New 8-Hydroxybriaranes from the Gorgonian Coral Junceella fragilis (Ellisellidae)
Abstract
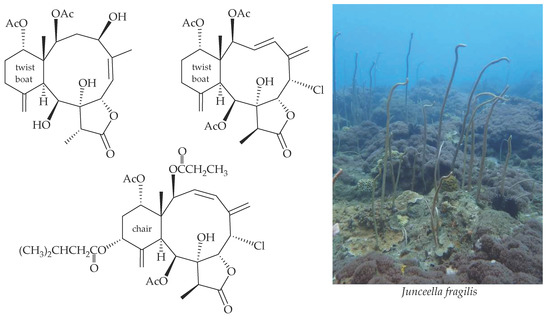
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. In Vitro Anti-inflammatory Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bayer, F.M. Key to the genera of octocorallia exclusive of Pennatulacea (Coelenterata: Anthozoa), with diagnoses of new taxa. Proc. Biol. Soc. Wash. 1981, 94, 902–947. [Google Scholar]
- Bayer, F.M.; Grasshoff, M. The genus group taxa of the family Ellisellidae, with clarification of the genera established by J.E. Gray (Cnidaria: Octocorallia). Senckenb. Biol. 1994, 74, 21–45. [Google Scholar]
- Chen, C.C.; Chang, K.H. Gorgonacea (Coelenterata: Anthozoa: Octocorallia) of Southern Taiwan. Bull. Inst. Zool. Acad. Sin. 1991, 30, 149–181. [Google Scholar]
- Chung, H.M.; Wang, Y.C.; Tseng, C.C.; Chen, N.F.; Wen, Z.H.; Fang, L.S.; Hwang, T.L.; Wu, Y.C.; Sung, P.J. Natural product chemistry of gorgonian corals of genus Junceella–Part III. Mar. Drugs 2018, 16, 339. [Google Scholar] [CrossRef] [PubMed]
- Sung, P.J.; Fan, T.Y. 9-O-Deacetylumbraculolide A, a new diterpenoid from the gorgonian Junceella fragilis. Heterocycles 2003, 60, 1199–1202. [Google Scholar] [CrossRef]
- Sung, P.J.; Wang, S.H.; Chiang, M.Y.; Su, Y.D.; Chang, Y.C.; Hu, W.P.; Tai, C.Y.; Liu, C.Y. Discovery of new chlorinated briaranes from Junceella fragilis. Bull. Chem. Soc. Jpn. 2009, 82, 1426–1432. [Google Scholar] [CrossRef]
- Lin, C.C.; Chen, W.F.; Lee, G.H.; Wen, Z.H.; Fang, L.S.; Kuo, Y.H.; Lee, C.Y.; Sung, P.J. Fragilides M–O, new triacetoxybriaranes from the gorgonian coral Junceella fragilis (Ellisellidae). Heterocycles 2019, 98, 984–993. [Google Scholar]
- Jean, Y.H.; Chen, W.F.; Sung, C.S.; Duh, C.Y.; Huang, S.Y.; Lin, C.S.; Tai, M.H.; Tzeng, S.F.; Wen, Z.H. Capnellene, a natural marine compound derived from soft coral, attenuates chronic constriction injury- induced neuropathic in rats. Br. J. Pharmacol. 2009, 158, 713–725. [Google Scholar] [CrossRef] [PubMed]
- Jean, Y.H.; Chen, W.F.; Duh, C.Y.; Huang, S.Y.; Hsu, C.H.; Lin, C.S.; Sung, C.S.; Chen, I.M.; Wen, Z.H. Inducible nitric oxide synthase and cyclooxygenase-2 participate in anti-inflammatory and analgesic effects of the natural marine compound lemnalol from Formosan soft coral Lemnalia cervicorni. Eur. J. Pharmacol. 2008, 578, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.C.; Lin, Y.Y.; Jean, Y.H.; Lu, Y.; Chen, W.F.; Yang, S.N.; Wang, H.M.D.; Jang, I.Y.; Chen, I.M.; Su, J.H.; et al. Anti-inflammatory and analgesic effects of the marine- derived compound comaparvin isolated from the crinoid Comanthus bennetti. Molecules 2014, 19, 14667–14686. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.; Figueiredo, C.A.; Brito, C.; Stavroullakis, A.; Prakki, A.; da Silva Velozo, E.; Nogueira-Filho, G. Effect of Allium cepa L. on lipopolysaccharide-stimulated osteoclast precursor cell viability, count, and morphology using 4′,6-diamidino-2-phenylindole-staining. Int. J. Cell Biol. 2014, 535789. [Google Scholar] [CrossRef]
- Cheng, W.; Li, X.; Yin, F.; van Ofwegen, L.; Lin, W. Halogenated briarane diterpenes with acetyl migration from the gorgonian coral Junceella fragilis. Chem. Biodiver. 2017, 14, e1700053. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Ji, M.; Li, X.; Ren, J.; Yin, F.; van Ofwegen, L.; Yu, S.; Chen, X.; Lin, W. Fragilolides A–Q, norditerpenoid and briarane diterpenoids from the gorgonian coral Junceella fragilis. Tetrahedron 2017, 73, 2518–2528. [Google Scholar] [CrossRef]
- Su, Y.D.; Su, J.H.; Hwang, T.L.; Wen, Z.H.; Sheu, J.H.; Wu, Y.C.; Sung, P.J. Briarane diterpenoids isolated from octocorals between 2014 and 2016. Mar. Drugs 2017, 15, 44. [Google Scholar] [CrossRef] [PubMed]

 ) correlations, selective HMBC (
) correlations, selective HMBC (  ), and selective protons with key NOESY correlations (
), and selective protons with key NOESY correlations (  ) of 1.
) of 1.
 ) correlations, selective HMBC (
) correlations, selective HMBC (  ), and selective protons with key NOESY correlations (
), and selective protons with key NOESY correlations (  ) of 1.
) of 1.
 ) correlations, selective HMBC (
) correlations, selective HMBC (  ), and selective protons with key NOESY correlations (
), and selective protons with key NOESY correlations (  ) of 2.
) of 2.
 ) correlations, selective HMBC (
) correlations, selective HMBC (  ), and selective protons with key NOESY correlations (
), and selective protons with key NOESY correlations (  ) of 2.
) of 2.
 ) correlations, selective HMBC (
) correlations, selective HMBC (  ), and selective protons with key NOESY correlations (
), and selective protons with key NOESY correlations (  ) of 3.
) of 3.
 ) correlations, selective HMBC (
) correlations, selective HMBC (  ), and selective protons with key NOESY correlations (
), and selective protons with key NOESY correlations (  ) of 3.
) of 3.
| Position | 1 a | 2 b | 3 a |
|---|---|---|---|
| 2 | 4.83 d (7.2) c | 5.47 d (9.5) | 6.33 d (10.8) |
| 3α/β | 1.91 ddd (15.6, 7.2, 5.4); 2.96 dd (15.6, 12.6) | 5.67 dd (16.0, 9.5) | 5.78 dd (12.0, 10.8) |
| 4 | 4.18 dd (12.6, 5.4) | 6.72 d (16.0) | 5.96 d (12.0) |
| 6 | 5.64 d (10.2, 1.2) | 5.20 s | 5.12 br s |
| 7 | 5.97 d (10.2) | 4.97 br s | 4.88 d (4.2) |
| 9 | 4.22 dd (5.4, 3.6) | 5.53 s | 5.59 s |
| 10 | 3.20 d (3.6) | 3.47 s | 4.08 s |
| 12α/β | 2.22 m | 2.24 m; 2.38 m | 5.37 dd (5.4, 3.0) |
| 13α/β | 1.96 m; 1.81 m | 1.84 m; 1.74 m | 2.06 m |
| 14 | 4.74 dd (4.2, 1.8) | 4.84 dd (2.5, 2.0) | 4.94 dd (4.2, 3.0) |
| 15 | 1.25 s | 1.18 s | 1.16 s |
| 16a/b | 2.09 d (1.2) | 5.49 s; 5.32 s | 5.66 s; 5.48 s |
| 17 | 3.11 q (7.2) | 2.93 q (7.5) | 2.90 q (7.2) |
| 18 | 1.14 d (7.2) | 1.18 d (7.5) | 1.22 d (7.2) |
| 20a/b | 4.97 s; 4.88 s | 4.93 s; 4.76 s | 5.33 s; 4.72 s |
| OH-8 | - | 3.11 s | 2.70 s |
| OH-9 | 2.09 d (5.4) | - | - |
| Acetoxy groups | 1.94 s 1.99 s | 2.01 s 2.04 s 2.09 s | 2.00 s 2.16 s |
| Propionoxy group | - | - | 1.08 t (7.2) 2.26 m |
| Isovaleroxy group | - | - | 0.96 d (6.6) 0.98 d (6.6) 2.10 m 2.15 m |
| Position | 1 a | 2 b | 3 a |
|---|---|---|---|
| 1 | 48.2, C c | 47.5, C | 47.7, C |
| 2 | 73.3, CH | 76.9, CH | 70.4, CH |
| 3 | 39.7, CH2 | 129.7, CH | 130.4, CH |
| 4 | 71.4, CH | 135.4, CH | 128.7, CH |
| 5 | 146.9, C | 142.6, C d | 137.5, C |
| 6 | 123.1, CH | 66.0, CH d | 64.5, CH |
| 7 | 76.4, CH | 79.5, CH | 76.9, CH |
| 8 | 83.4, C | 81.8, C | 80.3, C |
| 9 | 74.1, CH | 76.3, CH | 74.8, CH |
| 10 | 43.0, CH | 41.8, CH | 37.0, CH |
| 11 | 152.3, C | 149.3, C | 146.3, C |
| 12 | 29.0, CH2 | 29.1, CH2 | 74.3, CH |
| 13 | 27.8, CH2 | 26.0, CH2 | 31.4, CH2 |
| 14 | 74.2, CH | 74.4, CH | 73.0, CH |
| 15 | 15.6, CH3 | 16.4, CH3 | 14.7, CH3 |
| 16 | 26.2, CH3 | 118.8, CH2 d | 116.4, CH2 |
| 17 | 43.8, CH | 49.3, CH | 50.7, CH |
| 18 | 6.6, CH3 | 9.1, CH3 | 8.7, CH3 |
| 19 | 176.7, C | 174.1, C d | 173.9, C |
| 20 | 111.3, CH2 | 111.4, CH2 | 115.2, CH2 |
| Acetoxy groups | 21.0, CH3 170.8, C 21.3, CH3 170.8, C | 21.2, CH3 170.4, C 21.2, CH3 170.1, C 21.2, CH3 169.2, C | 20.7, CH3 169.7, C 21.3, CH3 169.6, C |
| Propionoxy group | - | - | 8.8, CH3 27.8, CH2 172.0, C |
| Isovaleroxy group | - | - | 22.6, CH3 22.8, CH3 25.0, CH 43.5, CH2 172.6, C |
| Compound | iNOS | COX-2 | β-Actin | n |
|---|---|---|---|---|
| Expression (% of LPS) | ||||
| Negative Control | 1.80 ± 0.21 | 1.04 ± 0.35 | 110.02 ± 5.23 | 2 |
| LPS | 100.01 ± 5.06 | 100.06 ± 0.43 | 100.07 ± 8.4 | 4 |
| 1 | 104.11 ± 16.63 | 100.51 ± 6.11 | 105.70 ± 7.05 | 4 |
| 2 | 61.21 ± 9.61 | 100.01 ± 5.11 | 99.29 ± 11.29 | 3 |
| 3 | 100.91 ± 24.08 | 96.36 ± 21.31 | 115.29 ± 3.4 | 4 |
| Dexamethasone | 5.54 ± 1.72 | 8.15 ± 5.13 | 105.21 ± 15.57 | 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-Y.; Fang, L.-S.; Chen, Y.-H.; Peng, B.-R.; Su, T.-P.; Huynh, T.-H.; Lin, F.-Y.; Hu, C.-C.; Lin, N.-C.; Wen, Z.-H.; et al. New 8-Hydroxybriaranes from the Gorgonian Coral Junceella fragilis (Ellisellidae). Mar. Drugs 2019, 17, 534. https://doi.org/10.3390/md17090534
Chen Y-Y, Fang L-S, Chen Y-H, Peng B-R, Su T-P, Huynh T-H, Lin F-Y, Hu C-C, Lin N-C, Wen Z-H, et al. New 8-Hydroxybriaranes from the Gorgonian Coral Junceella fragilis (Ellisellidae). Marine Drugs. 2019; 17(9):534. https://doi.org/10.3390/md17090534
Chicago/Turabian StyleChen, You-Ying, Lee-Shing Fang, Yu-Hsin Chen, Bo-Rong Peng, Tung-Pin Su, Thanh-Hao Huynh, Feng-Yu Lin, Chiung-Chin Hu, Nai-Cheng Lin, Zhi-Hong Wen, and et al. 2019. "New 8-Hydroxybriaranes from the Gorgonian Coral Junceella fragilis (Ellisellidae)" Marine Drugs 17, no. 9: 534. https://doi.org/10.3390/md17090534
APA StyleChen, Y.-Y., Fang, L.-S., Chen, Y.-H., Peng, B.-R., Su, T.-P., Huynh, T.-H., Lin, F.-Y., Hu, C.-C., Lin, N.-C., Wen, Z.-H., Chen, J.-J., Lee, C.-Y., Wang, J.-W., & Sung, P.-J. (2019). New 8-Hydroxybriaranes from the Gorgonian Coral Junceella fragilis (Ellisellidae). Marine Drugs, 17(9), 534. https://doi.org/10.3390/md17090534