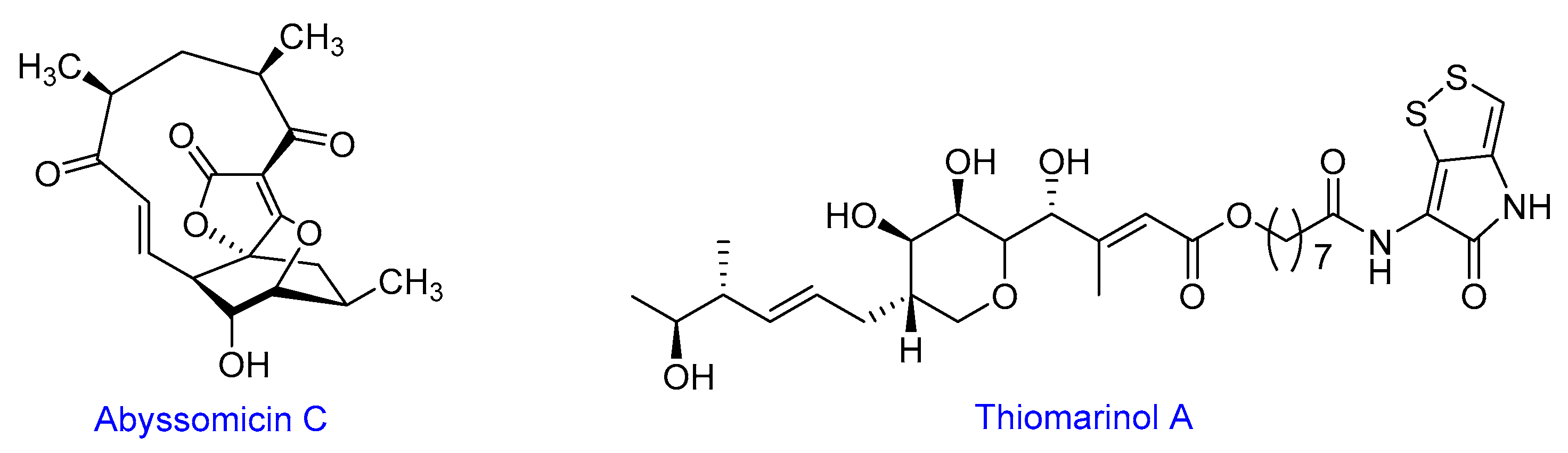
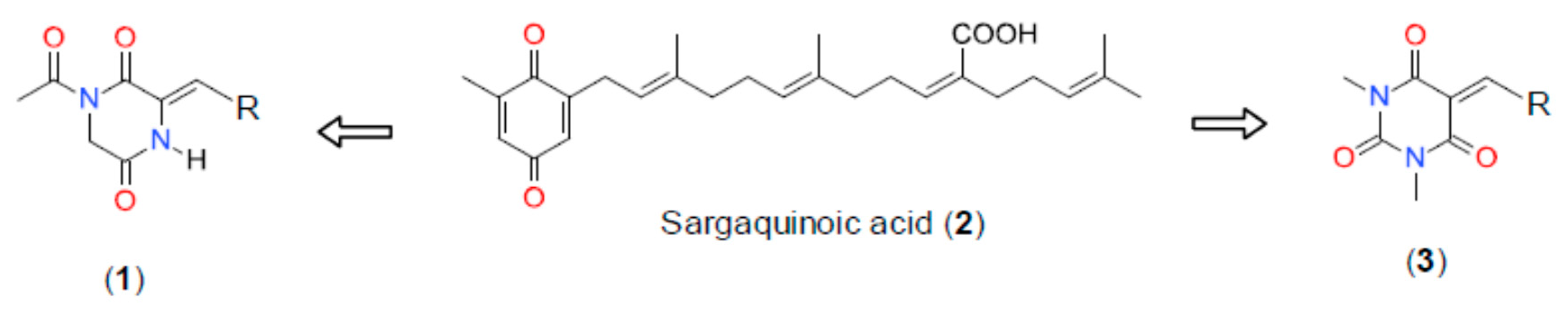
Theme 1. Chemical Ecology and Ecosystem Functioning
Comparative Studies of the Two Sea Cucumber Genera Bohadschia and Actinopyga: Biological Activities and Structural–Activity Differences
Elham Kamyab 1,
Matthias Kellermann 1,
Sven Rohde 1,
Eike Steinmann 2,
Matthias Köck 3,
Kathrin Mohr 3
and
Peter J. Schupp 1,4
1
Institute for Chemistry and Biology of the Marine Environment, Carl-von-Ossietzky University Oldenburg, Schleusenstraße 1, 26382 Wilhelmshaven, Germany
2
Institute of Experimental Virology Group Virus Transmission (TWINCORE), Centre for Experimental and Clinical Infection Research, 30625 Hannover, Germany
3
Hemholtz-Zentrum für Infektionsforschung GmbH (HZI), Inhoffenstraße 7, 38124 Braunschweig, Germany
4
Helmholtz Institute for Functional Marine Biodiversity at the University of Oldenburg (HIFMB), Ammerländer Heerstrasse 231, D-26129 Oldenburg, Germany
Marine organisms provide a large diversity of Natural Products (NPs) with unique structures and functions that increase organisms’ survival and fitness. Sea cucumbers are slow moving organisms that do not have significant escape behaviour. Although some morphological defence mechanisms have been reported, they rely mainly on their chemical defences to deter predators or competitors.
In this study we analysed the chemical defense of 5 holothurian in two genera, Bohadschia (i.e., Bohadschia argus, Bohadschia vitiensis, Bohadschia sp.) and Actinopyga (i.e., Actinopyga echinites, Actinopyga mauritiana). We used an activity-guided fractionation in B. argus to elucidate the structural-activity relationships of saponins. Crude organic extracts of different genera of family Holothuriidae showed that Actinopyga and Bohadschia contained high concentrations of saponins. However, while all species deterred feeding of the puffer fish Canthigaster solandri, Bohadschia extracts demonstrated significantly higher feeding deterrence compared to Actinopyga and also had higher anti-bacterial activities against (non) pathogenic bacteria. Furthermore, Bohadschia extracts (mainly from B. argus) showed higher cytotoxicity based on a brine shrimp assay, although the anti-fouling activities of both holothurid genera against the benthic diatom C. closterium were similar. Separation of the crude extract of B. argus by column chromatography yielded a series of active fractions against the Hepatitis Virus C (HCV), the bacteria Rhodococcus glutinis, Staphylococcus aureus, and Mucor hiemalis and the fungus Candida albicans. To detect and describe the structure-activity relationships of saponins, purification and identification of the active and non-active fractions are currently ongoing using various methods of high performance liquid chromatography (HPLC), high resolution liquid chromatography mass spectrometry (LC/MS) and nuclear magnetic resonance (NMR). We emphasize that a combination of biological and chemical screenings are an effective approach for selecting bioactive organisms to discover pharmacologically active natural products.
Location Explains Variation in the Metabolic Production of the Marine Sponge Xestospongia sp.
Lina M. Bayona 1,
Gemma van Leeuwen 1,
Özlem Erol 1,
Thomas Swierts 2,
Esther Van Der Ent 2,
Nicole de Voogd 2,3
and
Young Hae Choi 1,4
1
Natural Products Laboratory, Institute of Biology, Leiden University, 2333 BE Leiden, The Netherlands
2
Marine Biodiversity, Naturalis Biodiversity Center, 2333 CR Leiden, The Netherlands
3
Global Change and Marine Ecosystems, Institute of Environmental Sciences, Leiden University, 2300 RA Leiden, The Netherlands
4
College of Pharmacy, Kyung Hee University, Seoul 02447, Korea
The giant barrel sponge (Xestospongia sp.) has been widely studied due to their biological and chemical importance. From an ecological perspective, their relatively large size allows them to play an essential role in providing resources and protection to other organisms in the reef. Additionally, the wide range of chemical compounds that have been isolated from the sponge, such as terpenoids, alkaloids, brominated fatty acids, and sterols, have attracted significant attention from the scientific community.
Unlike other sponges, the giant barrel sponge can be found in a broad geographical range; they are situated from the red sea to the Indo-Pacific Ocean and Australia as X. testudinaria and in the Atlantic Ocean as the sister species X. muta. However, recent studies have shown the presence of cryptic species, and specimens found in the same location are not necessarily genetically related.
In this study, to correlate geographical location and metabolic variation, 139 specimens of Xestospongia sp., collected in four different locations: Martinique, Curacao, Taiwan, and Tanzania were investigated from a holistic approach. Using a multiplatform metabolomics methodology (NMR, LC-MS, and HPTLC), we aimed to gain insight into the metabolome of samples collected in different locations. It was found that samples collected in each location displayed characteristic metabolites and based on their chemical fingerprint it was possible to group the samples according to their geographical location. This indicates that environmental factors are a driving force in the production of metabolites and they can be just as important as genetic factors.
A Chemical and Ecological Approach Sheds Light on the Urticating System of Marine Fireworms
Sara Righi 1,2,
Martina Savioli 2,
Luca Forti 1,
Daniela Prevedelli 1
and
Roberto Simonini 1
1
Department of Life Sciences, University of Modena and Reggio Emilia, 41125 Modena, Italy
2
Department of Chemical and Geological Sciences, University of Modena and Reggio Emilia, 41125 Modena, Italy
Marine fireworms (Annelida, Amphinomidae) hold stinging dorsal bristles (chaetae) that cause injuries to divers and bathers. Hermodice carunculata is the most notorious species and it has recently attracted interest as a potentially invasive fireworm with few predators and uncharacterized defensive capacities. To date, the only acute inflammation inducer isolated from an amphinomid is “complanine”, a trimethylammonium compound. The main goal of this study was to promote an ecological understanding of H. carunculata defences through a multidisciplinary approach. The occurrence of complanine within tissues and its mode of delivery were assessed combining chromatographic steps and high resolution LC-MS/MS. The exact mass and retention time of complanine were detected in the extracts of the main ectodermal (dorsal body wall, gills, dorsal chaetae, ventral chaetae) and endodermal (gut, pharynx) tissues of H. carunculata. The role of complanine in trophic interactions was assessed offering the ectodermal tissues towards a predator (the fish Chromis viridis) and two relevant prey species (the anemones Anemonia viridis and Aiptasia diaphana). Only the dorsal chaetae were effective against predators and prey: they strongly deterred fishes and induced paralysis in the anemone tentacles. Dorsal chaetae treated with organic solvents lost their deterrence against fish predators and an inner hollow cavity suitable to vehicle toxins could be viewed by ESEM. These findings support a synergy between the mechanical injury of dorsal chaetae penetration and the release of complanine. This unique feature could support the success of fireworms in marine benthic environments and significantly improves knowledge on the chemical ecology of amphinomids.
Can Trait Evolution and Phylogenetics Predict the Discovery of New Toxins from Marine Invertebrates?
Carolina Madeira 1,2,
Cátia Gonçalves 1,
Jorge Lobo 3
and
Pedro M. Costa 1
1
UCIBIO—Applied Molecular Biosciences Unit, Departamento de Ciências da Vida, Faculdade de Ciências e Tecnologia, Universidade NOVA de Lisboa, 2829-516 Caparica, Portugal
2
MARE—Marine and Environmental Sciences Centre, Departamento de Biologia Animal, Faculdade de Ciências da Universidade de Lisboa, 1749-016 Lisboa, Portugal
3
Instituto Português do Mar e da Atmosfera, I.P. (IPMA), Rua Alfredo Magalhães Ramalho, 6, 1495-006 Lisboa, Portugal
Due to their extraordinary biodiversity, marine invertebrates can become one of the most important sources of novel bioactive compounds (BCs), especially toxins. As recent incentives to blue biotechnology have led to a significant increase in bioprospecting efforts in the marine realm, there is a need to improve our ability to predict the distribution of such BCs along the marine animal tree-of-life. We envisage that evolutionary ecology offers solutions for this grand challenge by integrating systematics, life-history traits and the secretion of specialised chemical compounds into phylogenetic models. Using intertidal Polychaeta from the Portuguese coast as a case study, our research aimed at predicting BCs occurrence in these marine invertebrates. Using Bayesian inference-based phylogenetics we combined: (i) molecular systematics (through COI gene sequencing) with (ii) morphoanatomical features (presence vs absence of tentacles in the proboscis and/or of mandibulae and venom glands) and (iii) ecological characteristics (trophic level) to construct a phylogenetic tree to identify potentially venom-producing polychaetes. Results allowed us to map several families within Order Phyllodocida, such as Glyceridae, Phyllodocidae, Nephtyidae and Nereidae that have evolved to produce complex toxin mixtures as defence or predation strategies. Harvesting secretions from mucus and glands, plus toxicity testing with natural prey (e.g., mussels) assisted validation of findings by disclosing tissue and cell-level toxicopathological alterations like cell death and DNA damage. Altogether, frontline bioinformatics and multi-trait phylogenetics can be combined into powerful tools to locate new species of interest secreting BCs, therefore contributing to turn marine bioprospecting for biotechnological purposes from random to systematic.
Acknowledgments
“Fundação Para a Ciência e Tecnologia” (project WormALL—PTDC/BTA-BTA/28650/2017) and UCIBIO (strategic project—UID/Multi/04378/2019) for funding this research.
Toxin Profile of Ostreopsis cf. ovata from Continental Portuguese Coast and Selvagens Islands (Madeira, Portugal)
Lucía Soliño 1,2,
María García-Altares 3,
Lia Godinho 1,
Alexandra Silva 1
and
Pedro Reis Costa 1,2
1
IPMA—Instituto Português do Mar e da Atmosfera, Rua Alfredo Magalhães Ramalho, 6, 1495-006 Lisbon, Portugal
2
CCMAR—Centre of Marine Sciences, University of Algarve, Campus of Gambelas, 8005-139 Faro, Portugal
3
Department of Biomolecular Chemistry, Hans-Knöll-Institut (HKI), Adolf-Reichwein-Straße 23, 07745 Jena, Germany
The toxigenic dinoflagellate Ostreopsis cf. ovata is known to produce a range of palytoxin (PLTX)—related compounds named ovatoxins (OVTX). O. cf ovata presents a wide variability in toxin production and its toxic profile is strain-specific. Several OVTX, denominated from -a to -l have been reported from different strains of this benthic microalgae up to now, mainly in Mediterranean isolates. However, less is known about the toxin profile of the strains present in the Atlantic coasts of Europe. In this work, two strains of toxigenic O. cf ovata isolated from the South coast of Portugal mainland (Algarve) and Selvagens Island (Madeira, Portugal) were cultured and tested for toxicity by haemolytic assay. Toxin profiles were qualitatively elucidated by Liquid Chromatography-High Resolution Mass Spectrometry (LC-HRMS). The strain from Algarve presented lower toxic potency than the strain from Selvagens island (12.29 against 54.79 pg of PLTX equivalents per cell) showing in both cases the characteristic toxin profile of Mediterranean strains. The major component, OVTX-a, was concomitant with OVTX from -b to -g and isobaric PLTX. Regarding the morphological and molecular characteristics of both strains as well as their toxin fingerprint, it is likely that both strains are closely related to those from Mediterranean coasts. The present study reports for the first time the occurrence of several OVTX congeners and iso-PLTX in O. cf ovata from Portuguese waters. Further research on quantitative toxin production of these and newly isolated strains are currently ongoing, to characterize the risk of OVTXs- related outbreaks in Portugal.
Mesophotic Marine Habitats: Inspiring Understudied Biodiversity
Yehuda Benayahu 1,
Erez Shoham 1,
Ronen Liberman 1,
Shai Tamir 1,
Carolina Alonso 2,
Pedro Álvarez 2,
Suchana Chavanich 3,
Anne Bialecki 4,
Géraldine Le Goff 5
and
Jamal Ouazzani 5
1
Tel Aviv University, 69978 Tel Aviv, Israel
2
iMare Natural, 18600 Granada, Spain
3
Chulalongkorn University, Bangkok10330, Thailand
4
Université de la Réunion, 97715, Ile de la Réunion, France
5
ICSN-CNRS, 91198 Gif sur Yvette, France
Studies have revealed the bewildering diversity on shallow reefs, comprising plethora of invertebrates. Until the past decade most biodiversity surveys have been restricted to the upper ~30 m. The mesophotic coral-reef ecosystem (MCE) has been defined as comprising the light-dependent communities (30 to <150 m) in tropical and subtropical regions. Remotely-operated vehicles (ROVs) and technical diving have now facilitated the investigation of MCEs. Consequently, they have become available for research with an increasing interest in their yet unexplored bio-resources, keeping in mind holobiont concept. The scarce data available on non-scleractinian MCE fauna in the Red Sea, Andaman Sea, Gulf of Thailand and the Western Mediterranean Sea intrigued us to conduct thorough surveys on the MCE fauna for bioprospecting purposes. The results revealed diverse species assemblages associated with a variety of micro-symbionts, including species new to science and new zoogeographical records. The findings highlight the possibility that MCEs host depth generalists, along with unique depth specialists uniquely found in MCEs. In addition, this ecosystem might include species also found below the deepest fringes of the MCEs. The evidence suggests that octocorals, sponges along with other invertebrates are the major benthic organisms in MCE, being far more diverse than has been envisioned. The results also raise pressing issues concerning the importance of conservation policies aiming at protecting the MCE biodiversity and its possible function as refugia for impoverished shallow reef habitats.
Acknowledgments
TASCMAR project (
www.tascmar.eu) is funded by the European Union in the frame of H2020 (GA No 634674).
Strategic Defense Potential of a Sponge Associated with Sandy Bottom in the Western South Atlantic Ocean
Beatriz G. Fleury 1,2,
Amanda G. Silva 1,2,
Ana Lea D. Lopes 1,
Carolina G. Amorim 1,
Juliana M. Araujo 1,2,
Tamires S. R. Braga 1,
Victor R. Amaral 1
and
Yollanda C. S. F. Vançato 1,2
1
Departamento de Ecologia, IBRAG, Universidade do Estado do Rio de Janeiro, PHLC sala 220, Rua São Francisco Xavier 524, Maracanã, Rio de Janeiro 20550-900, Brazil
2
Programa de Pós-Graduação em Ecologia e Evolução, IBRAG, Universidade do Estado do Rio de Janeiro, Rio de Janeiro 20550-013, Brazil
Porifera evolved different strategies, physical and chemical, to defend themselves against threats from their environment. They produce biologically functional natural products, such allelopathic substances and other chemical defenses against predation, fouling and pathogens. However, few studies of the different sponge defense strategies have been carried out to date in the South Atlantic Ocean. The aim of this study was to investigate the chemical and physical defenses responses of the abundant Iotrochota arenosa (Demospongiae) from Ilha Grande Bay, RJ-Brazil. The interactions between I. arenosa and invasive Tubastraea corals was evaluated, in situ, with competition trials using physical barriers to separate chemical and physical effects. The antifouling activity of mucus and organic extracts were tested in the lab through substrate preference (treatment and filter paper control) in the ‘mussel test’. Anti-predation bioassays against the crab Pachygrapsus transversus used mucus, extracts and powdered sponge, added separately to artificial food based on agar+squid (treatments). The competition results indicated that I. arenosa uses physical and chemical mechanisms of defense, to surround and later override the invasive Tubastraea, probably causing choking. The antifouling results indicated that I. arenosa mucus was more efficient (p = 0.047) to inhibit the production of byssal threads. Deterrent activity was effective with powdered sponge, mucus and extract (p = 0.01; n = 23). This work is unprecedented in the defensive evaluation of I. arenosa, suggesting that the physical and chemical weapons may be driving the observed success of this sponge in the environment.
Bioactive Metabolites from the Algal Garden of the Limpet Scutellastra mexicana
Carolina de los Reyes 1,
Benjamín Yáñez 2,
J. Luis Carballo 2
and
Eva Zubía 1
1
Departamento de Química Orgánica, Facultad de Ciencias del Mar y Ambientales, Universidad de Cádiz, 11510-Puerto Real/Cádiz, Spain
2
Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Apdo. 811-Mazatlan, Sinaloa 82000, Mexico
The giant limpet Scutellastra mexicana is the only known true patellid living on tropical east Pacific coasts, from Mexico to Peru, and nowadays is among the most endangered marine invertebrates [1]. S. mexicana lives on rocky substrates in the shallow sublittoral, showing homing and algal gardener behaviour. Thus, each limpet creates a home scar on the rock where returns after feeding and around that scar grows an algal garden of about 5–10 cm length [2].
Although limpet foraging has been the topic of extensive scientific research [3], there are no data on the involvement of chemical compounds in the relationship between the limpet and the algae growing around. Interestingly, no other invertebrate seems to invade the algal garden around each limpet, which suggests a deterrent role of the algae.
As a part of our ongoing multidisciplinary approach to the study of the threatened species S. mexicana, we have performed a chemical analysis of the algal garden of this limpet, aimed to determine the presence of bioactive metabolites.
Samples of the algae were collected from rocks populated with S. mexicana at Maria Cleofas Island (Pacific Ocean, Mexico). The algal extract was subjected to different chromatographic separation steps, allowing the isolation of a series of compounds whose structures have been determined by NMR and MS analysis. From a biological/ecological point of view, the isolated compounds are endowed with osmoregulatory, antifeeding or antifouling properties.
These results represent the first characterization of natural compounds produced by the algae thriving around S. mexicana that could have a key role in the chemical defence of this giant limpet.
References
Esqueda, M.C.; Ríos-Jara, E.; Michel-Morfín, J.E.; Landa-Jaime, V. Vertical distribution and diversity of gastropods molluscs from intertidal hbitats of the Ratnagiri Coast Mahararashtra, India. Rev. Biol. Trop. 2000, 48, 765–775.
Espinosa, F.; Rivera-Ingraham, G.A. Biological Conservation of Giant Limpets: The Implications of Large Size. Adv. Mar. Biol. 2017, 76, 105–155.
Burgos-Rubio, V.; De la Rosa, J.; Altamirano, M.; Espinosa, F. The role of patellid limpets as omnivorous grazers: a new insight into intertidal ecology. Mar. Biol. 2015, 162, 2093–210.
Benthic Cyanobacteria from Tropical Mangroves as Producers of Antimicrobials
Sébastien Duperron 1,2,
Mehdi A. Beniddir 3,
Sylvain Durand 1,
Arlette Longeon 1,
Charlotte Duval 1,
Olivier Gros 4,
Cécile Bernard 1
and
Marie-Lise Bourguet-Kondracki 1
1
Molécules de Communication et Adaptation des Microorganismes, UMR 7245 MCAM, Muséum National d’Histoire Naturelle, 57 rue Cuvier (CP 54), 75005 Paris, France
2
Institut Universitaire de France, 75005 Paris, France
3
Équipe “Pharmacognosie-Chimie des Substances Naturelles” BioCIS Univ. Paris-Sud, CNRS, Université Paris-Saclay 5 rue J.-B. Clément, 92290 Châtenay-Malabry, France
4
UMR 7205 ISYEB et Université des Antilles, Pointe à Pitre, 97157 Guadeloupe, France
Cyanobacteria have emerged as a target group of interest in the search for new types of bioactive compounds [1]. The benthic species, especially in tropical zones, that may form dense biofilms on various types of substrates are particularly interesting [2] but still poorly known, compared to pelagic species. In this context and in the framework of a French CNRS research project (X-Life CABMAN 2018–2019) aiming to explore benthic cyanobacteria in mangrove ecosystems, a sampling program was conducted in two distinct areas, Guadeloupe (Caribbean) and Mayotte (Indian Ocean) that resulted in isolation of a collection of eighty new cyanobacterial strains. Our first investigations on Guadeloupe strains have combined phylogenetic, chemical and biological studies in order to better understand the taxonomic diversity as well as the chemical ecology of these cyanobacteria through the role of their specialized metabolites. Therefore, in addition to the description of new cyanobacteria species by polyphasic approach, chemical analyses using LC-MS/MS data allowed to set up a molecular network, which was enriched by antimicrobial activities evaluation against human pathogenic and environmental ichtyopathogenic bacterial strains. Results focused on the chemical biodiversity of cyanobacteria isolated from tropical mangrove in French overseas territories will be presented and discussed.
References
Shah, S.A.A.; Akhter, N.; Auckloo, B.N.; Khan, I.; Lu, Y.; Wang, K.; Wu, B.; Guo, Y.-W. Structural diversity, biological properties and applications of natural products from Cyanobacteria. A review. Mar. Drugs 2017, 15, 354.
Alvarenga, D.O.; Rigonato, J.; Branco, L.H.Z.; Fiore, M.F. Cyanobacteria in mangrove ecosystems. Cyanobacteria in mangrove ecosystems. Biodivers. Conserv. 2015, 24, 799–817.
Metagenomic Studies of Microbial Sulphur Mats, an Unexplored Natural Product Resource
Chandrashekhar Padhi 1,
Hans-Joachim Ruscheweyh 1,
Mrutyunjay Suar 2,
Shinichi Sunagawa 1
and
Jörn Piel 1
1
Institute of Microbiology, ETH Zurich, 8093 Zurich, Switzerland
2
School of Biotechnology, KIIT University, Odisha 751024, India
Bacterial natural products are the basis of most known antibiotic families, but have traditionally been isolated from a taxonomically and ecologically limited range of prokaryotic life. Based on the hypothesis that chemical novelty will be found in poorly studied, high-diversity, interaction-rich microbiomes in oligotrophic habitats, we have studied one such community, sulphur mats from a brackish coastal lagoon. These algal mats follow an annual cycle of formation and degradation, and in the later stage of this cycle release the sulphur-based odorous compounds from which they earn their name. Unlike cyanobacterial mats, sulphur mats have not been investigated in context of their natural product potential. In this study, we used metagenomic methods to investigate the biosynthetic potential of the complex microbial mat community. To overcome the challenges of bacterial DNA being overwhelmed and masked by algal and other eukaryotic DNA in the community during metagenome sequencing, we applied an array of binning platforms. The result was high-quality prokaryotic bins that could be assigned to bacterial taxa with relatively high confidence. In-depth analysis of the biosynthetic gene clusters (BGCs) revealed a high abundance of putative ribosomally synthesized and post-translationally modified peptides (RiPPs) and terpenes; a finding that was consistent among spatially distant samples. In contrast, metagenomes from other lake ecosystems have shown a moderate number of polyketide and non-ribosomal peptide BGCs in addition to terpene genes. The high diversity of RiPP systems identified in the mat ecosystem provides a rich discovery resource for bioactive peptides with putative functions in chemical defense.
Toxins from an Unsuspected Invertebrate: A Poisonous Cocktail from a Jawless Predatory Polychaete
Ana P. Rodrigo,
Ana Grosso,
Pedro V. Baptista,
Alexandra R. Fernandes
and
Pedro M. Costa
UCIBIO—Research Unit on Applied Molecular Biosciences, Departamento de Ciências da Vida, Faculdade de Ciências e Tecnologia da Universidade Nova de Lisboa, 2829-516 Caparica, Portugal
Animal venoms are complex mixtures of toxins and other substances with immense biotechnological potential due to their ability to interfere with specific biological pathways. The extraordinary biodiversity of marine invertebrates offers a wide range of novel toxins that are difficult to isolate and characterise in little-known animals with reduced genomic annotation. It is the case for Eulalia viridis, a predatory phyllodocid that is believed to secrete noxious substances to assist its predatorial behaviour. Combining RNAseq and microscopy techniques, our work disclosed that Eulalia possesses several transcripts that were annotated and found to code for peptides with sequence similarities from previously reported venom components of other species, being secreted in specialised cells located in the proboscis. Specifically, through homology search and phylogenetic analyses, several noxious substances, such as hyaluronidases and cysteine-rich peptides, were found to be closely related with those typically present in venomous animals, such as Hymenoptera, Conus and Serpentes, highlighting the diversity and complexity of this cocktail. Among the venomous cocktail of Eulalia, proteins with well-preserved domains, such as ankyrin motifs and EGF were also found, common in venom proteins of both invertebrates and vertebrates. These proteins in Eulalia venomous secretions can play various functions, such as tissue permeabilization and interference with neuronal calcium channels, as well as having anticoagulant properties. These fit Eulalia’s behaviour, which is a jawless worm, feeding by suction after immobilising prey. Altogether, the results indicate that Eulalia secretes toxins, rendering it from an apparently inoffensive worm to a fierce predator of the intertidal.
Acknowledgments
The authors acknowledge “Fundação para a Ciência e Tecnologia” for funding project GreenTech (PTDC/MAR-BIO/0113/2014) and the Ph.D. fellowship SFRH/BD/109462/2015 to A.P.R. This work was supported by UCIBIO, financed by national funds from FCT/MCTES (UID/Multi/04378/2019).
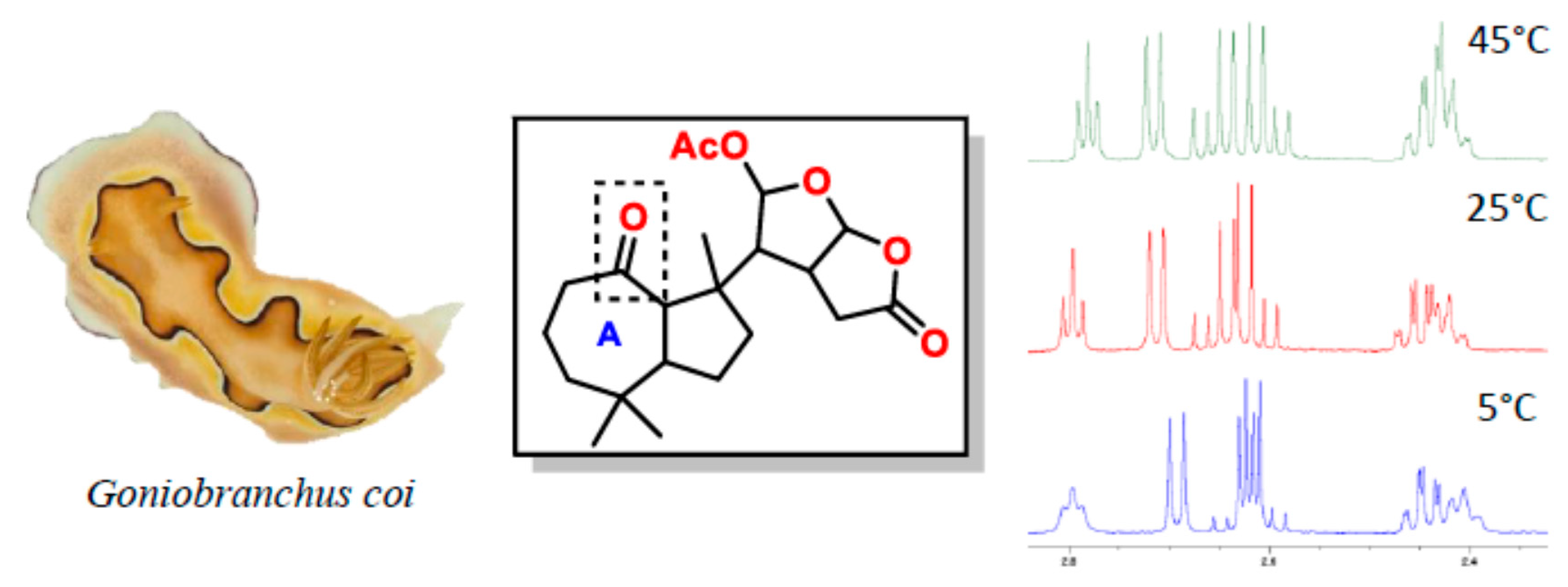
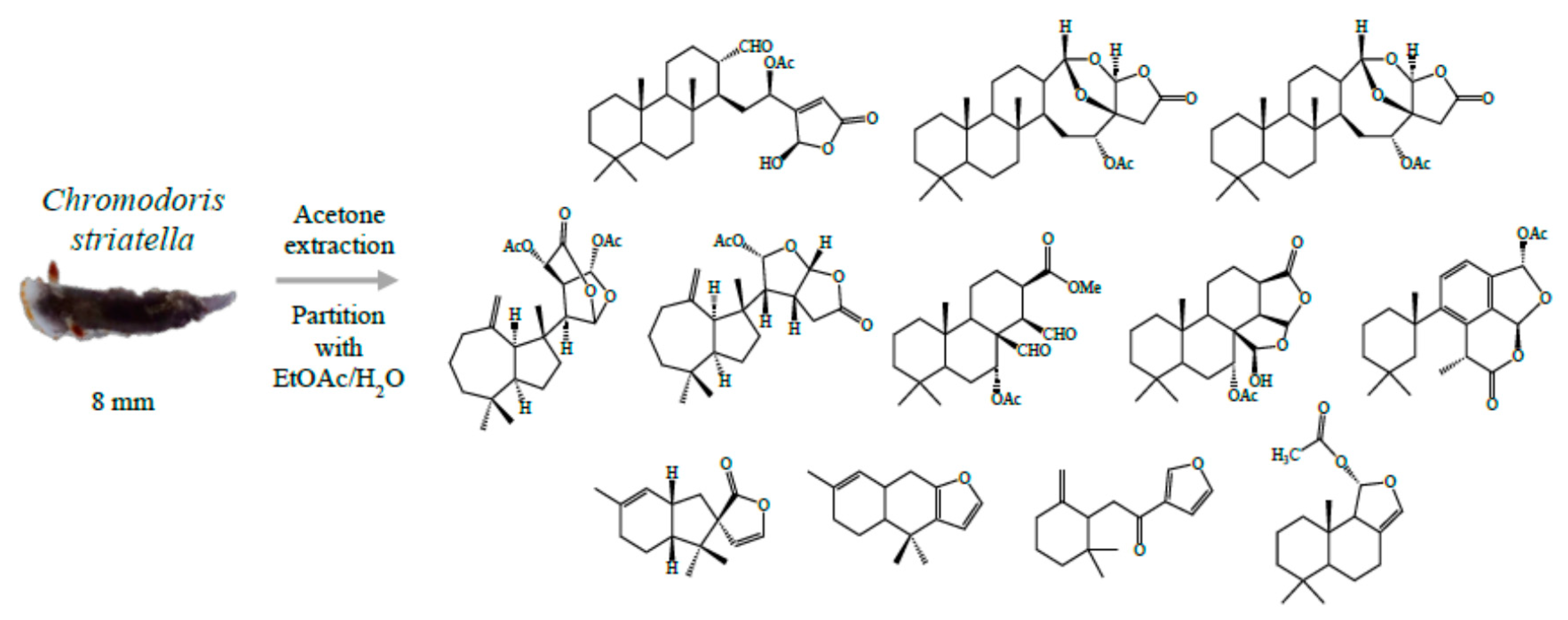
The Chemical Ecology of Chromodoris Nudibranchs
Weili Chan 1,
Mary J. Garson 1
and
Karen L. Cheney 2,3
1
School of Chemistry and Molecular Biosciences, University of Queensland, Brisbane 4072, Australia
2
School of Biological Sciences, University of Queensland, Brisbane 4072, Australia
3
Queensland Brain Institute, University of Queensland, Brisbane 4072,, Australia
Nudibranchs (Mollusca: Gastropoda: Opisthobranchia) that feed on marine sponges have evolved to acquire and re-purpose noxious sponge metabolites for their own defence. Many chemically protected nudibranchs also display bright colourations and patterns to advertise their unprofitability to predators, a phenomenon known as aposematism (apo: away; sema: sign). In particular, a few aposematic
Chromodoris species had demonstrated a highly selective sequestration of a toxic macrolide, latrunculin A, in the mantle rim [1]. This contrasts other
Chromodoris nudibranchs that utilize a complex mixture of compounds in the mantle border for defence. A recent study found that
Chromodoris nudibranchs display flexible colour patterns. [2] suggesting that this group is involved in a Müllerian mimicry ring whereby conspicuously coloured species mimic one another to increase the efficacy of the aposematic signals to their common predators. Species within a mimicry group may possess different chemical profiles and therefore have unequal levels of chemical defence. Non-chemically protected species can also mimic the colour patterns of a well defended species (Batesian mimicry). This study explores the chemistry of the newly suggested
Chromodoris mimics, as well as the different chemical strategies employed by chromodorid nudibranchs (
Figure 15).
References
Cheney, K.L.; White, A.; Mudianta, I.W.; Winters, A.E.; Quezada, M.; Capon, R.J.; Mollo, E.; Garson, M.J. Choose Your Weaponry: Selective Storage of a Single Toxic Compound, Latrunculin A, by Closely Related Nudibranch Molluscs. PLoS ONE 2016, 11, e0145134.
Layton, K.K.S.; Gosliner, T.M.; Wilson, N.G. Flexible colour patterns obscure identification and mimicry in Indo-Pacific Chromodoris nudibranchs (Gastropoda: Chromodorididae). Mol. Phylogenet. Evol. 2018, 124, 27–36.
Bugs from Slugs: Exploring the Bacterial Diversity of Nudibranchs by Metagenomics
Sheila Pimentel-Elardo 1,
Karen Cheney 2,
Mary Garson 3
and
Justin Nodwell 1
1
Department of Biochemistry, University of Toronto, Toronto, ON M5G 1M1, Canada
2
School of Biological Sciences, University of Queensland, Brisbane 4072 QLD, Australia
3
School of Chemistry and Molecular Biosciences, University of Queensland, Brisbane 4072 QLD, Australia
Nudibranchs commonly called sea slugs are marine gastropod mollusks that shed their shells during the larval stage. Due to their lack of physical protection, nudibranchs have evolved different defense mechanisms to deter predators. Several species sequester chemical defenses from their diet and store different secondary metabolites from their prey to render their bodies distasteful to potential predators. In some species, these compounds are selectively localized in different body parts such as the rim as their primary line of defense. Although several defensive secondary metabolites identified in nudibranchs are suspected to be of microbial origin, no one has investigated these so far.
This study aims to look at the microbiome of different nudibranch species and compare the bacterial composition of different body parts in selected species. We used 16S rRNA gene-based metagenomics to characterize the bacterial composition of 24 different sea slugs mostly nudibranchs, but also including some sacoglossans, sea hares and headshield slugs collected from the Great Barrier Reef, Australia. Analysis of the core microbiome revealed that Mycoplasma, Ruegeria and Alteromonas are the prevalent genera while Proteobacteria as the most abundant phylum. In a few nudibranchs, some bacterial species dominate the entire microbiome (>98%) while in selected species, the rim is dominated by a unique bacterial taxon not found in other parts of the animal. This is the first extensive study looking at the microbiome of diverse nudibranchs. With shotgun metagenomics underway, we hope to correlate the microbial producers of the defensive metabolites found in these remarkable animals.
Potential of Metabolomics in the Integrative Systematics of Octocorals, Case Study in the Tropical Eastern Pacific
Karla B. Jaramillo 1,2,
Mehdi A. Beniddir 3,
Rubén Abad 1,
Jenny Rodriguez 1,
Juan A. Sanchez 4,
Grace McCormack 2
and
Olivier P. Thomas 5
1
Escuela Superior Politécnica del Litoral, ESPOL. Centro Nacional de Acuicultura e Investigaciones Marinas, CENAIM. Campus Gustavo Galindo Km. 30.5 Vía Perimetral, P.O. Box 09-01-5863 Guayaquil, Ecuador
2
Zoology, School of Natural Sciences and Ryan Institute, National University of Ireland Galway, University Road, H91 TK33 Galway, Ireland
3
Équipe “Pharmacognosie-Chimie des Substances Naturelles” BioCIS, Univ. Paris-Sud, CNRS, Université Paris-Saclay, 5 rue J.-B. Clément, 92290 Châtenay-Malabry, France
4
Universidad de los Andes, Departamento Ciencias Biológicas, Laboratorio de Biología Molecular Marina (BIOMAR), Cra. 1 #18a 12, Bogotá 111711, Colombia
5
Marine Biodiscovery, School of Chemistry and Ryan Institute, National University of Ireland Galway, University Road, H91 TK33 Galway, Ireland
Soft corals (Cnidaria, Anthozoa, Octocorallia) represent a benthic group of marine invertebrates that inhabit some ecosystems of the oceans and they are found highly abundant in the Tropical Eastern Pacific. In this marine ecoregion, Ecuador is well-recognized as a marine biodiversity hotspot and has attracted experts in the taxonomy of octocorals. The identification of octocorals in the Eastern Pacific has usually been supported by sclerite characterization and DNA barcoding but despite the valuable contributions of molecular data, many discrepancies remain especially at species level.
As soft corals are also known to produce a diverse array of secondary metabolites, an integrative approach was conducted to better define the systematics of this group using three methods: morphological characterization of sclerites, phylogenetic analyses (based on two mitochondrial markers COI and MutS markers) and a metabolomic examination (combining LC-MS analyses and MS/MS molecular networking). The untargeted metabolomic approach using UHPLC-HRMS was proven to be useful as a complementary tool in the systematics of these species especially at the genus level. Interestingly, the MS/MS molecular networking revealed key biomarkers for an interspecific discrimination between the 5 different genera studied. Our first insight into the octocorals diversity at El Pelado Marine Protected Area—Ecuador led to the identification of eleven species. These preliminary results demonstrate the high potential of metabolomics for a more general application to the octocoral group.
Theme 2. Isolation and Structure Elucidation of Marine Natural Products
Isolation of Fungi Using the Diffusion Chamber Device FIND
Benjamin Libor,
Henrik Harms,
Stefan Kehraus,
Ekaterina Egereva
and
Gabriele M. König
Institute for Pharmaceutical Biology, University of Bonn, Nussallee 6, 53115 Bonn, Germany
A general problem in trying to obtain axenic cultures of environmental microorganisms is the “great plate count anomaly”, meaning that 99% of all microbes in environmental samples cannot be isolated. In the current study we aimed to improve the isolation of fungal strains with the help of the Fungal one-step IsolatioN Device (FIND) technology. In general, the FIND technology is a multi-chambered micro agar plate, where initially in each chamber only one fungal part is located. After inoculation the device is placed back into the original natural environment of sample collection, to ensure favourable growth conditions.
Figure 16 displays the procedure of a FIND experiment. We carried out experiments with terrestrial soil and marine sediment, as well as sea water samples to test this method, and were able to obtain axenic cultures of 12 different filamentous fungal strains, one of them being the marine
Heydenia cf.
alpina. The latter yielded two new terpenoid structures, which are the first secondary metabolites from this genus.
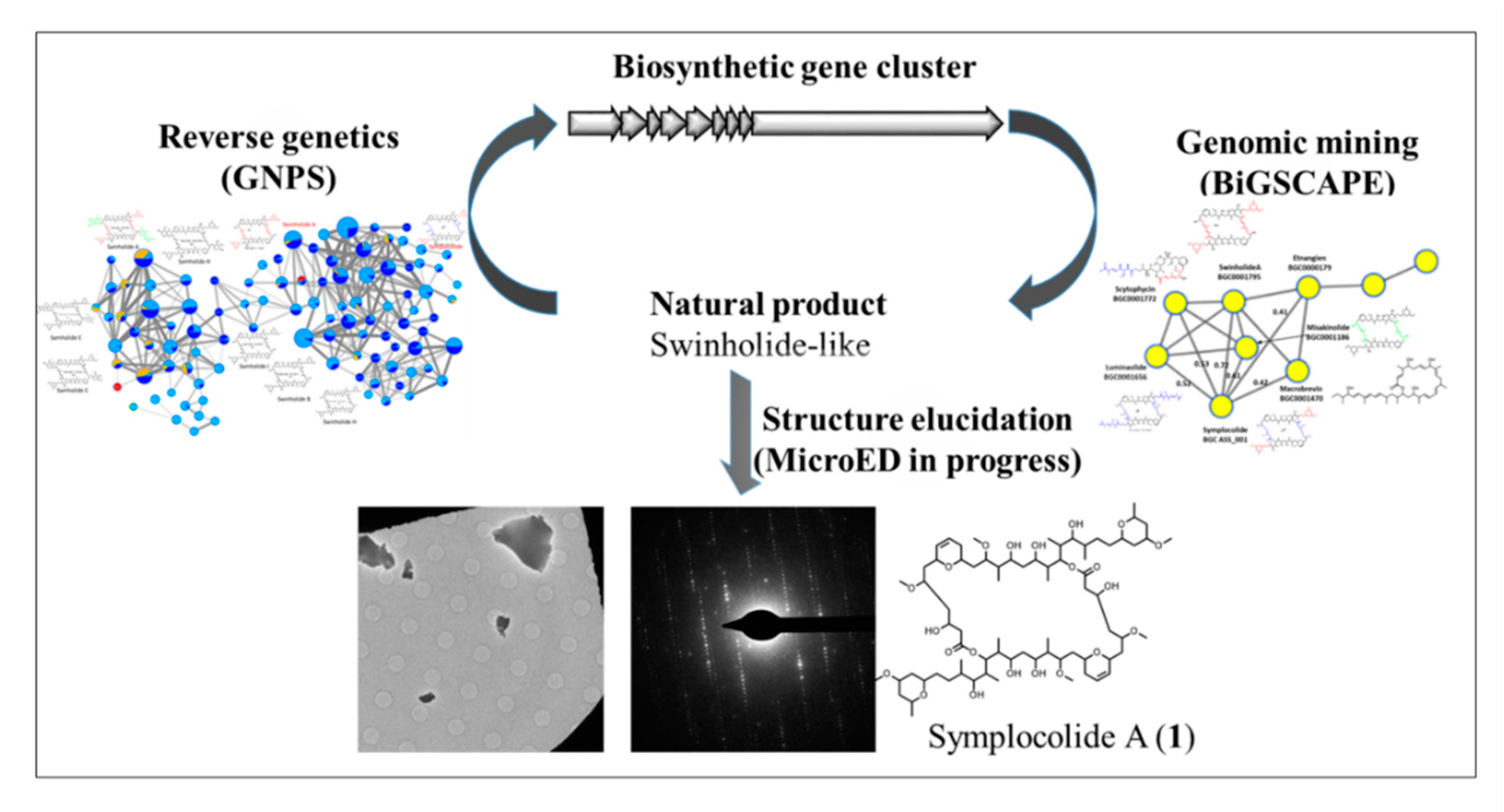
Connecting Molecules to Gene and Back—Can Three Distinct Classes of Swinholide-Like Macrolides Be Produced by the Same Biosynthetic Gene Cluster of a Symploca sp.?
Raphael Reher 1,
Tiago Ferreira-Leão 1,
Nathan A. Moss 1,
Bahar Teke 1,
Gary Arevalo 1,
Brent Nannenga 2,
Lena Gerwick 1
and
William H. Gerwick 1,3
1
Center for Marine Biotechnology and Biomedicine, Scripps Institution of Oceanography, University of California, La Jolla, CA 92093, USA
2
Center for Applied Structural Discovery, The Biodesign Institute, Arizona State University, Tempe, AZ 85281, USA
3
Skaggs School of Pharmacy and Pharmaceutical Sciences, University of California, La Jolla, CA 92093, USA
Tropical marine filamentous cyanobacteria of the genus Symploca have been described as prolific producers of bioactive natural products such as the antineoplastic agent dolastatin 10 or the actin cytoskeleton disruptor swinholide A. An environmental collection of the American Samoan cyanobacterium Symploca sp. ASS-20JUL14-1 was fractionated guided by both MS2-based molecular networking and cytotoxicity against NCI-H460 human lung cancer cells and led to the detection of swinholide A-J as well as samholide A-I. The planar structures of the latter, occuring in nanomolar quantities, were proposed by manual MS2 analysis and remain to be confirmed by the cryoEM method MicroED, a novel cutting-edge technique for the structure elucidation of small molecules with drastically reduced compound and analysis time requirements.
Further, we isolated and elucidated the structure of a new swinholide-like macrolide, symplocolide A (1), a structural hybrid of swinholide A and luminaolide B. This raised interest in the biosynthesis of 1; after genome sequencing and assembly using the MiBIG reference biosynthetic gene cluster (BGC) “swi” and initial mining of the biosynthetic pathways, we located a 98 kB BGC, “sym” that putatively encodes for 1. Preliminary biosynthetic analysis suggests that sym might not only be responsible for the production of 1, but intriguingly, for the aforementioned swinholides and samholides as well. BiGSCAPE analysis of the sym BGC versus all cyanobacterial genomes from NCBI and the Gerwick lab, as well as use of the bioinformatic tool transATor, support this hypothesis, paving the way for decrypting the mechanism of biosynthesis of these divergent swinholide-like metabolites.
Bisindole Alkaloids Isolated from the Irish Coastal Sponge, Spongosorites calcicola, as Part of the National Marine Biodiscovery Project, NMBLI
Laurence K. Jennings 1,2,
Navdeep Kaur 1,2,
Daniel Rodrigues 1,2,
Jeffrey Fisher 2
and
Olivier P. Thomas 1
1
Marine Biodiscovery, School of Chemistry and Ryan Institute, National University of Ireland Galway (NUI Galway), University Road, H91 TK33 Co. Galway, Ireland
2
Marine Biodiscovery, Marine Institute, Rinville West, H91 R673 Co. Galway, Ireland
Over the last 60 years, marine natural products have been reported with a unique chemical diversity not observed in terrestrial natural products. However, there still remains significant areas of largely unexplored diverse marine habitats. The Beaufort Marine Biodiscovery project established the basis for marine biodiscovery in Ireland. Currently, the national marine biodiscovery laboratory in Ireland project (NMBLI) is continuing this marine natural products discovery campaign, with a primary goal to examine Irish coastal invertebrates to identify bioactive marine natural products.
Following initial chemical and biological screening of Irish marine invertebrates, fractions of the sponge
Spongosorites calcicola were found to exhibit strong anti-tumor activities and a large number of brominated metabolites. Herein, we describe the isolation, structure elucidation and novel biological evaluation of one new bisindole alkaloid, Calcicamide (
1), from
S. calcicola (
Figure 17). Calcicamide is biosynthetically related to a number of other common marine natural products including the topsentins, spongotines and hamacanthins. We will also present the effects of Calcicamide as well as five other known bisindole analogues on the expression of the telomeric protein TRF2, which exhibits potent pro-oncogenic properties.
The Structure and Biological Activity of Heinamides from the Cyanobacterium Nostoc sp. UHCC0702
L. M. P. Heinilä,
A. Jortikka,
J. Jokela,
M. Wahlsten,
D. P. Fewer
and
K. Sivonen
Department of Microbiology, University of Helsinki, Helsinki 00014, Finland
Cyanobacteria are a rich source of natural products many of which have potent antifungal activity. Here we report the discovery of heinamides from the filamentous diazotrophic cyanobacterium
Nostoc sp. UHCC 0702. Heinamides are new macrocyclic peptides belonging to two separate structural classes that both exhibitted antifungal activity against
Aspergillus flavus. The chemical structures of the compounds were elucidated with UPLC-MS and NMR. The isolated compounds were named heinamides A1–A3 and B1–B4 (
Figure 18) and exhibited structural similarity to the laxaphycin A (11 residue) and laxaphycin B (12 residue) families of macrocyclic peptides. The two classes of peptides exhibited synergistic antifungal effect when one compound from both groups was present. The antifungal acitivity was minimal when tested with individual compounds and with multiple compounds of the same size group. We obtained a genome sequence for
Nostoc sp. UHCC 0702 and identified the biosynthetic genes for heinamide production. This study demonstrates that the production of antifungal laxaphycin A and B peptide pairs that act in synergy is widespread in cyanobacteria.
Isotopologue-Guided Identification of Halogenated Anilines from a Benthic Diatom
Tim U. H. Baumeister 1,
Mona Staudinger 2
and
Georg Pohnert 1,2
1
Max Planck Institute for Chemical Ecology, Fellow Group Plankton Community Interaction, Hans-Knöll-Straße 8, 07745 Jena, Germany
2
Friedrich Schiller University Jena, Institute for Inorganic and Analytical Chemistry, Department of Bioorganic Analytics, Lessingstr. 8, 07743 Jena, Germany
The intriguing isotope pattern produced by brominated/chlorinated compounds is a useful property to identify those compounds in an extract by chromatography-coupled mass spectrometry. The introduction of high resolution accurate mass (HRAM) mass spectrometry further allowed for using the mass difference between light and heavier isotopes as a property for identification. In an integrated approach, DeltaMS, an open source R app, uses the mass difference and the isotope pattern to highlight potential candidate ions. As a proof of concept, extracts from the marine benthic diatom Nitzschia cf. pellucida, known for its possession of haloperoxidases, were obtained and analyzed on a HRAM GC-Orbitrap mass spectrometer. Several brominated compounds could be detected, whereby the detection of a tribrominated compound drew our attention. Structure elucidation by MS and a co-injection confirmed the presence of 2,4,6-tribromoaniline which was so far only known as an anthropogenic pollutant. But not only tribromoaniline, but also the trichloroaniline, and the mixed trihalogenated (Cl/Br) anilines could be identified. Stable isotope labeling, using Na15NO3, and NaH13CO3 as sole carbon and nitrogen source in the growth medium, confirmed the biosynthesis of the halogenated anilines by the diatom. Intra- and extracellular monitoring of the halogenated anilines at different time points during the algal growth showed an accumulation of those compounds. Intracellular amounts exceeded the extracellular by many times, indicating that those compounds could act as a deterrent against grazers. This is the first study that shows the presence of biosynthesized trihalogenated anilines.
Spinochromes of Pacific Sea Urchins: Distribution and Bioactivity
Elena A. Vasileva,
Natalia P. Mishchenko
and
Sergey A. Fedoreyev
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far-Eastern Branch of the Russian Academy of Sciences, 690022 Vladivostok, Russia
Marine hydrobionts, such as sea urchins, and specifically their gonads, are a valuable renewable food resource. At the same time, they can serve as a unique source of various natural compounds, which can be the basis for the creation of various biomaterials, effective medicinal and parapharmaceutical preparations, as well as functional food products. After the removal of the gonads, large amounts of sea urchin shells are left as waste. This shell material is rich in bioactive quinonoid pigments, principally spinochromes. Although this class of compounds has been known for over 100 years, there is still not so much data on their chemical and biological properties. The most common spinochrome—echinochrome A is the active substance in the Russian antioxidant drug Histochrome® that is being used successfully in cardiology and ophthalmology. Recent findings revealed new properties of echinochrome A—anti-diabetic, anti-allergic, gastroprotective, mitochondria-protective and other activities. Seeing echinochrome A to exhibit so many different effects it is interesting to study the distribution and biological activity of other spinochromes.
Using validated HPLC-DAD-MS method we investigated spinochrome composition of 21 Pacific sea urchin species. Ten spinochromes containing different substituents in 1,4-naphthoquinone core were isolated for the structure-activity relationship studies. All these compounds were tested for in vitro antioxidant activity using several standard assays, in vitro cardioprotective activity on the model of doxorubicin-induced oxidative stress in human cardiomyocytes, and for their ability to prevent cisplatin-induced oxidative stress in mice kidneys. The most potent antioxidants appeared to be echinamine B, spinochromes D and E and 7,7’-anhydroethylidene-6,6’-bis(2,3,7-trihydroxynaphthazarin).
Marine Sponges from Indian Ocean: A Highly Promising Source for the Discovery of Novel Bioactive Compounds to Fight against Ageing and Age-Related Diseases
Charifat Saïd Hassane 1,
Pierre-Eric Campos 1,
Florent Tintillier 1,
Patricia Clerc 1,
Jean-Bernard Boyer 1,
Nicole De Voogd 2,
Nikolas Fokialakis 3,
Ioannis P. Trougakos 4,
Konstantinos Gardikis 5,
Christine Wenzkowski 6,
Jérôme Bignon 7,
Géraldine Le Goff 7,
Céline Moriou 7,
Ali Al-Mourabit 7,
Laurent Dufosse 1,
Mireille Fouillaud 1,
Jamal Ouazzani 6
and
Anne Bialecki 1
1
LCSNSA, Université de la Réunion, Ile de la Réunion, 97744 Saint-Denis, France
2
Naturalis Biodiversity Center, Leiden, Darwinweg 2, 2333 CR Leiden, The Netherlands
3
Faculty of Pharmacy, National & Kapodistrian University of Athens, 15771 Athens, Greece
4
Faculty of Biology, National & Kapodistrian University of Athens, 15784 Athens, Greece
5
APIVITA SA, Research and Development Department, Industrial Park of Markopoulo, Markopoulo Mesogaias, 19003 Athens, Greece
6
Crelux GmbH, 82152 Martinsried, Germany
7
ICSN-CNRS, 91190 Gif sur Yvette, France
Ageing is commonly defined as the accumulation of diverse deleterious changes occurring in cells and tissues with advancing age that are responsible for the increased risk of pathologies such as Alzheimer’s disease, cardiovascular diseases, neurodegeneration or cancers. As the population of developed countries is ageing, the prevalence of a variety of age-related diseases is increasing. In order to counteract this major healthcare challenge, marine natural products represent an extraordinary reservoir of structurally diverse bioactive metabolites which may offer anti-ageing properties with pharmaceutical, cosmeceutical and nutraceutical applications.
Taking into consideration the aforementioned issues, the H2020 European project TASCMAR explores marine invertebrates and symbionts from under-investigated marine biodiversity hotspots and develops innovative approaches for the discovery and production of compounds with anti-ageing activity. The Chemistry Laboratory of Natural Substances and Food Sciences (LCSNSA, University of La Reunion) located at Reunion island is involved in this ambitious research program and this communication will therefore provide an outline of the contribution made by the LCSNSA to TASCMAR. The laboratory has collected a total of 112 samples of sponges from Mayotte and Rodrigues (Indian Ocean). The samples were extracted and the crude extracts obtained were submitted to a biological evaluation against a wide range of different targets involved in ageing or age-related diseases. These targets include catalase, sirtuin 1, CDK7, proteasome, Fyn kinase, tyrosinase and elastase. Twenty-nine (29) crude extracts have shown promising results. The chemical investigation of these 29 extracts for the discovery of molecules with anti-ageing effects will be discussed.
MS Dereplication for Rapid Discovery of Structurally New or Novel Natural Products
Jioji N. Tabudravu 1,2,
Léonie Pellissier 2,
Alan James Smith 2,
Richard Kid 3,
Edward J. Milton 4,
Hai Deng 2,
Rainer Ebel 2,
Carmela Gissi 5,6,
Bruce F. Milne 7,
Gabriela Cimpan 4
and
Marcel Jaspars 2
1
School of Forensic and Applied Sciences, Faculty of Science & Technology, University of Central Lancashire, Preston, Lancashire PR1 2HE, UK
2
Marine Biodiscovery Centre, Department of Chemistry, University of Aberdeen, Scotland AB24 3UE, UK
3
Publisher, Data & Databases, Royal Society of Chemistry, Thomas Graham House, Science, Park, Milton Road, Cambridge CB4 0WF, UK
4
Advanced Chemistry Development, UK Ltd. Venture House, Arlington Square, Downshire Way, Bracknell, Berks RG12 1WA, UK
5
Department of Biosciences, Biotechnologies and Biopharmaceutics, University of Bari “A. Moro”, Via Orabona 4, 70125 Bari, Italy
6
IBIOM, Istituto di Biomembrane, Bioenergetica e Biotecnologie Molecolari, CNR, Via Amendola 165/A, 70126 Bari, Italy
7
CFisUC, Department of Physics, University of Coimbra, Rua Larga, 3004-516 Coimbra, Portugal
In order to accelerate the isolation and characterisation of structurally new or novel natural products, it is crucial to develop efficient strategies that prioritise samples with greatest promise early in the workflow so that resources can be utilised in a more efficient and cost-effective manner. Two complementary approaches have been developed: One is based on targeted identification of known compounds held in a database based on high resolution MS and predicted LC retention time data [1]. The second is an MS metrics-based approach where the software algorithm calculates metrics for sample novelty, complexity, and diversity after interrogating databases of known compounds, and contaminants. These metrics are then used to prioritise samples for isolation and structure elucidation work [2]. Both dereplication approaches have been validated using natural product extracts resulting in the isolation and characterization of new or novel natural products.
References
Chervin, J.; Stierhof, M.; Tong, M.H.; Peace, D.; Hansen, K.Ø.; Urgast, D.S.; Andersen, J.H.; Yu, Y.; Ebel, R.; Kyeremeh, K.; et al. Targeted Dereplication of Microbial Natural Products by High-Resolution MS and Predicted LC Retention Time. J. Nat. Prod. 2017, 80, 1370–1377, doi:10.1021/acs.jnatprod.6b01035.
Tabudravu, J.N.; Pellissier, L.; Smith, A.J.; Subko, K.; Autréau, C.; Feussner, K.; Hardy, D.; Butler, D.; Kidd, R.; Milton, E.J.; et al. LC-HRMS-Database Screening Metrics for Rapid Prioritization of Samples to Accelerate the Discovery of Structurally New Natural Products. J. Nat. Prod. 2019, 82, 211–220, doi:10.1021/acs.jnatprod.8b00575.
Cone Snails Natural Products: Isolation and Characterization of Toxins
Jorge L. B. Neves 1,2,
Julita S. Imperial 3,
Zhenjian Lin 3,
David Morgenstern 4,
Beatrix Ueberheide 4,
Joanna Gajewiak 3,
Samuel D. Robinson 3,
Samuel Espino 3,
Maren Watkins 3,
Agostinho Antunes 1,5,
Eric W. Schmidt 3,
Vitor Vasconcelos 1,5
and
Baldomero M. Olivera 3
1
CIIMAR/CIMAR, University of Porto, Terminal de Cruzeiros do Porto de Leixões, Avenida General Norton de Matos, S/N, 4450-208 Matosinhos, Portugal
2
Faculty of Engineering and Marine Science, University of Cabo Verde, Mindelo CP 163, Cabo Verde
3
Departments of Medicinal Chemistry and Biology, University of Utah, Salt Lake City, UT 84112, USA
4
Langone Medical Center, Department of Biochemistry and Molecular Pharmacology, New York University, New York, NY 10016, USA
5
Faculty of Sciences, University of Porto, Rua do Campo Alegre, 4169-007 Porto, Portugal
The natural products from Cone Snails (Conus spp.) venoms have received much attention over the last decades due to the biological activity, extraordinary diversity, and molecular studies that opened a window for biomedicine research [1]. Venomous Conus are highly specialized venomous predators that may produce up to 100,000 small compounds and it is estimated over 2,000,000 natural products to be present in the venoms of venomous marine snails. Conotoxins, are currently being developed as analgesics for the treatment of neuropathic pain. In December 2004, the synthetic version of the peptide ω-conotoxin MVIIA (commercial name Prialt®) from C. magus has been approved by the US-FDA to treat chronic pain in humans [2,3].
Our research has been focused on Cabo Verde venomous marine snails particularly those that belong to the genus Conus. This project includes the isolation and structure elucidation of compounds from the venom of two species, one endemic—Conus ateralbus, and one non-endemic Conus genuanus; using MALDI-TOF, LC-MSMS, and NMR data. We described the isolation and characterization of the first bioactive peptide from the venom of C. ateralbus. The 30Amino Acid (AA) venom peptide is named δ-conotoxin AtVIA. An excitatory activity was manifested by the peptide on a majority of mouse lumbar dorsal root ganglion neurons and homology which include conserved sequence elements with δ-conotoxins (pharmacology family) from another worm and fish-hunters [4]. On the C. genuanus we did the chemical characterization of a novel small molecule, a guanine derivative with unprecedented features; we named it genuanine. Genuanine was neuroactive when injected into mice having paralytic activity [5].
References
Terlau, H.; Olivera, B.M. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004, 84, 41–68.
Jimenez, E.C.; Shetty, R.P.; Lirazan, M.; Rivier, J.; Walker, C.; Abogadie, F.C.; Yoshikami, D.; Cruz, L.J.; Olivera, B.M. Novel excitatory Conus peptides define a new conotoxin superfamily. Nurochem 2003, 85, 610.
Olivera, B.M.; Russell, W. Molecular Interventions; University of Utah: Salt Lake City, UT, USA, 2007; pp. 251–260.
Neves, J.L.B.; Lin, Z.; Imperial, J.S.; Antunes, A.; Vasconcelos, V.; Olivera, B.M.; Schmidt, E.W. Small Molecules in the Cone Snail Arsenal. Org. Lett. 2015, 17, 4933–4935.
Neves, J.L.B.; Imperial, J.S.; Morgenstern, D.; Ueberheide, B.; Gajewiak, J.; Antunes, A.; Robinson, S.D.; Espino, S.; Watkins, M.; Vasconcelos, V.; et al. Characterization of the First Conotoxin from Conus ateralbus, a Vermivorous Cone Snail from the Cabo Verde Archipelago. Mar. Drugs 2019, 17, 432.
The Prospects of Microbial Natural Products as a Source of Possible Drug Prototypes for the Neglected African Disease: Ghana, a Case Study
Kwaku Kyeremeh 1,2,
Hai Deng 2
and
Marcel Jaspars 2
1
Marine and Plant Research Laboratory of Ghana, Department of Chemistry, School of Physical and Mathematical Sciences, University of Ghana, P.O. Box LG 56 Legon-Accra, Ghana
2
Marine Biodiscovery Centre, Department of Chemistry, University of Aberdeen, Old Aberdeen AB24 3UE, Scotland, UK
The sub-Saharan Africa (SSA) region is burdened with a high incidence of infections, schistosomiasis, trypanosomiasis, leishmaniasis and cancer. There is current rapid widespread development of resistance to the available treatments for these diseases. More striking a fact is that; for the parasitic diseases, the available number of drugs for treatment is exceptionally low and each of these have been under prescription for periods not less than 30 years. In fact, an average of the prescription periods from time of discovery to-date for the top 10 antiparasitics (Praziquantel or oxamniquine, amphotericine, pentavalent antimonials, paromomycin, miltefosine, pentamidine, fluconazole/itraconazole, melarsoprol, eflornithine and nifurtimox) that is likely to be prescribed to you in the clinic today is 56 years. Interestingly, there is a rising expertise of drug research scientists in SSA who have received training from Europe and other parts of the world and this coupled with the huge natural product diversity of the region necessitates the discovery of new drug scaffolds. Microbial natural products provide the largest chemical and biological diversity in any drug discovery screening program compared to other natural sources like plants and invertebrates with the resupply problems mostly overcome with large scale fermentation and heterologous expression. In the last six years, many interesting molecules such as butremycin, butrepyrazinone, butrecitrinadin, paenidigyamycins, legonmycins, legonindolizidines, legonmaleimides and legonaridines and many interesting enzymes like the Talented Solo Legon C have been discovered from novel Ghanaian microbes. This presentation outlines what is indeed the first major attempt to discover new drug scaffolds from SSA microbes.
“Being Penicillium ubiquetum”: Metabolomics and Molecular Networking for the Discovery of Rare New Natural Products from a Marine-Sourced Fungus
Thi Phuong Thuy Hoang 1,2,
Catherine Roullier 1,3,
Jean-François Gallard 4,
Yves François Pouchus 1,
Mehdi A. Beniddir 5
and
Olivier Grovel 1,3
1
Nantes Université, Faculty of Pharmacy, MMS, 9 Rue Bias, 44035 Nantes, France
2
Phu Tho College of Pharmacy, Phu Tho 290000, Vietnam
3
Corsaire-ThalassOMICS Metabolomics Facility, Biogenouest, Université de Nantes, 44035 Nantes, France
4
Institut de Chimie des Substances Naturelles, CNRS UPR 2301, University of Paris-Saclay, 91198 Gif-sur-Yvette, France
5
BioCIS, Université Paris-Sud, CNRS, Université Paris-Saclay, 92290 Châtenay-Malabry, France
Marine microorganisms, including fungi, are the source of ever more original molecules that can be used therapeutically. Nevertheless, their discovery requires the use of elicitation strategies of cryptic biosynthetic pathways to reveal their metabolic potential. These methods, such as co-cultivation, OSMAC or epigenetic remodeling, sometimes lead to the production of new major metabolites. In a much more frequent way they induce an over-expression of many original molecules produced in very small quantities, therefore very difficult to detect. For this, metabolomic and dereplicative tools such as molecular networks allow an exhaustive investigation of metabolic capacities and their variations.
In this way, in the goal of the discovery of new marine fungi natural products, we have investigated various marine-sourced Penicillium sp. strains including a P. ubiquetum, a species for which no chemical works have been reported so far. The study of variations of its metabolic expression in response to the cultural conditions has been carried out by LC-HRMS/MS. By combining metabolomics analyses, dereplication and molecular networking, we highlighted the ability of P. ubiquetum to direct its metabolism—under specific conditions and in the presence of seawater—toward the production of an abundant series of minor complex meroterpenoids and some unusual C25-steroids. MS-guided purification led to the discovery of new isobaric analogs in these two rare chemical families, showing the power of new bioinformatics tools for natural products discovery.
Egyptian Fungal Antibiotic Metabolites—From Pharaohs to Modern Textiles
Sylvia Soldatou 1,
M. Mallique Qader 2,
Coralie Pavesi 1,
Kevin Jace Miranda 1,
Oluwatofunmilay A. Diyaolou 1,
Ahmed A. Hamed 3,
Mostafa E. Rateb 2
and
Rainer Ebel 1
1
Marine Biodiscovery Centre, Department of Chemistry, University of Aberdeen, Aberdeen AB24 3UE, Scotland, UK
2
School of Computing, Engineering & Physical Sciences, University of the West of Scotland, Paisley PA1 2BE, UK
3
Microbial Chemistry Department, National Research Center, 33 El-Buhouth Street, P.O. Box 12622, Dokki, Giza 12622, Egypt
Since the discovery of penicillin, fungi have been in the spotlight as a prolific source of bioactive agents with many examples in the literature emphasising their importance in drug discovery. In our efforts towards the discovery of new bioactive fungal metabolites from niche ecosystems, several fungal strains were isolated from marine organisms and plants collected from Hurghada (Red Sea) and Wadi El Natrun valley, respectively. Based on the antimicrobial screening against a panel of pathogenic microorganisms, fifteen endophytic or invertebrate-associated fungal isolates were prioritised for further analysis. Chemical investigation was carried out for organic extracts obtained from small-scale fermentations. Specifically, analysis of the MS2 data through the GNPS platform revealed several known compounds which clustered with unidentified parent ions, suggesting the presence of new secondary metabolites. Fungal fermentation on rice afforded sufficient biomass for fractionation and separation which led to the isolation of a suite of new and known compounds belonging to various classes. In particular, itaconic acid and kojic acid derivatives were isolated from a Cladosporium sp., whereas a new cyclo-peptide and a new morpholine-2,5-dione were isolated from an Epicoccum sp. and Alternaria sp., respectively. Moreover, potentially new analogues of emericellamide A were identified in the molecular network of the crude extract of a marine Aspergillus sp. An addtional Aspergillus strain produced a suite of butyrolactone derivatives. The pure metabolites were screened for antibiotic and cytotoxic activity. The ultimate goal of this interdisciplinary project is to incorporate the new antimicrobial compounds onto textile substrates to enhance their functionality.
Targeted Co-Cultivation of Baltic Marine fungi with Phytopathogens for Discovery of Novel Natural Agrochemicals
Ernest Oppong-Danquah,
Martina Blümel
and
Deniz Tasdemir
GEOMAR Centre for Marine Biotechnology, Research Unit Marine Natural Product Chemistry, GEOMAR Helmholtz Centre for Ocean Research Kiel, Am Kiel-Kanal 44, 24106 Kiel, Germany
Resistance of phytopathogens to pesticides threatens global food security, necessitating discovery of novel, ecofriendly crop-protection agents. Fungi have been prolific sources of natural agrochemicals. However, genomic studies point out a discrepancy between the often high number of biosynthetic gene clusters (BGCs) and low number of molecules obtained from fungi, because many BGCs remain silent in monoculture conditions. Co-cultivation of two microorganisms is a highly efficient method to awaken cryptic BGCs and enhance chemical diversity. Herein we adopted a unique co-cultivation approach involving marine fungi and plant pathogens to induce the targeted production of natural antibiotics against phytopathogens. Towards this aim, 123 marine-adapted fungi from the Baltic Sea were isolated and identified. 21 selected isolates were co-cultivated with economically relevant bacterial (
Pseudomonas syringae,
Ralstonia solanacearum) and fungal (
Magnaporthe oryzae,
Botrytis cinerea) phytopathogens. UPLC-QToF-MS/MS-based untargeted metabolomics approach using molecular networking (GNPS) was performed for comparative metabolome analyses of mono- and co-cultures. Co-cultivation of the marine fungus
Cosmospora sp. and the phytopathogen
M. oryzae led to production of 3 novel coumarans in the inhibition zone (
Figure 19) that were absent in the monocultures. These compounds, plus several known naphtho-γ-pyrones and isochromans were purified by HPLC and characterized by spectroscopic means. The new and known compounds showed antifungal activities (up to IC
50 0.8 μg/mL against
M. oryzae). These results render phytopathogens useful elicitors for inducing novel chemistry in fungal co-culture studies for discovery of crop protection agents.
Dereplication of Natural Products Using Diffusion Ordered Spectroscopy (DOSY)
Guy Kleks 1,2,
Vicky M. Avery 2
and
Anthony R. Carroll 1,2
1
Environmental Futures Research Institute, Griffith University, Gold Coast QLD 4222, Australia
2
Griffith Institute for Drug Discovery, Griffith University, Brisbane QLD 4111, Australia
One of the challenges in natural product discovery is the re-isolation of known compounds. Dereplication is the process used to quickly identify these known compounds, thus saving time and effort directed at their re-isolation and structure determination. MS and/or NMR methods have been used in dereplication and each technique generates its own unique set of data (either mass or functional group based). Diffusion-ordered spectroscopy (DOSY) is a powerful tool that allows for the spectroscopic separation of components of a mixture by their diffusion coefficient. This separation is determined by the size and shape of the molecule in solution and thus provides an opporuntiy to generate molecular weight data directly from NMR spectra. We have generated DOSY diffusion coefficents for a library of known natural products and developed a correlation matrix to predict molecular weights by NMR (
Figure 20). This approach have been successfully applied to identify known natural products in complex mixtures and to predict the molecular weight of unknown compounds in a mixture. The DOSY approach therefore provides a useful dereplication tool that bridges the gap between hyphenated MS/NMR dereplication methods. The biggest disadvatange of 2D DOSY is signal overlap, which leads to inaccurate estimation of diffusion coefficients resulting in large errors in the molecular weight prediction. Since signal overlap is unavoidable for a complex mixture such as an extract, we also utilzed 3D DOSY experiments. Here we demonstrate the dereplication of natural products using 2D and 3D DOSY.
Halogenated Tyrosine Derivatives from the Pacific Zoantharian Antipathozoanthus hickmani
Paul O. Guillen 1,2,
Karla B. Jaramillo 1,3,
Laurence Jennings 2,
Grégory Genta-Jouve 4,5,
Mercedes de la Cruz 6,
Bastien Cautain 6,
Fernando Reyes 6,
Jenny Rodríguez 1
and
Olivier P. Thomas 2
1
ESPOL Escuela Superior Politécnica del Litoral, ESPOL, Centro Nacional de Acuacultura e Investigaciones Marinas, Campus Gustavo Galindo km. 30.5 vía Perimetral, P.O. Box 09-01-5863 Guayaquil, Ecuador
2
Marine Biodiscovery, School of Chemistry and Ryan Institute, National University of Ireland Galway (NUI Galway), University Road, H91 TK33 Galway, Ireland
3
Zoology, School of Natural Sciences and Ryan Institute, National University of Ireland Galway (NUI Galway), University Road, H91 TK33 Galway, Ireland
4
Équipe C-TAC, UMR CNRS 8038 CiTCoM—Université Paris Descartes, 4 Avenue de l’Observatoire, 75006 Paris, France
5
Unité Molécules de Communication et Adaptation des Micro-Organismes (UMR 7245), Sorbonne Universités, Muséum National d’Histoire Naturelle, CNRS, 75231 Paris, France
6
Fundación MEDINA, Centro de Excelencia en Investigación de Medicamentos Innovadores en Andalucía, Avda. del Conocimiento 34, Parque Tecnológico de Ciencias de la Salud, E-18016 Armilla, Granada, Spain
Zoantharians (Cnidaria: Hexacorallia) are sessile invertebrates commonly found in all marine ecosystems. Despite their wide distribution, chemical investigation on these organisms has been generally overlooked and they are limited to species belonging to the genera Zoanthus and Palythoa. Most reports of their chemical diversity deal with species collected from the Indo-Pacific and Atlantic Ocean, while species from the Tropical Eastern Pacific have been poorly investigated. Antipathozoanthus hickmani is one of the most representative zoantharians from this ecosystem, and it was first reported from the Galapagos Islands where it overgrows the black coral Anthipathes galapagensis.
Herein, we report the isolation and structure elucidation of four halogenated dipeptides named Valdiviamides A-D isolated from A. hickmani collected in the Marine Protected Area El Pelado, Santa Elena-Ecuador. Valdiviamides A-D are halogenated tyrosine dipeptides characterized by the presence of bromine and iodine atoms on the phenol ring. Additionally, we propose halogenated tyrosine derivatives as chemical markers for species of the family Parazoanthidae.
Novel Zwitterionic Metabolites from Marine Diatoms
Simona Fenizia 1,2
and
Georg Pohnert 1,2
1
Institute for Inorganic and Analytical Chemistry, Friedrich Schiller University, Lessingstrasse 8, 07743 Jena, Germany
2
Max Planck Institute for Chemical Ecology, Hans-Knöll-Straße 8, 07745 Jena, Germany
Zwitterions are characterized by the presence of both a positive and a negative charge within one molecule. They play an important role in the environment, since they are involved in the modulation of the global sulphur cycle in the atmosphere and since they mediated many interactions among marine species.
The identification and classification of zwitterionic metabolites has been problematic until our development of novel chromatographic and mass spectrometric methods. These analytical methods show the presence of many zwitterions in microalgae that have not been recognized or characterized previously. In this talk, I will present how liquid chromatography coupled with mass spectrometry is utilized to assign novel hitherto unknown components in the “zwittermetabolome” of diatoms. The physiological functions of novel key metabolites is introduced and ecological implications are discussed. I report studies on three diatom species, Phaeodactylum tricornutum, Skeletonema costatum and Thalassiosira weissflogii that have emerged as model organisms in phycological studies.
Quimioprospecting for Streptomyces from the South Pacific: Genomic and Metabolic Study of Novel Compounds
N. Serna,
N. Zamorano
and
B. Cámara
Laboratorio de Microbiología Molecular y Biotecnología Ambiental, Departamento de Química & Centro de Biotecnología DAL, Universidad Técnica Federico Santa María, Avenida España 1680, Casilla 110-V, Valparaíso, Chile
The indiscriminate use of antibiotics has enhanced the favourable conditions for the selected microorganisms. The scientific community is focusing on the search for new antibiotic compounds, which implies the need to identify new bioactive molecules. Specifically, marine actinomycetes have aroused interest because they are emerging sources of antibiotic compounds.
Our group has been working on the bioprospecting of Chilean marine actinomycetes, in order to investigate their biotechnological potential to produce active secondary metabolites against pathogenic strains model, in this exploration we obtained a collection of 30 genera of actinomycetes and a possible novel genera belonging to the Nocardiopsaceae family.
Antimicrobial activity assay of extracts crude of our actinobacteria obtained for OSMAC strategy was tested against pathogenic strain models like Staphylococcus aureus, Pseudomonas aeruginosa, and Saprolegnia parasitica. The promissory active extracts were selected to determine chemical profile using liquid chromatography high resolution mass spectrometry (LC-HRMS) that allowed dereplicated secondary metabolite and builds a hierarchical cluster, which enabled to choose those strains with new chemical entities and where further purification and identification efforts are being achieved. In addition, the fermentation extracts analyzed showed that 37% of the metabolites do not coincide with the fragmentation patterns of known metabolites reported in the Natural Product Dictionary (NPD). As an auxiliary tool in the search and elucidation of the chemical structure of novel natural products, the analysis and study of the genomes of our actinobacteria has allowed the identification of interesting cluster of biosynthetic genes that would be associated with secondary metabolites not reported in NPD.
Bioactivity and Metabolome Profile of Marine Microorganisms Isolated from Arctic Deep-Sea Sediments
Florent Magot 1,
Gwendoline Van Soen 1,
Martina Blümel 1,
Thomas Soltwedel 2
and
Deniz Tasdemir 1,3
1
GEOMAR Centre for Marine Biotechnology (GEOMAR-Biotech), Research Unit Marine Natural Products Chemistry, GEOMAR Helmholtz Centre for Ocean Research Kiel, Am Kiel-Kanal 44, 24106 Kiel, Germany
2
Alfred-Wegener-Institute Helmholtz Center for Polar and Marine Research, Dept. Deep-Sea Ecology and Technology, Am Handelshafen 12, 27570 Bremerhaven, Germany
3
Kiel University, Christian-Albrechts-Platz 4, 24118 Kiel, Germany
The deepsea (>1000 m water depth) constitutes more than 60% of the ocean’s biosphere and harbors an unparalleled biodiversity. Because of the high pressure, darkness and low nutrient availability, the deepsea represents an extreme environment for organisms, requiring excellent adaptation capability. Microorganisms that have the ability to thrive in deep-sea environment are regarded promising for biodiscovery. Herein, we studied microorganisms obtained from Arctic deep-sea sediment as a source of new bioactive metabolites. 70 bacterial and 7 fungal strains were isolated and identified from sediment samples collected by a ROV in the Fram Strait, Arctic Ocean (2432 m water depth) during RV Polarstern expedition PS108 in 2017. In order to activate different biosynthetic gene clusters and enhance chemical diversity, the strains were cultivated in four different solid media. The cultures were extracted with EtOAc and the extracts were analyzed by UPLC-MS/MS. Molecular networking was used successfully for comparative metabolomics of microorganisms grown in different media. The crude extracts were screened against a panel of clinically relevant microbial pathogens and six cancer cell lines. Two fungi and two bacteria showed selective activity against the yeast Candida albicans, the Gram-negative bacterium Pseudomonas aeruginosa and the melanoma cancer cell line A375. Interestingly, for three of the strains, the bioactivity occurred only when grown in one specific media, showing the importance of culture conditions. This presentation will outline the metabolome and bioactivity analyses of the Artic deep-sea sediment microorganisms.
Acknowledgments
Funding was provided in frame of EU-H2020 as part of the MSCA-ITN project “MarPipe”.
Can Well Studied Geographic Locations Still Provide Opportunities for Natural Product Discovery?—An Australian Case Study
Sylvia Urban
and
James Lever
Marine and Terrestrial Natural Products Research Group, School of Science (Applied Chemistry and Environmental Science), RMIT University, GPO Box, Melbourne, VIC 2476, Australia
Southern Australian shores are home to more than 140 species of green algae, 240 species of brown algae and 800 species of red algae, making this region one of the most species diverse coastlines in the world [1]. Port Phillip Bay, located on the southern shore of Australia (
Figure 21), has an area of approximately 2000 square kilometres and an average depth of 13 m. It has approximately 120 different species of macroalgae, represented by all three major phyla of brown, red and green algae. Since the first study to date 47% of these species, yielding an array of natural products, have been studied for phytochemical purposes.
In the field of natural products discovery, sampling species from well studied geographical locations potentially decreases opportunity for the discovery of new bioactive secondary metabolites. Does this hypothesis hold true for Port Phillip Bay? A review of the discovery of natural products from marine algae studied in this region, region has been conducted [2]. Case studies demostrating the impact of new methodologies and technologies on the discovery rate will be presented to better understand what we might expect from this biodiverse region in the future.
References
Edgar, G. Australian Marine Life: The Plants and Animals of Temperate Waters; Reed New Holland: Sydney, NSW, Australia, 2000.
Lever, J.; Brkljača, R.; Urban, S. Natural Products of the Common Marine Algae of Port Phillip Bay, Australia. (Manuscript in preparation).
Stereochemical Study of Spongosoritin A by Vibrational Circular Dichroism and Quantum Chemical Calculations
Andrea N. L. Batista 1,
Fernando M. dos Santos, Jr. 1,
Joao M. Batista, Jr. 2
and
Alessandra L. Valverde 1
1
Chemistry Institute, Fluminense Federal University, Niteroi, Rio de Janeiro 24020-141, Brazil
2
Institute of Science and Technology, Federal University of Sao Paulo, Sao Jose dos Campos, Sao Paulo 12231-280, Brazil
The polyketide spongosoritin A (
1) was first reported in 2005 from
Plakortis angulospiculatus and
Spongosorites sp. marine sponges [1,2]. This compound is moderately cytotoxic against colon cancer cells (HCT-116) with time-dependent activity [3]. It has been suggested that structural features, such as relative and absolute configurations, could affect the selectivity of the reported cytotoxicity by the activation of different pathways [3]. To date, the relative and absolute configuration of natural spongosoritin A has been suggested to be
syn-(6
R,8
R) based on the comparison of its optical rotation values with that of others marine polyketides with furanylidene moiety as well as the synthetic
1 [4]. However, the determination of absolute configuration based on optical rotation values measured in single wavelengths may result in misassignments [5,6]. Thus, in this work we have applied a direct and efficient method for the determination of the absolute configuration of the natural spongosoritin A using vibrational circular dichroism, NMR and optical rotation, all associated with quantum chemical calculations (
Figure 22).
References
Epifanio, R.A.; Pinheiro, L.S.; Alves, N.C. Polyketides from the marine sponge Plakortis angulospiculatus. J. Braz. Chem. Soc. 2005, 16, 1367.
Capom, R.J.; Singh, S.; Ali, S.; Sotheeswaran, S. Spongosoritin A: A New Polyketide from a Fijian Marine Sponge, Spongosorites sp. Aust. J. Chem. 2005, 58, 18.
Santos, E.A.; Quintela, A.L.; Ferreira, E.G.; Sousa, T.S.; Pinto, F.D.C.L.; Hajdu, E.; Carvalho, M.S.; Salani, S.; Rocha, D.D.; Wilke, D.V.; et al. Cytotoxic Plakortides from the Brazilian Marine Sponge Plakortis angulospiculatus. J. Nat. Prod. 2015, 78, 996.
Alcock, L.J.; Norris, M.D.; Perkins, M.V. Total synthesis and structural elucidation of spongosoritin A. Org. Biomol. Chem. 2018, 16, 1351.
Joyce, L.A.; Nawrat, C.C.; Sherer, E.C.; Biba, M.; Brunskill, A.; Martin, G.E.; Cohen, R.D.; Davies, I.W. Beyond optical rotation: what’s left is not always right in total synthesis. Chem. Sci. 2018, 9, 415.
Nakahashi, A.; Yaguchi, Y.; Miura, N.; Emura, M.; Monde, K. A Vibrational Circular Dichroism Approach to the Determination of the Absolute Configurations of Flavorous 5-Substituted-2(5H)-furanones. J. Nat. Prod. 2011, 74, 707.
Secondary Metabolites from Marine Antarctic Fungus Arthrinium sp. and their Photoprotective and Antioxidant Potential
A. C. Jordão 1,
L. R. Gaspar 1,
C. M. Marzocchi-Machado 1,
N. C. Canicoba 1,
P. Colepicolo 2
and
H. M. Debonsi 1
1
School of Pharmaceutical Sciences of Ribeirão Preto, University of São Paulo, Ribeirão Preto, São Paulo 14040-903, Brazil
2
Institute of Chemistry of University of São Paulo, São Paulo 05508-000, Brazil
The conditions of the Antarctic continent are extreme; therefore, the organisms produce secondary metabolites necessary for survival in these severe conditions, which comprises in a rich source of new natural products. This study aimed to isolate and identify the secondary metabolites from the extract of the fungus
Arthrinium sp., besides the biological potential evaluation. The fungus was cultivated in PDB medium and artificial sea water. The extract was obtained in AcOEt, and then submitted to vacuum chromatographic column, using a polarity gradient solvent system Hex: AcOEt. The procedures for the substances’ isolation were carried out using chromatographic techniques, such as TLC and HPLC. The metabolites identification was performed by NMR, spectroscopy in the Infrared and Ultraviolet regions, and mass spectrometry. The photoprotection and photodegradation of the material were determined by analyzing the absorption spectra in a spectrophotometer (range of 200–400 nm). The antioxidant activity was analyzed by the effect of the sample on the production of reactive oxygen species (ROS) by neutrophils, measured by chemiluminescence assay. The results can be found in
Figure 23A,B. Among the isolated six substances, one fraction was identified as 2,3,4,6,8-pentahydroxy-1-methylxanthone. The extract and some fractions showed a potential photoprotective activity having good absoption in the UVB (280–320 nm), and UVA (320–400 nm) regions. The extract also showed antioxidant activities, in a concentration 100 μg/mL with a viability of 94.5%. The evaluation potential is being carrying out for the isolated substances.
Genome Mining and Chemical Analysis of Lipopeptides from a Bacillus Strain Isolated from a New Spongia Species
Antigoni-Angeliki Kyritsi 1,
Marla Trindade 2,
Fernando Reyes 3,
Rainer Ebel 1
and
Marcel Jaspars 1
1
Marine Biodiscovery Centre, Department of Chemistry, University of Aberdeen, Aberdeen AB24 3UE, UK
2
Institute for Microbial Biotechnology and Metagenomics (IMBM), Department of Biotechnology, University of the Western Cape, Bellville, Cape Town 7535, South Africa
3
Fundación MEDINA, Centro de Excelencia en Investigación de Medicamentos Innovadores en Andalucía, 18916 Granada, Spain
Natural products play a key role when it comes to the discovery of novel bioactive compounds. With terrestrial resources gradually becoming exhausted efforts have focused on expanding the isolation sources to the marine environment instead [1,2]. The world’s ocean covers 70% of the Earth’s surface and provides habitat to a variety of marine organisms that represent a vast untapped resource of novel bioactive natural products. Within them, Bacillus species has proven to be a prolific source of a distinguished class of cyclic lipopeptides (CLPs), which are known for their broad spectrum of antibacterial activity and low toxicity and can be mainly divided into three groups, surfactins, iturins and fengycins [3]. In the present work, Bacillus strain PE8-15 which was isolated from the sponge, Spongia sp. 001RSASPN sampled from low profile rocky reefs in Algoa bay White sands reef (South Africa) at depths of 23–25 metres, was originally subjected to PCR screening at Prof. Marla Trindade’s laboratory, revealing the presence of a putative NRP-PKS hybrid pathway, assumed to be responsible for the biosynthesis of a new lipopeptide. Fractionation by column chromatography of a crude extract obtained using HP-20 solid phase extraction, followed by HR-LCMS analysis of the produced fractions, indicated the presence of several lipopeptides. Further HPLC fractionation led to the isolation of several of them, among others two with m/z 1031.5146 and 1045.5301, corresponding to the molecular formulae C48H74N6O19, and C49H76N6O19, respectively, which indicates that they could represent bacillomycin D analogues. 2D NMR and LC-MS/MS experiments are currently being performed on them to determine their structures
References
Bernan, V.S.; Greenstein, M.; Maiese, W.M. Marine microorganisms as a source of new natural products. Adv. Appl. Microbiol. 1997, 43, 57–90.
Imhoff, J.F.; Labes, A.; Wiese, J. Bio-mining the microbial treasures of the ocean: New natural products. Biotechnol. Adv. 2011, 29, 468–482.
Xu, B.-H.; Lu, Y.-Q.; Ye, Z.-W.; Zheng, Q.-W.; Wei, T.; Lin, J.-F.; Guo, L.Q. Genomics-guided discovery and structure identification of cyclic lipopeptides from the Bacillus siamensis JFL15. PLoS ONE 2018, 13, e0202893.
Fractions Isolated from Octopus (Paraoctopus vulgaris): Identification and Chemopreventive Studies
Armando Burgos-Hernández 1,
Susana-Gabriela Cruz-Ramírez 1,
Carmen-María López-Saiz 1,
Joel-Said García-Romo 1,
Ema-Carina Rosas-Burgos 1,
Francisco-Javier Cinco-Moroyoqui 1,
Carlos Velázquez 2
and
Javier Hernández 3
1
Departamento de Investigación y Posgrado en Alimentos, Universidad de Sonora, Hermosillo, Sonora 83000, Mexico
2
Departamento de Ciencias Químico-Biológicas, Universidad de Sonora, Hermosillo, Sonora 83000, Mexico
3
Unidad de Servicios de Apoyo en Resolución Analítica, Universidad Veracruzana, Xico, Veracruz 94294, Mexico
The chemopreventive potential of hexanic (HP) and methanolic (MP) phases from a hexane-soluble extract of Paraoctopus vulgaris was studied. The HP was found to be antimutagenic in the Ames assay using Salmonella typhimurium TA98 and TA100 tester strains and aflatoxin B1 as control mutagen. After silica gel-column chromatography, 7 fractions (F1–F7) resulted from HP, finding F6 and F7 to be antimutagenic in both tester strains. Since a high antimutagenic activity (85–97% reversion inhibition) was observed in TA98 strain at 3 μg/mL, F6 and F7 were analyzed using HPLC/photodiode array. A peak at 9.42 min was observed in both fractions suggesting a single compound in common. After 1H-NMR and 13C-NMR analysis, bis (2-ethylhexyl) phthalate (DEHP) was suggested as the potential bioactive compound. Based on the above, a chemopreventive bioassay using ENU-induced mammary tumor Sprague-Dawley female rats was performed using commercially acquired DEHP. Results suggested that DEHP might have a chemopreventive activity in the model used; however, further investigation must be carried out for full bioactivity assessment.
Omics Integration for Bioguided Natural Product Isolation from Serratia proteamaculans
Begrem Simon 1,2,
Passerini Delphine 2,
Delbarre-Ladrat Christine 2
and
Grovel Olivier 1
1
Nantes Université, Faculty of Pharmacy, MMS, 9 Rue Bias, 44035 Nantes, France
2
Ifremer, Rue de l’Île d’Yeu, 44311 Nantes, France
Antimicrobial resistance has become a major health concern over the world, and new antimicrobial drugs with new mechanisms of action are urgently needed. Natural products from marine bacteria are a recognised and still promising source of bioactive compounds. One of the way to reach the discovery of such products consists in studying chemical interactions within complex microbial ecosystems such as the marine microbiome. In this way, following an initial screening of bacterial strains isolated from seafood products, we performed co-cultures of active bacteria against pathogens in order to induce the activation of cryptic biosynthetic gene clusters (BGCs) encoding antimicrobial compounds.
A marine Serratia proteamaculans strain has been selected, which exhibited antimicrobial activity against both bacterial and fungal pathogens, such as Staphylococcus epidermidis, Aerococcus salmonicidae, Aspergillus fumigatus and Candida albicans. In order to detect putatively original bioactive natural products produced by this understudied species, we engaged a strategy combining genome mining and biological assays, together with metabolomics analysis of co-cultures of S. proteamaculans with target bacteria. In silico genome mining of S. proteamaculans allowed the detection of 9 BGCs among which 6 are undescribed, showing the high metabolic potential of the strain in terms of structural novelty. LC-HRMS metabolic profiles of Serratia/Staphylococcus co-cultures were compared to those of individual pure cultures of the two bacteria: metabolomics analyses exhibited an increased production of several metabolites but particularly the de-novo induction of 2 non-dereplicated compounds in co-culture samples. Further purification of these compounds and co-cultivation with other organisms are ongoing.
New Scalarane-Type Sesterterpenoids from Marine Sponge Lendenfeldia sp.
Bo-Rong Peng 1,2,3,
Chang-Yih Duh 4
and
Ping-Jyun Sung 3,5
1
Doctoral Degree Program in Marine Biotechnology, National Sun Yat-Sen University, Lien-Hai Road, Kaohsiung 804, Taiwan
2
Doctoral Degree Program in Marine Biotechnology, Academia Sinica, Academia Road, Taipei 115, Taiwan
3
National Museum of Marine Biology & Aquarium, Pingtung 944, Taiwan
4
Department of Marine Biotechnology and Resources, National Sun Yat-Sen University, Lien-Hai Road, Kaohsiung 804, Taiwan
5
Graduate Institute of Marine Biotechnology, National Dong Hwa University, Pingtung 944, Taiwan
Marine sponge-derived natural products possess many classes of secondary metabolites. For instance, there were 18 identified chemical classes of the isolated new compounds during the last decade, including acid, alkaloid, ester, fatty acid, glycoside, ketone, lipid, macrolide, alcohol, peptide, peroxide, polyketide, quinone, steroid, sterol, terpene, terpenoid and unclassified. Among them, scalarane-type sesterterpenoids emerged as an interesting group of terpenoids isolated from marine sponges and shell-less mollusks. Scalarane sesterterpenoids demonstrated a wide spectrum of interesting biological properties, such as anti-inflammation, cytotoxicity, anti-feedant, anti-microbial, anti-fungal, ichthyotoxicity, anti-tubercular, anti-HIV, anti-fouling, inhibition of platelet-aggregation, inhibition of transactivation for the nuclear hormone receptor (FXR, farnesoid X-activated receptor), and stimulation of nerve growth factor synthesis. In the further study of metabolites from marine sponge
Lendenfeldia sp, four new 24-homoscalarane analogues, lendenfeldarane A–D (
1–
4) (
Figure 24) and eight known scalarane-type sesterterpenoids were isolated. The structures of scalaranes
1–
12 were elucidated on the basis of spectroscopic analysis. Cytotoxicity of scalaranes
1–
12 against the proliferation of a limited panel of tumor cell lines was evaluated.
Ubiquitin-Proteosome Modulating Dolabellanes from Formosan Marine Soft Corals
Chang-Yih Duh 1,
Xue-Hua Ling 1
and
Shang-Kwei Wang 2
1
Department of Marine Biotechnology and Resources, National Sun Yat-Sen University, Kaohsiung 80441, Taiwan
2
Department of Microbiology and Immunology, Kaohsiung Medical University, Kaohsiung 80708, Taiwan
Marine soft corals have evolved unique characteristics in metabolic and physiological capabilities to produce secondary metabolites that may function in defense, food capture, interference competition. Soft coral-derived metabolites exhibit diverse biological activities such as cytotoxicity, inhibition of inflammatory reaction, anti-microbial and anti-viral activities. The ubiquitin-proteasome system (UPS) is a major intracellular, nonlysosomal proteolytic system that is involved in many important cellular pathways, including protein quality control, protein homeostasis, DNA repair, cell cycle progression, pathogen infection, transcriptional regulation, cellular differentiation, and immune modulation. Therapeutic drugs, Bortezomib, Carfilzomib and Ixazomib, target UPS have been licensed in treating multiple myeloma. Moreover, UPS inhibitors are demonstrated to attenuate the progression of neural degenerative disease. Therapeutic prospect of UPS inhibitor is promising and valuable. HCMV UL76 interacts with proteasome regulatory subunits of 26S proteasome. Fluorescence intensities and the phenotypic behaviors of UL76-aggresome are used as markers for proteasome inhibition. We have established a cell-based high-content drug screening assay direct-acting UPS and assessed dolabellanes isolated from Formosan soft corals. Four of them were shown to modulate EGFP-UL76 high-content profile in comparative to proteasome inhibitors MG132 and bortezomib.
Antimicrobial Peptides and Peptidyl Analogues from Marine Microorganisms
Chih-Chuang Liaw 1,2,
Ya-Chu Lien 1
and
Mau-Xuan Hung 1
1
Department of Marine Biotechnology and Resources, National Sun Yat-sen University, Kaohsiung 80424, Taiwan
2
Doctoral Degree Program of Marine Biotechnology, National Sun Yat-sen University, Kaohsiung 80424, Taiwan
The indiscriminate worldwide overuse and misuse of antibiotics has led to high rates of microbial resistance so that the search of novel antimicrobial molecules to overcome the resistance phenomenon is urgently needed to human health. Based on the evolution view, antimicrobial peptides (AMPs) and their analogues are powerful weapons in either unicellular organism, such as bacteria, or multicellular organisms, such as fungi, plants, and animals to serve a protection role for themselves. Therefore, naturally-occurring AMPs and peptidyl analogues from marine microorganims could be considered as suitable templates for the development of alternatives to conventional antibiotics. By the global natural product social molecular networking (GNPS) analysis, we find a series of new AMPs, peptaibols, from the marine-derived fungus, Trichoderma sp. and peptidyl analogues, pesudopeptides, from Vibrio sp. All the structures of these peptide analogues are elucidated by 1D and 2D NMR, MS, and CD spectra. Their antimicrobial activity of these compounds is under investigation.
Approaches for Accessing and Studying the Full Encoded Natural Product Potential of Marine Proteobacteria
Avena C. Ross
Department of Chemistry, Queen’s University, Kingston, ON K7L 3N6, Canada
Bacteria have long been an excellent source of bioactive natural product molecules that find application in therapeutics and drug development. Marine microbes in particular represent a promising and relatively untapped reservoir of natural products, however, there are still several challenges in the field of bacterial natural products discovery. Difficulties associated with cultivation of microbes in the artificial laboratory environment and also challenges gaining access to the full biosynthetic potential of bacteria in the lab remain bottlenecks in the effective and expedient exploitation of natural products as drug leads. We are currently developing ways to not only facilitate growth of marine bacteria but also to expand the range of compound classes and number of molecules they produce during lab cultivation. This presentation will discuss approaches we are taking to prioritize the most fruitful strains to study, cultivation based strategies to facilitate molecule production/discovery and genetic methods for inducing expression of silent biosynthetic gene clusters to allow access to the full scope of encoded metabolites.
Search for Bioactive Compounds from Marine-Derived Fungus Emericellopsis maritima through Osmac Techniques
Cristina Pinedo-Rivilla,
María J. Núñez,
Carlos Millán,
Josefina Aleu,
Rosa Durán
and
Isidro G. Collado
Department of Organic Chemistry, Facultad de Ciencias, Campus Universitario Río San Pedro s/n, Torre sur, 4º planta, Universidad de Cádiz, 11510 Puerto Real, Cádiz, Spain
The wide marine biodiversity and the relative lack of research on its resources have promoted in recent years the search for new compounds with biological activity, especially antibiotics due to the resistance acquired by several microorganisms to those already available in the market.
Marine derived fungi are proving to be an important source of compounds with interesting biologial activities. Furthermore, from the chemical point of view, they can be used as biocatalysts, producers of enzymes and bioremediators.
In this study, the secondary metabolism of the marine-derived fungus Emericellopsis maritima, isolated from sediments samples collected along an intertidal gradient of Bay of Cádiz, was studied as possible source of new compounds with pharmacological activity [1].
A new approach using metabolomic analysis was incorporated to target the isolation of the bioactive compounds. Following the OSMAC (One Strain, Many Compounds) [2] approach, the fungus was grown using different cultivation conditions. The purification of the crude extracts afforded the sesquiterpenes with eremophilane skeleton
1–
8 (
Figure 25), which are known to show important biological activities [3,4].
References
Inostroza, A.; Lara, L.; Paz, C.; Perez, A.; Galleguillos, F.; Hernandez, V.; Becerra, J.; González Rocha, G.; Silva, M. Antibiotic activity of Emerimicin IV isolated from Emericellopsis minima from Talcahuano Bay, Chile. Nat. Prod. Res. 2018, 32, 1361–1364.
Romano, S.; Jackson, S.A.; Patry, S.; Dobson, A.D.W. Extending the “One Strain Many Compounds” (OSMAC) Principle to Marine Microorganism. Mar. Drugs 2018, 16, 244.
Daengrot, C.; Rukachaisirikul, V.; Tansakul, C.; Thongpanchang, T.; Phongpaichit, S.; Bowornwiriyapan, K.; Sakayaroj, J. Eremophilane Sesquiterpenes and Diphenyl Thioethers from the Soil Fungus Penicillium copticola PSU-RSPG138. J. Nat. Prod. 2015, 78, 615–622.
Shi, X.; Zhang, S.; Wang, J.; Li, T.; Liu, J.; Hou, Y. Synthesis and Bioactivity of Natural Occurring Petasin-Like Derivatives as Antitumor Agents. Open J. Med. Chem. 2015, 5, 23–31.
GC- and UHPLC-MS Profiles as a Tool to Valorizate the Red Alga Asparagopsis armata
Marie L. Lesenfants 1,
Ana M. L. Seca 1,2,3,
Artur M. S. Silva 3
and
Diana C. G. A. Pinto 3
1
Faculty of Sciences and Technology, University of Azores, Rua Mãe de Deus, 9501-321 Ponta Delgada, Portugal
2
cE3c-Centre for Ecology, Evolution and Environmental Changes/Azorean Biodiversity Group, Rua Mãe de Deus, 9501-321 Ponta Delgada, Portugal
3
QOPNA & LAQV-REQUIMTE, Department of Chemistry, University of Aveiro, Campus de Santiago, 3810-193 Aveiro, Portugal
Asparagopsis armata is considered a biological invader and this red alga is in the last few years one of the worst nightmares for Azores coast biodiversity. So efforts to find an economically valuable application are welcome. In this context biological evaluations of its extracts, such as anti-aging, antioxidant and anticholinesterasic activities, were recently presented [1,2]. Naturally, the knowledge of this species chemical composition is utmost importance not only to find some valuable utilization but also to discovery its mechanisms of defence that can explain its invasive behaviour. In our effort to contribute to this problem solution we establish the GC-MS and UHPLC-MS profiles of both the non-polar and polar extracts. The main compounds in the lipophilic extract were palmitic acid and 1-monopalmitin and brominated compounds dominate both extracts. The detailed results will be presented and discussed in the presentation.
Acknowledgments
This study was financed by ASPAZOR project (DRCT: ACORES- 01-0145-FEDER-00060- ASPAZOR); Portuguese National Funds, through FCT –Fundação para a Ciência e a Tecnologia, and as applicable co-financed by the FEDER within the PT2020 Partnership Agreement by funding the Organic Chemistry Research Unit (QOPNA) (UID/QUI/00062/2013) and the cE3c centre (FCT Unit funding (Ref. UID/BIA/00329/2013, 2015–2018) and UID/BIA/00329/2019).
References
Lesenfants, M.L.; Rosa, G.P.; Barreto, C.; Seca, A.M.L. Asparagopsis armata dichloromethane extract: Biological activity and chemical composition. In Proceedings of the 1st Seaweed for Health Conference, Galway, Ireland, 24–27 June 2018; p. 13.
Fonseca, A.R.; Ponte, J.; Barreto, C.; Pinto, D.C.G.A.; Seca, A.M.L. Polar extracts of Asparagopsis armata: Anti-aging activity and chemical composition by LC-MS. In Proceedings of the 1st Seaweed for Health Conference, Galway, Ireland, 24–27 June 2018; p. 50.
Polyketides and a Dihydroquinolone Alkaloid from a Marine-Derived Strain of the Fungus Metarhizium marquandii
Dina H. El-Kashef 1,2,
Georgios Daletos 1,
Malte Plenker 3,
Rudolf Hartmann 3,
Attila Mándi 4,
Tibor Kurtán 4,
Horst Weber 5,
Wenhan Lin 6,
Elena Ancheeva 1
and
Peter Proksch 1
1
Institut für Pharmazeutische Biologie und Biotechnologie, Heinrich-Heine-Universität Düsseldorf, Universitätsstrasse 1, 40225 Düsseldorf, Germany
2
Department of Pharmacognosy, Faculty of Pharmacy, Minia University, Minia 61519, Egypt
3
Institute of Complex Systems: Strukturbiochemie, Forschungszentrum Jülich GmbH, ICS-6, 52425 Jülich, Germany
4
Department of Organic Chemistry, University of Debrecen, POB 400, 4002 Debrecen, Hungary
5
Institut für Pharmazeutische und Medizinische Chemie, Heinrich-Heine-Universität Düsseldorf, Universitätsstrasse 1, 40225 Düsseldorf, Germany
6
State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Beijing 100191, China
Three new natural products (1–3), including two butenolide derivatives (1 and 2) and one dihydroquinolone derivative (3), together with nine known natural products were isolated from a marine-derived strain of the fungus Metarhizium marquandii. The structures of the new compounds were unambiguously deduced by spectroscopic means including HRESIMS and 1D/2D NMR spectroscopy, ECD, VCD, OR measurements and calculations. The absolute configuration of marqualide (1) was determined by a combination of modified Mosher’s method with TDDFT-ECD calculations at different levels. The (3R,4R) absolute configuration of aflaquinolone I (3), determined by, O.R.; ECD and VCD calculations, was found to be opposite to the (3S,4S) absolute configuration of the related aflaquinolones A-G. The absolute configuration of the known natural product, terrestric acid hydrate (4), was likewise determined for the first time in this study. TDDFT-ECD calculations allowed determination of the absolute configuration of its chirality center remote from the stereogenic unsaturated γ-lactone chromophore.
Acknowledgments
D.H.E.-K. gratefully acknowledges the Egyptian Government (Ministry of Higher Education) for awarding the doctoral scholarship. Financial support by the Manchot Foundation to P.P. is gratefully acknowledged.
Diketopiperazines, Tetralones and Two Novel Valerolactam Derivatives from Endophytic Fungi Associated to the Marine Red Alga Asparagopsis taxiformis
Dulce Silva 1,
Iatã Mendonça 1,
Victor Rufino 1,
Alana Honório 1,
Rebeca Medina 1
and
Nair Yokoya 2
1
Institute of Chemistry, São Paulo State University—UNESP, Araraquara 14800-060, Brazil
2
Institute of Botany, SMA, Sao Paulo 0401-902, Brazil
Marine organisms from Brazilian coast and their endophytes have received growing attention during the past few decades. Our recent work on endophytic fungi from marine algae has confirmed their potential as interesting sources of bioactive compounds as well as their marked chemodiversity. Two fungal strains, Hypoxylon investiens and Humicola fuscoatra, were isolated from the marine red alga Asparagopsis taxiformis, collected at Fortaleza beach, in Ubatuba (SP, Brazil). After surface sterilization, fragments of the alga were spread on PDA or Czapek medium plates, and seawater or ultrapure water was used for fungal strains cultivation.
Subsequent micelia separation and partition of each extract, led to aqueous (AqFr), acetonitrile (MeCNFr) and hexane (HexFr) fractions, which were then assayed and showed moderate cytotoxic and anticholinesterase bioactivities. Fractionation of the Humicola fuscoatra extract over C-18 silica-gel CC, gradient mode, yielded 7 fractions, which were purified by HPLC-DAD-UV and afforded two isocoumarins, the diketopiperazine cycle (Phe-Pro), in addition to two novel valerolactam derivatives, with moderate cytotoxic activity. HRESIMS and detailed NMR spectral analyses, including 1H-15N HMBC experiments, indicated its molecular formulae, C12H17NO4, consistent with a novel dienone-valerolactam derivative. Hypoxylon investiens extract fractionation over Sephadex LH-20 and purification by HPLC afforded 5-methylmelein, nodulisporone, isosclerone, two diketopiperazines, in addition to a novel phenolic benzofuran derivative. Their structures were established from UV and detailed NMR spectral analyses, and confirmed from HRESI-TOF-MS data. Such results highlight the outstanding chemodiversity of marine-derived fungi, which may contribute for bioprospection efforts and sustainable exploration of Brazilian marine biodiversity.
Bioactive Bromotyrosine Metabolites from the Marine Sponge Aplysinella rhax
Emmanuel Oluwabusola 1,
Jioji Tabudravu 1,
Klaus Feussner 2,
Freddie Annang 3,
Fernando Reyes 3,
Guiomar Pérez-Moreno 4,
Luis Ruiz-Pérez 4,
Dolores González-Pacanowska 4,
Rainer Ebel 1
and
Marcel Jaspars 1
1
Marine Biodiscovery Centre, Department of Chemistry, University of Aberdeen, Aberdeen AB24 3UE, Scotland, UK
2
Institute of Applied Sciences, Faculty of Science, Technology and Environment, University of the South Pacific, Suva, Fiji
3
Fundación MEDINA, Parque Tecnológico de Ciencias de la Salud, Avenida del Conocimiento, 34.18016-Armilla, 18016 Granada, Spain
4
Instituto de Parasitología y Biomedicina “López-Neyra”, Consejo Superior de Investigaciones Científicas, Parque Tecnológico de Ciencias de la Salud, Avenida del Conocimiento, s/n, 18016-Armilla, 18016 Granada, Spain
Marine sponges are highly diverse and capable of biosynthesizing a greater variety of natural bioactive products than other invertebrate phyla. Those belonging to the family Verongida are known producers of brominated secondary metabolites. The methanolic extract of the Fijian marine sponge
Aplysinella rhax collection was fractionated and purified by reversed phase HPLC to yield 9 bromotyrosine derivatives (
Figure 26) including two that have not been described in the literature before: psammaplin O (
5) and 3-bromo-2-hydroxyl-5-(methoxycarbonyl)benzoic acid (
6), alongside psammaplins A-D (
1–4), bisaprasin (
9), (3-bromo-4-hydroxyphenyl)-acetonitrile (
7) and bromohydroxybenzoates (
8). The structures of the new compounds were elucidated by HRMS, and 1D and 2D NMR spectroscopic analysis. The two new compounds exhibited no significant activity against
Trypanosoma cruzi Tulahuen C4 parasites and
Plasmodium falciparum 3D7. Psammaplin A and D showed moderate activity against both
T. cruzi Tulahuen C4 and
P. falciparum 3D7 with IC
50 values of 30–43 μM and 60–67 μM respectively. Additionally, compounds
3 and
8 displayed weak activity against
P. falciparum 3D7 with IC
50 values of 131 and 115 μM respectively.
Secondary Metabolites from Vietnamees Strain of Marine-Derived Fungus Aspergillus flocculosus
Elena V. Ivanets 1,
Olga F. Smetanina 1,
Ekaterina S. Menchinskaya 1,
Trinh Phan 2,3
and
Anton N. Yurchenko 1
1
G.B. Elyakov Pacific Institute of Bioorganic Chemistry Far Eastern Branch of Russian Academy of Sciences, 690022 Vladivostok, Russia
2
Nhatrang Institute of Technology Research and Application, Vietnam Academy of Science and Technology, Nha Trang 650000, Vietnam
3
Graduate University of Science and Technology, Vietnam Academy of Science and Technology, Ha Noi 150000, Vietnam
The marine fungi are promising sources of bioactive compounds [1]. Marine habitat affects the biosynthetic processes in the organisms inhabited it, therefore, marine fungi can produce compounds unique in structure. In addition, the coast of Vietnam is an insufficiently studied area of South China Sea, since most researches are focus on the coast of China and Taiwan Strait.
In this research work the metabolite composition of marine-derived fungus
Aspergillus flocculosus (sediments, Nha Trang Bay, South China Sea) was investigated. As a result, a new merpterpenoid 12-
epi-aspertetranone D (
1) (
Figure 27) together with its known epimer aspertetranone D (
2) [2], two new sesquiterpenoids (
3–
4) and their known
p-nitrobenzoate derivatives (
5–
6) [3,4], a new cyclic tetrapeptide (
7), a new tetraketide aspilactonol G (
8) and two its known isomers aspilactonol F (
9) and dihydroaspyrone [5], as well as a known diketopiperazine mactanamide [6] were isolated. The structures of isolated compounds were established by combination of 1D and 2D
1H and
13C NMR and HRESIMS data.
Compound 5 showed cytotoxic activity against mice neuroblastoma Neuro2A cells and human breast cancer cells MCF-7 with IC50 4.9 and 59.6 μM, respectively, while compound 6 was inactive. Also compounds 3, 5 and 7 showed cytotoxic activity against human prostate cancer cells 22Rv1 with IC50 35.3, 3.0 and 38.7 μM, respectively.
Acknowledgments
The study was supported by Russian Foundation for Basic Research (Grants No 18-34-00621 for chemical study and 18-34-00737 for bioassays).
References
Wang, K.W.; Ding, P. New Bioactive Metabolites from the Marine-derived Fungi Aspergillus. Mini-Rev. Med. Chem. 2018, 18, 1072–1094.
Wang, J.; Wei, X.; Qin, X.; Tian, X.; Liao, L.; Li, K.; Zhou, X.; Yang, X.; Wang, F.; Zhang, T.; et al. Antiviral Merosesquiterpenoids Produced by the Antarctic Fungus Aspergillus ochraceopetaliformis SCSIO 05702. J. Nat. Prod. 2016, 79, 59–65.
Belofsky, G.N.; Jensen, P.R.; Renner, M.K.; Fenical, W. New cytotoxic sesquiterpenoid nitrobenzoyl esters from a marine isolate of the fungus Aspergillus versicolor. Tetrahedron 1998, 54, 1715–1724.
Fang, W.; Lin, X.; Zhou, X.; Wan, J.; Lu, X.; Yang, B.; Ai, W.; Lin, J.; Zhang, T.; Tu, Z.; et al. Cytotoxic and antiviral nitrobenzoyl sesquiterpenoids from the marine-derived fungus Aspergillus ochraceus Jcma1F17. MedChemComm 2014, 5, 701–705.
Fuchser, J.; Zeeck, A. Secondary metabolites by chemical screening, 34: Aspinolides and aspinonene/aspyrone Co-metabolites, new pentaketides produced by Aspergillus ochraceus. Liebigs Ann. 1997, 1, 87–95.
Lorenz, P.; Jensen, P.R.; Fenical, W. Mactanamide, a new fungistatic diketopiperazine produced by a marine Aspergillus sp. Nat. Prod. Lett. 1998, 12, 55-60.
Meroditerpenoids Isolated from the North-Eastern Atlantic Macroalga, Halidrys siliquosa
Elliot Murphy 1,
Kevin Calabro 1,
Perrine Lasserre 1,
Marianela Zanolla 2,
Dagmar B. Stengel 2
and
Olivier P. Thomas 1
1
Marine Biodiscovery, School of Chemistry and Ryan Institute, National University of Ireland Galway University Road, H91TK33 Galway, Ireland
2
Botany and Plant Science, School of Natural Sciences and Ryan Institute, National University of Ireland Galway University Road, H91TK33 Galway, Ireland
Macroalgae have evolved to produce various defence mechanisms including a chemical defence in the form of small metabolites. Halidrys siliquosa, a species of brown algae native to the Atlantic coasts across western Europe, has been shown to contain secondary metabolites exhibiting biological activities in combating various biofilms [1].
The aim of our study was to isolate and characterise the structure of the chemical diversity produced by
H. siliquosa using a set of analytical techniques. The chemical structures of an outstanding diversity of meroterpenoids present have been first elucidated with 1D and 2D NMR spectroscopic analysis (
Figure 28). The question of the absolute configuration was addressed with circular dichroism. A molecular network built on GNPS has also been included in our study to better describe the algae’s metabolome.
References
Culioli, G.; Ortalo-Magné, A.; Valls, R.; Hellio, C.; Clare, A.S.; Piovetti, L. Antifouling activity of meroditerpenoids from the marine brown alga Halidrys siliquosa. J. Nat. Prod. 2008, 71, 1121–1126.
Search for Marine Natural Products from the Mixtures of Marine Invertebrates in the Twilight Zone
Fumiaki Nakamura 1
and
Yoichi Nakao 1,2
1
Graduate School of Advanced Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku-ku, Tokyo 169-8555, Japan
2
Research Institute for Science and Engineering, Waseda University, 3-4-1 Okubo, Shinjuku-ku, Tokyo 169-8555, Japan
The twilight zone, also known as the mesopelagic zone between a depth of 100–1000 m, where sunlight hardly reaches [1]. The extreme environments different from the terrestrial were changed the metabolism in the inhabiting organisms. As the result, secondary metabolites from the twilight zone retain unique structures and bioactivities [2]. However, most of these precious samples are obtained as the crushed and fragmented small pieces that were difficult to be classified because of the collecting way by dredging. Therefore, we focused on the unclassified small fragment mixtures from the twilight zone and searched for new natural products from the extract of the mixture [3]. The fragment mixture (24.6 kg, wet wt.) collected by dredging at Yaku-shinsone (166–167 m), Kagoshima prefecture, Japan, was extracted and tested for the cytotoxicity using the MTT assay. Bioassay guided fractionation of the extracts identified several bioactive substances. On the other sides, metabolome analysis of the extracts from 99 classified dredging samples was performed using LC-MS that lead to the identification of the source organisms of the isolated substances.
References
Lee, C. Transformations in the “Twilight Zone” and beyond. Mar. Chem. 2004, 92, 87–90.
Skropeta, D. Deep-sea natural products. Nat. Prod. Rep. 2008, 25, 1131–1166.
Machida, K.; Abe, T.; Arai, D.; Okamoto, M.; Shimizu, I.; de Voogd, N.J.; Fusetani, N.; Nakao, Y. Cinanthrenol A, an estrogenic steroid containing phenanthrene nucleus, from a marine sponge Cinachyrella sp. Org. Lett. 2014, 16, 1539–1541.
Breakthrough Innovative Technologies in Marine Natural Products Investigation
Géraldine Le Goff 1,
P. Vlachou 2,
C. Alonso 3,
P. A. Álvarez 3,
J.-F. Gallard 1,
N. Fokialakis 2,
D. Felezeu 4,
A. Touron 4,
C. Allegretbordon 4
and
J. Ouazanni 1
1
Institut de Chimie des Substances Naturelles ICSN, Centre National de la Recherche Scientifique, Avenue de la Terrasse, 91198 Gif-sur-Yvette, France
2
Department of Pharmacognosy & Natural Products Chemistry, Faculty of Pharmacy, National and Kapodistrian University of Athens, 15771 Athens, Greece
3
iMARE Natural S.L. Avda. Habana, 10, 18600 Motril, Spain
4
Pierre Guerin Technologies, 79000 Niort, France
TASCMAR is an H2020 EU funded research project that investigates the chemical potential of the ocean’s mesophotic zone. The goal is to develop sustainable methods for discovering chemical compounds that can be used for application in diverse fields such as health/nutrition, depollution and nature-based cosmetics. In the context of TASCMAR, two breakthrough innovations were recently patented. These technologies are dedicated to the optimal production of microbial metabolites and the trapping of invertebrate metabolites in their natural habitat: UNIFERTEX (UNIversal FERmenTor EXpert) [1] offers a unique pilot-scale fermentor to carry out solid and liquid state fermentations in parallel, in a highly controlled environment with dedicated remote supervision and control software. SOMARTEX (Self Operating MARine Trapping EXtractor) [2] is the first technology to trap invertebrate/holobionts molecules in their natural habitat at any location and depth, without harvesting the organism or impacting their surrounding ecosystem. The proof of concept was established in-aquarium with XAD-amberlite solid-phase extraction. We reported the elicitation of guanidine alkaloids production of Crambe crambe in the presence of Anemonia sulcata, both collected from the Mediterranean Sea. Besides the previously reported crambescidin 359, and crambescidin acid, three new compounds were isolated; one carboxylated analog named crambescidin 401, and two analogs of crambescin B, crambescin B 281 and crambescin B 253 [3].
References
Ouazzani, J.; Le Goff, G.; Felezeu, D.; Touron, A.; Allegret-Bourdon, C. Unifertex, Universal Fermentor Expert, Device for Microbial Cultivation. CNRS/Pierre Guerin Technologies. PCT/EP2018/086882. 2018. Available online:
https://youtu.be/sfXKRaAl-HU (accessed on May 27 2019).
Ouazzani, J.; Le Goff, G. Somartex, Self Operating MARine Trapping Extractor. CNRS, PCT/EP2019/051912. 2019. Available online:
https://youtu.be/1Tmh8dFqZ34 (accessed on May 27 2019).
Vlachou, P.; Le Goff, G.; Alonso, C.; Álvarez, P.; Gallard, J.F.; Fokialakis, N.; Ouazzani, J. Innovative Approach to Sustainable Marine Invertebrate Chemistry and a Scale-Up Technology for Open Marine Ecosystems. Mar. Drugs 2018, 16, 152.
New Ophiobolins, Cytotoxic Sesterterpenes from the Marine Fungus Aspergillus flocculosus
Hee Jae Shin 1,2,
Byeoung-kyu Choi 1,2
and
Jong Soon Kang 3
1
Department of Marine Biotechnology, University of Science and Technology (UST), 217 Gajungro, Yuseong-gu, Daejeon 34113, Korea
2
Marine Natural Products Chemistry Laboratory, Korea Institute of Ocean Science and Technology, 385 Haeyang-ro, Yeongdo-gu, Busan 49111, Korea
3
Laboratory Animal Resource Center, Korea Research Institute of Bioscience and Biotechnology, 30 Yeongudanjiro, Cheongju 28116, Korea
Five new sesterterpenes (1–5) together with four known ophiobolins (6–9) were isolated from the marine fungus Aspergillus flocculosus and their structures were elucidated by extensive NMR and MS data analysis and the comparison of chemical shift and optical rotation data with those reported in the literature. Among these ophiobolins, five new ophiobolins were first isolated as the ophiobolin derivatives consisting of three double bonds in the side chain and Z-conformation at C-14/C-15. Isolated ophiobolins were evaluated for cytotoxic activity against six cancer cell lines, HCT-15, NUGC-3, NCI-H23, ACHN, PC-3 and MDA-MB-231. All the compounds showed potent cytotoxicity with GI50 values ranging from 0.14~2.01 μM against all the cell lines.
Cadiolide J–M, Antibacterial Polyphenyl Butenolides from the Korean Tunicate Pseudodistoma antinboja
Weihong Wang 1,
Hiyoung Kim 2,
Rahul S. Patil 1,
Awadut G. Giri 1,
Dong Hwan Won 1,
Dongyup Hahn 1,
Youjung Sung 1,
Jusung Lee 1,
Hyukjae Choi 1,
Sang-Jip Nam 1
and
Heonjoong Kang 1
1
Center for Marine Natural Products and Drug Discovery, School of Earth and Environmental Sciences, Seoul National University, Seoul 08826, Korea
2
Department of Biomedical Science & Engineering, Konkuk University, 120 Neungdong ro, Gwangjin gu, Seoul 05029, Korea
The tunicates (ascidians) of the genus
Pseudodistoma (family
Pseudodistomidae) comprise more than 20 species and are widely distributed in tropical as well as in temperate waters. Although previous investigations of the chemical constituents and bioactivity of tunicates of this genus have led to the discovery of a diverse array of nitrogenous secondary metabolites encompassing piperidine, alkyl amine, amino alcohol, β-carboline, and quinoline alkaloids, fewer examples of metabolites with interesting biological activities have been reported. Recently a chemical investigation conducted by our research group identified a series of antibacterial non-nitrogenous metabolites from the Korean tunicate
Pseudodistoma antinboja. Activity-guided fractionations of the tunicate
Pseudodistoma antinboja yielded four new compounds of the cadiolide class (cadiolides J–M,
1,
3–
5) along with a known one (cadiolide H,
2) (
Figure 29). The structures were defined by spectroscopic methods including X-ray crystallographic analysis. These compounds were evaluated for their antibacterial activity and exhibited potent antibacterial activity against all of the drug resistant strains tested with MICs comparable to those of marketed drugs such as vancomycin and linezolid.
Gas Chromatography and Mass Spectrometry Analyses of Volatiles from Marine Algae
I. Jerković 1,
Z. Marijanović 1,
M. Kranjac 1,
S. Jokić 2
and
M. Roje 3
1
Faculty of Chemistry and Technology, University of Split, R. Boškovića 35, Split 21000, Croatia
2
Josip Juraj Strossmayer University of Osijek, Faculty of Food Technology Osijek, Franje Kuhača 20, Osijek 31000, Croatia
3
Institure Rudjer Boskovic, Bijenicka Cesta 54, Zagreb 10000, Croatia
Marine secondary metabolites possess outstanding structural and functional diversity related to their different metabolic pathways. While the volatile organic compounds (VOCs) of terrestrial plants have attracted attention for a long time, the VOCs of marine algae have been much less investigated. The most common VOCs released by terrestrial plants are: “green leaf volatiles” (GLVs) which consist almost exclusively of C6 aldehydes and alcohols, other aliphatic compounds, monoterpenes, sesquiterpenes, phenylpropane derivatives, others. This is in remarkable contrast to marine VOCs that show much higher structural diversity: e.g., only among marine sesquiterpenes 54 different skeletal types of tricyclic sesquiterpenes were found. The best method for the VOCs analysis is gas chromatography coupled to mass spectrometry (GC-MS). However, before the analysis adequate preparative methods should be applied to marine algae samples such as headspace solid-phase microextraction (HS-SPME) or hydrodistillation (HD) that can be applied on the fresh or dried samples. Different examples of marine algae VOCs chemical profiles will be presented containing terpene compounds (mostly sesquiterpenes), sulphur containing compounds, non-isoprenoid C11-compounds, others. VOCs of marine organisms can have multiple functions in ecosystem in intraspecific (pheromones) and interspecific (kairomones) communication and in activated defences. In addition, these compounds (usually present in the extracts) may exhibit other biological activities, e.g., antibacterial, cytotoxic and antitumor.
Bromophenolics from the Southern Australian Marine Alga Polysiphonia decipiens
James Lever,
Robert Brkljača
and
Sylvia Urban
Marine and Terrestrial Natural Product Research Group, School of Science (Applied Chemistry & Environmental Science), RMIT University, GPO Box 2476, Melbourne, Vic 3001, Australia
Marine red algae of the family Rhodomelaceae are known to produce bromophenolic type secondary metabolites that display antifeedant, antioxidant and anti-inflammatory properties [1–3]. Reported herein is the extraction, isolation and characterisation of four previously reported bromophenolic compounds (
1–
4) (
Figure 30), from the red alga
Polysiphonia decipiens, all of which displayed no appreciable antibacterial properties. Also reported is the isolation, characterisation and derivatisation of the known natural product rhodomelol (
5) which was previously isolated from other members of the family Rhodomelaceae
Polysiphonia lanosa and
Osmundaria colensoi. Detailed here is the confirmation of the relative configuration of rhodomelol (
5) which has been achieved for the first time. This involved chemical derivatisation followed by conducting single irradiation NOE NMR spectroscopy experiments. Finally, we were also able to secure the structure of an unprecedented structural derivative of rhodomelol via extensive 1D and 2D NMR spectroscopy. This work represents the first study of the phytochemical constituents of
P. decipiens.
References
Kurata, K.K.; Taniguchii, K.; Takashima, K.; Hayashi, I.; Suzuki, M. Feeding deterrent bromophenols from odonthalia corymbifera. Phytochemistry 1997, 45, 485–487.
Li, K.; Li, X.M.; Ji, N.Y.; Wang, B.G. Bromophenols from the Marine Red Alga Polysiphonia urceolata with DPPH Radiacal Scavenging Activity. J. Nat. Prod. 2008, 71, 28–30.
Choi, Y.K.; Ye, B.-R.; kim, E.-A.; Kim, J.; Kim, M.-S.; Lee, W.W.; Ahn, G.-N.; Kang, N.; Jung, W.-K.; Heo, S.-J. Bis (3-bromo-4,5-dihydroxybenzyl) ether, a novel bromophenol from the marine red alga Polysiphonia morrowii that suppresses LPS-induced inflammatory response by inhibiting ROS-mediated ERK signaling pathway in RAW 264.7 macrophages. Biomed. Pharmacother. 2018, 103, 1170–1177.
Discovery of Two New Sorbicillinoids by Overexpression of the Global Regulator Laea in Penicillium dipodomyis YJ-11
Jing Yu 1,
Xianyan Zhang 1,
Chuanteng Ma 1,
Chunxiao Sun 1,
Qian Che 1,
Qianqun Gu 1,
Tianjiao Zhu 1,2,
Guojian Zhang 1,2
and
Dehai Li 1,2
1
Key Laboratory of Marine Drugs, Chinese Ministry of Education, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, China
2
Laboratory for Marine Drugs and Bioproducts of Qingdao Pilot National Laboratory for Marine Science and Technology, Qingdao 266237, China
For decades, various secondary metabolites produced by filamentous fungi continued to be heard since they are important sources of bioactive natural products. As the sequencing technology developed, a great many biosynthetic genes were found not expressed under the ordinary laboratorial conditions, which were also referred to as “silent” or “cryptic” genes. To up-regulate these gene clusters in order to mine new compounds, a variety of approaches have been developed to affect the biosynthetic process. As a part of our ongoing work of searching diversified secondary metabolites from marine derived fungi, we recently isolated one filamentous fungi Penicillium dipodomyis YJ-11 from a marine sediment sample collected in Jiaozhou Bay of Qingdao. In order to activate the silent genes and obtain novel secondary metabolites, we overexpressed the native global regulator PdLaeA in P. dipodomyis YJ-11 and the mutant showed obvious changes both in morphologies and metabolic products in contrast to the wild type strain. Further chemical studies on mutant strain led to the isolation of two new compounds together with four known sorbicillin analogues. This is the first report on application of global regulator LaeA in P. dipodomyis with the purpose to tap up the silent genes and the result supported the truth that overexpression of the global regulator LaeA could be a feasible tool in activating silent biosynthetic gene clusters and producing new natural product structures.
Nucleosides from the Pacific Bryozoan Nelliella nelliiformis
Joe Bracegirdle 1,2,
Dennis P Gordon 3,
Joanne E. Harvey 1,2
and
Robert A. Keyzers 1,2
1
School of Chemical and Physical Sciences, Victoria University of Wellington, 6012 Wellington, New Zealand
2
Centre for Biodiscovery, Victoria University of Wellington, 6012 Wellington, New Zealand
3
National Institute of Water & Atmospheric Research (NIWA), P.O. Box 893, Nelson, New Zealand
Marine organisms are a valuable source of bioactive natural products. Bryozoans, or sea mosses, are an understudied class of marine invertebrate, yet still have been the source of a variety of medicinally relevant compounds, such as the well-known bryostatins. Here, the secondary metabolites of the Pacific bryozoan Nelliella nelliiformis were investigated using an NMR-guided isolation procedure, leading to the purification of two new nucleosides termed nelliellosides A and B. The structures, including absolute configurations, were solved by spectroscopic and chromatographic techniques. Their total synthesis, along with a panel of four similar analogues, was also achieved, and a biological evaluation is reported herein.
Discovery of New Anti-Inflammatory Cembranoids from a Formosan Soft Coral Sarcophyton cherbonnieri
Chia-Chi Peng 1,
Chiung-Yao Huang 1,
Atallah F. Ahmed 2,
Tsong-Long Hwang 3
and
Jyh-Horng Sheu 1,4
1
Department of Marine Biotechnology and Resources, National Sun Yat-sen University, Kaohsiung 804, Taiwan
2
Department of Pharmacognosy, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia
3
Graduate Institute of Natural Products, College of Medicine, Chang Gung University, Taoyuan 333, Taiwan
4
Frontier Center for Ocean Science and Technology, National Sun Yat-sen University, Kaohsiung 804, Taiwan
We have previously discovered seven cembranoids including an anti-inflammatory biscembrane peroxide from Formosan soft coral
Sarcophyton cherbonnieri [1]. In order to detailedly investigate the secondary metabolites for understanding the biosynthetic capability and potential bioactivity of natural products from
S. cherbonnieri, we continued our chemical investigation of this marine organism. This study led to the isolation of seven natural compounds, cherbonolides F–L (
1–
7) (
Figure 31). The structures and relative configurations of these metabolites had been established by detailed spectroscopic analyses including 1D and 2D NMR (COSY, HSQC, HMBC and NOESY) data associated with MS experiments. The anti-inflammatory activities of compounds (
1–
7) on neutrophil pro-inflammatory responses were assayed by measuring their ability in suppressing fMLF/CB-induced superoxide anion generation and elastase release in human neutrophils. From the results, cherbonolide G (
2) significantly exhibited elastase release (48.2 ± 6.2%) and cherbonolide H (
3) showed stronger inhibition (44.5 ± 4.6%) toward superoxide anion generation at 30 µM, than other metabolites.
References
Peng, C.-C.; Huang, C.-Y.; Ahmed, A.F.; Hwang, T.-L.; Dai, C.-F.; Sheu, J.-H. New Cembranoids and a Biscembranoid Peroxide from the Soft Coral Sarcophyton cherbonnieri. Mar. Drugs 2018, 16, 276.
Retrospective Metabolomics Using GNPS Molecular Networking for the Identification and Isolation of Bioactive Secondary Metabolites from Extreme Environment Microorganisms
Kevin Jace A. Miranda 1,
Ahmed A. Hamed 2,
Rainer Ebel 1
and
Marcel Jaspars 1
1
Marine Biodiscovery Centre, Department of Chemistry, University of Aberdeen, Meston Building, Meston Walk, Aberdeen AB24 3UE, Scotland, UK
2
Microbial Chemistry Department, National Research Centre, 33 El-Buhouth Street, P.O. Box 12622, Dokki, Giza, Egypt
The evolution of natural products research and drug discovery from bioassay guided isolation to genomic and statistical approaches like metabolomics has paved the way to the isolation and characterization of new and novel compounds. One such statistical approach that has gained increasing attention is Global Natural Product Social Molecular Networking (GNPS) molecular networking, relying on MS/MS fragmentation to evaluate similarities and differences in complex data sets. In this study, we employed what we term retrospective metabolomics to evaluate the secondary metabolites produced by microorganisms living in two different extreme environments. We analyzed the isolated compounds from two strains of bacteria,
Halomonas sulfadaestris and
Pseudomonas sp. from the South Shetland Trench, Antarctica and two strains of fungi,
Aspergillus sp. M113 and
Penicillium sp. 7S9 from Hurghada, Egypt. High resolution electrospray ionisation mass spectrometry data from each sample was enterered into GNPS, along with its internal database, to construct molecular networks evaluating known and unknown compounds. Prior to molecular networking, we isolated a cyclic depsipeptides such as Emericellamide A from the fungal sample and bacillomycin from the bacterial sample. Emericellamide A showed a distinct cluster in the molecular network that suggests several new analogs are present based on dereplication and MS/MS fragmentation patterns. These clusters identified from the molecular network will serve as a guide for further fractionation, isolation and structure elucidation. (
Figure 32)
Isolation and Antimycobacterial Activity of New Secondary Metabolites from a South African Marine Streptomyces Species MUIZ4Y
Kojo S. Acquah 1,
Denzil Beukes 2,
Digby F. Warner 3,4,5,
Paul R. Meyers 6,
Suthananda N. Sunassee 1,
Rainer Ebel 7,
Hai Deng 7,
Marcel Jaspars 7
and
David W. Gammon 1
1
Department of Chemistry, University of Cape Town, Rondebosch 7701, South Africa
2
School of Pharmacy, University of the Western Cape, Bellville 7535, South Africa
3
SAMRC/NHLS/UCT Molecular Mycobacteriology Research Unit & DST/NRF Centre of Excellence for Biomedical TB Research, Department of Pathology, Faculty of Health Sciences, University of Cape Town, Observatory 7925, South Africa
4
Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Observatory 7925, South Africa
5
Wellcome Centre for Clinical Infectious Diseases Research in Africa, University of Cape Town, Rondebosch 7701, South Africa
6
Department of Molecular and Cell Biology, University of Cape Town, Rondebosch 7701, South Africa
7
Marine Biodiscovery Centre, Department of Chemistry, University of Aberdeen, Old Aberdeen AB24 3UE, Scotland, UK
Tuberculosis (TB) is the leading cause globally of mortality owing to an infectious disease. The rapid emergence of multidrug-resistant strains of the causative agent, Mycobacterium tuberculosis, coupled with the HIV-AIDS co-pandemic, necessitates the urgent discovery of potent anti-TB drugs with novel mechanisms of action. Natural products have historically proved a major source of drugs with microorganisms including actinobacteria serving as prolific producers of bioactive molecules. Here, we report the isolation of a
Streptomyces strain Muiz4Y, from marine sediment collected along the False Bay coastline between Muizenberg and St. James in Cape Town, South Africa. The organic extract of a liquid culture of this strain exhibited antimycobacterial activity against
Mycobacterium aurum A+ and
Mycobacterium tuberculosis H37Rv. An activity guided isolation scheme including sequence of column chromatography, preparative TLC and reverse phase HPLC resulted in the isolation of four compounds comprising a β-carboline alkaloid (
1), peptide (
2) (
Figure 33), diketopiperazine (
3) and triphenyl hexane (
4). Although
3 and
4 are known compounds
1 and
2 are new natural products. Only
4 exhibited antimycobacterial activity against
Mycobacterium tuberculosis H37Rv with an MIC of 5.764 µg/mL.
Exploring Marine Micromonospora Species as Phocoenamicins Producers
Maria Kokkini,
Mercedes Perez-Bonilla,
Daniel Oves-Costales,
Mercedes de la Cruz,
Jesús Martin,
Francisca Vicente,
Olga Genilloud
and
Fernando Reyes
Fundación MEDINA, Avda. del Conocimiento 34, Parque Tecnológico de Ciencias de la Salud, 18016 Granada, Spain
Marine actinomycetes are considered as a drug treasure house with respect to great potential regarding their secondary metabolites. Within the “rare actinomycetes” group, Micromonospora is the most prolific in producing metabolites, accounting for more than 700 compounds to date, including pharmaceutically important chemical classes [1]. Spirotetronates are a growing family of actinomyces-derived polyketides that possess complex structures and exhibit potent and unexplored bioactivities [2]. As part of the PharmaSea and MarPipe projects, phocoenamicins B and C, together with the known spirotetronate polyketide phocoenamicin, were isolated from cultures of Micromonospora sp. and demonstrated antimicrobial activity against methicillin-resistant Staphylococcus aureus (MRSA) and Mycobacterium tuberculosis H37Ra [3]. In this work, we cultivated Micromonospora sp. using an OSMAC approach, selected the most promising fermentation media to scale up their production and detected that EtOAc extracts produce several structurally related compounds not disclosed before, depending on the culture medium. Herein we report the diverse production, isolation and structural elucidation of new additional phocoenamicins isolated from culture broths of this actinomycete. Three strains belonging to the Micromonospora genus (CA-214671, CA-218877 and CA-214658), isolated from marine invertebrate and sediments collected in the Canary Islands were grown on carefully selected fermentation media (FR23-M, RAM2-P V2 and M-016). The Liquid-Liquid extraction of culture broths with EtOAc afforded organic crude extracts which were subjected to reversed-phase preparative HPLC and yielded several new minor analogs of the previously mentioned spirotetronates. Structural elucidation of the compounds was based on 1D and 2D NMR and High Resolution Mass Spectrometry (ESI-TOF).
References
Igarashi, Y.; Ogura, H.; Furihata, K.; Oku, N.; Indananda, C.; Thamchaipenet, A. Maklamicin, an antibacterial polyketide from an endophytic Micromonospora sp. J. Nat. Prod. 2011, 74, 670–674.
Braddock, A.A.; Theodorakis, E.A. Marine Spirotetronates: Biosynthetic Edifices That Inspire Drug Discovery. Mar. Drugs 2019, 17, 232.
Pérez-Bonilla, M.; Oves-Costales, D.; de la Cruz, M.; Kokkini, M.; Martín, J.; Vicente, F.; Genilloud, O.; Reyes, F. Phocoenamicins B and, C.; New Antibacterial Spirotetronates Isolated from a Marine Micromonospora sp. Mar. Drugs 2018, 16, 95.
Isolation and Structure Elucidation of Ficiformylenes Isolated from the Italian Marine Sponges of Petrosia ficiformis
Yasumoto Oyadomari 1,
Koshi Machida 2,
Leontine E. Becking 3,4,
Giorgio Bavestrello 5,
Maria Valeria D’Auria 6,
Angela Zampella 7,
Nobuhiro Fusetani 8
and
Yoichi Nakao 1,2
1
Graduate School of Advanced Science and Engineering, Waseda University, Shinjuku-ku, Tokyo 169-8555, Japan
2
Research Institute for Science and Engineering, Waseda University, Shinjuku-ku, Tokyo 169-8555, Japan
3
Wageningen Marine Research, Wageningen University & Research Centre, 1781 AG Den Helder, The Netherlands
4
Marine Animal Ecology, Wageningen University & Research Centre, Wageningen, The Netherlands
5
DiSTAV (Department of Earth, Environmental and Life Sciences), University of Genoa, Corso Europa 26, 16132 Genova, Italy
6
Department of Pharmacy, University of Naples “Federico II”, 1-80131 Naples, Italy
7
Department of Pharmacy, University of Naples “Federico II”, Via D. Montesano 49, 80131 Naples, Italy
8
Fisheries and Oceans Hakodate, Hakodate, Hokkaido 041-8611, Japan
Marine sponges are a rich source of secondary metabolites containing unique structures and bioactivities. Sponges are very fertile host animals for diverse symbiotic microorganisms which are believed to produce most of the sponge secondary metabolites. However, there are only a limited number of studies on the difference in sponge metabolites from the different habitats. The marine sponges of the genus Petrosia have been reported from various parts in the world and are known to contain a variety of polyacetylene compounds. During our study to compare sponge secondary metabolites of the same species, we found that the extracts of three marine sponge specimens of Petrosia ficiformis collected at the different sites in Italy exhibited different compositions of the secondary metabolites. As the result of the LC/MS-guided fractionation of the extracts prepared from P. ficiformis collected at Paraggi and Ischia, a new polyacetylene, ficiformylene A (1), was isolated as the characteristic compound of the collection at Paraggi. The pseudomolecular ion peak of 1 was detected only in the extract of P. ficiformis collected at Paraggi, while the related ficiformylene B (2) was detected in all the extracts of P. ficiformis collections. The structures of ficiformylens were elucidated on the basis of NMR and MS analyses. Ficiformylene A (1) indicated cytotoxicity against P388 cells with an IC50 value of 11.2 μM, while ficiformylene B (2) exhibited no cytotoxicity.
The Chemical Constituents and Biological Activities of Cladosiphon okamuranus
Kun-Ching Cheng 1,2
and
Tian-Shung Wu 3
1
Taiwan Sugar Research Institute, Tainan 701, Taiwan
2
Department of Chemistry, National Cheng Kung University, Tainan 701, Taiwan
3
School of Pharmacy, National Cheng Kung University, Tainan 701, Taiwan
C. okamuranus is a filamentous brown alga that mainly grows in Okinawa, Japan and the sub-tropical reef area of the Ryukyu islands. Recently, many experiments have confirmed that since C. okamuranus is rich in bio-active substances such as alginic acid and sulfated polysaccharides (fucoidan). They have a wide spectrum of activity in biological systems. Besides their well-known anticoagulant, anti-tumor, anti-hyperlipidemia and anti-thrombotic activity, fucoidans modulate inflammation, possess anti-proliferative and anti-adhesive effects on cells, protect cells from viral infection, and interfere with mammalian fertilization. C. okamuranus are now used as raw material for development of drugs and are also widely used as a health-promoting food component. In the present study, we aimed to purify anti-inflammatory consitiuents from C. okamuranus and further to develop anti-inflammatory food supplement.
Isolation and Absolute Configuration of the Cyanobacterial Secondary Metabolite Nocuolin A
Marco A. C. Preto 1,
M. Lígia Sousa 1,2,
Ralph Urbatzka 1,
Joana R. Almeida 1,
Pedro N. Leão 1
and
Vitor Vasconcelos 1,2
1
CIIMAR—Interdisciplinary Centre of Marine and Environmental Research, Terminal de Cruzeiros de Leixões, Av. General Norton de Matos s/n, 4450-208 Matosinhos, Portugal
2
FCUP—Faculty of Sciences, University of Porto, Rua do Campo Alegre 1021/1055, 4169-007 Porto, Portugal
Cyanobacteria are ancient organisms that have been evolving surviving strategies for more than 3500 million of years. These diverse survival strategies translate into chemical diversity, originating compounds with wide variety of activities and very diverse chemical templates. As such, cyanobacteria have been proving to be a valuable resource for new chemical entities, with unique structural features. A recently discovered secondary metabolite, nocuolin A [1], was found to also be produced by a strain from LEGE-CC collection [2]. In this work, the isolation of nocuolin A was carried out using a bioassay guided procedure. The planar structure of the isolated metabolite was confirmed via 1D and 2D NMR and LC-MS/MS experiments. The determination of the absolute configuration of nocuolin A’s single stereogenic centre was performed by using ECD spectroscopy coupled with computational simulations. In addition, clues regarding the intriguing biosynthesis of this compound were collected from feeding experiments employing 13C labelled sodium acetate. The biological activity of nocuolin A has also been explored [3].
Acknowledgments
Support for this work was provided by the Strategic Funding UID/Multi/04423/2013 through national funds provided by the Foundation for Science and Technology (FCT) and the European Regional Development Fund (ERDF), in the framework of the programme PT2020 and funding was provided by the project NASCEM PTDC/BTA-BTA/31422/2017 (POCI-01-0145-FEDER-031422), financed by the FCT, COMPETE2020 and PORTUGAL2020.
References
Voráčová, K.; Hájek, J.; Mareš, J.; Urajová, P.; Kuzma, M.; Cheel, J.; Villunger, A.; Villunger, A.; Kapuscik, A.; Bally, M.; et al. The cyanobacterial metabolite nocuolin a is a natural oxadiazine that triggers apoptosis in human cancer cells. PLoS ONE 2017, 12, e0172850, doi:10.1371/journal.pone.0172850.
Ramos, V.; Morais, J.; Castelo-Branco, R.; Pinheiro, Â.; Martins, J.; Regueiras, A.; Pereira, A.L.; Lopes, V.R.; Frazão, B.; Gomes, D.; et al. Cyanobacterial diversity held in microbial biological resource centers as a biotechnological asset: the case study of the newly established LEGE culture colection. J. Appl. Phycol. 2018, doi:10.1007/s10811-017-1369-y.
Sousa, M.L.; Preto, M.; Vasconcelos, V.; Linder, S.; Urbatzka, R. Antiproliferative Effects of the Natural Oxadiazine Nocuolin A Are Associated With Impairment of Mitochondrial Oxidative Phosphorylation. Front. Oncol. 2019, 9, doi:10.3389/fonc.2019.00224.
Stylissa aff. Carteri from Futuna Islands as a Source of Pyrrole-Imidazole Alkaloids
Maria Miguel-Gordo 1,
Kevin Calabro 1,
Laurence Jennings 1,
Christine Morrow 2
and
Olivier P. Thomas 1
1
Marine Biodiscovery, School of Chemistry and Ryan Institute, National University of Ireland Galway (NUI Galway), University Road, H91 TK33 Galway, Ireland
2
Department of Natural Sciences, National Museums Northern Ireland, 153 Bangor Road, BT18 0EU Cultra, Northern Ireland
The Tara Pacific expedition (2016–2018) explored the Pacific Ocean with the aim of conducting an integrated study of the marine biodiversity of coral reefs facing climate and demographic changes. In this context, an inventory of marine sponges was undertaken for the first time around the Futuna Islands situated in the Central Indo-Pacific Ocean with the objective of describing their associated chemical diversity. A priority list of the collected sponges was created based on the taxonomy, biomass available, chemical profiles and bioassay results. The sponge
Stylissa aff.
carteri was selected for further investigation leading to the isolation of 8 brominated pyrrole-imidazole alkaloids (PIAs). Three new oroidin derivatives were identified, a new bicyclic dimer with a novel pyrrolo[1,2-
c]imidazole core (
1) and two new hexacyclic dimers, a dibrominated carteramine (
2) and a dibrominated konbu’acidin (
3) analogues (
Figure 34). The 2D structures of
1–
3 were elucidated from extensive analysis of NMR and HRMS spectroscopic data. The absolute configuration of the new molecules was assigned through computational and ECD experiments. Further, investigation through molecular networking applied to the fractions unveiled a large diversity of analogues.
Antiproliferative and Anti-inflammatory Potential of Isolated Compounds from Octopus Ink (Octopus vulgaris)
Martin Samuel Hernandez-Zazueta 1,
Ángel Antonio Carbonell-Barrachina 2,
Pablo Taboada-Antelo 3,
Ema Carina Rosas-Burgos 1,
Josafat Marina Ezquerra-Bauer 1,
Joel Said Garcia-Romo 1,
Rocío Campos-Vega 4,
Edgar Sandoval-Petris 5,
María Guadalupe Burboa-Zazueta 5,
Javier Hernández-Martínez 6
and
Armando Burgos-Hernández 1
1
Department of Research and Postgraduate in Food, University of Sonora, Sonora 83000, Mexico
2
Department of Agrifood Technology, University of Miguel Hernandez de Elche, 03202 Alicante, Spain
3
Department of Particle Physics, University of Santiago de Compostela, 15703 Santiago de Compostela, Spain
4
Department of Chemistry, The Autonomous University of Querétaro. Santiago de Querétaro, Querétaro 76010, Mexico
5
Department of Scientific and Technological Research, University of Sonora, Sonora 83000, Mexico
6
Support Services Unit in Analytical Resolution, Universidad Veracruzana, Veracruz 91190, Mexico
The purpose of this study was to take advantage of the by-products of fishing, where compounds have been identified as a product of the secondary metabolism of marine organisms with potential pharmacological application, especially against chronic degenerative diseases such as cancer. In turn, persistent chronic inflammation processes have been linked to carcinogenesis and the development of the disease. Therefore, we used freeze-dried octopus ink (Octopus vulgaris) for the search, isolation, and characterization of the main compounds present in the ink, with anti-inflammatory and anti-proliferative potential. The results exhibited a compounds present in fraction eluted with Hex/AcOEt (open column) with IC50 of 47 μg/mL on nitric oxide production in stimulated macrophage cells with LPS from E. coli; also, the evaluation of inflammatory proteins modulation (Mouse Cytokine Array Panel A, of Proteome Profiler, Antibody Array, R&D Systems), suggest a regulation of different cytokine implicated in the pro-inflammatory response (IL-2, IL-7, IL-16, IL-23 and CCL-17). It should be noted that, this interaction with membrane proteins (Il-23 receptors) is linked to was observed (on the basis of microscopic immunofluorescence) when colon (GI50 of 52 μg/mL) and prostate (GI50 of 27 μg/mL) cancer cells were treated with the same compounds, exhibiting morphological membrane changes and apoptotic bodies, suggesting death by apoptosis. On the other hand, 1H and 13C NMR analysis suggested the presence of different sterol base, pyrrole, and indole compounds. Consequently, it is proposed to investigate the mechanism of action for the use of this type of compounds as possible drugs in the treatment of this disease.
Bacterial-Derived Extracellular Electron Shuttle
Emily Mevers 1,
Lin Su 2,
Gleb Pishchany 1,3,
Jose Cornejo 2,
Moshe Baruch 2,
Caroline Ajo-Franklin 2
and
Jon Clardy 1
1
Department of Biological Chemistry & Molecular Pharmacology, Harvard Medical School, Boston, MA 02115, USA
2
Molecular Foundry, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
3
Department of Microbiology and Immunobiology, Harvard Medical School, Boston, MA 02115, USA
Shewanella oneidensis MR-1 is a facultative anaerobic γ-proteobacterium that has the ability to utilize a diverse suite of terminal electron acceptors, including insoluble solid metal oxides. The mechanisms underlying how MR-1 indirectly shuttles electrons to these solid substrates are poorly understood. In 2000 chemical analyses of MR-1’s spent supernatant revealed that MR-1 excretes a small labile molecule that has the ability to recover anaerobic respiration of mutants on solid substrates, but the active metabolite was never identified. Revisiting this lack of identity with specialized resin, HR-LCMS analysis, and total synthesis, led to its identification as 2-amino-3-carboxy-naphthoquinone (ACNQ). ACNQ is derived from DHNA (1,4-dihydroxy-2-naphthoic acid) in a non-enzymatic process that frustrated genetic approaches to identify the shuttle. Both ACNQ and DHNA robustly restore reduction of AQDS under anaerobic growth in menaquinone-deficient mutants. Electrochemistry analyses reveal that ACNQ is contributing to the extracellular electron transfer (EET) as an electron shuttle, without altering any menaquinone generation or EET related cytochrome c expression.
Anti-Bacterial Biofilm Activity and Fatty Acids Composition from Saudi Red Sea Organisms
Usama W. Hawas,
Fekri Shaher,
Mohamed Ghandourah,
Lamia T. Abou El-Kassem,
Sathianeson Satheesh
and
Abdul Mohsin A. Al-Sofyani
Marine Chemistry Department, Faculty of Marine Sciences, King Abdulaziz University, Jeddah 21589, Saudi Arabia
Biofilm formation is the primary step in biofouling growth on substrates submerged in the sea. This study aimed to evaluate the antibiofilm activity of metabolites of some Red Sea organisms against three biofilm-bacterial strains isolated from Jeddah coast. Free fatty acids (FAs) and other lipoidal matters were extracted from the sea cucumber Holothuria atra, green alga Avrainvillea amadelpha, and costal plant Salicornia fruticosa. The composition of lipoidal fractions were analyzed by GC-MS which showed A. amadelpha is rich by saturated FAs with 74%, while sea cucumber H. atra revealed high content of unsaturated FAs with 60%. Palmetic acid is the major FAs composition in all species ranged from 14.5% to 26.7%. Phytol, sterols and hydrocarbons (C8–C29) were represented in the alga A. amadelpha as high contents with values 25.8%, 21.9% and 18.5%, respectively. The metabolites of extracts and lipoidal contents showed biofilm inhibitory activity against the isolated bacterial strains, where the lipoidal (unsaponified) fraction of S. fruticosa exhibited higher inhibitory activity against Planomicrobium sp. at a concentration of 200 μg/mL. Concentration dependent activity was not uniform among the extracts or the fractions.
Polyoxygenated Steroids from the Soft Coral Sinularia polydactyla Collected in the Egyptian Red Sea
Mohamed Tammam 1,2,
Aldoushy Mahdy 3,
Efstathia Ioannou 1
and
Vassilios Roussis 1
1
Section of Pharmacognosy and Chemistry of Natural Products, Department of Pharmacy, National and Kapodistrian University of Athens, 15771 Athens, Greece
2
Biochemistry Department, Faculty of Agriculture, Fayoum University, Fayoum 63514, Egypt
3
Department of Zoology, Faculty of Science, Al-Azhar University (Assiut Branch), Assiut 71524, Egypt
Steroidal molecules comprise a very significant class of natural product owing to their capability to cross the lipophilic membrane of the cell, and after binding to the steroid receptors, articulate their detailed physiological functions. Soft corals are considered a prolific source of often structurally complex steroids, showing diversified nuclei or functionalized side chains. Steroids of marine origin and especially polyoxygenated sterols have exhibited a diverse array of pharmacological activities, such as antimicrobial, cytotoxic, antifouling, ichthyotoxic and antiinflammatory. In the course of our investigations on the chemistry of marine organisms, specimens of the gorgonian Sinularia polydactyla were collected by SCUBA diving from Hurghada in the Red Sea (Egypt). The soft coral tissues were extracted with mixtures of CH2Cl2/MeOH and the organic residue was submitted to a series of chromatographic separations, leading to the isolation of 24 steroids, among which 6 are new natural products. The structures and the relative configurations of the isolated natural products were established mainly on the basis of extensive analysis of 1D and 2D NMR and MS data.
Acknowledgments
Financial support to M.T. from the non-profit organization.
A Novel Chlorophyll Derivative, 132-Hydroxy-Pheofarnesin A, from a Marine Cyanobacteria with Lipid Reducing Activity
Natália Gonçalves Silva 1,
Maria Lígia Sousa 1,
Mariana Alves Reis 1,
Vítor Vasconcelos 1,2
and
Ralph Urbatzka 1
1
Interdisciplinary Center of Marine and Environmental Research (CIIMAR/CIMAR), University of Porto, Terminal de Cruzeiros de Leixões, Av. General Norton de Matos s/n, 4450-208 Matosinhos, Portugal
2
Faculty of Sciences, University of Porto, Rua do Campo Alegre 1021/1055, 4169-007 Porto, Portugal
Research has focused on natural product discovery for health treatments, such as obesity and related co-morbidities, and cyanobacteria are regarded as a prolific source of biologically active natural compounds. In this work, a bioassay-guided isolation approach was used to discover secondary metabolites from the Blue Biotechnology and Ecotoxicology Culture Collection (LEGEcc) with lipid reducing activity. Cyanobacterial strain Nodosilinea sp. LEGE 06001 was cultured and harvested. The freeze-dried biomass was extracted by repeated percolation with mixtures of DCM/MeOH. The resultant organic crude extract was fractionated by normal-phase vacuum liquid chromatography, and after several rounds of fractionation a novel chlorophyll derivative was successfully isolated. The structure elucidation of 132-hydroxy-pheofarnesin a (hfa) was established based on one- and two-dimensional NMR spectroscopy and mass spectrometry. Hfa showed significant neutral lipid-reducing activity in the zebrafish Nile red fat metabolism assay after 48 h of exposure with an EC50 value of 15.5 ± 1.3 μM and did not cause any general toxicity or malformations. In an attempt to establish the structure-activity relationships, chlorophylls a and b were analysed using the same assay. Since did not show any effect, these results highlight the importance of the structural differences of hfa for the lipid reducing activity. This bioactivity was additionally confirmed in differentiated 3T3-L1 multicellular spheroids of murine preadipocytes. Hfa may be developed as a nutraceutical with lipid reduction activity, however future studies are needed to clarify its mechanisms of action.
Acknowledgments
This work was supported by the European ERA-NET Marine Biotechnology project CYANOBESITY (ERA-MBT/0001/2015), financed by national funds through FCT (Foundation for Science and Technology, Portugal).
A National Marine Biodiscovery Laboratory in Ireland
Navdeep Kaur 1,2,
Laurence K. Jennings 1,2,
Daniel Rodrigues 1,2,
Jeffrey Fisher 2,
Dick FitzGerald 3,
Alan Dobson 4,
Dagmar B. Stengel 5,
Marianela Zanolla 5,
Grace McCormack 6
and
Olivier P. Thomas 1
1
Marine Biodiscovery, School of Chemistry and Ryan Institute, National University of Ireland Galway (NUI Galway), University Road, H91 TK33 Co. Galway, Ireland
2
Marine Biodiscovery, Marine Institute, Rinville West, H91 R673 Co. Galway, Ireland
3
Department of Biological Sciences, Faculty of Science & Engineering, University of Limerick, V94 T9PX Co. Limerick, Ireland
4
Environmental Research Institute, University College Cork, College Road, T12 K8AF Co. Cork, Ireland
5
Botany and Plant Science, School of Natural Sciences and Ryan Institute, National University of Ireland Galway (NUI Galway), University Road, H91 TK33 Co. Galway, Ireland
6
Zoology, School of Natural Sciences and Ryan Institute, National University of Ireland Galway (NUI Galway), University Road, H91 TK33 Co. Galway, Ireland
The marine environment is a source of an underexplored diversity of macro and microorganisms which are known to produce unique specialized metabolites for communication, defence, and competition. Some marine ecoregions of the oceans have not been inventoried and only sparsely chemically studied like the coasts of the island of Ireland in the North Eastern Atlantic Ocean.
The NMBLI aims to promote marine biodiscovery in Ireland and to bring Ireland to the forefront of global marine research in Europe. Therefore, the NMBLI has developed an Irish marine repository containing freeze-dried biomass, voucher specimens, and fractionated extracts from Irish marine invertebrates. Our repository is organized around a state-of-the-art web-database for storage of data and dereplication of known natural products. The fractionated extracts are chemically screened with UHPLC-HRMS/MS and biologically screened against a broad panel of microbial pathogens and tumour cell lines. These data profiles are uploaded on our web-database and our linked study on the open-access EMBL-EBI’s ‘Metabolights’. This process quickly leads us to the identification of marine organisms for an in-depth chemical investigation. Herein, we present the first results from the dereplication of marine natural products from our marine repository as well as some in-depth chemical investigations that have been performed on promising Irish marine invertebrates.
Insights into Eudistoma vannamei Holobiome: Microbial and Chemical Diversities
Anelize Bauermeister 1,2,
Luciana Costa Furtado 1,
Elthon G. Ferreira 3,
Paula Christine Jimenez 4,
Welington Luiz Araújo 1,
Luiz Ricardo Olchnheski 1,
Tito Monteiro da Cruz Lotufo 5,
Leticia Veras Costa-Lotufo 1
and
Norberto Peporine Lopes 2
1
Instituto de Ciências Biomédicas, Universidade de São Paulo, São Paulo 05508-000, Brazil
2
Departamento de Física e Química, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Ribeirão Preto, São Paulo14040-903, Brazil
3
Departamento de Química Orgânica e Inorgânica, Universidade Federal do Ceará, CE, 60451-970 Fortaleza, Brazil
4
Departamento de Ciências do Mar, Universidade Federal de São Paulo, Santos, SP 11.070-100, Brazil
5
Instituto Oceanográfico, Universidade de São Paulo, São Paulo 05508-120, Brazil
Eudistoma vannamei is a colonial ascidian endemic to the Brazilian northeastern coast considered a prolific source of secondary metabolites. This ascidian also hosts bioactive compounds-producing actinomycetes. To understand the relationship between the associated-microbiota and the ascidian, herein, E. vannamei was studied as a holobiont model using MS-based metabolomics and 16S rRNA gene diversity analyses. Gene sequencing analysis of E. vannamei showed that most of the detected OTUs belong to bacterial phyla, in which Proteobacteria was the most abundant (>50%), followed by Planctomycetes, Actinobacteria, Bacteroidetes, among others. Interestingly, the three analyzed samples shared about 50% of the observed OTUs, but the Metacoder analysis showed that, while there is specimens’ uniqueness concerning the microbial content, especially related to Actinomycetes and Bacteroidetes, groups belonging to the core community are undoubtably more abundant. The analyses by molecular network indicated the presence of staurosporines, a well-known chemical class present in the genus Eudistoma, but recognized as microbial metabolites. Aminoacids and phospholipids were also observed. Bacteria were also recovered from the ascidian tissue, and cultured in liquid and solid culture media to evaluate their metabolic potential to produce different chemical structures. The protocol employed herein led to the isolation of bacteria mostly from the Actinobacteria phylum, including Streptomyces and Micomonospora. The liquid culture of these strains provided a greater number of metabolites than the solid cultures, and the molecular network analysis allowed the rapid identification of compounds from the desferrioxamine, erythromycin, and marineosin chemical classes, which are interesting compounds from a biotechnological point of view.
Acknowledgments
Financial support from FAPESP 2015/17177-6 and2017/17648-4.
First Insights into Health Issues Related to Blooms of the Cyanobacterium Lyngbya Cf. majuscula in New Caledonia
Olivier P. Thomas 1,
Hiren Solanki 1,
Mayalen Zubia 2,
Sandra Gegunde 3,
Kevin Calabro 1,
Georges Kakue 4,
Amparo Alonso 3,
Luis M. Botana 3
and
Claude Payri 5
1
Marine Biodiscovery, School of Chemistry and Ryan Institute, National University of Ireland (NUI Galway), University Road, H91 TK33 Galway, Ireland
2
UMR EIO 241, Labex CORAIL, University of French Polynesia, BP6570, 98702 Faa’a Tahiti, French Polynesia, France
3
Departamento de Farmacología, Facultad de Veterinaria, Universidade de Santiago de Compostela, 27002 Lugo, Spain
4
Direction du Développement Durable et de la Recherche Appliquée—Province des îles Loyauté, Qaneno, Drehu, New Caledonia, France
5
UMR ENTROPIE (IRD, UR, CNRS), Institut de Recherche pour le Développement, B.P. A5, 98848 Nouméa CEDEX, New-Caledonia, France
Cyanobacterial blooms are becoming more recurrent in the Pacific Ocean leading to health but also economic concerns among the local communities. Recently, closures of beaches have resulted from the yearly occurrence of blooms of a cyanobacterium related to Lyngbya majuscula. The project CYCLADES was recently supported by the Pacific Fund to give some insights into recent events of dermatitis associated with these blooms in the Drehu island of New Caledonia.
We report the first results on the chemical diversity identified in specimens overgrowing corals in the affected beach. New linear and cyclic modified peptides were isolated as major metabolites together with the known dolastatin 3. Additionally, some modified fatty acids are reported for the first time and all metabolites were biologically tested.
Marine-Derived Microorganisms as Potential Sources of Neurotrophin Mimetics for the Treatment of Neurodegeneration and Neuroinflammation
Paolo Giaccio 1,
Marco Destro 2,
Pamela J. Shaw 2,
Laura Ferraiuolo 2,
Vassilios Roussis 1
and
Efstathia Ioannou 1
1
Section of Pharmacognosy and Chemistry of Natural Products, Department of Pharmacy, National and Kapodistrian University of Athens, 15771 Athens, Greece
2
Sheffield Institute for Translational Neuroscience, University of Sheffield, Sheffield S10 2HQ, UK
Among neurodegenerative diseases, Amyotrophic Lateral Sclerosis (ALS) is a rare fatal heterogeneous disorder characterized by the progressive degeneration of motoneurones. ALS is a non-cell-autonomous disorder in which also astrocytes are affected and contribute to the motoneuronal death. ALS patients experience rapid deterioration in muscle function, with an average lifespan of 2–3 years after diagnosis. Currently, the most effective therapy extends lifespan only by a few months, thus highlighting the need for new and improved therapies.
In the framework of EuroNeurotrophin, marine microorganisms from the East Mediterranean basin will be investigated aiming at the discovery of new potential neurotrophin mimetics to treat neurodegenerative diseases. To this end, the organic extracts of 85 bacterial and 15 fungal marine-derived strains were submitted to a High-Throughput Screening (HTS) for the preliminary bioactivity evaluation and prioritization of extracts capable of inhibiting the cell death of cultured motoneurones. HTS was performed using the in vitro models of human astrocytes derived from a panel of patient biosamples carrying different ALS-associated mutations and neuronal co-cultures with end point neuroprotection of motor neurons. According to the results of the preliminary evaluation, three bacterial extracts exhibited significantly higher activity than that of the positive control. The results on the isolation and structure elucidation of the metabolites of the currently under chemical investigation large-scale extracts of the most promising strains will be presented.
Acknowledgments
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 765704 (
www.euroneurotrophin.eu).
New Antiinflammatory Secoeunicellins from Octocoral Cladiella sp.
Zhi-Jun Zhang 1,2,
Tsong-Long Hwang 3,4
and
Ping-Jyun Sung 1,2
1
National Museum of Marine Biology and Aquarium, Pingtung 944, Taiwan
2
Graduate Institute of Marine Biology, National Dong Hwa University, Pingtung 944, Taiwan
3
Research Center for Chinese Herbal Medicine, Research Center for Food and Cosmetic Safety, Graduate Institute of Healthy Industry Technology, College of Human Ecology, Chang Gung University of Science and Technology, Taoyuan 333, Taiwan
4
Graduate Institute of Natural Products, College of Medicine, Chang Gung University, Taoyuan 333, Taiwan
Two novel 6,7-secoeunicellins, cladieunicellins W (
1) and X (
2) (
Figure 35), were isolated from an octocoral identified as
Cladiella sp. The structures of secoeunicellins
1 and
2 were established by spectroscopic methods. Eunicellin
1 is the first secoeunicellin possessing two tetrahydrofuran moieties and
2 represents the first 6,7-secoeunicellin possessing a γ-lactone ring. In anti-inflammatory testing, secoeunicellin 1 dispalyed inhibitory effects on the release of elastase (IC
50 = 7.83 ± 0.83 µM) and the generation of superoxide anions (IC
50 = 7.18 ± 1.20 µM). These results implied that the methoxy group at C-6 in 1 played important role in determining the activity of the compounds.
Fucoidans from Brown Algae: Diversity of Structure and Anticancer Activity in Vitro
Roza Usoltseva,
Natalia Shevchenko,
Valeriy Surits,
Olesya Malyarenko
and
Svetlana Ermakova
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far Eastern Branch of the Russian Academy of Sciences P. 104, 690022, Vladivostok, Russia
Brown algae produce valuable biologically active sulfated polysaccharides—fucoidans possessing different kinds of biological activity, for example, anticancer, antiviral, anticoagulant, immunomodulatory. These polysaccharides have a various structures: fucoidans can contain galactose, mannose, xylose, uronic acids residues, acetyl groups, branches; also the types of bonds between monosaccharide residues are different. The aim of the present work is comparison of structure of fucoidans from some kinds of brown algae and their anticancer activity in vitro. The structure of fucoidan fractions, isolated from Laminaria longipes, Saccharina cichorioides (Laminariaceae, Laminariales), Sargassum duplicatum and Sargassum feldmannii (Sargassaceae, Fucales), Dictyota dichotoma and Padina boryana (Dictyotaceae, Dictyotales) were investigated by chemical and instrumental methods. It was shown that the fucoidans from L. longipes and S. cichorioides were alfa-L-fucans, from S. duplicatum and S. feldmannii—galactofucans, from Dictyota dichotoma and Padina boryana—heteropolysaccharides with complex structure. All obtained fucoidans were differed significantly in details of structure. So, fucoidan from S. cichorioides was predominantly 1,3-linked alfa-L-fucan with small amount of branches at C2, while fucan from L. longipes mainly contained alternating 1,3-, 1,4- and 1,2-linked fucose residues. The both galactofucans from S. duplicatum and S. feldmannii had the main chains from fucose and galactose, but the backbone of polysaccharide from S. feldmannii was 1,3-linked, from S. duplicatum—1,4-linked. The branches of galactofucans also were different. The investigations of anticancer activity of obtained fucoidans revealed that these polysaccharides inhibited formation of colonies and growth of colon cancer cells in different manner.
Acknowledgments
This work was supported by the RFBR Grant 19-54-54005.
Implementing Metabolomics Tools to Optimise the Production of Anti-Biofilm Metabolites of Endophytic Fungi from Scottish Seaweeds
Saif Aldeen Jaber 1,2,
Louise Young 1,
Kirsty Black 3,
Rothwelle Tate 1
and
RuAngelie Edrada-Ebel 1
1
Strathclyde Institution of Pharmacy and Biomedical Sciences, University of Strathclyde, 161 Cathedral Street, Glasgow G4 0RE, UK
2
Middle East University, Amman 11610, Jordan
3
Marine Biopolymers Ltd., Unit 54, Heathfield Industrial Park, Boundary Road, Ayr KA8 9DJ, UK
An increasing number of compounds with new structures and various bioactivities are recently being isolated from marine fungal sources. A metabolomics approach was used to aid media selection for the growth algal endophytic fungi and optimum production of their anti-biofilm metabolites. Anti-biofim activity has played an important role in multi-resistance of pathogenic bacteria like those of Staphylococcus aureus, Klebsiella pneumoniae, and Pseudomonas aeruginosa. The endophytic fungus Dendryphilla salina obtained from marine Scottish seaweed Laminaria hyperborea, was grown on nine different media namely: malt extract, rice, oat, and Wickerham media either incorporated with or without sea salt and in marine broth as well. Incubation periods were accomplished at three different growth phases. Fungal extracts were then prepared from the different fermentation conditions and their chemical profiles were analysed through nuclear magnetic resonance (NMR) spectroscopy and HPLC-coupled high-resolution mass spectrometry (HPLC-HRMS). The spectral data were processed and dereplicated using the Dictionary of Natural Products (DNP) database. The processed data were also subjected to multivariate analysis to target the bioactive metabolites and establish the optimum conditions for their production. The extracts were tested for anti-biofilm activity by Alamar Blue and planktonic solution bioassays. Three fungal extracts obtained from malt media both with and without sea salt as well as the oat media with sea salt were found active affording 100% inhibition of biofilm formation at concentration of 100 μg/mL. From the multivariate data analysis, three new metabolites were targeted for isolation work.
Chemodiversity of the Irish Deep-Sea Sponge Characella pachastrelloides
Sam Afoullouss 1,2,
Kevin Calabro 1,
Christine Morrow 2,
Louise Allcock 2
and
Olivier P. Thomas 1
1
Marine Biodiscovery, School of Chemistry and Ryan Institute, National University of Ireland Galway University Road, H91TK33 Galway, Ireland
2
Zoology, School of Natural Sciences and Ryan Institute, NUI Galway, University Road, H91 TK33 Galway, Ireland
As part of a program aimed at characterizing hotspots of biological and chemical diversity in the deep sea of the North Eastern Atlantic, samples of marine sponges were collected from biogenic and geogenic reefs from a depth between 1000–3000 m. The extreme physical conditions of the deep sea force these organisms to develop unique specialized metabolites with original chemical structures and characteristics. Based on a preliminary chemical screening process, we decided to focus on the Tetractinellida sponge Characella pachastrelloides (Carter, 1876). We report herein the isolation and structure elucidation of four novel glycolipopeptides named characellides. These compounds (867 Da) consist of three separate moieties: a tripeptide (O-Me-Tyr, Asp, Thr), a nine/ten-member alkyl chain capped with a dimethyl substituted cyclic tetrahydropyran bound to the threonine and a 2-α-aminopyranuronamide sugar connected to the tripeptide via a O-glycosidic linkage with the threonine also. Characellides A and B are epimers, which only differ in their sugar moieties where the 2-α-glucuronamide replaces a 2-α-galacturonamide, with a ten member alkyl chain, while characellides C and D are epimers with a nine member alkyl chain. Additionally, 6 methylhercynine, two new poecillastroside saponins and cyanocobalamin were also isolated from this sponge.
GNPS-Guided Isolation of Cyanobacterial Secondary Metabolites from the LEGE Culture Collection
Sara R. Freitas 1,
Pedro N. Leão 1
and
Vitor Vasconcelos 1,2
1
Interdisciplinary Centre of Marine and Environmental Research (CIIMAR/CIMAR), University of Porto, Avenida General Norton de Matos, s/n, 4450-208 Matosinhos, Portugal
2
FCUP, Faculty of Science, Department of Biology, University of Porto, Rua do Campo Alegre, 4169-007 Porto, Portugal
Cyanobacteria are a highly diversified group of photosynthetic ancestral bacteria that have adapted to virtually all environments on Earth where light is available. Their biological and lifestyle diversity is associated with the production of a plethora of secondary metabolites, many with potent bioactivities. From genome data, we know that only a small fraction of cyanobacterial natural products has been isolated so far, therefore there are ample opportunities for discovery within this bacterial phylum.
In this work we focused on the isolation of new natural products from a selected group of cyanobacteria isolated from the Atlantic Portuguese coast and maintained in the culture collection LEGE cc, from CIIMAR research centre. The cyanobacterial strains were selected based on the perceived potential for being secondary metabolite-rich, which in turn was inferred from their phylogenetic positioning. In parallel, a bioactivity screening (anticancer and antimicrobial) was conducted to prioritize the cyanobacterial strains for new secondary metabolite isolation, complemented with GNPS-based molecular networking analysis. Fractions derived from an organic extract of a Desmonostoc muscorum strain revealed antibacterial activity against Bacillus subtilis and, in addition, showed a conspicuous molecular network cluster that was not found in other cyanobacteria extracts. These masses were not represented in the natural products databases, therefore the major compound in the cluster was selected for isolation and structure elucidation, the details of which are presented herein.
Intra-Clade Molecular Networking to Target the Isolation of Anti-Proteasome Metabolites from Salinispora sp. from the Madeira Archipelago
Anelize Bauermaister 1,
Tiago Dias 2,
Patrícia Valentão 3,
Paula Andrade 3,
Norberto P. Lopes 1,
David Pereira 3
and
Susana P. Gaudêncio 2
1
Faculdade de Ciências Farmacêuticas, Universidade de São Paulo, Núcleo de Pesquisa em Produtos Naturais e Sintéticos (NPPNS), Departamento de Física e Química, Ribeirão Preto, São Paulo 14040-903, Brazil
2
UCIBIO, Department of Chemistry, Blue Biotechnology and Biomedicine Lab, Faculdade de Ciências e Tecnologia da Universidade NOVA de Lisboa, 2829-516 Caparica, Portugal
3
REQUIMTE/LAQV, Laboratório de Farmacognosia, Departamento de Química, Faculdade de Farmácia, Universidade do Porto, 4050-313 Porto, Portugal
Ocean environments constitute a major source of biodiversity, harboring marine life forms capable of producing a variety of molecules with unique features, unmatched biochemical diversity and structural complexity. Thirty-four marine obligate actinomycetes strains Salinispora sp. (33 S. pacifica, 1 S. arenicola), isolated from sediments collected off the Madeira Archipelago [1], were screened for anti-proteasome activity. Their metabolites were dereplicated using MS/MS Molecular Networking [2–4], and the data available in literature for the genus Salinispora. The 26S proteasome is a catalytic complex that constitutes the main non-lysosomal pathway of protein degradation in eukaryotic cells. This complex plays a key role in cellular protease as it is involved in the degradation of poorly bounded proteins. Since degradation of these proteins is critical to the survival of cancer cells, inhibition of the proteasome is currently a prominent therapeutic strategy. The potent anti-proteasome Salinosporamide A (Marizomib) discovered in 2003 from S. tropica, which in Phase III of clinical trials for the treatment of multiple myeloma highlights the importance of Salinispora genus as a source of anti-cancer agents.
Molecular Networking LC-MS/MS metabolomics profiling unveiled the absence of previously isolated anti-proteasome metabolites from MAR1. Three S. pacifica strains exhibited high anti-proteasome activity (inhibition > 90%). Based on the findings of this study, the Northeast Atlantic marine-derived MAR1 Salinispora sp. are revealed as a potential source of novel anti-proteasome agents.
Acknowledgments
This work was supported by the Applied Molecular Biosciences Unit—UCIBIO which is financed by national funds from FCT/MCTES (UID/Multi/04378/2019). FCT/MCTES through grants PTDC/QUIQUI/119116/2010 and IF/00700/2014.
References
Prieto-Davo, A.; Dias, T.; Gomes, S.E.; Rodrigues, S.; Parera-Valadez, Y.; Borralho, P.M.; Pereira, F.; Rodrigues, C.M.P.; Santos-Sanches, I.; Gaudêncio, S.P. The Madeira Archipelago As a Significant Source of Marine-Derived Actinomycete Diversity with Anticancer and Antimicrobial Potential. Front. Microbiol. 2016, 7, 1594, doi:10.3389/fmicb.2016.01594.
Gaudêncio, S.P.; Pereira, F. Dereplication: racing to speed up the natural products discovery process. Nat. Prod. Rep. 2015, 32, 779–810.
Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837.
Bauermeister, A.; Velasco-Alzate, K.; Dias, T.; Macedo, H.; Ferreira, E.G.; Jimenez, P.C.; Lotufo; T.M.C.; Lopes, N.P.; Gaudêncio, S.P.; Costa-Lotufo, L.V. Metabolomic Fingerprinting of Salinispora From Atlantic Oceanic Islands. Front. Microbiol. 2019, 9, 3021, doi:10.3389/fmicb.2018.03021.
Antibacterial Polyketides from Antarctic Sponge Derived Fungus Penicillium citrinum HDN151010
Chunxiao Sun,
Zichao Sun,
Qian Che,
Guojian Zhang,
Qianqun Gu,
Dehai Li
and
Tianjiao Zhu
Key Laboratory of Marine Drugs, Chinese Ministry of Education, School of Medicine and Pharmacy, Ocean University of China, Qingdao 266003, China
The sponge-associated microorganisms have been an attractive resource for novel structural bioactive compounds. In our on-going search for bioactive secondary metabolites from Antarctic sponge-associated microorganisms, two new polyketides, named demethyldihydrocitrinone (1) and trimethylphenylacetate A (2), together with five biogenetically related known citrinin derivatives (3–7), were isolated from the crude extract of the fungus Penicillium citrinum HDN151010, which was isolated from an unidentified sponge collected in the Prydz bay (depth 624 m), Antarctic. Their structures, including absolute configurations, were established on basis of, M.S.; NMR, and ECD analysis. As a well-known class of polyketides, citrinins possess regular skeletons and substituted groups. Distinguished from the previously reported citrinin derivatives, compound 1 was the first case with the C-3 methyl group lost, indicating that unique geographical features of Antarctica may promote the unique metabolic pathways of the microorganisms that living in it. Compound 1 showed promising activities against Escherichia coli, Vibrio Parahemolyticus, and Proteus vulgaris with MIC values of 0.8, 3.1 and 6.3 μM, respectively, while compounds 3–7 were found to inhibit the growth of Mycobacterium phlei for the first time, indicating anti-tuberculousis potential.
New Starfish Glycosides: Structure and Anticancer Activity
Timofey V. Malyarenko,
Natalia V. Ivanchina,
Alla A. Kicha
and
Valentin A. Stonik
G.B. Elyakov Pacific Institute of Bioorganic Chemistry, Far Eastern Branch of the Russian Academy of Sciences, Pr. 100-let Vladivostoku 159, 690022 Vladivostok, Russia
The studies of polar steroidal compounds from starfish by our research group led to the isolation of new substances with anticancer activity. For example, six new steroidal biglycosides, cariniferosides A–F, were isolated from the starfish Asteropsis carinifera. Sulfated compounds demonstrated a significant inhibition of colony formation of human melanoma RPMI-7951 and breast cancer T-47D cells. A new monoglycoside, leptaochotensoside A, isolated from the Far Eastern starfish Leptasterias ochotensis, were found to prevent EGF-induced neoplastic cell transformation of mouse epidermal JB6 Cl41 cells and inhibit the colony formation of human breast cancer cells T-47D in a soft agar. The molecular mechanism of action of leptaochotensoside A was realized through regulation of MAPK signaling pathway. The new steroidal glycosides, anthenosides V–X, and seven previously known anthenosides were isolated from the extract of the tropical starfish Anthenea aspera. The mixture of known anthenosides J and K was shown to possess significant anticancer activity by inducing the apoptosis of colon cancer cells HT-29.
Recently eight new triterpene glycosides, pacificusosides A H, and one previously known triterpene glycoside, cucumarioside D, were obtained from the alcoholic extract of the Far Eastern starfish Solaster pacificus. Triterpene glycosides are not typical metabolites of starfishes but specific for sea cucumbers. It was established, that these glycosides effectively suppressed the EGF- and TPA-induced neoplastic cell transformation of JB6 Cl41 cells.
Acknowledgments
The study was supported by Grant No. 18-74-10028 from the RSF (Russian Science Foundation).
Isolation and Structure Determination of Trikoramide A from the Marine Cyanobacterium Symploca sp.
Yadanar Phyo Ma 1,2,
Chi Ying Gary Ding 1,
Ji Fa Marshall Ong 1
and
Lik Tong Tan 1
1
Natural Sciences and Science Education, National Institute of Education, Nanyang Technological University, 1 Nanyang Walk, Singapore 637616, Singapore
2
School of Biological Sciences, Nanyang Technological University, Singapore 637551, Singapore
Marine cyanobacteria are a treasure trove of unique bioactive secondary metabolites for drug discovery and development efforts. A majority of these compounds are nitrogen-containing and they belong to the hybrid polyketide-polypeptide structural class of molecules. Moreover, a number of these molecules possess potent biological activities, ranging from anticancer to anti-inflammatory property. In this study, a novel cyclic depsipeptide, trikoramide A, has been isolated from samples of the marine cyanobacterium, Symploca sp., collected from east coast of Bintan Island. Its planar structure was deduced by extensive 1D and 2D NMR spectroscopy in conjunction with mass spectrometry. The initial analysis using mass spectral data revealed the partial structure of trikoramide A to consist of proline, leucine, valine and phenylalanine units. In addition, trikoramide A possessed in vitro cytotoxicity towards MOLT4 human leukemic cancer cell line with IC50 value of 5.75 μM.
Acknowledgments
This research is supported by the National Research Foundation, Prime Minister’s Office, Singapore under its Marine Science Research and Development programme (Award Nos. MSRDP-P15 and MSRDP-P34).
Fragilides M-O, New 2,9,14-Triacetoxybriaranes from Junceella fragilis
Chieh-Yu Lee 1,2,
You-Ying Chen 1,3,
Zhi-Hong Wen 3
and
Ping-Jyun Sung 1,2
1
National Museum of Marine Biology and Aquarium, Pingtung 944, Taiwan
2
Graduate Institute of Marine Biology, National Dong Hwa University, Pingtung 944, Taiwan
3
Department of Marine Biotechnology and Resources, National Sun Yat-sen University, Kaohsiung 804, Taiwan
Chemical investigation of the EtOAc-soluble fraction from the MeOH/DCM extract of a sea whip gorgonian coral
Junceella fragilis afforded four polyacetoxybriaranes, including a known metabolite, junceellin (
1), along with four new analogues, fragilides M–P (
2–
5). The absolute configuration of
1 was determined by X-ray analysis and the structures of
2–
5 were elucidated on the basis of spectroscopic methods (
Figure 36) The proton chemical shifts of briarane-type natural products containing an 11,20-exocyclic carbon-carbon double bond were summarized and the difference between the two olefin protons (H-20a/b) was smaller than 0.2 ppm, whereas the methylidenecyclohexane rings exhibited a twisted boat conformation. Owing to the chemical shifts of the C-20 methylene protons, the configurations of the methylidenecyclohexane rings in briaranes
4 and
5 were concluded to be a twisted boat conformation and this finding was further supported by the NOESY correlations between H-10/H-20b; and H-12/H3-15 in the NOESY spectra of
4 and
5. Anti-inflammatory activity of briaranes
1–
5 in terms of reducing the expressions of elastase and superoxide anions by human neutrophils were described.
Domhainone: A New Oxidized Steroid from the Irish Deep-Water Coral Jasonisis sp.
Ryan M. Young 1,
Trang Nguyen 2,
Xingmin Sun 2,
Mark P. Johnson 1,
Bill J. Baker 3
and
A. Louise Allcock 1
1
Ryan Institute, National University of Ireland, Galway, H91 CF50, Ireland
2
Molecular Medicine, College of Medicine, University of South Florida, Tampa, FL 33620, USA
3
Department of Chemistry, College of Arts & Science, University of South Florida, Tampa, FL 33620, USA
Submarine canyons are often biodiversity and biomass hotspots and the Whittard Canyon, located approximately 300 km off the Irish mainland, with steep cliffs inhabited by a plethora of corals and sponges is no exception. One species which populates these waters is a bamboo coral of the genus
Jasonisis. In a high-throughput screening effort, the fractions originating from a chemical extract of this coral were identified as active against the pathogenic bacterium
Clostridioides difficile. Infections of
C. difficile have emerged as a significant public health threat worldwide. In the United States,
C. difficile caused an estimated half a million infections and 29,000 deaths in 2012. The DCM extract of the bamboo coral was fractionated on C18 via VLC. The active fraction was subsequently purified via HPLC yielding the major metabolite domhainone (
Figure 37). The final structure was elucidated using 1D/2D NMR spectroscopy and HRMS.
(α-d-Glucopyranosyl-(1→2)-β-d-fructofuranoside 3)-l-Tryptophan Ester
Kwaku Kyeremeh 1,
Samuel Kwain 1,
Gilbert Mawuli Tetevi 1,
Anil Sazak Camas 2,
Mustafa Camas 2,
Aboagye Kwarteng Dofuor 3,
Hai Deng 4
and
Marcel Jaspars 4
1
Marine and Plant Research Laboratory of Ghana, Department of Chemistry, School of Physical and Mathematical Sciences, University of Ghana, P.O. Box LG 56 Legon-Accra, Ghana
2
Department of Bioengineering, Munzur University, 62000 Tunceli, Turkey
3
Department of Biochemistry, Cell and Molecular Biology, University of Ghana, Legon, Ghana
4
Marine Biodiscovery Centre, Department of Chemistry, University of Aberdeen, Old Aberdeen AB24 3UE, UK
The Mycobacterium sp. BRS2A-AR2 is an endophyte of the mangrove plant Rhizophora racemosa, which grows along the banks of the River Butre, in the Western Region of Ghana. Spectroscopy and spectrometry aided chemical profiling of fermentation extracts produced by the strain led to the isolation of the new compound, (α-d-Glucopyranosyl-(1→2)-β-d-fructofuranoside)-l-tryptophan ester (1). Compound 1 is an aminoglycoside consisting of a tryptophan moiety esterified to a disaccharide made up of β-d-fructofuranose and α-d-glucopyranose sugars. The full structure of 1 was determined using, U.V.; IR, 1D, 2D-NMR and HRESI-LC-MS data. When tested against Trypanosoma brucei brucei, 1 (IC50 11.25 μM) was just as effective as Coptis japonica (IC50 8.20 μM). It is possible that, compound 1 interferes with the normal uptake and metabolism of tryptophan in the Trypanosoma brucei brucei parasite.
Metabolomics at the Service of Natural Product Drug Discovery
Catherine Roullier
University of Nantes, EA 2160—MMS, 9 rue Bias, 44035 Nantes, France
In natural product drug discovery, several strategies have emerged to highlight the most promising compounds within complex mixtures (fractions or crude extracts) using tools from the metabolomics field. With the huge advances in analytical techniques and the development of bioinformatics, new horizons for natural products drug discovery open. Indeed, a great deal of interest has raised among the scientific community towards the use of metabolomics approaches to highlight, very early in the process, the most interesting compounds to circumvent the drawbacks of bioguided fractionation. In this presentation, the recent developments made by our lab in this area will be presented. In summary, based on LC-HRMS profiles, a complete workflow has been developed combining different freely-available and in-house bioinformatics tools to prioritize subsequent purification. It then allows to highlight from complex mixtures the most valuable molecules because of either:1 their potential novelty (based on an automated annotation process) [1, 2] the presence of the interesting pharmacophores Cl or Br (based on the R MeHaloCoA package) [2,3] a high score of importance in the bioactivity observed (based on the recently developed R FiBiCo script) [3]. These recent advances in the field will be presented together with examples of application to marine fungal extracts.
References
Bertrand, S.; Guitton, Y.; Roullier, C. Successes and pitfalls in automated dereplication strategy using liquid chromatography coupled to mass spectrometry data: A CASMI 2016 experience Phytochem. Lett. 2017, 21, 297–305, doi:10.1016/j.phytol.2016.12.025.
Roullier, C.; Guitton, Y.; Valery, M.; Amand, S.; Prado, S.; Robiou du Pont T.; Grovel O.; Pouchus Y.F. Automated Detection of Natural Halogenated Compounds from LC-MS Profiles-Application to the Isolation of Bioactive Chlorinated Compounds from Marine-Derived Fungi. Anal. Chem. 2016, 88, 9143–9150, doi:10.1021/acs.analchem.6b02128.
Ory, L.; Nazih, E.H.; Daoud, S.; Mocquard, J.; Bourjot, M.; Margueritte, L.; Delsuc, M.A.; Bard, J.M.; Pouchus, Y.F.; Bertrand, S.; et al. Targeting bioactive compounds in natural extracts—Development of a comprehensive workflow combining chemical and biological data. Anal. Chim. Acta 2019, 1070, 29–42, doi:10.1016/j.aca.2019.04.038.
Chemical Investigation of Two Sponges from Rodrigues Island (Indian Ocean) for the Discovery of Molecules with Healthy Ageing Promoting Activity
Pierre-Eric Campos 1,
Flavia Bollaro 1,
Patricia Clerc 1,
Jean-Bernard Boyer 1,
Nicole De Voodg 2,
Nikolas Fokialakis 3,
Ioannis P. Trougakous 4,
Konstantinos Gardikis 5,
Jérôme Bignon 6,
Géraldine Le Goff 6,
Céline Moriou 6,
Ali Al-Mourabit 6,
Jamal Ouazzani 6
and
Anne Bialecki 1
1
LCSNSA, Université de la Réunion, Ile de la Réunion, 97744 Saint-Denis, France
2
Naturalis Biodiversity Center, Darwinweg 2, 2333 CR Leiden, The Netherlands
3
Faculty of Pharmacy, National & Kapodistrian University of Athens, 15771 Athens, Greece
4
Faculty of Biology, National & Kapodistrian University of Athens, 15784 Athens, Greece
5
APIVITA SA, Research and Development Department, Industrial Park of Markopoulo, Markopoulo Mesogaias, 19003 Athens, Greece
6
ICSN-CNRS, Gif sur Yvette, 91190 Paris, France
As the population of developed countries is ageing, the prevalence of a variety of age-related diseases, such as Alzheimer’s disease, cardiovascular diseases, neurodegeneration or cancers is increasing. In order to counteract this major healthcare challenge, marine natural products represent an extraordinary reservoir of structurally diverse bioactive metabolites. In this regard, TASCMAR, a EU-funded Horizon 2020 project, aspires to develop new tools and strategies on discovering novel marine derived compounds with healthy ageing promoting activity. The LCSNSA laboratory, located at Reunion Island and involved in TASCMAR project, has explored marine invertebrates and symbionts from the South West part of Indian Ocean.
Thus, two sponges,
Agelas sp. and
Ernstia naturalis (
Figure 38), from Rodrigues have been investigated. The genus Agelas, already well studied and known to produce bioactive compounds, can be considered as a promising source of metabolites with anti-ageing activity. Sponges of the genus Ernstia on their side have never been studied for their chemical composition. The crude extract of
Agelas sp. was found to inhibit FYN kinase (alzheimer’s disease), CDK7 (cancer) and tyrosinase (skin ageing). The crude extract of Ernstia naturalis was found to inhibit proteasome (oncogenesis) and tyrosinase.
This communication will provide an outline of the methodology used for the chemical investigation of these two sponges. The study of Agelas sp. led to the isolation of five bromopyrrole alkaloids and investigation of Ernstia naturalis yielded to the isolation of four imidazole alkaloids. These nine compounds were characterized by HRMS and NMR. Bioassays on the pure compounds are in progress.
Isolation of Minutissamide Derivatives from a Marine Cyanobacteria Collected in Palau
Marta Pérez,
Laura Coello,
Rogelio Fernández
and
Carmen Cuevas
R&D, PharmaMar, S.A., Avda. de los Reyes 1, Pol. Ind. La Mina, 28770 Colmenar Viejo, Madrid, Spain
Blue-green algae, the most photosynthetic prokaryotes in nature, have been shown to be prolific producers of bioactive secondary metabolites, and a considerable number of these toxins have been developed as anticancer agents [1]. A major class of secondary metabolites from cyanobacteria are polyketide-conjugated nonribosomal peptides, commonly known as lipopeptides. A representative group of these structures are the minutissamides [2], which have been reported to exhibit antiproliferative activity against the MDA-MB-435 human melanoma cancer cell line. This study led to the identification of four new minutissamide analogs from an extract of a cyanobacteria collected in the north of Palau, where different ecosystems such as mangroves, channels and coral reefs coexist. The sample was collected under the Research Agreement of Palau International Coral Reef Center (PICRC)/Republic of Palau (ROP) and PharmaMar. The planar structures were determined using various spectroscopic techniques including HRESIMS and 1D and 2D NMR experiments. The absolute configurations of the amino acid residues were assigned using Marfey’s method after acid hydrolysis, while the relative stereochemistry of some centres was determined through JBC/ROESY analysis. Details of the isolation and spectroscopic data leading to the structure elucidation of the compounds, as well as their biological activities will be presented. The existence of numerous lipopeptides produced by cyanobacteria raises the question of their ecological relevance.
Acknowledgments
We gratefully acknowledge the help of E. Gómez for their valuable technical assistance. The present research was financed in part by Grants from Ministerio de Ciencia, Innovación y Universidades of Spain (AGL2015-63740-C2-2-R and RTC-2016 4611-1, Inmunotop project), cofunded by the FEDER Programme from the European Union.
References
Nunnery, J.K.; Mevers, E.; Gerwick, W.H. Biologically active secondary metabolites from marine cyanobacteria. Curr. Opin. Biothecnol. 2010, 21, 787–793.
Kahk, H.S.; Sturdy, M.; Krunic, A.; Kim, H.; Shen, Q.; Swanson, S.; Orjala, J. Minutissamides E–L, antiproliferative cyclic lipodecapeptides from the cultured freshwater cyanobacterium cf. Anabaena sp. Bioorg. Med. Chem. 2012, 20, 6134–6143.
Isolation of Serrawettin Analogs from a Marine Bacteria Vibrio harveyi with the Assistance of Molecular Networking
Patricia Cruz,
Juan José González,
Pilar Rodríguez,
Paz Zuñiga,
Raquel Altares,
Marta Pérez,
Fernando de la Calle
and
Carmen Cuevas
R&D, PharmaMar, S.A., Avda. de los Reyes 1, Pol. Ind. La Mina, 28770 Colmenar Viejo, Madrid, Spain
Serrawettins are non-ionic biosurfactant lipopeptides produced by the proteobacteria Serratia marcescens. Four molecular species, serrawettins W2, W4, W5 and W6 have also been reported from Serratias associated with roadkill mammals [1]. As part of our continuous efforts to find new secondary metabolites with anticancer properties, a bioactive extract of a cultured Vibrio harveyi (HEL-17) isolated from a bryozoan collected in the Pacific Ocean was selected for further scale up. The sample was collected under the Research Agreement of Palau International Coral Reef Center (PICRC)/Republic of Palau (ROP) and PharmaMar. A literature search indicated that there are only two reports concerning the composition of secondary metabolites from Vibrio harveyi. This fact encouraged us to characterize the compounds present in this extract using LC-MS/MS analysis combined with molecular networking and NMR. This study led to the isolation of six new serrawettin analogs together with other known families of glycolipids and fatty acids, showing the importance of combining different analytical methods. Details of the producer microorganism, isolation, and spectroscopic data leading to the structure elucidation of the new compounds, as well as their biological activity will be described in this communication.
Acknowledgments
The present research was financed in part by Grants from Ministerio de Ciencia, Innovación y Universidades of Spain (RTC-20164892-1, DESPOL project).
References
Motley, J.; Stamps, B.W.; Mitchell, C.A.; Thompson, A.T.; Cross, J.; You, J.; Powell, D.R.; Stevenson, B.S.; Cichewicz, R.H. Opportunistic Sampling of Roadkill as an Entry Point to Accessing Natural Products Assembled by Bacteria Associated with Non-anthropoidal Mammalian Microbiomes. J. Nat. Prod. 2017, 80, 598–608.
New Peptides Isolated from the Indonesian Sponge Theonella sp.
Rogelio Fernández,
Marta Pérez,
Santiago Bueno
and
Carmen Cuevas
R&D, PharmaMar, S.A., Avda. de los Reyes 1, Pol. Ind. La Mina, 28770 Colmenar Viejo, Madrid, Spain
Sponges of the genus Theonella sp. have been found to contain a vast variety of bioactive metabolites, including macrolides, cyclic and linear peptides, steroids and alkaloids. Most of them have exhibited significant cytotoxic activity [1].
In the course of our screening program to isolate novel compounds with antitumor properties from marine sources, we have isolated two new linear peptides from a Theonella specimen, which was collected off the coast of Seram in Indonesia under collaboration with Andalas University.
These compounds were obtained by bioassay-guided fractionation of an organic extract of the organism, using VLC RP-18 chromatography and reverse phase semi-preparative HPLC. Structure elucidation of these new metabolites was carried out by spectroscopic methods including MS, 1H, 13C and 2D-NMR. The stereochemistry of the amino acids was determined by hydrolysis followed by derivatization with Marfey’s reagent and comparison with commercial standards by HPLC-MS. The two new linear peptides isolated contain an unprecedented tribromopyrrol unit in their structure. This finding highlights Theonella and its microbiota as an endless source of novel structures.
Acknowledgments
We gratefully acknowledge the help of E. Gómez for their valuable technical assistance. The present research was financed in part by Grants from Ministerio de Ciencia, Innovación y Universidades of Spain (AGL2015-63740-C2-2-R and RTC-2016 4611-1, Inmunotop project), cofunded by the FEDER Programme from the European Union.
References
Micheal, C.; Wilson, M.C.; Mori, T.; Rückert, C.; Uria, A.R.; Helf, M.J.; Takada, K.; Gernert, C.; Steffens, U.A.E.; Heycke, N.; et al. An environmental bacterial taxon with a large and distinct metabolic repertoire. Nature 2014, 506, 58–62.