularia viridis on human myeloid leukemia (HL-60) cells. Biochem. Biophys. Res. Commun. 1985, 130, 515–523. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Guh, J.-H.; Shen, Y.-C.; Teng, C.-M. Investigation of anticancer mechanism of clavulone II, a coral cyclopentenone prostaglandin analog, in human acute promyelocytic leukemia. J. Biomed. Sci. 2005, 12, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Bader, T.; Yamada, Y.; Ankel, H. Antiviral activity of the prostanoid clavulone II against vesicular stomatitis virus. Antivir. Res. 1991, 16, 341–355. [Google Scholar] [CrossRef]
- Iguchi, K.; Kaneta, S.; Mori, K.; Yamada, Y.; Honda, A.; Mori, Y. Chlorovulones, new halogenated marine prostanoids with an antitumor activity from the stolonifer Clavularia viridis Quoy and Gaimard. Tetrahedron Lett. 1985, 26, 5787–5790. [Google Scholar] [CrossRef]
- Iguchi, K.; Kaneta, S.; Mori, K.; Yamada, Y. A new marine epoxy prostanoid with an antiproliferative activity from the stolonifer Clavularia viridis Quoy and Gaimard. Chem. Pharm. Bull. 1987, 35, 4375–4376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iguchi, K.; Kaneta, S.; Mori, K.; Yamada, Y.; Honda, A.; Mori, Y. Bromovulone I and iodovulone I, unprecedented brominated and iodinated marine prostanoids with antitumour activity isolated from the Japanese stolonifer Clavularia viridis Quoy and Gaimard. J. Chem. Soc. Chem. Commun. 1986, 981–982. [Google Scholar] [CrossRef]
- Shen, Y.-C.; Cheng, Y.-B.; Lin, Y.-C.; Guh, J.-H.; Teng, C.-M.; Ko, C.-L. New prostanoids with cytotoxic activity from Taiwanese octocoral Clavularia viridis. J. Nat. Prod. 2004, 67, 542–546. [Google Scholar] [CrossRef]
- Duh, C.-Y.; El-Gamal, A.A.H.; Chu, C.-J.; Wang, S.-K.; Dai, C.-F. New cytotoxic constituents from the Formosan Soft Corals Clavularia viridis and Clavularia violacea. J. Nat. Prod. 2002, 65, 1535–1539. [Google Scholar] [CrossRef]
- Iwashima, M.; Okamoto, K.; Iguchi, K. Clavirins, a new type of marine oxylipins with growth-inhibitory activity from the Okinawan soft coral, Clavularia viridis. Tetrahedron Lett. 1999, 40, 6455–6459. [Google Scholar] [CrossRef]
- Iwashima, M.; Terada, I.; Okamoto, K.; Iguchi, K. Tricycloclavulone and clavubicyclone, novel prostanoid-related marine oxylipins, isolated from the Okinawan Soft Coral Clavularia viridis. J. Org. Chem. 2002, 67, 2977–2981. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.J.; Okuda, R.K.; Yu, P.T.K.; Scheuer, P.J. Punaglandins: Halogenated antitumor eicosanoids from the octocoral Telesto riisei. J. Am. Chem. Soc. 1985, 107, 2976–2977. [Google Scholar] [CrossRef]
- Komoda, Y.; Kanayasu, T.; Ishikawa, M. Prostaglandin F 2 alpha from the Japanese coastal gorgonian, Euplexaura erecta. Chem. Pharm. Bull. 1979, 27, 2491–2494. [Google Scholar] [CrossRef] [PubMed]
- Carmely, S.; Kashman, Y.; Loya, Y.; Benayahu, Y. New prostaglandin (PGF) derivatives from the Soft Coral. Tetrahedron Lett. 1980, 21, 875–878. [Google Scholar] [CrossRef]
- Varvas, K.; Järving, I.; Koljak, R.; Vahemets, A.; Pehk, T.; Müürisepp, A.-M.; Lille, Ü.; Samel, N. In vitro biosynthesis of prostaglandins in the White Sea Soft Coral Gersemia fruticosa: Formation of optically active PGD2, PGE2, PGF2α and 15-keto-PGF2α from arachidonic acid. Tetrahedron Lett. 1993, 34, 3643–3646. [Google Scholar] [CrossRef]
- Järving, R.; Järving, I.; Kurg, R.; Brash, A.R.; Samel, N. On the evolutionary origin of cyclooxygenase (COX) isozymes: Characterization of marine invertebrate COX genes points to independent duplication events in vertebrate and invertebrate lineages. J. Biol. Chem. 2004, 279, 13624–13633. [Google Scholar] [CrossRef]
- Christ, E.J.; Van Dorp, D.A. Comparative aspects of prostaglandin biosynthesis in animal tissues. Biochim. Biophys. Acta (BBA)—Lipids Lipid Metab. 1972, 270, 537–545. [Google Scholar] [CrossRef]
- Ogata, H.; Nomura, T.; Hata, M. Prostaglandin biosynthesis in the tissue homogenates of marine animals. NIPPON SUISAN GAKKAISHI 1978, 44, 1367–1370. [Google Scholar] [CrossRef][Green Version]
- Levine, L.; Kobayashi, T. Detection of compounds immunologically related to arachidonic acid transformation products in extracts of invertebrates. Prostaglandins Leukot. Med. 1983, 12, 357–369. [Google Scholar] [CrossRef]
- Stanley-Samuelson, D.W. Physiological roles of prostaglandins and other eicosanoids in invertebrates. Biol. Bull. 1987, 173, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Tahara, D.; Yano, I. Maturation-related variations in prostaglandin and fatty acid content of ovary in the kuruma prawn (Marsupenaeus japonicus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 137, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Ogata, H.; Masao, I.T.O. Occurrence of prostaglandins in fish testis. Tohoku J. Agric. Res. 1973, 24, 138–144. [Google Scholar]
- Nylund, G.M.; Weinberger, F.; Rempt, M.; Pohnert, G. Metabolomic Assessment of induced and activated chemical defence in the invasive red alga Gracilaria vermiculophylla. PLoS ONE 2011, 6, e29359. [Google Scholar] [CrossRef] [PubMed]
- Cimino, G.; Crispino, A.; Di Marzo, V.; Spinella, A.; Sodano, G. Prostaglandin 1,15-lactones of the F series from the nudibranch mollusk Tethys fimbria. J. Organ. Chem. 1991, 56, 2907–2911. [Google Scholar] [CrossRef]
- Zambounis, A.; Gaquerel, E.; Strittmatter, M.; Salaün, J.P.; Potin, P.; Küpper, F.C. Prostaglandin A2 triggers a strong oxidative burst in Laminaria: A novel defense inducer in brown algae? ALGAE 2012, 27, 21–32. [Google Scholar] [CrossRef]
- Cabrera, D.M.; Janech, M.G.; Morinelli, T.A.; Miller, D.H. A thromboxane A(2) system in the Atlantic stingray, Dasyatis sabina. Gen. Comp. Endocrinol. 2003, 130, 157–164. [Google Scholar] [CrossRef]
- Thomson, M.; al-Hassan, J.M.; al-Saleh, J.; Fayad, S.; Ali, M. Prostanoid synthesis in whole blood cells from fish of the Arabian Gulf. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 119, 639–646. [Google Scholar] [CrossRef]
- Freas, W.; Grollman, S. Uptake and binding of prostaglandins in a marine bivalve, Modiolus demissus. J. Exp. Zool. 1981, 216, 225–233. [Google Scholar] [CrossRef]
- Hampson, A.J.; Rowley, A.F.; Barrow, S.E.; Steadman, R. Biosynthesis of eicosanoids by blood cells of the crab, Carcinus maenas. Biochim. Biophys. Acta 1992, 1124, 143–150. [Google Scholar] [CrossRef]
- Korotchenko, O.D.; Mishchenko, T.Y.; Isay, S.V. Prostaglandins of Japan Sea invertebrates—I. The quantitation of group B prostaglandins in echinoderms. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1983, 74, 85–88. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Nagle, D.G.; Proteau, P.J. Oxylipins from marine invertebrates. In Marine Natural Products—Diversity and Biosynthesis; Scheuer, P.J., Ed.; Springer: Berlin/Heidelberg, Germany, 1993; Volume 167, pp. 117–180. ISBN 978-3-540-56513-0. [Google Scholar]
- Ogata, H.; Nomura, T. Isolation and identification of prostaglandin E2 from gastrointestinal tract of shark Triakis scyllia. Biochim. Biophys. Acta 1975, 388, 84–91. [Google Scholar] [PubMed]
- Goetz, F.W.; Duman, P.; Ranjan, M.; Herman, C.A. Prostaglandin F and E synthesis by specific tissue components of the brook trout (Salvelinus fontinalis) ovary. J. Exp. Zool. 1989, 250, 196–205. [Google Scholar] [CrossRef]
- Anderson, A.A.; Fletcher, T.C.; Smith, G.M. Prostaglandin biosynthesis in the skin of the plaice Pleuronectes platessa L. Comp. Biochem. Physiol. C Comp. Pharmacol. 1981, 70, 195–199. [Google Scholar] [CrossRef]
- Rowley, A.F.; Barrow, S.E.; Hunt, T.C. Preliminary studies on eicosanoid production by fish leucocytes, using GC-mass spectrometry. J. Fish Biol. 1987, 31, 107–111. [Google Scholar] [CrossRef]
- Cagen, L.M.; Qureshi, Z.; Nishimura, H. Synthesis of prostaglandin E2 and prostaglandin F2α by toadfish red blood cells. Biochem. Biophys. Res. Commun. 1983, 110, 250–255. [Google Scholar] [CrossRef]
- Dang, T.H.; Lee, H.J.; Yoo, E.S.; Hong, J.; Choi, J.S.; Jung, J.H. The occurrence of 15-keto-prostaglandins in the red alga Gracilaria verrucosa. Arch. Pharm. Res. 2010, 33, 1325–1329. [Google Scholar] [CrossRef]
- Hsu, B.-Y.; Tsao, C.-Y.; Chiou, T.-K.; Hwang, P.-A.; Hwang, D.-F. HPLC determination for prostaglandins from seaweed. Food Control 2007, 18, 639–645. [Google Scholar] [CrossRef]
- Imbs, A.B.; Latyshev, N.A.; Svetashev, V.I.; Skriptsova, A.V.; Le, T.T.; Pham, M.Q.; Nguyen, V.S.; Pham, L.Q. Distribution of polyunsaturated fatty acids in red algae of the genus Gracilaria, a promising source of prostaglandins. Russ. J. Mar. Biol. 2012, 38, 339–345. [Google Scholar] [CrossRef]
- De Almeida, C.L.F.; Falcão, H.d.S.; Lima, G.R.d.M.; Montenegro, C.d.A.; Lira, N.S.; de Athayde-Filho, P.F.; Rodrigues, L.C.; de Souza, M.d.F.V.; Barbosa-Filho, J.M.; Batista, L.M. Bioactivities from marine algae of the genus Gracilaria. Int. J. Mol. Sci. 2011, 12, 4550–4573. [Google Scholar] [CrossRef]
- Kanamoto, H.; Takemura, M.; Ohyama, K. Identification of a cyclooxygenase gene from the red alga Gracilaria vermiculophylla and bioconversion of arachidonic acid to PGF(2α) in engineered Escherichia coli. Appl. Microbiol. Biotechnol. 2011, 91, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Bouarab, K.; Adas, F.; Gaquerel, E.; Kloareg, B.; Salaün, J.-P.; Potin, P. The innate immunity of a marine red alga involves oxylipins from both the eicosanoid and octadecanoid pathways. Plant Physiol. 2004, 135, 1838–1848. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.; Brodhun, F.; Feussner, I. Biosynthesis of oxylipins in non-mammals. Prog. Lipid Res. 2009, 48, 148–170. [Google Scholar] [CrossRef] [PubMed]
- Ianora, A.; Miralto, A. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: A review. Ecotoxicology 2010, 19, 493–511. [Google Scholar] [CrossRef] [PubMed]
- Levine, L.; Sneiders, A.; Kobayashi, T.; Schiff, J.A. Serologic and immunochromatographic detection of oxygenated polyenoic acids in Euglena gracilis var. bacillaris. Biochem. Biophys. Res. Commun. 1984, 120, 278–285. [Google Scholar] [CrossRef]
- Kafanova, T.V.; Busarova, N.G.; Isai, S.V.; Zvyagintseva, T.Y. Fatty acids and prostaglandins of thermal cyanobacteria. Chem. Nat. Compd. 1996, 32, 861–865. [Google Scholar] [CrossRef]
- Krüger, G.H.J.; Groenewald, E.G.; de Wet, H.; Botes, P.J. The occurrence of prostaglandin F2α in procaryotic organisms. S. Afr. J. Bot. 1990, 56, 150–153. [Google Scholar] [CrossRef]
- Narumiya, S.; Fukushima, M. Cyclopentenone prostaglandins: Anti-proliferative and anti-viral actions and their molecular mechanism. In Eicosanoids and Other Bioactive Lipids in Cancer and Radiation Injury: Proceedings of the 1st International Conference 11–14 October 1989, Detroit, MI, USA; Honn, K.V., Marnett, L.J., Nigam, S., Walden, T.L., Eds.; Developments in Oncology; Springer: Boston, MA, USA, 1991; pp. 439–448. ISBN 978-1-4615-3874-5. [Google Scholar]
- Conti, M. Cyclopentenone: A special moiety for anticancer drug design. Anticancer Drugs 2006, 17, 1017–1022. [Google Scholar] [CrossRef]
- Lis, L.G.; Zheldakova, T.A. New marine prostanoids, clavulones, halogenovulones, and punaglandins. Chem. Nat. Compd. 1993, 29, 259–274. [Google Scholar] [CrossRef]
- Imbs, A.B. Prostaglandins and oxylipins of corals. Russ. J. Mar. Biol. 2011, 37, 325–334. [Google Scholar] [CrossRef]
- Kikuchi, H.; Tsukitani, Y.; Iguchi, K.; Yamada, Y. Clavulones, new type of prostanoids from the stolonifer Clavularia viridis Quoy and Gaimard. Tetrahedron Lett. 1982, 23, 5171–5174. [Google Scholar] [CrossRef]
- Kitagawa, I.; Kobayashi, M.; Yasuzawa, T.; Son, B.W.; Yoshihara, M.; Kyogoku, Y. New prostanoids from Soft Coral. Tetrahedron 1985, 41, 995–1005. [Google Scholar] [CrossRef]
- Iwashima, M.; Okamoto, K.; Konno, F.; Iguchi, K. New marine prostanoids from the Okinawan Soft Coral, Clavularia viridis. J. Nat. Prod. 1999, 62, 352–354. [Google Scholar] [CrossRef] [PubMed]
- Corey, E.J.; D’Alarcao, M.; Matsuda, S.P.T.; Lansbury, P.T.; Yamada, Y. Intermediacy of 8-(R)-HPETE in the conversion of arachidonic acid to pre-clavulone a by Clavularia viridis. Implications for the biosynthesis of marine prostanoids. J. Am. Chem. Soc. 1987, 109, 289–290. [Google Scholar] [CrossRef]
- Corey, E.J.; Matsuda, S.P.T. Generality of marine prostanoid biosynthesis by the 2-oxidopentadienylcation pathway. Tetrahedron Lett. 1987, 28, 4247–4250. [Google Scholar] [CrossRef]
- Watanabe, K.; Sekine, M.; Iguchi, K. Isolation and structures of new halogenated prostanoids from the Okinawan Soft Coral Clavularia viridis. J. Nat. Prod. 2003, 66, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Sekine, M.; Iguchi, K. Isolation of three marine prostanoids, possible biosynthetic intermediates for clavulones, from the Okinawan Soft Coral Clavularia viridis. Chem. Pharm. Bull. 2003, 51, 909–913. [Google Scholar] [CrossRef][Green Version]
- Gerwick, W.H. Carbocyclic oxylipins of marine origin. Chem. Rev. 1993, 93, 1807–1823. [Google Scholar] [CrossRef]
- Munro, M.H.G.; Luibrand, R.T.; Blunt, J.W. The search for antiviral and anticancer compounds from marine organisms. In Bioorganic Marine Chemistry; Scheuer, P.J., Ed.; Springer: Berlin/Heidelberg, Germany, 1987; pp. 93–176. [Google Scholar]
- Baker, B.J.; Scheuer, P.J. The punaglandins: 10-chloroprostanoids from the octocoral Telesto riisei. J. Nat. Prod. 1994, 57, 1346–1353. [Google Scholar] [CrossRef]
- Suzuki, M.; Morita, Y.; Yanagisawa, A.; Baker, B.J.; Scheuer, P.J.; Noyori, R. Prostaglandin synthesis 15. Synthesis and structural revision of (7E)- and (7Z)-punaglandin 4. J. Org. Chem. 1988, 53, 286–295. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Wilson, N.H. Aspects of the thromboxane receptor system. Gen. Pharmacol. 1995, 26, 463–472. [Google Scholar] [CrossRef]
- Mustafa, T.; Srivastava, K.C. Prostaglandins (eicosanoids) and their role in ectothermic organisms. In Advances in Comparative and Environmental Physiology; Brouwer, M., Carr, W.E.S., Ellington, W.R., Engel, D.W., Gleeson, R.A., Korsgaard, B., Moerland, T.S., Mustafa, T., Prior, D.J., Sidell, B.D., et al., Eds.; Advances in Comparative and Environmental Physiology; Springer: Berlin/Heidelberg, Germany, 1989; pp. 157–207. ISBN 978-3-642-74510-2. [Google Scholar]
- Kayama, M.; Sadõ, T.; Iijima, N. The Prostaglandin synthesis in rainbow trout thrombocyte. NIPPON SUISAN GAKKAISHI 1986, 52, 925. [Google Scholar] [CrossRef][Green Version]
- Pettitt, T.R.; Barrow, S.E.; Rowley, A.F. Thromboxane, prostaglandin and leukotriene generation by rainbow trout blood. Fish Shellfish Immunol. 1991, 1, 71–73. [Google Scholar] [CrossRef]
- Hill, D.J.; Hallett, M.B.; Rowley, A.F. Effect of prostanoids and their precursors on the aggregation of rainbow trout thrombocytes. Am. J. Physiol. 1999, 276, R659–R664. [Google Scholar] [CrossRef] [PubMed]









| Prostaglandin | Producer Organism | Activity | Target Cells | Reference |
|---|---|---|---|---|
| (15R)-PGE2 (15R)-O-AcPGA2 | Plexaura homomalla | Anti-inflammatory | Leucocyte/TPA-induced mouse-ear edema | Reina et al., 2013 [43] |
| Clavulones I-III | Clavularia viridis | Anti-inflammatory | fertilized chicken eggs | Kikuchi et al., 1983 [44] |
| Clavulones I-III | Clavularia viridis | Anti-cancer | HL-60 | Honda et al., 1985 [45]; Huang et al., 2005 [46] |
| Clavulone II | Clavularia viridis | Anti-viral | VSV infected L929 | Bader et al., 1991 [47] |
| Chlorovulone I | Clavularia viridis | Anti-proliferative and cytotoxic | HL-60 | Iguchi et al., 1985 [48] |
| PGs Epoxy-prostanoid | Clavularia viridis | Anti-proliferative | HL-60 | Iguchi et al., 1987 [49] |
| Bromovulone I and Iodovulone I | Clavularia viridis | Anti-proliferative and cytotoxic | HL-60 | Iguchi et al., 1986 [50] |
| Bromovulone III | Clavularia viridis | Cytotoxic | PC-3/HT-29 | Shen et al., 2004 [51] |
| Chlorovulones II and III | Clavularia viridis | Cytotoxic | PC-3/HT-29 | Shen et al., 2004 [51] |
| Claviridenone F | Clavularia viridis | Cytotoxic | A549/HT-29/P-388 | Duh et al., 2002 [52] |
| Claviridenone G | Clavularia viridis | Cytotoxic | A549 | Duh et al., 2002 [52] |
| Clavirins I-II | Clavularia viridis | Growth-inhibition | HeLa S3 | Iwashima et al., 1999 [53] |
| Clavubicyclone | Clavularia viridis | Growth-inhibition | MCF-7/OVCAR-3 | Iwashima et al., 2002 [54] |
| Punaglandins I–IV | Telesto riisei | Cytotoxic | L1210 | Baker et al., 1985 [55] |
| Compound | Producer Organism | Biological Activities | Reference |
|---|---|---|---|
| PGF2α | Marsupenaeus japonicus | Ovarian maturation | Tahara et al., 2004 [64] |
| Thunnus thynnus | Contraction of smooth muscles during ejaculation and metabolism of testis | Nomura et al., 1973 [65] | |
| PGE1 | Marine Invertebrates | Thermoregulation and fever | Stanley-Samuelson, 1987 [63] |
| Laminaria digitata | Protection against stress conditions induced by copper excess | Ritter et al., 2008 [19] | |
| Salmo sp. | Contraction of smooth muscles during ejaculation and metabolism of testis | Chirst and Van Dorp, 1972 [60] | |
| PGE2 | Marsupenaeus japonicus | Ovarian maturation | Tahara et al., 2004 [64] |
| Paralichthys olivaceus and Thunnus thynnus | Contraction of smooth muscles during ejaculation and metabolism of testis | Nomura et al., 1973 [65] | |
| Gracilaria vermiculophylla | Wounding-activated chemical defense molecules | Nylund et al., 2011 [66] | |
| Laminaria digitata | Protection against stress conditions induced by copper excess | Ritter et al., 2008 [19] | |
| PGF2α- and PGF3α-1,15-lactones fatty acid esters (PLFE) | Tethys fimbria | Reproduction and multiple roles depending on body localization | Cimino et al., 1991 [67]; Di Marzo et al., 1991 [37] |
| PGF1α | Oncorhynchus keta | Contraction of smooth muscles during ejaculation and metabolism of testis | Nomura et al., 1973 [65] |
| 15-keto-PGE2 | Gracilaria vermiculophylla | Wounding-activated chemical defense molecules | Nylund et al., 2011 [66] |
| Laminaria digitata | Protection against stress conditions induced by copper excess | Ritter et al., 2008 [19] | |
| PGE2-1,15-lactone | Tethys fimbria | Reproduction and multiple roles depending on body localization | Cimino et al., 1991 [67]; Di Marzo et al., 1991 [37] |
| PGE3-1,15-lactone-11-acetate | Tethys fimbria | Reproduction and multiple roles depending on body localization | Cimino et al., 1991 [67]; Di Marzo et al., 1991 [37] |
| PGE3-1,15-lactone | Tethys fimbria | Reproduction and multiple roles depending on body localization | Cimino et al., 1991 [67]; Di Marzo et al., 1991 [37] |
| PGD1 | Laminaria digitata | Protection against stress conditions induced by copper excess | Ritter et al., 2008 [19] |
| PGA2 | Gracilaria vermiculophylla | Wounding-activated chemical defense molecules | Nylund et al., 2011 [66] |
| Laminaria digitata | Protection against copper stress and trigger of oxidative responses | Zambounis et al., 2012 [68] | |
| PGB2 | Laminaria digitata | Protection against stress conditions induced by copper excess | Ritter et al., 2008 [19] |
| PGJ2 | Laminaria digitata | Protection against stress conditions induced by copper excess | Ritter et al., 2008 [19] |
| Clavulones | Clavularia viridis | Suggested to be hypothetical repellents against other marine organisms | Honda et al., 1985 [45] |
| iTXB2 | Dayatis sabina | Blood clotting | Cabrera et al., 2003 [69] |
| TXB2 | Oncorhynchus mykiss | Vasodilator agent | Thomson et al., 1998 [70] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Costanzo, F.; Di Dato, V.; Ianora, A.; Romano, G. Prostaglandins in Marine Organisms: A Review. Mar. Drugs 2019, 17, 428. https://doi.org/10.3390/md17070428
Di Costanzo F, Di Dato V, Ianora A, Romano G. Prostaglandins in Marine Organisms: A Review. Marine Drugs. 2019; 17(7):428. https://doi.org/10.3390/md17070428
Chicago/Turabian StyleDi Costanzo, Federica, Valeria Di Dato, Adrianna Ianora, and Giovanna Romano. 2019. "Prostaglandins in Marine Organisms: A Review" Marine Drugs 17, no. 7: 428. https://doi.org/10.3390/md17070428
APA StyleDi Costanzo, F., Di Dato, V., Ianora, A., & Romano, G. (2019). Prostaglandins in Marine Organisms: A Review. Marine Drugs, 17(7), 428. https://doi.org/10.3390/md17070428








