The Sialic Acid-Dependent Nematocyst Discharge Process in Relation to Its Physical-Chemical Properties Is a Role Model for Nanomedical Diagnostic and Therapeutic Tools
Abstract
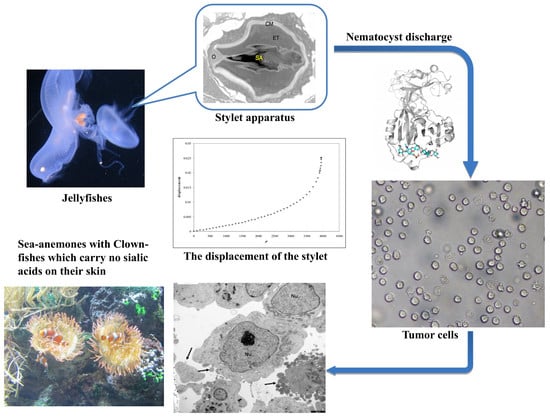
:1. Introduction
2. Results
2.1. Theoretical Model
2.2. Biophysical Experiments
2.3. QCM Analysis of Collagen Fragments from Cnidaria
2.4. Molecules Constituting the Nematocyst Membrane
3. Discussions
Physical Properties of Nematocysts and Their Potential Targets
4. Materials and Methods
4.1. Tumor Cells: Neuroblastoma Cells
4.2. Hydra Culture
4.3. Proteoglycans, Algae Polysaccharides and Collagen Fragments from Marine Organisms and Bacteria
4.4. Electron Microscopy
4.5. Measurements with a QCM System
4.6. Molecular Modelling
4.7. Preparation of FSNP-Sia
4.7.1. Synthesis of Fluorescent Silica Nanoparticles (FSNP)
4.7.2. Synthesis of Perfluorophenyl Azide (PFPA)-functionalized Fluorescent Silica Nanoparticles (FSNP-PFPA)
4.7.3. Synthesis of Sialic Acid-Conjugated FSNP (FSNP-Sia)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Turing, A.M. The chemical basis of morphogenesis. Bull. Math. Biol. 1990, 52, 153–197; discussion 119–152. [Google Scholar] [CrossRef]
- Von Neumann, J.; Burks, A.W. Theory of Self-Reproducing Automata; University of Illinois Press: Champagne, IL, USA, 1966; pp. 64–87. [Google Scholar]
- Gierer, A.; Berking, S.; Bode, H.; David, C.N.; Flick, K.; Hansmann, G.; Schaller, H.; Trenkner, E. Regeneration of Hydra from reaggregated cells. Nat. New Biol. 1972, 239, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Gierer, A.; Meinhardt, H. A theory of biological pattern formation. Kybernetik 1972, 12, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meinhardt, H. Turing’s theory of morphogenesis of 1952 and the subsequent discovery of the crucial role of local self-enhancement and long-range inhibition. Interface Focus 2012, 2, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Maini, P.K.; Woolley, T.E.; Baker, R.E.; Gaffney, E.A.; Lee, S.S. Turing’s model for biological pattern formation and the robustness problem. Interface Focus 2012, 2, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, S. Answering Descartes: Beyond Turing. In The Once and Future Turing: Computing the World; Cooper, S.B., Hodges, A., Eds.; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- ’t Hooft, G. Can the ultimate laws of nature be found? Under the spell of the gauge principle. In Advanced Series in Mathematical Physics; World Scientific Publishing Company: Calgary, AB, Canada, 1994; pp. 666–676. [Google Scholar]
- ’t Hooft, G. Playing with Planets. World Scientific News, October 2008. [Google Scholar] [Green Version]
- Holstein, T.W.; Benoit, M.; Herder, G.V.; David, C.N.; Wanner, G.; Gaub, H.E. Fibrous minicollagens in Hydra nematocysts. Science 1994, 265, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Weber, J. Nematocysts (stinging capsules of Cnidaria) as Donnan-potential-dominated osmotic systems. Eur. J. Biochem. 1989, 184, 465–476. [Google Scholar] [CrossRef] [PubMed]
- Morabito, R.; Marino, A.; Dossena, S.; La Spada, G. Nematocyst discharge in Pelagia noctiluca (Cnidaria, Scyphozoa) oral arms can be affected by lidocaine, ethanol, ammonia and acetic acid. Toxicon 2014, 83, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Todaro, D.; Watson, G.M. Force-dependent discharge of nematocysts in the sea anemone Haliplanella luciae (Verrill). Biol. Open 2012, 1, 582–587. [Google Scholar] [CrossRef] [Green Version]
- Nuchter, T.; Benoit, M.; Engel, U.; Özbek, S.; Holstein, T.W. Nanosecond-scale kinetics of nematocyst discharge. Curr. Biol. 2006, 16, R316–R318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holstein, T.W.; Hobmayer, E.; Technau, U. Cnidarians: An evolutionarily conserved model system for regeneration? Dev. Dyn. 2003, 226, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.C.G. Why polyps regenerate and we don’t: Towards a cellular and molecular framework for Hydra regeneration. Dev. Biol. 2007, 303, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.C.G.; Augustin, R.; Anton-Erxleben, F.; Fraune, S.; Hemmrich, G.; Zill, H.; Rosenstiel, P.; Jacobs, G.; Schreiber, S.; Leippe, M.; et al. Uncovering the evolutionary history of innate immunity: The simple metazoan Hydra uses epithelial cells for host defence. Dev. Comp. Immunol. 2009, 33, 559–569. [Google Scholar] [CrossRef] [PubMed]
- Fraune, S.; Augustin, R.; Anton-Erxleben, F.; Wittlieb, J.; Gelhaus, C.; Klimovich, V.B.; Samoilovich, M.P.; Bosch, T.C.G. In an early branching metazoan, bacterial colonization of the embryo is controlled by maternal antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2010, 107, 18067–18072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boehm, A.M.; Khalturin, K.; Anton-Erxleben, F.; Hemmrich, G.; Klostermeier, U.C.; Lopez-Quintero, J.A.; Oberg, H.H.; Puchert, M.; Rosenstiel, P.; Wittlieb, J.; et al. FoxO is a critical regulator of stem cell maintenance in immortal Hydra. Proc. Natl. Acad. Sci. USA 2012, 109, 19697–19702. [Google Scholar] [CrossRef] [PubMed]
- Augustin, R.; Fraune, S.; Franzenburg, S.; Bosch, T.C.G. Where simplicity meets complexity: Hydra, a model for host-microbe interactions. Adv. Exp. Med. Biol. 2012, 710, 71–81. [Google Scholar] [PubMed]
- Chapman, J.A.; Kirkness, E.F.; Simakov, O.; Hampson, S.E.; Mitros, T.; Weinmaier, T.; Rattei, T.; Balasubramanian, P.G.; Borman, J.; Busam, D.; et al. The dynamic genome of Hydra. Nature 2010, 464, 592–596. [Google Scholar] [CrossRef]
- Gabius, H.-J.; Siebert, H.-C.; André, S.; Jiménez-Barbero, J.; Rüdiger, H. Chemical biology of the sugar code. ChemBioChem 2004, 5, 740–764. [Google Scholar] [CrossRef]
- Siebert, H.-C.; Lu, S.Y.; Wechselberger, R.; Born, K.; Eckert, T.; Liang, S.; von der Lieth, C.-W.; Jiménez-Barbero, J.; Schauer, R.; Vliegenthart, J.F.G.; et al. A lectin from the Chinese bird-hunting spider binds sialic acids. Carbohydr. Res. 2009, 344, 1515–1525. [Google Scholar] [CrossRef] [Green Version]
- Siebert, H.-C.; Burg-Roderfeld, M.; Eckert, T.; Stötzel, S.; Kirch, U.; Diercks, T.; Humphries, M.J.; Frank, M.; Wechselberger, R.; Tajkhorshid, E.; et al. Interaction of the alpha 2A domain of integrin with small collagen fragments. Protein Cell 2010, 1, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Siebert, H.-C.; Scheidig, A.; Eckert, T.; Wienk, H.; Boelens, R.; Mahvash, M.; Petridis, A.K.; Schauer, R. Interaction studies of sialic acids with model receptors contribute to nanomedical therapies. J. Neurol. Disord. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Simon, P.; Baumner, S.; Busch, O.; Rohrich, R.; Kaese, M.; Richterich, P.; Wehrend, A.; Muller, K.; Gerardy-Schahn, R.; Muhlenhoff, M.; et al. Polysialic acid is present in mammalian semen as a post-translational modification of the neural cell adhesion molecule NCAM and the polysialyltransferase ST8SiaII. J. Biol. Chem. 2013, 288, 18825–18833. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, A.; Vivekanandan, S.; Eckert, T.; Burg-Roderfeld, M.; Wechselberger, R.; Romanuka, J.; Bächle, D.; Kornilov, A.V.; von der Lieth, C.-W.; Jiménez-Barbero, J.; et al. Why structurally different cyclic peptides can be glycomimetics of the HNK-1 carbohydrate antigen. J. Am. Chem. Soc. 2010, 132, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, Y.E.; Burg-Roderfeld, M.; Loers, G.; Arda, A.; Sukhova, E.V.; Khatuntseva, E.A.; Grachev, A.A.; Chizhov, A.O.; Siebert, H.-C.; Schachner, M.; et al. Synthesis and molecular recognition studies of the HNK-1 trisaccharide and related oligosaccharides. The specificity of monoclonal anti-HNK-1 antibodies as assessed by surface plasmon resonance and NMR. J. Am. Chem. Soc. 2012, 134, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Schadow, S.; Siebert, H.-C.; Lochnit, G.; Kordelle, J.; Rickert, M.; Steinmeyer, J. Collagen metabolism of human osteoarthritic articular cartilage as modulated by bovine collagen hydrolysates. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, H.; Gierer, A. Generation and regeneration of sequence of structures during morphogenesis. J. Theor. Biol. 1980, 85, 429–450. [Google Scholar] [CrossRef]
- Mason, P.E.; Uhlig, F.; Vanek, V.; Buttersack, T.; Bauerecker, S.; Jungwirth, P. Coulomb explosion during the early stages of the reaction of alkali metals with water. Nat. Chem. 2015, 7, 250–254. [Google Scholar] [CrossRef]
- Banerjee, S.; Mazumdar, S. Electrospray ionization mass spectrometry: A technique to access the information beyond the molecular weight of the analyte. Int. J. Anal. Chem. 2012, 2012, 1–40. [Google Scholar] [CrossRef]
- Lin, X.H.; Chen, H.Q.; Jiang, S.Y.; Zhang, C.B. A Coulomb explosion theoretical model of femtosecond laser ablation materials. Sci. China Technol. Sci. 2012, 55, 694–701. [Google Scholar] [CrossRef]
- Tursch, A.; Mercadante, D.; Tennigkeit, J.; Grater, F.; Özbek, S. Minicollagen cysteine-rich domains encode distinct modes of polymerization to form stable nematocyst capsules. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Feynman, R. Plenty of room at the bottom. Am. Phys. Soc. Pasadena 1959. [Google Scholar] [CrossRef]
- Eckert, T.; Stötzel, S.; Burg-Roderfeld, M.; Sewing, J.; Lütteke, T.; Nifantiev, N.E.; Vliegenthart, J.F.G.; Siebert, H.-C. In silico study on sulfated and non-sulfated carbohydrate chains from proteoglycans in cnidaria and interaction with collagen. Open J. Phys. Chem. 2012, 2, 123–133. [Google Scholar] [CrossRef]
- Stötzel, S.; Schurink, M.; Wienk, H.; Siebler, U.; Burg-Roderfeld, M.; Eckert, T.; Kulik, B.; Wechselberger, R.; Sewing, J.; Steinmeyer, J.; et al. Molecular organization of various collagen fragments as revealed by atomic force microscopy and diffusion-ordered NMR spectroscopy. ChemPhysChem 2012, 13, 3117–3125. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Eckert, T.; Lütteke, T.; Hanstein, S.; Scheidig, A.J.; Bonvin, A.M.; Nifantiev, N.E.; Kožár, T.; Schauer, R.; Enani, M.A.; et al. Structure-function relationships of antimicrobial peptides and proteins with respect to contact molecules on pathogen surfaces. Curr. Top. Med. Chem. 2016, 16, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Loers, G.; Schachner, M.; Boelens, R.; Wienk, H.; Siebert, S.; Eckert, T.; Kraan, S.; Rojas-Macias, M.A.; Lütteke, T.; et al. Molecular basis of the receptor interactions of polysialic acid (polySia), polySia mimetics, and sulfated polysaccharides. ChemMedChem 2016, 11, 990–1002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wu, L.; Eckert, T.; Burg-Roderfeld, M.; Rojas-Macias, M.A.; Lütteke, T.; Krylov, V.B.; Argunov, D.A.; Datta, A.; Markart, P.; et al. Lysozyme’s lectin-like characteristics facilitates its immune defense function. Q. Rev. Biophys. 2017, 50, e9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, N.; Mohri, M.; Wu, L.; Eckert, T.; Krylov, V.B.; Antosova, A.; Ponikova, S.; Bednarikova, Z.; Markart, P.; et al. Nanomedical relevance of the intermolecular interaction dynamics-Examples from lysozymes and insulins. ACS Omega 2019, 4, 4206–4220. [Google Scholar] [CrossRef] [PubMed]
- Sato, C.; Kitajima, K.; Tazawa, I.; Inoue, Y.; Inoue, S.; Troy, F.A. Structural diversity in the alpha 2-->8-linked polysialic acid chains in salmonid fish egg glycoproteins. Occurrence of poly(Neu5Ac), poly(Neu5Gc), poly(Neu5Ac, Neu5Gc), poly(KDN), and their partially acetylated forms. J. Biol. Chem. 1993, 268, 23675–23684. [Google Scholar]
- Rawnaq, T.; Quaas, A.; Zander, H.; Gros, S.J.; Reichelt, U.; Blessmann, M.; Wilzcak, W.; Schachner, M.; Sauter, G.; Izbicki, J.R.; et al. L1 is highly expressed in tumors of the nervous system: A study of over 8000 human tissues. J. Surg. Res. 2012, 173, 314–319. [Google Scholar] [CrossRef]
- Shpirer, E.; Chang, E.S.; Diamant, A.; Rubinstein, N.; Cartwright, P.; Huchon, D. Diversity and evolution of myxozoan minicollagens and nematogalectins. BMC Evol. Biol. 2014, 14, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckmann, A.; Xiao, S.; Muller, J.P.; Mercadante, D.; Nuchter, T.; Kroger, N.; Langhojer, F.; Petrich, W.; Holstein, T.W.; Benoit, M.; et al. A fast recoiling silk-like elastomer facilitates nanosecond nematocyst discharge. BMC Biol. 2015, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Chen, Y.; Cui, X.; Cai, S.; Chen, Z. High-resolution 1H NMR spectroscopy of fish muscle, eggs and small whole fish via Hadamard-encoded intermolecular multiple-quantum coherence. PLoS ONE 2014, 9, e86422. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.P.A. Are we quantum computers, or merely clever robots? Int. J. Mod. Phys. B 2017, 31, 1743001. [Google Scholar] [CrossRef]
- Theis, T.; Mishra, B.; von der Ohe, M.; Loers, G.; Prondzynski, M.; Pless, O.; Blackshear, P.J.; Schachner, M.; Kleene, R. Functional role of the interaction between polysialic acid and myristoylated alanine-rich C kinase substrate at the plasma membrane. J. Biol. Chem. 2013, 288, 6726–6742. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.C. Quartz crystal microbalance with dissipation monitoring: Enabling real-time characterization of biological materials and their interactions. J. Biomol. Tech. 2008, 19, 151–158. [Google Scholar] [PubMed]
- Schadow, S.; Simons, V.S.; Lochnit, G.; Kordelle, J.; Gazova, Z.; Siebert, H.-C.; Steinmeyer, J. Metabolic response of human osteoarthritic cartilage to biochemically characterized collagen hydrolysates. Int. J. Mol. Sci. 2017, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, B.; Bernhardt, A.; Lode, A.; Heinemann, S.; Sewing, J.; Klinger, M.; Notbohm, H.; Gelinsky, M. Jellyfish collagen scaffolds for cartilage tissue engineering. Acta Biomater. 2014, 10, 883–892. [Google Scholar] [CrossRef]
- Sewing, J.; Klinger, M.; Notbohm, H. Jellyfish collagen matrices conserve the chondrogenic phenotype in two- and three-dimensional collagen matrices. J. Tissue Eng. Regen. Med. 2015, 11, 916–925. [Google Scholar] [CrossRef]
- Hwang, J.S.; Takaku, Y.; Momose, T.; Adamczyk, P.; Özbek, S.; Ikeo, K.; Khalturin, K.; Hemmrich, G.; Bosch, T.C.G.; Holstein, T.W.; et al. Nematogalectin, a nematocyst protein with GlyXY and galectin domains, demonstrates nematocyte-specific alternative splicing in Hydra. Proc. Natl. Acad. Sci. USA 2010, 107, 18539–18544. [Google Scholar] [CrossRef]
- Benkert, P.; Biasini, M.; Schwede, T. Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 2011, 27, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Watanabe, Y.; Lee, M.S.; Ogawa, T.; Muramoto, K. Structure of rhamnose-binding lectin CSL3: Unique pseudo-tetrameric architecture of a pattern recognition protein. J. Mol. Biol. 2009, 391, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, P.; Zenkert, C.; Balasubramanian, P.G.; Yamada, S.; Murakoshi, S.; Sugahara, K.; Hwang, J.S.; Gojobori, T.; Holstein, T.W.; Özbek, S. A non-sulfated chondroitin stabilizes membrane tubulation in cnidarian organelles. J. Biol. Chem. 2010, 285, 25613–25623. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Beck, K.F.; Raslik, I.; Walpen, S.; Mihalik, D.; Micegova, M.; Macakova, K.; Schonherr, E.; Seidler, D.G.; Varga, G.; et al. Biglycan, a nitric oxide-regulated gene, affects adhesion, growth, and survival of mesangial cells. J. Biol. Chem. 2003, 278, 26227–26237. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Babelova, A.; Kiss, E.; Hausser, H.J.; Baliova, M.; Krzyzankova, M.; Marsche, G.; Young, M.F.; Mihalik, D.; Gotte, M.; et al. The matrix component biglycan is proinflammatory and signals through Toll-like receptors 4 and 2 in macrophages. J. Clin. Investig. 2005, 115, 2223–2233. [Google Scholar] [CrossRef] [PubMed]
- Roedig, H.; Nastase, M.V.; Frey, H.; Moreth, K.; Zeng-Brouwers, J.; Poluzzi, C.; Tzung-Harn Hsieh, L.; Brandts, C.; Fulda, S.; Wygrecka, M.; et al. Biglycan is a new high-affinity ligand for CD14 in macrophages. Matrix Biol. 2019, 77, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Engel, U.; Pertz, O.; Fauser, C.; Engel, J.; David, C.N.; Holstein, T.W. A switch in disulfide linkage during minicollagen assembly in Hydra nematocysts. EMBO J. 2001, 20, 3063–3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, U.; Özbek, S.; Engel, R.; Petri, B.; Lottspeich, F.; Holstein, T.W. Nowa, a novel protein with minicollagen Cys-rich domains, is involved in nematocyst formation in Hydra. J. Cell Sci. 2002, 115, 3923–3934. [Google Scholar] [CrossRef] [PubMed]
- Pokidysheva, E.; Milbradt, A.G.; Meier, S.; Renner, C.; Haussinger, D.; Bachinger, H.P.; Moroder, L.; Grzesiek, S.; Holstein, T.W.; Özbek, S.; et al. The structure of the Cys-rich terminal domain of Hydra minicollagen, which is involved in disulfide networks of the nematocyst wall. J. Biol. Chem. 2004, 279, 30395–30401. [Google Scholar] [CrossRef]
- Ozacmak, V.H.; Thorington, G.U.; Fletcher, W.H.; Hessinger, D.A. N-acetylneuraminic acid (NANA) stimulates in situ cyclic AMP production in tentacles of sea anemone (Aiptasia pallida): Possible role in chemosensitization of nematocyst discharge. J. Exp. Biol. 2001, 204, 2011–2020. [Google Scholar]
- Charlton, L.M.; Pielak, G.J. Peeking into living eukaryotic cells with high-resolution NMR. Proc. Natl. Acad. Sci. USA 2006, 103, 11817–11818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muralikrishna, G.; Reuter, G.; Peter-Katalinic, J.; Egge, H.; Hanisch, F.G.; Siebert, H.-C.; Schauer, R. Identification of a new ganglioside from the starfish Asterias rubens. Carbohydr. Res. 1992, 236, 321–326. [Google Scholar] [CrossRef]
- Zenkert, C.; Takahashi, T.; Diesner, M.O.; Özbek, S. Morphological and molecular analysis of the Nematostella vectensis cnidom. PLoS ONE 2011, 6, e22725. [Google Scholar] [CrossRef] [PubMed]
- Petersen, H.O.; Höger, S.K.; Looso, M.; Lengfeld, T.; Kuhn, A.; Warnken, U.; Nishimiya-Fujisawa, C.; Schnölzer, M.; Krüger, M.; Özbek, S.; et al. A comprehensive transcriptomic and proteomic analysis of Hydra head regeneration. Mol. Biol. Evol. 2015, 32, 1928–1947. [Google Scholar] [CrossRef] [PubMed]
- Berking, S.; Herrmann, K. Formation and discharge of nematocysts is controlled by a proton gradient across the cyst membrane. Helgol. Mar. Res. 2006, 60, 180–188. [Google Scholar] [CrossRef]
- Ogawa, T.; Watanabe, M.; Naganuma, T.; Muramoto, K. Diversified carbohydrate-binding lectins from marine resources. J. Amino Acids 2011, 2011, 838–914. [Google Scholar] [CrossRef] [PubMed]
- Datta, D.; Talapatra, S.N.; Swarnakar, S. An overview of lectins from freshwater and marine macroinvertebrates. World Sci. News 2016, 46, 77–87. [Google Scholar]
- Technau, U.; Steele, R.E. Evolutionary crossroads in developmental biology: Cnidaria. Development 2011, 138, 1447–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, R.E.; David, C.N.; Technau, U. A genomic view of 500 million years of cnidarian evolution. Trends Genet. 2011, 27, 7–13. [Google Scholar] [CrossRef] [Green Version]
- Raspanti, M.; Congiu, T.; Alessandrini, A.; Gobbi, P.; Ruggeri, A. Different patterns of collagen-proteoglycan interaction: A scanning electron microscopy and atomic force microscopy study. Eur. J. Histochem. 2000, 44, 335–343. [Google Scholar]
- Kar, R.K.; Gazova, Z.; Bednarikova, Z.; Mroue, K.H.; Ghosh, A.; Zhang, R.; Ulicna, K.; Siebert, H.-C.; Nifantiev, N.E.; Bhunia, A. Evidence for Inhibition of lysozyme amyloid fibrillization by peptide fragments from human lysozyme: A combined spectroscopy, microscopy, and docking study. Biomacromolecules 2016, 17, 1998–2009. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, H.S.; Jayawardana, K.W.; Chen, X.; Yan, M. Maltoheptaose promotes nanoparticle internalization by Escherichia coli. Chem. Commun. 2013, 49, 3034–3036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Ramström, O.; Yan, M. Glyconanomaterials: Emerging applications in biomedical research. Nano Res. 2014, 7, 1381–1403. [Google Scholar] [CrossRef] [Green Version]
- Sundhoro, M.; Park, J.; Jayawardana, K.W.; Chen, X.; Jayawardena, H.S.N.; Yan, M. Poly(HEMA-co-HEMA-PFPA): Synthesis and preparation of stable micelles encapsulating imaging nanoparticles. J. Colloid Interface Sci. 2017, 500, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ramström, O.; Yan, M. Dynamic light scattering as an efficient tool to study glyconanoparticle–lectin interactions. Analyst 2011, 136, 4174–4178. [Google Scholar] [CrossRef] [PubMed]
- Jayawardana, K.W.; Jayawardena, H.S.; Wijesundera, S.A.; De Zoysa, T.; Sundhoro, M.; Yan, M. Selective targeting of Mycobacterium smegmatis with trehalose-functionalized nanoparticles. Chem. Commun. 2015, 51, 12028–12031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondepudi, D.P.; Prigogine, I. Modern Thermodynamics: From Heat Engines to Dissipative Structures, Chapter 19.5: Turing Structures and Propagating Waves; Wiley Chichester: Chichester, UK, 1998; pp. 444–450. [Google Scholar]
- Bushman, J.; Mishra, B.; Ezra, M.; Gul, S.; Schulze, C.; Chaudhury, S.; Ripoll, D.; Wallqvist, A.; Kohn, J.; Schachner, M.; et al. Tegaserod mimics the neurostimulatory glycan polysialic acid and promotes nervous system repair. Neuropharmacology 2014, 79, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Loers, G.; Saini, V.; Mishra, B.; Papastefanaki, F.; Lutz, D.; Chaudhury, S.; Ripoll, D.R.; Wallqvist, A.; Gul, S.; Schachner, M.; et al. Nonyloxytryptamine mimics polysialic acid and modulates neuronal and glial functions in cell culture. J. Neurochem. 2014, 128, 88–100. [Google Scholar] [CrossRef]
- Siebert, H.-C.; André, S.; Lu, S.Y.; Frank, M.; Kaltner, H.; van Kuik, J.A.; Korchagina, E.Y.; Bovin, N.; Tajkhorshid, E.; Kaptein, R.; et al. Unique conformer selection of human growth-regulatory lectin galectin-1 for ganglioside GM1 versus bacterial toxins. Biochemistry 2003, 42, 14762–14773. [Google Scholar] [CrossRef]
- Siebert, H.-C.; Born, K.; André, S.; Frank, M.; Kaltner, H.; von der Lieth, C.-W.; Heck, A.J.; Jiménez-Barbero, J.; Kopitz, J.; Gabius, H.-J. Carbohydrate chain of ganglioside GM1 as a ligand: Identification of the binding strategies of three 15 mer peptides and their divergence from the binding modes of growth-regulatory galectin-1 and cholera toxin. Chem. Eur. J. 2006, 12, 388–402. [Google Scholar] [CrossRef]
- André, S.; Kaltner, H.; Lensch, M.; Russwurm, R.; Siebert, H.-C.; Fallsehr, C.; Tajkhorshid, E.; Heck, A.J.; von Knebel Doeberitz, M.; Gabius, H.-J.; et al. Determination of structural and functional overlap/divergence of five proto-type galectins by analysis of the growth-regulatory interaction with ganglioside GM1 in silico and in vitro on human neuroblastoma cells. Int. J. Cancer 2005, 114, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Singh, T.; Liu, J.H.; Krzeminski, M.; Russwurm, R.; Siebert, H.-C.; Bonvin, A.M.; André, S.; Gabius, H.-J. Activity-structure correlations in divergent lectin evolution: Fine specificity of chicken galectin CG-14 and computational analysis of flexible ligand docking for CG-14 and the closely related CG-16. Glycobiology 2007, 17, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Singh, T.; Liu, J.H.; André, S.; Lensch, M.; Siebert, H.-C.; Krzeminski, M.; Bonvin, A.M.; Kaltner, H.; Wu, J.H.; et al. Adhesion/growth-regulatory galectins: Insights into their ligand selectivity using natural glycoproteins and glycotopes. Adv. Exp. Med. Biol. 2011, 705, 117–141. [Google Scholar] [PubMed]
- Siebert, H.-C.; Tajkhorshid, E.; Dabrowski, J. Barrier to rotation around the C-sp(2)-C-sp(2) bond of the ketoaldehyde enol ether MeC(O)CH=CH-OEt as determined by 13C NMR and ab initio calculations. J. Phys. Chem. A 2001, 105, 8488–8494. [Google Scholar] [CrossRef]
- Van Lenthe, J.H.; den Boer, D.H.W.; Havenith, R.W.A.; Schauer, R.; Siebert, H.-C. Ab initio calculations on various sialic acids provide valuable information about sialic acid-specific enzymes. J. Mol. Struct. THEOCHEM 2004, 677, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Schwarzkopf, M.; Knobeloch, K.P.; Rohde, E.; Hinderlich, S.; Wiechens, N.; Lucka, L.; Horak, I.; Reutter, W.; Horstkorte, R. Sialylation is essential for early development in mice. Proc. Natl. Acad. Sci. USA 2002, 99, 5267–5270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, H.; Fujisawa, T.; Holstein, T.W. Cnidarians and the evolutionary origin of the nervous system. Dev. Growth Differ. 2009, 51, 167–183. [Google Scholar] [CrossRef]
- Siebert, H.-C.; von der Lieth, C.-W.; Dong, X.; Reuter, G.; Schauer, R.; Gabius, H.-J.; Vliegenthart, J.F.G. Molecular dynamics-derived conformation and intramolecular interaction analysis of the N-acetyl-9-O-acetylneuraminic acid-containing ganglioside GD1a and NMR-based analysis of its binding to a human polyclonal immunoglobulin G fraction with selectivity for O-acetylated sialic acids. Glycobiology 1996, 6, 561–572. [Google Scholar]
- Yeh, S.C.; Wang, P.Y.; Lou, Y.W.; Khoo, K.H.; Hsiao, M.; Hsu, T.L.; Wong, C.H. Glycolipid GD3 and GD3 synthase are key drivers for glioblastoma stem cells and tumorigenicity. Proc. Natl. Acad. Sci. USA 2016, 113, 5592–5597. [Google Scholar] [CrossRef] [Green Version]
- Sackmann, E.; Keber, F.; Heinrich, D. Physics of cellular movements. Annu. Rev. Conden. Matter 2010, 1, 257–276. [Google Scholar] [CrossRef]
- Haseley, S.R.; Vermeer, H.J.; Kamerling, J.P.; Vliegenthart, J.F.G. Carbohydrate self-recognition mediates marine sponge cellular adhesion. Proc. Natl. Acad. Sci. USA 2001, 98, 9419–9424. [Google Scholar] [CrossRef] [Green Version]
- Vilanova, E.; Santos, G.R.; Aquino, R.S.; Valle-Delgado, J.J.; Anselmetti, D.; Fernandez-Busquets, X.; Mourao, P.A. Carbohydrate-carbohydrate interactions mediated by sulfate esters and calcium provide the cell adhesion required for the emergence of early metazoans. J. Biol. Chem. 2016, 291, 9425–9437. [Google Scholar] [CrossRef]
- Lai, C.H.; Hutter, J.; Hsu, C.W.; Tanaka, H.; Varela-Aramburu, S.; De Cola, L.; Lepenies, B.; Seeberger, P.H. Analysis of carbohydrate-carbohydrate interactions using sugar-functionalized silicon nanoparticles for cell imaging. Nano Lett. 2016, 16, 807–811. [Google Scholar] [CrossRef]
- Lombardi, G.; Della Puppa, A.; Zustovich, F.; Pambuku, A.; Farina, P.; Fiduccia, P.; Roma, A.; Zagonel, V. The combination of carmustine wafers and fotemustine in recurrent glioblastoma patients: A monoinstitutional experience. Biomed. Res. Int. 2014, 2014, 1–4. [Google Scholar] [CrossRef]
- Rahman, R.; Hempfling, K.; Norden, A.D.; Reardon, D.A.; Nayak, L.; Rinne, M.L.; Beroukhim, R.; Doherty, L.; Ruland, S.; Rai, A.; et al. Retrospective study of carmustine or lomustine with bevacizumab in recurrent glioblastoma patients who have failed prior bevacizumab. Neuro-Oncology 2014, 16, 1523–1529. [Google Scholar] [CrossRef] [Green Version]
- Sieren, J.C.; Quelle, D.; Meyerholz, D.K.; Rogers, C.S. Porcine cancer models for translational oncology. Mol. Cell. Oncol. 2014, 1, e969626. [Google Scholar] [CrossRef] [Green Version]
- Lenhoff, H.M.; Brown, R.D. Mass culture of Hydra: An improved method and its application to other aquatic invertebrates. Lab. Anim. 1970, 4, 139–154. [Google Scholar] [CrossRef]
- Krylov, V.B.; Grachev, A.A.; Ustyuzhanina, N.E.; Ushakova, N.A.; Preobrazhenskaya, M.E.; Kozlova, N.I.; Portsel, M.N.; Konovalova, I.N.; Novikov, V.Y.; Siebert, H.-C.; et al. Preliminary structural characterization, anti-inflammatory and anticoagulant activities of chondroitin sulfates from marine fish cartilage. Russ. Chem. B 2011, 60, 746–753. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2010, 23, 543–597. [Google Scholar] [CrossRef]
- Van Zundert, G.C.P.; Rodrigues, J.; Trellet, M.; Schmitz, C.; Kastritis, P.L.; Karaca, E.; Melquiond, A.S.J.; van Dijk, M.; de Vries, S.J.; Bonvin, A. The HADDOCK2.2 Web Server: User-Friendly Integrative Modelling of Biomolecular Complexes. J. Mol. Biol. 2016, 428, 720–725. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. YASARA View–molecular graphics for all devices-from smartphones to workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef]
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modelling: Four approaches that performed well in CASP8. Proteins 2009, 77 (Suppl. 9), 114–122. [Google Scholar] [CrossRef]
- Jayawardena, H.S.; Wang, X.; Yan, M. Classification of lectins by pattern recognition using glyconanoparticles. Anal. Chem. 2013, 85, 10277–10281. [Google Scholar] [CrossRef]
- Szczepanek, S.; Cikala, M.; David, C.N. Poly-gamma-glutamate synthesis during formation of nematocyst capsules in Hydra. J. Cell Sci. 2002, 115, 745–751. [Google Scholar]
- Soriano, J.; Rudiger, S.; Pullarkat, P.; Ott, A. Mechanogenetic coupling of Hydra symmetry breaking and driven Turing instability model. Biophys. J. 2009, 96, 1649–1660. [Google Scholar] [CrossRef]
- Nobrega, F.L.; Costa, A.R.; Kluskens, L.D.; Azeredo, J. Revisiting phage therapy: New applications for old resources. Trends Microbiol. 2015, 23, 185–191. [Google Scholar] [CrossRef]
- Schauer, R.; Kamerling, J.P. Sialic acids, Part I: Historical background and development, and chemical synthesis. Adv. Carbohydr. Chem. Biochem. 2018, 75, 1–354. [Google Scholar]
- Morabito, R.; Dossena, S.; La Spada, G.; Marino, A. Heavy metals affect nematocysts discharge response and biological activity of crude venom in the jellyfish Pelagia noctiluca (Cnidaria, Scyphozoa). Cell. Physiol. Biochem. 2014, 34, 244–254. [Google Scholar] [CrossRef]
- Abdullah, N.S.; Saad, S. Rapid detecion of N-acetylneuraminic acid from false clownfish using HPLC-FLD for symbiosis to host sea anemone. Asian J. Sci. Technol. 2015, 3, 858–864. [Google Scholar]








| Δν, Hz | 0 | 300 | 250 | 300 | −300 | 300 | 0 | −300 | −100 | 150 |
| T, °C | 15 | 30 | 45 | 40 | 15 | 45 | 30 | 15 | 15 | 15 |
| t, sec | >0 | >1000 | >1500 | >2000 | >2500 | >4000 | >4500 | >5000 | >5500 | >6000 |
| Δν, Hz | 0 | 700 | 300 | 100 | 700 | 300 | 100 | 700 | 300 | 100 |
| T, °C | 15 | 37 | 25 | 15 | 37 | 25 | 15 | 37 | 25 | 15 |
| t, sec | 0 | 1500 | 2000 | 2500 | 3500 | 4000 | 4500 | 6000 | 6500 | 7000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Jin, L.; Zhang, N.; Petridis, A.K.; Eckert, T.; Scheiner-Bobis, G.; Bergmann, M.; Scheidig, A.; Schauer, R.; Yan, M.; et al. The Sialic Acid-Dependent Nematocyst Discharge Process in Relation to Its Physical-Chemical Properties Is a Role Model for Nanomedical Diagnostic and Therapeutic Tools. Mar. Drugs 2019, 17, 469. https://doi.org/10.3390/md17080469
Zhang R, Jin L, Zhang N, Petridis AK, Eckert T, Scheiner-Bobis G, Bergmann M, Scheidig A, Schauer R, Yan M, et al. The Sialic Acid-Dependent Nematocyst Discharge Process in Relation to Its Physical-Chemical Properties Is a Role Model for Nanomedical Diagnostic and Therapeutic Tools. Marine Drugs. 2019; 17(8):469. https://doi.org/10.3390/md17080469
Chicago/Turabian StyleZhang, Ruiyan, Li Jin, Ning Zhang, Athanasios K. Petridis, Thomas Eckert, Georgios Scheiner-Bobis, Martin Bergmann, Axel Scheidig, Roland Schauer, Mingdi Yan, and et al. 2019. "The Sialic Acid-Dependent Nematocyst Discharge Process in Relation to Its Physical-Chemical Properties Is a Role Model for Nanomedical Diagnostic and Therapeutic Tools" Marine Drugs 17, no. 8: 469. https://doi.org/10.3390/md17080469
APA StyleZhang, R., Jin, L., Zhang, N., Petridis, A. K., Eckert, T., Scheiner-Bobis, G., Bergmann, M., Scheidig, A., Schauer, R., Yan, M., Wijesundera, S. A., Nordén, B., Chatterjee, B. K., & Siebert, H. -C. (2019). The Sialic Acid-Dependent Nematocyst Discharge Process in Relation to Its Physical-Chemical Properties Is a Role Model for Nanomedical Diagnostic and Therapeutic Tools. Marine Drugs, 17(8), 469. https://doi.org/10.3390/md17080469