Molluscan Compounds Provide Drug Leads for the Treatment and Prevention of Respiratory Disease
Abstract
:1. Introduction
1.1. Respiratory Disease Pathology and Epidemiology
| Respiratory Disease * | Disease Classification | Causative/Risk Factors | Predominant Symptoms | Estimated Worldwide Morbidity (Annual) (P: Prevalence, I: Incidence) | Estimated Worldwide Mortality (Annual) | Conventional Treatments | Trends | Ref |
|---|---|---|---|---|---|---|---|---|
| Infectious diseases (e.g., TB; NTM; influenza; pneumonia †; corona viruses) | - Communicable ‡ - Acute - May become chronic if unresolving or recurrent | - Opportunistic bacterial; viral; fungal or parasitic invasion - Risk factors: low immunization rates; overpopulation; conditions of compromised immunity | Inflammation; cough; increased mucus; fever; dyspnea; tachypnea; malaise; muscle and join pain; sore throat; secondary infections | - TB: 10 million (P) - NTM: 40 per 100,000 population (P) - Influenza: 5–15% of population; 3–5 million severe cases (I) - RSV: 34 million child episodes (I) - COVID-19: >56 million § (P) | - >4 million total ¶ - TB: 1.5 million - Pneumonia: >1.3 million children <5 y (15% of all deaths) - Seasonal influenza: 290,000–650,000 - COVID-19: 1,500,000 § | - Antibiotics (bacterial) - Neuraminidase inhibitors (viral) - Symptomatic treatment - Immunization | - Consistently within top 3 causes of death - TB incidence declining by 2%/y, NTM increasing 40%/y - Increasing epidemics and drug resistant strains - Highest impact in developing countries | [2,26,27,28] |
| Chronic obstructive pulmonary disease (COPD) | - Non-communicable ‡ - Chronic with acute episodes - Progressive - Irreversible | - Tobacco smoke and other inhaled environmental pollutants - Frequent/chronic lower respiratory infections, asthma and abnormal lung development - Genetic factors | Chronic parenchymal and airway inflammation; persistent airflow restriction; dyspnea; wheeze; cough; decreased airway elasticity; airway remodeling; mucociliary dysfunction; co-morbidities | - >250 million (P) - Usually becomes apparent in people >40 yes of age | - 3.2 million - Third leading cause of death | - Cessation of smoking - Symptomatic treatment - Inhaled corticosteroids; long-acting β agonists; leukotriene modifiers - Immunization against infectious diseases | - Increasing prevalence and mortality rates | [2,27,29] |
| Asthma | - Non-communicable - Chronic with acute episodes - Reversible | - Genetic factors - Environmental triggers - Airway hyperresponsiveness | Airflow restriction; wheeze; dyspnea; cough; airway remodeling | - >339 million (P) - 24.8 million DALYs lost | - Relatively low mortality rate - 420,000 (2016) | - Medications for: rescue (e.g., fast-acting β agonists), maintenance (e.g., inhaled corticosteroids; long-acting β agonists; leukotriene modifiers) and allergies - Avoidance of triggers | - Increasing incidence - Highest mortality (80%) in developing countries | [2,5,27,30,31] |
| Lung cancer | - Non-communicable - Chronic - Progressive | - Tobacco smoke and other inhaled environmental pollutants - Physical carcinogens (e.g., ionizing radiation) - Other chronic respiratory diseases - Genetic factors | Dyspnea; hoarseness; hemoptysis; pain; loss of appetite; weight loss; fatigue; persistent cough | - 2.09 million (P) - 1.8 million (I) | - 1.76 million | - Surgery - Radiation and chemotherapy - Palliative care and psycho-social support | - 15% of diagnosed cancers - Most fatal cancer - 19% of deaths | [2,6,27] |
| Acute respiratory distress syndrome (ARDS)/acute lung injury (ALI) | - Non-communicable - Acute | - Trauma - Pulmonary infection - Non-pulmonary sepsis - Certain medical procedures | Severe dyspnea and tachypnea; pulmonary hemorrhage; edema; hypertension; hypoxemia; tissue damage; fibrosing alveolitis | - ARDS: 58.7–75 per 100,000 people (I) - ALI: 78.9 per 100,000 people (I) | - In-hospital mortality 38% for ALI; up to 46.1% for ARDS | - Corticosteroids and other anti-inflammatories; vasodilators - Mechanical ventilation - Hemodynamic management - Surfactant therapy | - 10% of all patients in intensive care treated for ARDS - Survival rates improving | [11,19] |
| Cystic fibrosis | - Non-communicable - Chronic with acute episodes - Progressive | - Autosomal recessive genetic factors (CFTR mutation) - Exacerbated by environmental triggers and infection # | Bronchiecstasis; persistent airway infection and inflammation; excessive, thick mucus and poor clearance; pneumothorax; hemoptysis; tissue damage; gastrointestinal, metabolic and reproductive manifestations | - 90,000 (P) (likely underestimated) - 1000 (I) | - More than half of patients die before the age of 18 | - Antibiotics - Immunisations - Nebulised hypertonic saline; dornase alfa; mannitol - CFTR modulators (e.g., Ivacaftor) - O2 therapy; pulmonary rehab. - Management of co-morbidities and nutrition - Lung transplant | - Survival rates improving | [32] |
1.2. Conventional Treatments and the Need for Alternatives
1.3. Molluscs: A Wealth of Potential Therapeutic Compounds
2. Literature Search Methods and Evaluation
3. Uses of Molluscs in Traditional Medicines for Respiratory Disease
3.1. Traditional Molluscan Respiratory Medicines
| Respiratory Disease or Symptom | Words/Phrases Used in the Literature to Describe Symptom or Disease | No. of Remedies * | No. of Species | Mollusc Parts Used | Cultures/Traditional Medicine Systems | Ref. |
|---|---|---|---|---|---|---|
| Allergy | Allergy; hypersensitivity; ENT or pulmonary allergies | 10 | 2 | Egg masses; flesh; whole animal; ink; shell | Europe; China | [104,118,119,120] |
| Asthma | Asthma; shortness of breath; dyspnea; wheeze; asthmatic cough; dyspnea with cough | 19 | 46 | Body; foot; shell; pearl; eggs | China; India; South America; Middle East | [78,91,110,113,114,115,118,119,120,121,122,123,124,125] |
| Cancer | Cancer; tumor; neoadjuvant treatment | 4 | 17 | Flesh; shell; operculum | India; South America; Egypt; China | [104,113,114,126,127] |
| Cough | Cough; chesty cough; croup; hemoptysis; laryngismus; whooping cough; cough associated with infection or fever; cough associated with inflammatory conditions; nervous cough; cough with chest stuffiness and dyspnea; xeropulmonary cough; cough and regurgitation | 28 | 127 | Adductor muscle; egg masses; flesh; mucus; pearl; shell | China; Europe; India; South America; Middle East; Nigeria | [91,113,114,123,124] |
| Ear problems | Ear problems; ear pain; ear inflammation; ear ache; ottorhoea; otitis media; parotid gland swelling and hearing loss; ear and eye diseases | 9 | 14 | Flesh; mucus; shell; operculum | China; Europe; India; Egypt; Nigeria | [104,109,114,118,119,120,123,126,127] |
| Fever | Fever; high fever; low fever; fever in children; fever and convulsion in children; high fever; feverish sensation in chest; night sweating; heat; heat toxicity | 15 | 59 | Adductor muscle; flesh; pearl; shell; whole animal | China; Europe; India; Korea | [91,104,109,114,128] |
| Low immunity | Strengthens immune system | 3 | 3 | Flesh; shell | Europe | [109] |
| Infection † | Infection; pneumonia; measles; flu; bronchitis; anthrax; upper respiratory tract infections in children; infectious diseases; bronchitis; measles; conjunctive congestion with swelling and pain | 18 | 56 | Flesh; shell; mucus; whole animal | China; Europe; South America; India | [91,109,113,114,118,119,120,129] |
| Respiratory inflammation | Inflammation; sinus inflammation; inflammatory conditions; parotid gland swelling; acute and chronic chest ailments; edema; swelling and pain; acute and chronic sinusitis | 10 | 36 | Flesh; shell; mucus; whole animal; operculum | China; Europe; India | [91,109,114,123] |
| Mucus | Mucus; excessive mucus; phlegm; congestion; nasal congestion; used as expectorant; retention of phlegm and fluid; phlegmatic heat; retention of fluid in chest | 22 | 101 | Adductor muscle; flesh; operculum; pearl; shell | China; Europe; India | [109,114,118,119,120] |
| Sore throat | Sore throat; pharyngitis; hoarseness; tonsillitis; tracheitis; pharynalgia | 10 | 21 | Flesh; mucus; shell; whole animal; pearl | China; Europe; South America; India | [91,114,130] |
| Tuberculosis ‡ | Tuberculosis; pthisis; scrofula; pulmonary tuberculosis; tuberculosis of lymph nodes | 47 | 237 | Adductor muscle; egg masses; flesh; shell; mucus; whole animal; pearl | China; Europe; India; South America | [91,113,114,115] |
| Other § | Chest and abdomen heat and pain; pain in sternum; bleeding from five aperture or subcutaneous tissue (e.g., eye; ear; nose; teeth; tongue) | 6 | 35 | Flesh; shell; pearl | China | [104] |
3.2. Supporting Evidence for the Bioactivity of Traditional Molluscan Respiratory Medicines
3.3. Taxonomic and Geographic Trends
| Number of Different Mollusc Families Represented in Each Literature Type | ||||||
|---|---|---|---|---|---|---|
| Traditional Medicines | Biomedical Studies | |||||
| TCMs * | OTMs | In Vitro | In Vivo | Clinical Trials | Model Antigen † | |
| Mollusc class | ||||||
| Gastropoda | 20 | 15 | 49 | 7 | 3 | 2 |
| Bivalvia | 23 | 6 | 9 | 3 | 1 | 0 |
| Cephalopoda | 1 | 3 | 4 | 0 | 0 | 0 |
| Polyplacophora | 2 | 0 | 1 | 0 | 0 | 0 |
| Aplacophora | 0 | 0 | 0 | 0 | 0 | 0 |
| Monoplacophora | 0 | 0 | 0 | 0 | 0 | 0 |
| Scaphopoda | 0 | 0 | 0 | 0 | 0 | 0 |
| Habitat type | ||||||
| Marine | 46 | 17 | 55 | 8 | 3 | 1 |
| Freshwater | 0 | 3 | 2 | 0 | 0 | 0 |
| Terrestrial | 0 | 4 | 5 | 2 | 1 | 1 |
4. Chemistry, Bioactivity and Biomedical Applications of Molluscan Extracts and Compounds Relevant to Respiratory Disease
4.1. Overview
| Type of Study | In Vitro | In Vivo Models | Clinical Trials | Model Antigen * | Total Studies | % of Studies |
|---|---|---|---|---|---|---|
| No. of studies | 54 | 25 | 11 | 16 | 106 † | |
| No. of compounds/extracts ‡ | 327 | 15 | 5 | 3 | ||
| No. of studies reporting bioactivity § | ||||||
| Anticancer | 13 | 7 | 3 | 23 | 22 | |
| Antibacterial | 33 | 1 | 34 | 32 | ||
| Antiviral | 4 | 4 | 4 | |||
| Antifungal | 7 | 7 | 7 | |||
| Anti-inflammatory ‖ | 1 | 5 | 3 | 9 | 8 | |
| Antitussive | 1 | 1 | 2 | 2 | ||
| Immunogenic ¶ | 2 | 15 | 4 | 16 | 37 | 35 |
4.2. Antimicrobial Activity
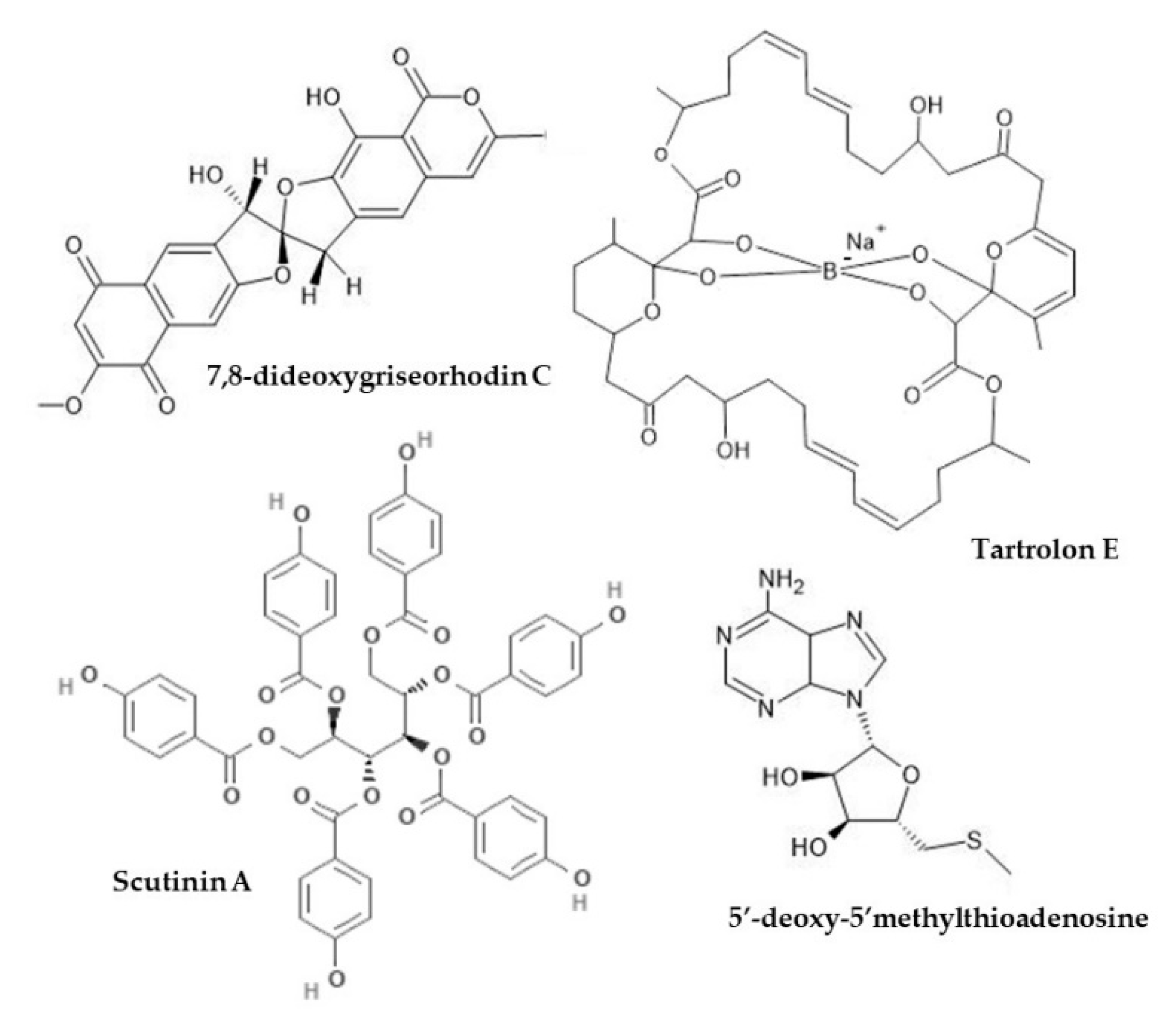
4.3. Anti-Inflammatory Activity
4.4. Anticancer Activity
| Mollusc Class Family | Derivative Part | Specific Extract/ Compound | Microbial or Cellular Target | Effective Concentrations * | Other Important Findings | Ref |
|---|---|---|---|---|---|---|
| Bivalvia | ||||||
| Mytilidae | Sperm | Crude perchloric acid extract (CE) and 3 isolated protamine-like (PL) proteins | Clinical and lab strains: Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae; human lymphocytes and red blood cells | MICs 7.8–250 μg/mL; MBCs (μg/mL): CE: 15.7–125, PL-II 15.7–125, PL-III 62.5–250, PIV 62.5–250 | Digested and non-digested PL-proteins had same effect; low toxicity to lymphocytes (80–90% viability), no sig. hemolysis; effect re protein membrane binding, cytosolic intrusion and nucleotide leakage | [187] |
| Hemolymph (hemocytes) | Myticin C and 9 peptide fragments | P. aeruginosa, S. aureus, Micrococcus lysodeikticus | MICs >64 μM for P. aeruginosa; MIC 32 μM of 3 peptide fragments for S. aureus | [168] | ||
| Hemolymph (hemocytes, plasma) | Myticin A and B peptides | M. luteus, Bacillus megaterium, S. aureus (clinical strain), Listeria monocytogenes (G+); P. aeruginosa (clinical strain), Brucella suis (G−); Fungi: Fusarium oxysporum | MBCs (μM): G+ 2.25- >20 Myt A, 1- >20 Myt B; G− >20 Myt A and B; fungi >20 Myt A, 5–10 Myt B | [167] | ||
| Ostreidae | Hemolymph | Cellular (c) and acellular (a) hemolymph fractions (0.2 μm filtration) | Human adenovirus (respiratory strain AdV-5) cultured in Vero and HEp-2 cell lines | CC50 0.19–0.36 mg/mL; EC50 0.05–0.16 mg/mL | Crassostrea rhizophorae cellular fraction showed best (64%) viral inhibition, particularly w post-infection treatment; virus preincubation w both fractions protected >90% of cells indicating virucidal activity at non-cytotoxic concs | [196] |
| Teredinidae | Gill (symbiotic Teredinibacter turnerae) | Tartrolon E | P. aeruginosa, methicillin-sensitive and methicillin resistant S. aureus (MSSa, MRSa) | MICs (mg/mL): 0.31 for P. aeruginosa, 0.08 for MSSa, 1.25 for MRSa | [169] | |
| Cephalopoda | ||||||
| Octopodidae | Suckers | Peptide (OctoPartenopin) (crude + 6 HPLC fractions + 5 synthetized fractions) | S. aureus, P. aeruginosa | MIC80 (μg/mL) 50–200 S. aureus, 50- >300 P. aeruginosa; 80 μM peptides inhibit and eradicate up to 60% biofilm formation | Also antifungal activity; improved activity with synthetized peptides | [170] |
| Sepiidae † | Shell | Chitosan | Bacteria: K. pneumoniae, Bacillus cereus (G+), S. aureus, M. luteus (G−); Fungi: Aspergillus niger, Fusarium sp. | 50 mg/mL (preliminary) | S. officinalis chitosan stronger activity than shrimp and crab chitosan; ZIs similar to gentamycine for G−, > cyclohemimide for fungi | [135] |
| Shell | Chitosan | Streptococcus pneumoniae, S. aureus (G+), P. aeruginosa, K. pneumoniae (G−) | MIC (μg/mL): 60–100 (G−), 100 (G+) | Higher MICs for phosphorylated chitosan | [136] | |
| Salivary glands | PSG toxin (glycopeptide) | K. pnemoniae, Streptococcus pyogenes | 1–50 μM (preliminary) | Little difference between 1–50 μM concs; low toxicity to zebrafish embryo | [290] | |
| PSG toxin (glycopeptide) | K. pnemoniae, S. aureus, P. aeruginosa | 25–100% (preliminary) | Susceptibility: S. aureus > K. pneumonia > P. aeruginosa; ZIs comparable to Ciprofloxacin | [291] | ||
| Sepiidae, Octopodidae | Body | Crude CH3OH extracts | Clinical bacterial strains: P. aeruginosa, K. pnemoniae, S. aureus, S. pneumoniae, Streptococcus sp., Vibrio alginolyticus; Fungi: Pencillium italicum, Alternaria alternata (allergen), Fusarium equisetii | MIC range 60–100 mg/mL | Extract from S. kobiensis showed the best/broadest spectrum activity; no positive control or toxicity data | [185] |
| Gastropoda | ||||||
| Achatinidae | Mucus | Mytimycin-AF (antimicrobial peptide) | S. aureus, B. megaterium, K. pneumoniae | MIC (μg/mL) S. aureus 1.9, B. megaterium 15, K. pneumoniae 30 | Better activity than human AMP control for S. aureus; minimal hemolysis (max 3.9% at 329 μg/mL) | [292] |
| Achatinidae, Helicidae | Mucus | Crude mucus and 4 size-separated fractions | K. pneumoniae, P. aeruginosa (3 strains), S. aureus, S. pyogenes, Acinetobacter sp. (clinical), Serratia marcescens (clinical) | 1:3 crude mucus:PBS (preliminary) | Crude H. aspera mucus inhibited S. aureus and P. aeruginosa; A. fulica mucus inhibited S. aureus; other microorganisms unsusceptible | [174] |
| Babyloniidae | Body | Crude extracts ‡ | P. aeruginosa, K. pneumoniae, S. aureus, S. pneumoniae; Fungi: A. flavus | Crude extract (preliminary) | Ethanol extract had highest antimicrobial activity; most effective against P. aeruginosa, least effective against S. aureus | [175] |
| Clathurellidae | Hepatopancreas (symbiotic Streptomyces sp.) | CH3OH extract (lobophorin compounds) | Mycobacterium tuberculosis, P. aeruginosa, Burkholderia cepacia; CEM-TART cell line | MIC90: 1.3–24 μM for M. tuberculosis, >100 for P. aeruginosa and B. cepacia | Strong cytotoxicity at similar MIC concentrations (0.3–100 μM) therefore not a suitable therapeutic candidate | [117] |
| Conidae | Venom | Conotoxin MVIIA and 9 analogues | S. aureus | MICs: >500 μM MVIIA, 7–78 μM analogues | MVIIA considered inactive, different activity among analogues re. cyclic structure and side chain modification | [293] |
| Cypraeidae ‖ | Shell | Powder | Micrococcus sp. | 4–5% w/v shell powder in distilled water (preliminary) | Dose-dependent antipyretic effect in vivo (not sig) | [134] |
| Dorididae | Sperm (also in egg masses) | 5’-deoxy-5’-methylthio-adenosine (MTA) and two natural analogues (xylo-MTA and xylo-A) | S. aureus, Corynebacterium diphtheriae; Vero and C8166 cell line | MICs: MTA 33 μM, xylo-MTA 200 μM, xylo-A 18 μM | MICs always higher than minimum non-toxic concentrations; xylo-A most toxic, xylo-MTA least toxic; no positive control | [172] |
| Fissurellidae | Body | Scutinin A and B | P. aeruginosa | MIC: 30 μg/mL scutinin A, 100 μg/mL scutinin B | [171] | |
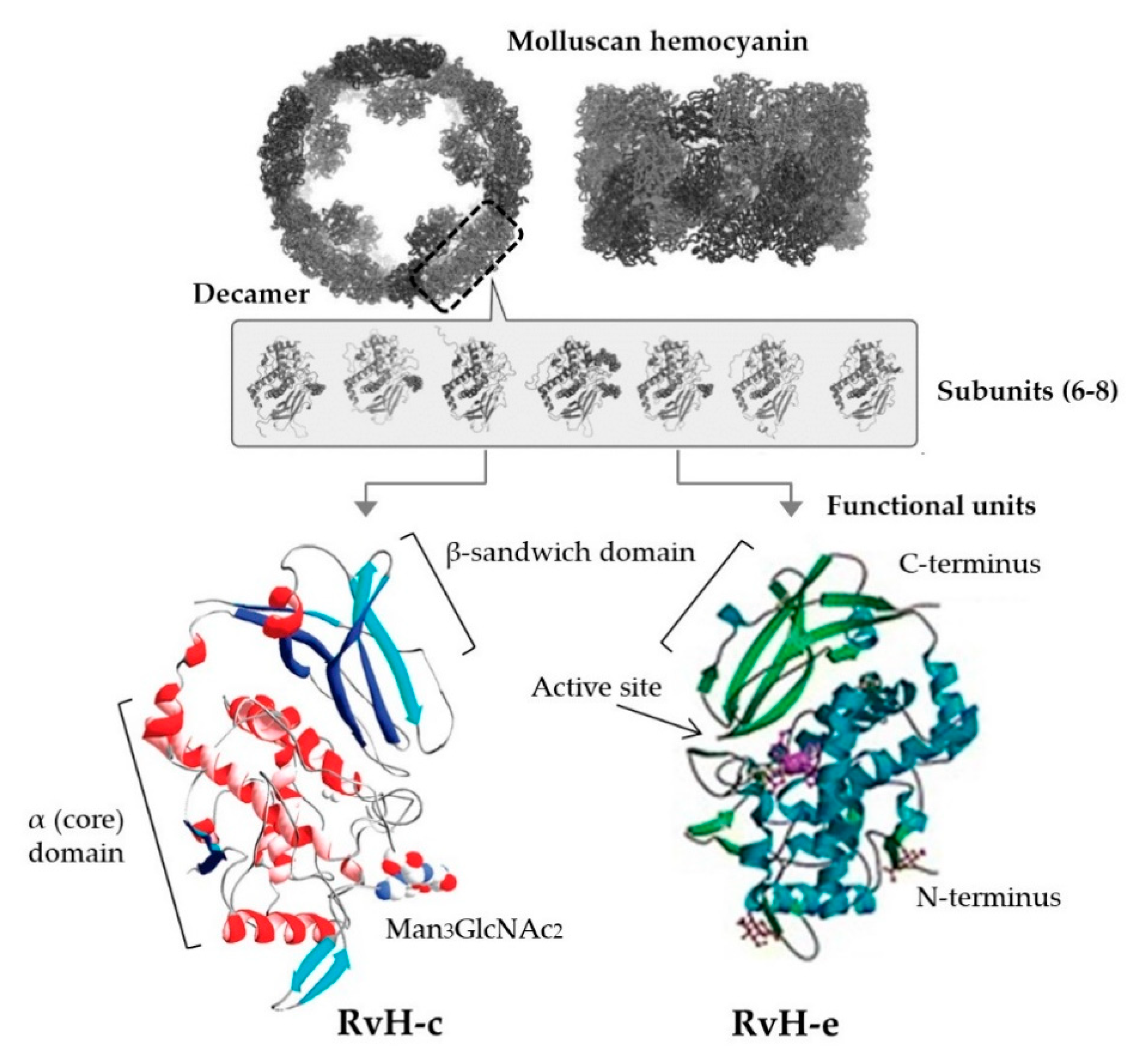
| Helicidae, Muricidae | Hemolymph | Experimentally purified Hc (βc-HaH subunit + 8 FUs, RvH1 + 4 FUs) | S. aureus, S. pyogenes, P. aeruginosa | MIC: 6.5 μM βc-HaH; MIC not calculated for RvH1 (1.25–10 μM range) | HaH more effective than RvH; native Hc more effective than subunits from both species; βc-HaH S. aureus and S. pyogenes 60 and 51% inhibition respectively, RvH1 35% inhibition, relative to control; limited activity against P. aeruginosa | [73] |
| Muricidae | Hemolymph | Experimentally purified Hc (RvH), glycosylated (RvH-c) and non-glycosylated (RvH-b) subunits | Respiratory synctial virus (RSV), cultured in Hep-2 cell line | RvH-c 1 mg/mL | RvH-c effective against replication of RSV (71.4% inhibition at 1 mg/mL), no effect on other tested viruses (poliovirus, cocksackie virus); native RvH and RvH-b no antiviral activity; no cytotoxic effect at highest concs | [197] |
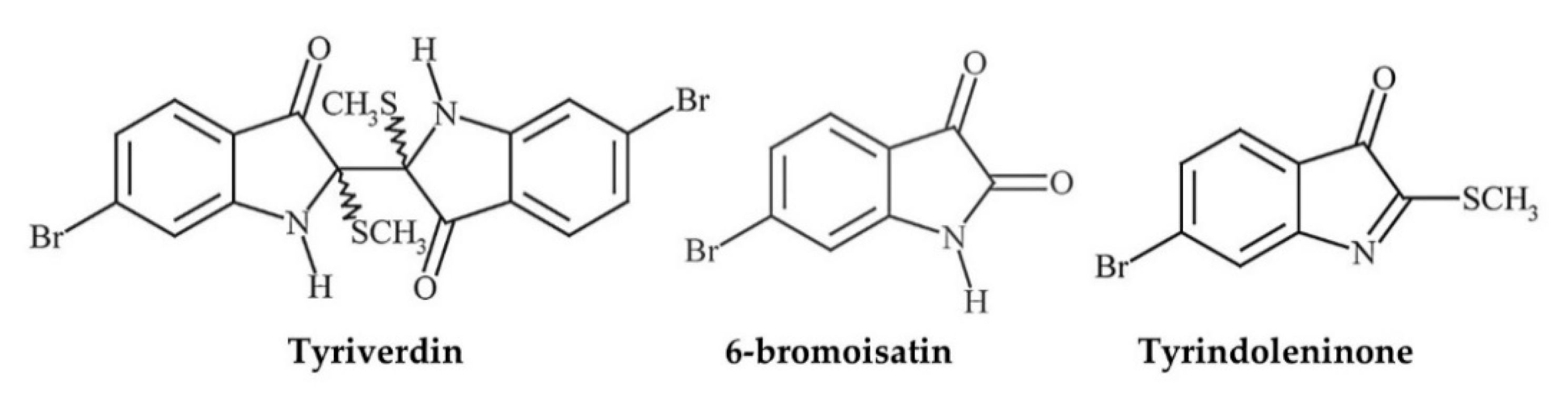
| Egg masses | Crude extracts (de, eth, CHCl3, CH3OH-H2O) # and isolated ty, tv, Tp, 6-b § | P. aeruginosa (G−), S.aureus (G+) | MICs (mg/mL): 0.0005 tv, 0.5–1.0 ty, 0.1–1.0 6-b, >1 Tp, 1.0–10 CHCl3, 0.1 de, 10 eth, >50 CH3OH-H2O | Lipophilic extracts had better activity; tv bacteriostatic, ty bactericidal | [149] | |
| Hemolymph | Experimentally purified Hc (11 protein fractions) | S. aureus, K. pneumoniae | 113–598 μg/mL | Peptides 8, 9, 10 and 11 showed >90% inhibition; S. aureus more susceptible; different proteins more/less active against different bacteria; longer protein chains more effective | [294] | |
| Body | Crude extracts ** | K. pneumoniae, P. aeruginosa, S. pneumoniae, Citrobacter sp., B. cereus | MICs 0.05–0.12 mg | Acetone extract most effective; similar effectiveness against other (non-resp) pathogens | [184] | |
| Olividae | Body | Acid-acetone peptide extract | S. aureus, P. aeruginosa, K. pneumoniae | MIC (mg/mL): 2.5 S. aureus, 0.039 P. aeruginosa, 1.25 K. pneumoniae; MBC (mg/mL): 2.5 S. aureus, 1.25 P. aeruginosa, >2.5 K. pneumoniae | Protein ZIs comparable to control antibiotics; ciprofloxacin and cefotaxime MICs reduced by >100% w protein extract, metronidazole and erythromycin MICs increased; effects re changes in membrane porosity/permeability | [179] |
| Body | Acid-acetone peptide extract | P. aeruginosa | MIC: 39.06 ug/mL (Gentamycin MIC 1.95 ug/mL) | Bacteriostatic; dose dependent reduction in virulence factors (pyoverdine, pyocyanin, protease)- peptide mix (69%) similar to gentamycin (72%) at 1/2 MIC; 50% reduction in biofilm formation at 39 ug/mL, 2.5 mg/mL required to degrade pre-formed biofilm | [178] | |
| Onchidiidae | Body | Dolabellanin B2 (AMP) | S. aureus, P. aeruginosa, K. pneumoniae | MICs: 10–25 ug/mL | Structure-function characterization; better activity against G+; compound identified as one previously isolated from Dolabella auricularia | [295] |
| Patellidae, Donacidae †† | Body | Acid-acetone extract | S. aureus, S. pneumoniae, K. pneumoniae, P. aeruginosa | MICs 17–20 mg/mL | ZI’s similar to ciprofloxacin; Galeta paradoxa extract stronger antibacterial than Patella rustica extract, which showed good antifungal activity | [296] |
| Pharidae | Body ‡‡ | 2 sialic acid-binding lectin recombinant proteins (rSgSABL-1, -2) | Staphylococcus aureus, Micrococcus luteus; Solen grandis (mollusc) hemocytes; mice (n = NA) immunised i.p. 2x w rSgSABL-1 (100 μg/mL) or rSgSABL-2 (180 μg/mL) in CFA ‖‖ | 100 μg/mL (phagocytosis), 90 μg/mL (microbe agglutination, and encapsulation); 100–180 μg/mL (Ab production) | High binding affinity to S. aureus peptidoglycan (PAMP) (also LPS, β-glucan); agglutination effect on M. luteus; enhanced phagocytosis and encapsulation ability (p < 0.05); antisera Ab reactivity with rSgSABL-1 and -2 | [188] |
| Plakobranchidae | Body | Kahalalide F and 8 analogues | Bacteria: P. aeruginosa, methicillin resistant S. aureus, M. tuberculosis (H37Rv), M. intracellulare; Fungi: Cryptococcus neoformans, A. fumigatus, Fusarium sp. | MIC 9.4–>16 μg/mL Kahalalide F analogues (M. tuberculosis); 30 μM antifungal | >90% inhibition of M. tuberculosis and up to 100% fungicidal activity comparable to controls; no activity against P. aeruginosa or S. aureus; high test concs- cytotoxicity test concs lower than MICs | [253] |
| Body | Kahalalide F, analogues KZ1 and KZ2 | Aspergillus sp., Fusarium sp. | 20 mg KZ1 and KZ2 | Analogue bioactivity profiles comparable to KF; antifungal activity comparable to ketonazole | [257] | |
| Strombidae | Body | Crude extracts ## | S. aureus, P. aeruginosa | 1–100 μg/mL H2O extract (preliminary) | Stronger activity against P. aeruginosa over S. aureus | [297] |
| Truncatellidae | Body (symbiotic Streptomyces sp.) | 7,8-dideoxygriseorhodin C (DC) | Methicillin-resistant S. aureus; MDCK and AA8 cell lines | MICs (μg/mL): 0.08–0.12 DC, 1.59–6.24 oxacillin; combination DC 0.01–0.02 DC and 0.02–0.298 Oxacillin | DC stronger than oxacillin as single agents; reduction MICs w combination; no cytotoxicity (IC50 15.84 μg/mL and >49.5 μg/mL for MDCK and AA8 cells, respectively) | [176] |
| Veronicellidae | Mucus | Concentrated crude mucus and 4 fractions (PUFA 39, 40, 49, 50) | Measles virus (Edmonston wild-type), cultured in Vero cell line | 60–220 ng/mL mucus/fraction 39 inhibition of viral replication; 2% mucus/fraction 39 inhibition of CPE | Effect attributed to disruption of the virus’ lipoprotein envelope; not cytotoxic to Vero cells (IC50 41 μL crude, 92.6 μL fraction 39) | [154] |
| Mucus | Crude concentrated mucus and 3 fractions (PUFAs 39, 40, 49) | Influenza A (H1N1) virus, cultured in MDCK cell line | 2% or 60–80 ng/mL crude mucus and fraction 39 | Inhibition of viral replication and >80% decrease in viral load in infected cells w crude mucus and frac 39; not cytotoxic (although IC50 NA); may interfere w binding of virus to host cell receptor | [153] | |
| 23 families §§ | Egg masses | Crude homogenised egg material and extracts ††† | P. aeruginosa, S. aureus | 1–10 mg/mL CHCl3 and CH3OH-H2O extracts (preliminary) | S. aureus and P. aeruginosa inhibited by 79% and 72% of tested egg masses, respectively; no dif in activity between tough and gelatinous egg masses | [65] |
| 16 families *** | Body | CHCl3 extracts | Bacteria: S. aureus, P. aeruginosa, K. pneumoniae; Fungi: A. fumigatus; chicken red blood cells and brine shrimp | Crude extracts (preliminary) | Positive antimicrobial activity; best result w extract of Conus betulinus; low toxicity to brine shrimp (LC50 12–42 μg/mL); 10/25 sp. extracts showed hemolytic activity; no antibiotic controls | [298] |
| 5 families ‡‡‡ | Body, gill and mantle (GM), digestive gland (DG) | 10, 40 and 80% SPE fractions of acidic (HCl) extract in sterile water | M. luteus and B. megaterium; Vero cell line | Most effective MICs (μg/mL): 43 80% DG extract both bacteria, 63 80% DG extract M. luteus, 40 80% G+M extract B. megaterium, 2560 M. luteus) | 40 and 80% fractions from all sp. effective; Crassostrea edule extracts showed best activity (also against non resp viruses); <50% cytotoxicity; positive controls (lysozyme and polymyxine B) more effective than extracts | [45] |
| Mollusc Class Family | Derivative Part | Specific Extract/ Compound | Microbial or Cellular Target | Effective Concentrations | Other Important Findings | Ref |
|---|---|---|---|---|---|---|
| Bivalvia | ||||||
| Mactridae * | Body | Spisulosine | SW1573 (human alveolar carcinoma) cell line (and 4 other human tumor cell lines) | GI50: 1.3 μM | Spisulosine most effective of tested compounds- >positive controls Cisplatin (3.0 μM) and Etoposide (15.0 μM); GI50′s 0.7–2.6 μM for range of cell lines- SW1573 intermediate sensitivity; selective CK1ε inhibition | [299] |
| Ostreidae | Hemolymph (hemocytes) | Tumor necrosis factor (CgTNF-2) | A549 (human alveolar carcinoma) | 200 ng/mL recombinant CgTNF-2 | CgTNF-2 expression upregulated in response to bacterial PAMPs (incl. Staphylococcus aureus), and serum lysosome activity, NO content and antibacterial activity (non-resp) increased (p < 0.05) | [247] |
| Veneridae | Body | (NH4)2SO4 fractionated peptide (‘Mere15′) | A549 and range of other non-respiratory cancer cell lines (breast, cervical, colorectal, pancreatic, liver); benign cells NIH 3T3 and MCF-10A | IC50: 31.8 μg/mL | A549 most susceptible among cancer types, therefore used in subsequent assays and animal model; not cytotoxic to benign cells (IC50 > 120 μg/mL) | [252] |
| Cephalopoda | ||||||
| Loliginidae † | Digestive gland/liver | Lipid extract | A549 and Vero cell lines | 70% growth inhibition at 960 μL/mL, 55% at 480 μL/mL; CC50 260 μg/mL for A549 (NA for Vero) | Better growth inhibition of A549 (max 70%) compared to Vero (max 7%) | [249] |
| Gastropoda | ||||||
| Ampullariidae | Body | “Polysaccharide extract” ‡ | A549 cell line | 20–200 μg/mL | 24-h reduction in tumor growth: 31% at 20 mg/mL, 43% at 50 mg/mL, 46%, at 100mg/mL, 57% at 200 μg/mL; 84% antioxidant at 5 mg/mL | [250] |
| Aplysiidae | Body | Dolastatin-10 | Human SCLC cell lines (NCI-H69, NCI-H82, NCI-H446, NCI-H510) | IC50 range 0.03–0.184 nM | >50% G2/M phase arrest, bcl-2 phosphorylation; pro-apoptotic mechanism | [254] |
| Chilondontidae | Body | Crude CHCl3 extract (1.25%) in Hanks Balanced Solution | A549 cell line | 5–20 μg/mL | 30–40% cytotoxicity at 5–20 μg/mL; apoptosis at 10 μg/mL, not increasing w higher doses or exposure time; wound area reduced by 28.3% at 5 μg/mL; all results p < 0.05; inhibitory effect on matrix metalloproteinase | [248] |
| Helicidae | Mucus | Helicidine formula (glycoproteins) (purified NaCl extract) | Tracheas dissected from Dunkin-Hartley guinea pigs; epithelium (E+) and epithelium-free (E−) strips prepared | 0.005–0.5 mg/mL (min-max effective) | Dose dependent reduction of contraction by 35% in E+ and 25% in E−; PGE2 higher post treatment (p < 0.01); related to COX inhibition (p < 0.01) | [79] |
| Hemolymph | Experimentally purified Hc (HpH) | Influenza (H3N2) immunisation model using Balb/c mice (n = 5–8/group) immunised w 50 μg influenza peptide (IP), IP w CFA, IP w alum, or IP w HpH (16, 40, 100 μg) | 100 μg HpH; 50 μg antigen + 100 μg HpH | Ex vivo spleenocytes of mice treated w IP+100 μg HpH showed stronger cytotoxicity against infected cells in vitro compared to all other groups (p < 0.0005) | [83] | |
| Plakobranchid-ae | Body | Kahalalide F (KF) and 8 analogues | A549 and NCI-H322M (human bronchioalveolar carcinoma) and Vero cell lines | GI50: 0.131–13.7 μM (compound-tumor specific e.g., for A549 analogues 8 and 16 IC50 0.166 and 13.189 μM, 0.165 and 0.167 µM for NCI-H322M) | Some compounds showed higher potency than Paclitaxel; similar anticancer activity among other tested cancer cell lines; no cytotoxicity at 4.76 μg/mL | [253] |
| Body (originally) | KF (synthetic) | 4x human NSCLC cell lines (A549, SW1573, NCI-H292 and NCI-H460) | IC50 0.1–7.0 μM | A549 and H292 particularly sensitive, H460 least sensitive; inhibition of ErbB andPI3K-Akt signaling at IC50 concs and necrosis-like cell death | [256] | |
| Body (originally) | PM02734 (elisidepsin trifluoroacetate; synthetic KF3 derivative) | HOP62 (human lung adenocarcinoma), A549 (human alveolar carcinoma), DV90 (human metastatic pleural carcinoma) cell lines | Elisidepsin IC50 ~4 μM for HOP62, <0.25 μM for A549, ~0.3 μM for DV90 | Downregulation of ErbB3, Akt and MAPK pathways in all cell lines; synergistic/additive effects w other cisplatin, paclitaxel and gemcitabine in all cell lines- combination therapy to improve clinical efficiency | [258] | |
| Body (originally) | PM02734 (elisidepsin trifluoroacetate; synthetic KF3 derivative) | 8 x human NSCLC cell lines (H322, A549, H661, H1299, H1975, H358, H460, H1650) | IC50 0.3 μM to >5 μM (0.58 μM for A549) | All cell lines sensitive to PM02734, only 2 cell lines sensitive to erlotinib; positive correlation between ErbB expression and sensitivity to PM02734; erlotinib inhibited EGFR, AKT and ERK1/2 phosphorylation whereas PM02734 strongly inhibited phosphorylation of ErbB3 and AKT and, to a lower extent, EGFR and ERK1/2 hence the efficacy of combined treatment in vivo | [263] | |
| Mucus (and body) | KF, analogues KZ1 and KZ2, crude CHCl3-CH3OH extract | A549 (and other non-respiratory cancer) cell lines | A549 IC50: 1 μM KZ1, 3 μM KZ2, 1 μM | Analogue bioactivity comparable to KF; low IC50 values for lung cancer relative to other tested cancer cell lines; mucus extracts stronger | [257] | |
| 7 families ‖ | Body | 95% C2H5OH extract | A549 cell line; mouse spleenocytes | 0.25–1 mg/mL | 73–96% tumor growth inhibition at 1 mg/mL, 63–89% at 0.25 mg/mL; molluscan extracts showed stronger anti-tumor properties than other invertebrate extracts and had strong promotion activity on T and B lymphocytes (+25% at 1 μg/mL); low toxicity (not quantified) | [251] |
| Mollusc Class Family | Derivative Part | Specific Extract/ Compound | Model Design * | Main Findings | Effective Concentrations | Ref |
|---|---|---|---|---|---|---|
| Bivalvia | ||||||
| Mytilidae | Body | Lipid extract (‘Lyprinol’) | Murine model of allergic airway disease using Balb/c mice (n = 3–8/group) fed a low-fat background diet treated w 200 uL Lyprinol (or fish oil control) p.o daily, 14 d prior to challenge w i.n. OVA (10 mg in 0.9% saline) (or PBS alone) on days 12–15 | Lyprinol group had lower eosinophil counts and fewer mucus-secreting cells (p < 0.05), other inflammatory cells lower (not sig); no sig dif. in Ab levels between fish oil and Lyprinol; lower IL-13, higher IL-4 and IFN-y in Lyprinol group (p < 0.05); both fish oil and Lyprinol suppressed airway resistance (p < 0.05); Lyprinol efficacy suggested re. synergistic effects between multiple nutritional components/PUFA profile | 200 μL | [208] |
| Pectinidae | Body | Protease extracted polysaccaride heparin sulfate analog (HS) | Lung metastasis model using mice (n = 9) treated w 8 mg/kg HS i.v. (or mammalian heparin or chondroitin controls) 10 min before challenge w i.v. Lewis lung carcinoma cells; separate model of P-selectin-mediated tumor cell-platelet association using labelled LLC cells w or w/out pre-treatment w 200 μg HS † | Molluscan HS inhibited lung metastasis (10 foci/lung control vs. 1 foci/lung HS; p < 0.05) w markedly smaller tumor size, and reduced heparinase activity (p < 0.05); reduced tumor–platelet complex to 30% (control 70%); similar effect to mammalian heparin at lower molar concentration; blocks both P-selectin-mediated interactions and heparinase activity blunting metastasis and inflammation | 8 mg/kg | [274] |
| Pharidae | Body ‡ | 2 sialic acid-binding lectin recombinant proteins (rSgSABL-1, -2) | Non-specific pathogen immunisation model using mice (n = NA) immunised i.p. 2x w rSgSABL-1 (100 μg/mL) or rSgSABL-2 (180 μg/mL) in CFA | Antisera antibodies reactive with rSgSABL-1 and -2 in Western Blot Analysis; strong Staphylococcus aureus peptidoglycan (PAMP) binding affinity (p < 0.05 compared to PBS and pre-serum) | 100–180 μg/mL | [188] |
| Veneridae | Body | (NH4)2SO4 fractionated peptide (‘Mere15′) | Human lung cancer (A549) xenograft model using Balb/c mice (n = 6/group) immunised s.c. w Mere15 12.5, 25.0 or 50.0 mg/kg (or cyclophosphamide [CTX] 50 mg/kg or normal saline) each day for 10 d | Mere15 at 25 and 50 mg/kg doses displayed 51% and 69% growth inhibition (p < 0.01), respectively; comparable to (though not sig dif than) CTX causing 53% inhibition; A549 most susceptible among cancer types in vitro therefore used in subsequent assays and animal model | 25–50 mg/kg | [252] |
| Gastropoda | ||||||
| Aplysiidae | Body | Dolastatin-10 | Human small cell lung cancer (SCLC) (NCI-H446) xenograft model using CB-17 SCID mice (n = 8–10/group) treated w 450 μg/kg dolastatin 10 i.v. 26 and 36 d (or 7 and 17 d) after tumor inoculation | Treatments at 7 and 17 d completely inhibited tumor formation and increased survival (median 59 d control, >214 d treatment); treatments at 26 and 36 d (after tumor formation) caused tumor shrinkage (mass 1635 mg control, 44 mg treatment), growth delay, and increased survival (median 42 control, 91 treatment); pro-apoptotic mechanism | 450 μg/kg | [254] |
| Body | TZT-1027, (dolastatin 10 derivative) | Human LX-1 lung carcinoma xenograft model using Balb/c mice treated w 0.5, 1 and 2 mg/kg TZT-1027 (and Cisplatin- 5 and 10 mg/kg) administered i.v. after tumor established at 7 d, or both 7 and 14 d ‖ | 1–2 treatments caused tumor regression of 84–98% at 1 mg/kg, 99% at 2 mg/kg (> cisplatin: 49–52% at 5 mg/kg, 83% at 10 mg/kg); greater regression of lung cancer compared to breast cancer; 10% and 80% de-polymerisation of microtubule proteins 10% at 1.0 μM, 80% at 10 μM | 1–2 mg/kg | [255] | |
| Limacidae | Body | Aqueous Limax extract in MEM | COPD model using C57BL/6J mice (n = 8/group) treated w A) normal air + 2.18 g/kg extract; B) cigarette smoke (CS) + purified water; C) CS + 2.18 g/kg extract; CS = 9 cigarettes/h, 4 h per d, 6 d per wk in whole body exposure chamber for 90 d; extract given i.g. 0.5 h before daily CS exposure; cytotoxicity assay 0.01 μg/mL–10 mg/mL extract | CS-exposed Limax-treated mice improved pulmonary function compared to untreated mice (p < 0.05); less visual symptoms (e.g., weakness, wheezing), reduced lung damage, hyperplasia, inflammation, alveolar intercept and airway thickness (p < 0.01); reduced BALF inflammatory cell count (p < 0.01), inflammatory cytokines (p = 0.01–0.05) and Muc5AC secretion/expression (p < 0.01); suppression of inflammatory signaling cascades (p < 0.05); no sig cytotoxicity at effective doses; PPAR-γ enhancement and P38 MAPK pathway suppression | 2.18 g/kg | [78] |
| Body | Limax lyophilized powder H2O suspension | Allergic asthma model using guinea pigs (n = 15/group); sensitization using AlOH2 and egg albumin, treated w Limax (189, 63, 21 mg/kg/d) (or Aminophylline 80 mg/kg/d control); inhalation challenge after 7 d | Reduced asthma onset time, mortality, inflammatory markers (BALF/peripheral blood leukocyte count, eosinophil infiltration, IL-2 and IL-4) (p < 0.05); 63 mg/kg more effective than Aminophylline at reducing onset time (p < 0.05) | 63 mg/kg | [140] ¶ | |
| Body | Limax powder in H2O | Lewis lung carcinoma model using mice (n = 10/group); treatments 800–2500 mg/kg | Inhibitory effect on tumor growth (47% inhibition at 800 mg/kg) and prolonged survival (p < 0.01) | 800 mg/kg | [111] # | |
| Muricidae | Hypobranchial gland (HBG) | Crude CHCl3-CH3OH extracts, 6-bromoisatin | Acute lung injury/inflammation model using C57Black/6 mice (n = 5–6/group) treated w HBG extract (0.5 or 0.1 mg/g), or 6-bromoisatin (0.05 or 0.1 mg/g) in 100 μL grape seed carrier oil (or PBS/carrier controls) administered p.o. 48 h, 24 h and 1 h prior to challenge w i.n. LPS (E. coli-derived) (1.25 mg/kg in 50 μL PBS) | Lower BALF total cells, neutrophils, TFN-α, IL-1β, and total protein in all treatments (p < 0.0001); 6-bromoisatin generally stronger effect (and lower concs used) but no sig difference between treatments; all doses of each compound significantly minimised all indicators of acute inflammatory damage to the lungs (p < 0.0001); positive correlation between histopathological scores and inflammatory markers (particularly TNF-α, IL-1β and neutrophils) in BALF (R2 0.53–0.77, p < 0.0001) | 0.5–0.1 mg/g HBG extract; 0.05–0.1 mg/g 6-bromoisatin | [77] |
| Muricidae **, Helicidae | Hemolymph | Experimentally purified Hc (RvH or HpH) | Colon cancer (C-26) model measuring lung metastasis in Balb/c mice (n = 20/group) sensitised i.p. w 200 μg RvH or HpH 2 wks before tumor inoculation and 100 μg weekly i.t.t. after solid tumor formation (sensitised) or 100 μg weekly i.t.t. only (non-sensitised); controls: PBS+challenge, RvH/HpH only no challenge | Lower surface lung metastases count in sens RvH, sens HpH and non-sens HpH groups; no sig dif. in cytokine profiles between groups; >anti-C-26 antibodies (p < 0.05); higher % survival in sens groups; >body weight in unsens groups (p = 0.001-0.05); reduced C-26 tumor size (p < 0.01) although all developed small tumors; HpH/RvH control survival NA | 100 μg (w/w-out 200 μg dose pre-tumor formation) | [76] |
| Plakobranchid-ae | Mucus (originally) | PM02734 (Elisidepsin- synthetic KF3 derivative) | NSCLC (A549) model using NUR-NU-F-M mice (n = 5–7/group) treated w PM02734 (0.1 mg/kg × 3/wk for 2 wk i.v.), Erlotinib (50 mg/kg × 5/wk for 2 wks p.o.), combination (PM02734 i.v. 0.1 mg/kg × 3/wk for 2 wks i.v + Erlotinib p.o. 50 mg/kg × 5/wk for 2 wks p.o.), or no treatment; in vitro component used PM02734 0–10 uM | Combination treatment enhanced survival (>150 d) compared to PM02734 (54 d), Erlotinib (39) and control (23) (p = 0.0003–0.002); Erlotinib inhibited EGFR, AKT and ERK1/2 phosphorylation in vitro whereas PM02734 inhibited phosphorylation of ErbB3 and AKT and, to a lower extent, EGFR and ERK1/2 hence the efficacy of combined treatment | PM02734 0.1 mg/kg w or w/out 50 mg/kg Erlotinib | [263] |
| Mollusc Class Family | Derivative Part | Specific Extract/ Compound | Study Type and Design * | Main Findings | Effective Concentrations | Ref |
|---|---|---|---|---|---|---|
| Gastropoda | ||||||
| Helicidae | Mucus | Helicidine | Double-bind, placebo-controlled, parallel-group clinical trial involving 30 COPD patients w history of chronic bronchitis and stabilised nocturnal cough (>20 cough episodes/night) treated w 2x 15-mL doses of 10% helicidine syrup (or placebo syrup) p.o. 3x daily for 3 d over 5-d observation period | Frequency of cough episodes/night reduced: 4.7–5.1 pre-treatment, 2.7–4.9 placebo, 1.3 helicidine group (p < 0.05); duration of cough period (during sleep and awakening) also reduced (p < 0.05); no sig difference between subjective endpoints (Spiegel questionnaire, CGI) | 15-mL 10% | [80] |
| Plakobranchidae | Body (originally) | Kahalalide F (KF) | Phase I clinical trial and pharmacokinetic study involving 38 cancer patients (13 w lung cancer) administered i.v. 50 μg/mL kahalalide F weekly starting at 266 μg/m2 increasing between 25–100% over 21–109 cycles | Tumor shrinkage by 25–50% or stable disease in lung cancer patients; mild-moderate side effects w severe blood transaminase activity being the dose-limiting factor (3 cases); 650 μg/m2 recommended for future studies | 650 μg/m2 | [260] |
| Body (originally) | KF | Non-randomised, multi-centre phase II clinical trial of KF as a second line therapy in 31 patients w advanced non-small cell lung cancer (NSCLC) administered i.v. 650 μg/m2 for 1 h/wk | One partial response observed; stable disease reported in 8 patients; majority of clinical benefit seen in patients with squamous cell carcinoma | 650 μg/m2 | [261,262] | |
| Body (originally) | PM02734 (Elisidepsin- synthetic KF3 derivative) | Phase 1 clinical trial and pharmacokinetic study involving 42 cancer patients (16 w lung cancer) administered i.v. 0.5 mg/m2 escalated at 100% increments (depending on grade of toxicity; median 2 cycles/patient, 3.2 mg/wk) | Disease stabilization in 12 patients (none w lung cancer), 1 patient (with metastatic esophageal adenocarcinoma) complete response; mild-moderate grade toxicities in ~17% of patients, grade 3 toxicities (hematologic, biochemical [transaminase]) in ~15% of patients lasting 7–14 d; necrosis-like cell-death | Max tolerable dose: 6.8 mg/m2 | [259] | |
| Bivalvia | ||||||
| Mytilidae | Body | Lipid extract (‘Lyprinol’) | Double blind, randomised placebo-controlled parallel-group clinical trial involving 23 atopic asthma (mild-mod) patients (and 23 healthy subjects) treated w 2x 150 mg Lyprinol (or olive oil) capsules p.o. 2x daily for 8 weeks | Mean daytime wheeze and exhaled H2O2 sig reduced and morning PEF sig higher w Lyprinol treatment (p < 0.05); no differences in night awakenings and use of short-acting B2 agonist meds; inhibition of 5’-lipoxygenase and cyclo-oxygenase pathways responsible for production of eicosanoids | 150 mg Lyprinol (50 mg extract in 100 mg olive oil) | [210] |
| Body | Lipid extract (‘Lyprinol’) | Double blind, randomised placebo-controlled clinical trial using 73 (71 completed) children aged 6–13yrs treated w 2x 150 mg Lyprinol (or olive oil) capsules p.o. 2x daily for 7 months | Reduction in Fluticasone use (< 57.8 μg/d vs. 42.8 μg/d; p = 0.27), rescue β-agonist use (42.6% vs. 53.8%; p = 0.67); fewer asthma exacerbations (annualised rate of exacerbation 0.5 Lyprinol vs. 0.86 control); higher % reporting little/no trouble w their asthma (97 vs. 76%; p = 0.057); many other clinically important though non-significant improvements | 150 mg Lyprinol (50 mg extract in 100 mg olive oil) | [209] | |
| Body | Lipid extract PCSO-524 (‘Lyprinol/OmegaXL’) | Double blind, randomised placebo-controlled clinical trial involving 20 patients w asthma and hyperpnea-induced bronchoconstriction treated w 8x 150 mg Lyprinol capsules p.o. daily for 8 weeks, followed by 2 weeks washout phase (usual diet) followed by 3-week special PCSO-524 diet phase (or usual diet control) | After the final phase, Lyprinol treatment and specific diet caused reduction in bronchodilator use and increase in mean morning and evening PEF (p < 0.05); no sif dif. in asthma symptom scores or FEV1; lower expired breath NO and urinary markers (p < 0.05) | 150 mg Lyprinol (50 mg extract in 100 mg olive oil) | [207] |
5. Molluscan Hemocyanins as Therapeutic Adjuvants and Model Antigens
6. Sustainable Supply and Traditional Knowledge Considerations
7. Conclusions: Promising Molluscan Extracts and Compounds for the Treatment and Prevention of Respiratory Disease
- This review highlights that there is a paucity of research on the bioactivity of molluscan extracts and compounds, considering the high diversity of species in this phylum and their merit as traditional medicines. Here, we have demonstrated the links between the anti-inflammatory, antimicrobial, anticancer, and immunomodulatory activity of molluscan extracts and compounds and their therapeutic potential in the prevention and treatment of respiratory diseases.
- At least 100 traditional medicines incorporating over 300 species of Mollusca have been used to treat respiratory diseases for thousands of years. Most of these are yet to receive research attention, and those few that have shown interesting bioactivities that validate some applications. There is a continued need to develop an evidence base toward the integration of quality-controlled traditional medicines.
- We identified particular incentive for biomedical research that elucidates anti-inflammatory factors from mollusc species/families comprising traditional medicines as there is likely to be some chemical basis consistent with their extensive use for alleviating inflammatory symptoms. Shell extracts are widely used but understudied and worthy of further investigation, as are certain taxonomic classes and families used in traditional medicines. The Polyplacophora and Scaphopoda, a wider range of Bivalvia including Veneridae, and shelled Gastropoda including Muricidae are of interest. Respiratory disease-focused ethnomedical studies would also be useful.
- Based on biomedical data, we expect that studies using molluscan compounds isolated from specialized glands, reproductive organs, microbial symbionts, and hemolymph would prove worthwhile.
- The exploration of novel molluscan Hcs holds good potential for the discovery of new antiviral and immunomodulatory agents, and therapeutic alternatives to KLH. Hcs could be tested in combination with other antiviral factors (e.g., zinc) from the same mollusc. Snail mucus should also be further investigated for anti-spasmodic, anti-inflammatory and anti-viral activities.
- Molluscan compounds may be valuable in the treatment of biofilm-associated respiratory infection and improve the efficacy of antibiotics. Further biofilm inhibition/disruption studies are needed and should be inclusive of S. pneumoniae and P. aeruginosa.
- Derivatives of KF and dolastatin 10 are the most potent molluscan anticancer compounds and of continued interest. Several compounds show anticancer activity and need to be tested against respiratory cancers (e.g., brominated indole/isatin derivatives; others in [66] and structural modifications). In vivo models of various cancer types should include measures of lung metastases and cytotoxicity to healthy cells.
- Compounds with bioactivities relevant to a range of respiratory diseases (e.g., anti-inflammatory activity) should be further explored, as well as combinations of compounds (molluscan-molluscan and molluscan-standard agents) to improve treatment efficacy for a single disease and address issues of chemotherapeutic and antimicrobial resistance.
- Overall, there is a need for more targeted research based on specific hypotheses related to respiratory disease using extracts and compounds derived from molluscs, with consideration to the sustainability of supply and attribution of traditional knowledge.
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
Common Abbreviations
| TCMs | traditional Chinese medicines |
| OTMs | other traditional medicines |
| Hc | Hemocyanin |
| COPD | chronic obstructive pulmonary disease |
| ARDS | acute respiratory distress syndrome |
| NSAIDs | non-steroidal anti-inflammatory drugs |
| MIC/MBC | minimum inhibitory/bactericidal concentration |
| IC50/GI50/CC50 | concentration causing 50% inhibition/growth inhibition/cytotoxicity |
| PUFAs | polyunsaturated fatty acids |
| FDA | United States Food and Drug Administration |
| Abs | antibodies |
References
- Amin, K.A.M. Allergic respiratory inflammation and remodeling. Turk. Thorac. J. 2015, 16, 133–140. [Google Scholar] [CrossRef] [PubMed]
- FIRS. The Global Impact of Respiratory Disease, 2nd ed.; European Respiratory Society: Sheffield, UK, 2017; p. 42.
- Jardins, T.D.; Burton, G.G. Clinical Manifestations and Assessment of Respiratory Disease, 7th ed.; Mosby Elsevier: St. Louis, MO, USA, 2016; p. 648. [Google Scholar]
- Ferkol, T.; Schraufnagel, D. The global burden of respiratory disease. Ann. Am. Thorac. Soc. 2014, 11, 404–406. [Google Scholar] [CrossRef] [PubMed]
- Global Asthma Network. The Global Asthma Report 2018; Global Asthma Network: Auckland, New Zealand, 2018; p. 92. [Google Scholar]
- Forum of International Respiratory Societies. Respiratory Diseases in the World; European Respiratory Society: Sheffield, UK, 2013; p. 35. [Google Scholar]
- Ksiazek, T.G.; Erdman, D.; Goldsmith, C.S.; Zaki, S.R.; Peret, T.; Emery, S.; Tong, S.; Urbani, C.; Comer, J.A.; Lim, W.; et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003, 348, 1953–1966. [Google Scholar] [CrossRef] [PubMed]
- De Groot, R.J.; Baker, S.C.; Baric, R.S.; Brown, C.S.; Drosten, C.; Enjuanes, L.; Fouchier, R.A.M.; Galiano, M.; Gorbalenya, A.E.; Memish, Z.A.; et al. Middle east respiratory syndrome coronavirus (MERS-CoV): Announcement of the coronavirus study group. J. Virol. 2013, 87, 7790–7792. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [Green Version]
- Moldoveanu, B.; Otmishi, P.; Jani, P.; Walker, J.; Sarmiento, X.; Guardiola, J.; Saad, M.; Yu, J. Inflammatory mechanisms in the lung. J. Inflamm. Res. 2009, 2009, 1–11. [Google Scholar]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [CrossRef]
- Murrow, E.J.; Oglesby, F.M. Acute and chronic illness: Similarities, differences and challenges. Orthop. Nurs. Natl. Assoc. Orthop. Nurses 1996, 15, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.S.; Alon, N.O.A.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.I.; Plavsic, N.; Chou, J.L.; et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef]
- Proud, D.; Chow, C.W. Role of viral infections in asthma and chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2006, 35, 513–518. [Google Scholar] [CrossRef]
- Umeki, S. Re-evaluation of eosinophilic pneumonia and its diagnostic criteria. Arch. Intern. Med. 1992, 152, 1913–1919. [Google Scholar] [CrossRef]
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [Green Version]
- Kaminska, B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy: From molecular mechanisms to therapeutic benefits. Biochim. Biophys. Acta 2005, 1754, 253–262. [Google Scholar] [CrossRef]
- Reber, L.L.; Hernandez, J.D.; Galli, S.J. The pathophysiology of anaphylaxis. J. Allergy Clin. Immunol. 2017, 140, 335–348. [Google Scholar] [CrossRef]
- Matthay, M.A.; Zemans, R.L.; Zimmerman, G.A.; Arabi, Y.M.; Beitler, J.R.; Mercat, A.; Herridge, M.; Randolph, A.G.; Calfee, C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers 2019, 5, 18. [Google Scholar] [CrossRef]
- Engels, E.A. Inflammation in the development of lung cancer: Epidemiological evidence. Expert Rev. Anticancer Ther. 2008, 8, 605–615. [Google Scholar] [CrossRef]
- Bhatia, M.; Moochhala, S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J. Pathol. 2004, 202, 145–156. [Google Scholar] [CrossRef]
- Störmann, P.; Lustenberger, T.; Relja, B.; Marzi, I.; Wutzler, S. Role of biomarkers in acute traumatic lung injury. Injury 2017, 48, 2400–2406. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [Green Version]
- MacNee, W. Oxidative stress and lung inflammation in airways disease. Eur. J. Pharmacol. 2001, 429, 195–207. [Google Scholar] [CrossRef]
- Mizgerd, J.P. Acute lower respiratory tract infection. N. Engl. J. Med. 2008, 358, 716–727. [Google Scholar] [CrossRef] [Green Version]
- Worldometer COVID-19 Coronavirus Pandemic. Available online: https://www.worldometers.info/coronavirus/ (accessed on 18 November 2020).
- World Health Organisation Fact Sheets. Available online: https://www.who.int/news-room/fact-sheets (accessed on 15 October 2020).
- Koh, W.J. Nontuberculous Mycobacteria-Overview. Microbiol Spectr 2017, 5. [Google Scholar] [CrossRef]
- Price, D.; Yawn, B.; Brusselle, G.; Rossi, A. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim. Care Respir. J. 2013, 22, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Weller, F.R.; Kallenberg, C.G.M.; Jansen, H.M.; Torensma, R.; Klaassen, R.J.L.; Weller, H.H.; Orie, N.G.M.; The, T.H. The primary immune response in bronchial asthma. I. A kinetic study of Helix pomatia hemocyanin-specific IgE, IgG, IgA, and IgM antibody responses in patients with asthma and in matched controls. J. Allergy Clin. Immunol. 1985, 76, 29–34. [Google Scholar] [CrossRef]
- Vissers, J.L.M.; van Esch, B.C.A.M.; Hofman, G.A.; van Oosterhout, A.J.M. Macrophages induce an allergen-specific and long-term suppression in a mouse asthma model. Eur. Respir. J. 2005, 26, 1040–1046. [Google Scholar] [CrossRef]
- Bell, S.C.; Mall, M.A.; Gutierrez, H.; Macek, M.; Madge, S.; Davies, J.C.; Burgel, P.-R.; Tullis, E.; Castaños, C.; Castellani, C.; et al. The future of cystic fibrosis care: A global perspective. Lancet Respir. Med. 2020, 8, 65–124. [Google Scholar] [CrossRef] [Green Version]
- Geddes, D. The history of respiratory disease management. Medicine 2016, 44, 393–397. [Google Scholar] [CrossRef]
- Acemoglu, D.; Johnson, S. Disease and development: The effect of life expectancy on economic growth. J. Political Econ. 2007, 115, 925–985. [Google Scholar] [CrossRef] [Green Version]
- Gonzales, R.; Malone, D.C.; Maselli, J.H.; Sande, M.A. Excessive antibiotic use for acute respiratory infections in the United States. Clin. Infect. Dis. 2001, 33, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Laine, L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 2001, 120, 594–606. [Google Scholar] [CrossRef]
- Oyston, P.; Robinson, K. The current challenges for vaccine development. J. Med. Microbiol. 2012, 61, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Vane, J.R.; Botting, R.M. Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 1998, 47, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Poole, K. Efflux-mediated antimicrobial resistance. J. Antimicrob. Chemother. 2005, 56, 20–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mader, J.S.; Hoskin, D.W. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin. Investig. Drugs 2006, 15, 933–946. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Balasubramanian, S.; Oelschlaeger, T.A.; Grkovic, T.; Pham, N.B.; Quinn, R.J.; Hentschel, U. Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect. Dis. 2017, 17, e30–e41. [Google Scholar] [CrossRef]
- Santos, L.H.; Araújo, A.N.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M.C.B.S.M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef] [Green Version]
- Corcoran, J.; Winter, M.J.; Tyler, C.R. Pharmaceuticals in the aquatic environment: A critical review of the evidence for health effects in fish. Crit. Rev. Toxicol. 2010, 40, 287–304. [Google Scholar] [CrossRef]
- Australian Government. Australia’s National Antimicrobial Resistance Strategy-2020 and Beyond; Department of Health/Department of Agriculture, Water and the Environment: Canberra, Australia, 2019; p. 18.
- Defer, D.; Bourgougnon, N.; Fleury, Y. Screening for antibacterial and antiviral activities in three bivalve and two gastropod marine molluscs. Aquaculture 2009, 293, 1–7. [Google Scholar] [CrossRef]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [Green Version]
- Harvey, A.L. Natural products as a screening resource. Curr. Opin. Chem. Biol. 2007, 11, 480–484. [Google Scholar] [CrossRef]
- Esmaeelian, B.; Benkendorff, K.; Le Leu, R.K.; Abbott, C.A. Simultaneous assessment of the efficacy and toxicity of marine mollusc–derived brominated indoles in an in vivo model for early stage colon cancer. Integr. Cancer Ther. 2018, 17, 248–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, T.B.; Liu, L.; Kotiw, M.; Benkendorff, K. Review of anti-inflammatory, immune-modulatory and wound healing properties of molluscs. J. Ethnopharmacol. 2018, 210, 156–178. [Google Scholar] [CrossRef]
- Jaspars, M.; De Pascale, D.; Andersen, J.H.; Reyes, F.; Crawford, A.D.; Ianora, A. The marine biodiscovery pipeline and ocean medicines of tomorrow. J. Mar. Biol. Assoc. UK 2016, 96, 151–158. [Google Scholar] [CrossRef] [Green Version]
- Abadines, I.B.; Le, K.; Newman, D.J.; Glaser, K.B.; Mayer, A.M. The marine pharmacology and pharmaceuticals pipeline in 2018. FASEB J. 2019, 33, 255–265. [Google Scholar]
- Mayer, A.M.; Glaser, K.B.; Cuevas, C.; Jacobs, R.S.; Kem, W.; Little, R.D.; McIntosh, J.M.; Newman, D.J.; Potts, B.C.; Shuster, D.E. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol. Sci. 2010, 31, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Benkendorff, K. Molluscan biological and chemical diversity: Secondary metabolites and medicinal resources produced by marine molluscs. Biol. Rev. 2010, 85, 757–775. [Google Scholar] [CrossRef] [PubMed]
- Pyron, M.; Brown, K. Introduction to Mollusca and the class Gastropoda (ch. 18). In Ecology and General Biology: Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Thorp, J.H., Rogers, D.C., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 383–421. [Google Scholar]
- Bouchet, P. The magnitude of marine biodiversity. In The Exploration of Marine Biodiveristy, Scientific and Technological Challenges; Duarte, C.M., Ed.; Fundacion BBVA: Bilboao, France, 2006; pp. 33–64. [Google Scholar]
- Dang, V.T.; Benkendorff, K.; Green, T.; Speck, P. Marine snails and slugs: A great place to look for antiviral drugs. J. Virol. 2015, 89, 8114–8118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutellec, M.-A.; Caquet, T. Gastropod ecophysiological response to stress (ch. 9). In Physiology of Molluscs: A Collection of Selected Reviews (Vol. 1); Saleuddin, S., Mukai, S., Eds.; Academic Press: Cambridge, MA, USA; CRC Press: Boca Raton, FL, USA, 2016; p. 932. [Google Scholar]
- Ammerman, J.; Fuhrman, J.; Hagström, A.; Azam, F. Bacterioplankton growth in seawater: I. Growth kinetics and cellular characteristics in seawater cultures. Mar. Ecol. Prog. Ser. 1984, 18, 31–39. [Google Scholar] [CrossRef]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef] [Green Version]
- Raynaud, X.; Nunan, N. Spatial ecology of bacteria at the microscale in soil. PLoS ONE 2014, 9, e87217. [Google Scholar] [CrossRef] [Green Version]
- Hooper, C.; Day, R.; Slocombe, R.; Handlinger, J.; Benkendorff, K. Stress and immune responses in abalone: Limitations in current knowledge and investigative methods based on other models. Fish Shellfish. Immunol. 2007, 22, 363–379. [Google Scholar] [CrossRef] [PubMed]
- Cummins, S.F.; Nichols, A.E.; Schein, C.H.; Nagle, G.T. Newly identified water-borne protein pheromones interact with attractin to stimulate mate attraction in Aplysia. Peptides 2006, 27, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Bornancin, L.; Bonnard, I.; Mills, S.C.; Banaigs, B. Chemical mediation as a structuring element in marine gastropod predator-prey interactions. Nat. Prod. Rep. 2017, 34, 644–676. [Google Scholar] [CrossRef]
- Jiang, M.; Zhao, C.; Yan, R.; Li, J.; Song, W.; Peng, R.; Han, Q.; Jiang, X. Continuous inking affects the biological and biochemical responses of cuttlefish Sepia pharaonis. Front. Physiol. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Benkendorff, K.; Davis, A.R.; Bremner, J. Chemical defense in the egg masses of benthic invertebrates: An assessment of antibacterial activity in 39 mollusks and 4 polychaetes. J. Invertebr. Pathol. 2001, 78, 109–118. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Lefranc, F.; Carbone, M.; Mollo, E.; Gavagnin, M.; Betancourt, T.; Dasari, R.; Kornienko, A.; Kiss, R. Marine mollusk-derived agents with antiproliferative activity as promising anticancer agents to overcome chemotherapy resistance. Med. Res. Rev. 2017, 37, 702–801. [Google Scholar] [CrossRef]
- Benkendorff, K. Chemical diversity in molluscan communities: From natural products to chemical ecology. In Neuroecology and Neuroethology in Molluscs: The Interface between Behaviour and Environment; Cosmo, A.D., Winlow, W., Eds.; Nova Science Publishers: New York, NY, USA, 2014; pp. 13–41. [Google Scholar]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2017, 34, 235–294. [Google Scholar] [CrossRef] [Green Version]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.; Prinsep, M. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [Green Version]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [Green Version]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [Green Version]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2020, 37, 175–223. [Google Scholar] [CrossRef] [PubMed]
- Dolashka, P.; Dolashki, A.; Van Beeumen, J.; Floetenmeyer, M.; Velkova, L.; Stevanovic, S.; Voelter, W. Antimicrobial activity of molluscan hemocyanins from Helix and Rapana snails. Curr. Pharm. Biotechnol. 2016, 17, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Benkendorff, K.; Davis, A.R.; Rogers, C.N.; Bremner, J.B. Free fatty acids and sterols in the benthic spawn of aquatic molluscs, and their associated antimicrobial properties. J. Exp. Mar. Biol. Ecol. 2005, 316, 29–44. [Google Scholar] [CrossRef]
- Vine, K.L.; Locke, J.M.; Ranson, M.; Benkendorff, K.; Pyne, S.G.; Bremner, J.B. In vitro cytotoxicity evaluation of some substituted isatin derivatives. Bioorg. Med. Chem. 2007, 15, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Gesheva, V.; Chausheva, S.; Mihaylova, N.; Manoylov, I.; Doumanova, L.; Idakieva, K.; Tchorbanov, A. Anti-cancer properties of gastropodan hemocyanins in murine model of colon carcinoma. BMC Immunol. 2014, 15, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, T.B.; Rudd, D.; Benkendorff, K.; Mahdi, L.K.; Pratt, K.A.; Dooley, L.; Wei, C.; Kotiw, M. Brominated indoles from a marine mollusc inhibit inflammation in a murine model of acute lung injury. PLoS ONE 2017, 12, e0186904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, X.; Wang, J.; Guan, R.; Zhao, L.; Li, D.; Long, Z.; Yang, Q.; Xu, J.; Wang, Z.; Xie, J.; et al. Limax extract ameliorates cigarette smoke-induced chronic obstructive pulmonary disease in mice. Int. Immunopharmacol. 2018, 54, 210–220. [Google Scholar] [CrossRef]
- Pons, F.; Koenig, M.; Michelot, R.; Mayer, M.; Frossard, N. The bronchorelaxant effect of helicidine, a Helix pomatia extract, involves prostaglandin E2 release. Pathol. Biol. 1999, 47, 73–80. [Google Scholar] [CrossRef]
- Sergysels, R.; Art, G. A double-masked, placebo-controlled polysomnographic study of the antitussive effects of helicidine. Curr. Ther. Res. Clin. Exp. 2001, 62, 35–47. [Google Scholar] [CrossRef]
- Erspamer, V.; Glasser, A. The pharmacological actions of murexine (urocanylcholine). Br. J. Pharmacol. Chemother. 1957, 12, 176–184. [Google Scholar] [CrossRef] [Green Version]
- Badiu, D.L.; Luque, R.; Dumitrescu, E.; Craciun, A.; Dinca, D. Amino acids from Mytilus galloprovincialis (L.) and Rapana venosa molluscs accelerate skin wounds healing via enhancement of dermal and epidermal neoformation. Protein J. 2010, 29, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Gesheva, V.; Chausheva, S.; Stefanova, N.; Mihaylova, N.; Doumanova, L.; Idakieva, K.; Tchorbanov, A. Helix pomatia hemocyanin - a novel bio-adjuvant for viral and bacterial antigens. Int. Immunopharmacol. 2015, 26, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Theilacker, C.; Coleman, F.T.; Mueschenborn, S.; Llosa, N.; Grout, M.; Pier, G.B. Construction and characterization of a Pseudomonas aeruginosa mucoid exopolysaccharide-alginate conjugate vaccine. Infect. Immun. 2003, 71, 3875–3884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, A.M. Clinical Pipeline: Marine Pharmacology. Available online: https://www.midwestern.edu/departments/marinepharmacology/clinical-pipeline.xml (accessed on 30 June 2020).
- McGivern, J.G. Ziconotide: A review of its pharmacology and use in the treatment of pain. Neuropsychiatr. Dis. Treat. 2007, 3, 69–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, L.J. Brentuximab vedotin: A review in CD30-positive Hodgkin lymphoma. Drugs 2017, 77, 435–445. [Google Scholar] [CrossRef]
- Deeks, E.D. Polatuzumab vedotin: First global approval. Drugs 2019, 79, 1467–1475. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, J.E.; O’Donnell, P.H.; Balar, A.V.; McGregor, B.A.; Heath, E.I.; Yu, E.Y.; Galsky, M.D.; Hahn, N.M.; Gartner, E.M.; Pinelli, J.M.; et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J. Clin. Oncol. 2019, 37, 2592–2600. [Google Scholar] [CrossRef]
- Benkendorff, K.; Rudd, D.; Nongmaithem, B.D.; Liu, L.; Young, F.; Edwards, V.; Avila, C.; Abbott, C.A. Are the traditional medical uses of muricidae molluscs substantiated by their pharmacological properties and bioactive compounds? Mar. Drugs 2015, 13, 5237–5275. [Google Scholar] [CrossRef] [Green Version]
- Bonnemain, B. Helix and drugs: Snails for western health care from antiquity to the present. Evid. Based Complement. Altern. Med. 2005, 2, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Straus, S.E. Complementary and alternative medicine: Challenges and opportunities for American medicine. Acad. Med. 2000, 75, 572–573. [Google Scholar] [CrossRef]
- Cragg, G.M.; Boyd, M.R.; Cardellina, J.H., 2nd; Newman, D.J.; Snader, K.M.; McCloud, T.G. Ethnobotany and drug discovery: The experience of the US National Cancer Institute. Ciba Found. Symp. 1994, 185, 178–196. [Google Scholar] [PubMed]
- Lee, M.R. The history of Ephedra (ma-huang). J. R. Coll. Physicians Edinb. 2011, 41, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, N.V. Fatty acids of marine mollusks: Impact of diet, bacterial symbiosis and biosynthetic potential. Biomolecules 2019, 9, 857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [Green Version]
- Chatzi, L.; Kogevinas, M. Prenatal and childhood Mediterranean diet and the development of asthma and allergies in children. Public Health Nutr. 2009, 12, 1629–1634. [Google Scholar] [CrossRef]
- Sorlí-Aguilar, M.; Martín-Luján, F.; Santigosa-Ayala, A.; Piñol-Moreso, J.L.; Flores-Mateo, G.; Basora-Gallisà, J.; Arija-Val, V.; Solà-Alberich, R. Effects of Mediterranean diet on lung function in smokers: A randomised, parallel and controlled protocol. BMC Public Health 2015, 15, 74. [Google Scholar] [CrossRef] [Green Version]
- Hageman, J.H.; Hooyenga, P.; Diersen-Schade, D.A.; Scalabrin, D.M.F.; Wichers, H.J.; Birch, E.E. The impact of dietary long-chain polyunsaturated fatty acids on respiratory illness in infants and children. Curr. Allergy Asthma Rep. 2012, 12, 564–573. [Google Scholar] [CrossRef] [Green Version]
- Shahar, E.; Folsom, A.R.; Melnick, S.L.; Tockman, M.S.; Comstock, G.W.; Gennaro, V.; Higgins, M.W.; Sorlie, P.D.; Ko, W.-J.; Szklo, M. Dietary n-3 polyunsaturated fatty acids and smoking-related chronic obstructive pulmonary disease. N. Engl. J. Med. 1994, 331, 228–233. [Google Scholar] [CrossRef]
- Zanjani, N.T.; Saksena, M.M.; Dehghani, F.; Cunningham, A.L. From ocean to bedside: The therapeutic potential of molluscan hemocyanins. Curr. Med. Chem. 2018, 25, 2292–2303. [Google Scholar] [CrossRef]
- Harris, J.R.; Markl, J. Keyhole limpet hemocyanin (KLH): A biomedical review. Micron 1999, 30, 597–623. [Google Scholar] [CrossRef]
- Guan, H.S.; Wang, S.G. Chinese Marine Materia Medica; Shanghai Scientific and Technical Publishers: Shanghai, China; China Ocean Press: Beijing, China; Chemical Industry Press: Beijing, China, 2009; p. 371. [Google Scholar]
- WoRMS World Register of Marine Species. Available online: http://www.marinespecies.org/aphia.php?p=search (accessed on 15 October 2020).
- Asta Lakshmi, S. Wonder molluscs and their utilities. Int. J. Pharm. Sci. Rev. Res. 2011, 6, 30–33. [Google Scholar]
- Neto, N.A.L.; Voeks, R.A.; Dias, T.L.P.; Alves, R.R.N. Mollusks of Candomblé: Symbolic and ritualistic importance. J. Ethnobiol. Ethnomed. 2012, 8, 1–10. [Google Scholar]
- Herbert, D.; Hamer, M.; Mander, M.; Mkhize, N.; Prins, F. Invertebrate animals as a component of the traditional medicine trade in KwaZulu-Natal, South Africa. Afr. Invertebr. 2003, 44, 1–18. [Google Scholar]
- Voultsiadou Eleni, E. Therapeutic properties and uses of marine invertebrates in the ancient Greek world and early Byzantium. J. Ethnopharmacol. 2010, 130, 237–247. [Google Scholar] [CrossRef]
- Sun, B.N.; Shen, H.D.; Wu, H.X.; Yao, L.X.; Cheng, Z.Q.; Diao, Y. Determination of chemical constituents of the marine pulmonate slug, Paraoncidium Reevesii. Trop. J. Pharm. Res. 2014, 13, 2071–2074. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.F.; Wu, X.P.; Liu, F.Z. Antitumor effect of Limax in tumor-bearing mice. Chin. J. Mod. Dev. Tradit. Med. 1989, 9, 675–676. [Google Scholar]
- Barberis, I.; Bragazzi, N.L.; Galluzzo, L.; Martini, M. The history of tuberculosis: From the first historical records to the isolation of Koch’s bacillus. J. Prev. Med. Hyg. 2017, 58, E9–E12. [Google Scholar]
- Alves, R.R.; Alves, H.N. The faunal drugstore: Animal-based remedies used in traditional medicines in Latin America. J. Ethnobiol. Ethnomed. 2011, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Gopal, R.; Vijayakumaran, M.; Venkatesan, R.; Kathiroli, S. Marine organisms in Indian medicine and their future prospects. Nat. Prod. Radiance 2008, 7, 139–145. [Google Scholar]
- Jamir, N.; Lal, P. Ethnozoological practices among Naga tribes. Indian J. Tradit. Knowl. 2005, 4, 100–104. [Google Scholar]
- Shilabin, A.G.; Kasanah, N.; Wedge, D.E.; Hamann, M.T. Lysosome and HER3 (ErbB3) selective anticancer agent kahalalide F: Semisynthetic modifications and antifungal lead-exploration studies. J. Med. Chem. 2007, 50, 4340–4350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Koch, M.; Pond, C.D.; Mabeza, G.; Seronay, R.A.; Concepcion, G.P.; Barrows, L.R.; Olivera, B.M.; Schmidt, E.W. Structure and activity of lobophorins from a turrid mollusk-associated Streptomyces sp. J. Antibiot. 2014, 67, 121–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brieger, J.E. Calcarea carbonica or Ostrea edulis? Br. Homeopath. J. 1960, 49, 41–43. [Google Scholar] [CrossRef]
- Ramchandani, N.M. Homoeopathic treatment of upper respiratory tract infections in children: Evaluation of thirty case series. Complement. Ther. Clin. Pract. 2010, 16, 101–108. [Google Scholar] [CrossRef]
- Colin, P. Homeopathy and respiratory allergies: A series of 147 cases. Homeopathy 2006, 95, 68–72. [Google Scholar] [CrossRef]
- Alves, R.R.; Rosa, I.L. Zootherapeutic practices among fishing communities in North and Northeast Brazil: A comparison. J. Ethnopharmacol. 2007, 111, 82–103. [Google Scholar] [CrossRef]
- Meyer-Rochow, V.B. Therapeutic arthropods and other, largely terrestrial, folk-medicinally important invertebrates: A comparative survey and review. J. Ethnobiol. Ethnomed. 2017, 13, 9. [Google Scholar] [CrossRef] [Green Version]
- Alade, G.O.; Frank, A.; Ajibesin, K.K. Animals and animal products as medicines: A survey of Epie-Atissa and Ogbia people of Bayelsa State, Nigeria. J. Pharm. Pharmacogn. Res. 2018, 6, 483–502. [Google Scholar]
- Meyerhof, M.; Sobhy, G.P. The Abridged Version of “The Book of Simple Drugs”, of Ahmad Ibn Muhammad Al-Ghafiqi by Gregorius Abul-Farag; Al Ettemad Printing Press and Publising House: Cairo, Egypt, 1932; pp. 224–228. [Google Scholar]
- Prabhakar, A.K.; Roy, S.P. Ethno-medicinal uses of some shell fishes by people of Kosi river basin of North-Bihar, India. Stud. Ethno Med. 2009, 3, 1–4. [Google Scholar] [CrossRef]
- Nongmaithem, B.D.; Mouatt, P.; Smith, J.; Rudd, D.; Russell, M.; Sullivan, C.; Benkendorff, K. Volatile and bioactive compounds in opercula from Muricidae molluscs supports their use in ceremonial incense and traditional medicines. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lev, E.; Zohar, A. Practical Materia Medica of the Medieval Eastern Mediterranean according to the Cairo Genizah; Brill Online Books and Journals: Leiden, The Netherlands, 2008; Volume 7. [Google Scholar]
- Kim, H.; Song, M.J. Ethnozoological study of medicinal animals on Jeju Island, Korea. J. Ethnopharmacol. 2013, 146, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Chinlampianga, M.; Singh, R.K.; Shukla, A.C. Ethnozoological diversity of Northeast India: Empirical learning with traditional knowledge holders of Mizoram and Arunachal Pradesh. Indian J. Tradit. Knowl. 2013, 12, 18–30. [Google Scholar]
- Alves, R.R.; Rosa, I.L. Zootherapy goes to town: The use of animal-based remedies in urban areas of NE and N Brazil. J. Ethnopharmacol. 2007, 113, 541–555. [Google Scholar] [CrossRef]
- McHugh, J. Blattes de byzance in India: Mollusk opercula and the history of perfumery. J. R. Asiat. Soc. 2013, 23, 53–67. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [Green Version]
- HPUS. Homeopathic Materia Medica of the United States, 1st ed.; Duncan Brothers Publishers: Chicago, IL, USA, 1878. [Google Scholar]
- Immanuel, G.; Thaddaeus, B.J.; Usha, M.; Ramasubburayan, R.; Prakash, S.; Palavesam, A. Antipyretic, wound healing and antimicrobial activity of processed shell of the marine mollusc Cypraea moneta. Asian Pac. J. Trop. Biomed. 2012, 2 (Suppl. S3), S1643–S1646. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Rinaudo, M.; Jellouli, K.; Nasri, M. Characterization and in vitro evaluation of cytotoxicity, antimicrobial and antioxidant activities of chitosans extracted from three different marine sources. Appl. Biochem. Biotechnol. 2015, 177, 18–35. [Google Scholar] [CrossRef]
- Shanmugam, A.; Kathiresan, K.; Nayak, L. Preparation, characterization and antibacterial activity of chitosan and phosphorylated chitosan from cuttlebone of Sepia kobiensis (Hoyle, 1885). Biotechnol. Rep. 2016, 9, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Oakes, F.R. Non-Lethal Method for Extracting Crude Hemocyanin from Gastropod Molluscs. U.S. Patent No.6852338B2, 8 February 2005. [Google Scholar]
- Quevauviller, A.; Mainil, J.; Garcet, S. Le mucus d’Hélix pomatia L.-préparation, composition, propriétés thérapeutiques et pharmacodynamiques. Rev. Pathol. Gen. Physiol. Clin. 1953, 653, 1514–1538. [Google Scholar]
- Greenberg, A.K.; Basu, S.; Hu, J.; Yie, T.A.; Tchou-Wong, K.M.; Rom, W.N.; Lee, T.C. Selective p38 activation in human non-small cell lung cancer. Am. J. Respir. Cell Mol. Biol. 2002, 26, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.K.; Lin, G.Q.; Luo, Q.F.; Xie, J.K. Effect of Limax lyophilized powder on bronchial asthma. J. Chin. Med. Mater. 2011, 34, 1086–1089. [Google Scholar]
- Benkendorff, K. Natural product research in the Australian marine invertebrate Dicathais Orbita. Mar. Drugs 2013, 11, 1370–1398. [Google Scholar] [CrossRef] [Green Version]
- Roseghini, M.; Severini, C.; Erspamer, G.F.; Erspamer, V. Choline esters and biogenic amines in the hypobranchial gland of 55 molluscan species of the neogastropod Muricoidea superfamily. Toxicon 1996, 34, 33–55. [Google Scholar] [CrossRef]
- Westley, C.; Benkendorff, K. Sex-specific Tyrian purple genesis: Precursor and pigment distribution in the reproductive system of the marine mollusc, Dicathais Orbita. J. Chem. Ecol. 2008, 34, 44–56. [Google Scholar] [CrossRef] [Green Version]
- Cooksey, C.J. Tyrian purple: 6, 6′-dibromoindigo and related compounds. Molecules 2001, 6, 736–769. [Google Scholar] [CrossRef]
- Bailey, K.C. The Elder Pliny’s Chapters on Chemical Subjects; E. Arnold & Company: London, UK, 1932. [Google Scholar]
- Edwards, V.; Benkendorff, K.; Young, F. Marine compounds selectively induce apoptosis in female reproductive cancer cells but not in primary-derived human reproductive granulosa cells. Mar. Drugs 2012, 10, 64–83. [Google Scholar] [CrossRef]
- Esmaeelian, B. In Vitro and In Vivo Testing of Purified Muricid Mollusc Extract on Colorectal Cancer. Ph.D. Thesis, Flinders University, Adelaide, Australia, 2014. [Google Scholar]
- Sklirou, A.D.; Gaboriaud-Kolar, N.; Papassideri, I.; Skaltsounis, A.-L.; Trougakos, I.P. 6-bromo-indirubin-3′-oxime (6BIO), a Glycogen synthase kinase-3β inhibitor, activates cytoprotective cellular modules and suppresses cellular senescence-mediated biomolecular damage in human fibroblasts. Sci. Rep. 2017, 7, 11713. [Google Scholar] [CrossRef] [Green Version]
- Benkendorff, K.; Bremner, J.B.; Davis, A.R. Tyrian purple precursors in the egg masses of the Australian muricid, Dicathais orbita: A possible defensive role. J. Chem. Ecol. 2000, 26, 1037–1050. [Google Scholar] [CrossRef]
- Gesheva, V.; Idakieva, K.; Kerekov, N.; Nikolova, K.; Mihaylova, N.; Doumanova, L.; Tchorbanov, A. Marine gastropod hemocyanins as adjuvants of non-conjugated bacterial and viral proteins. Fish Shellfish Immunol. 2011, 30, 135–142. [Google Scholar] [CrossRef]
- Ahmad, T.B.; Rudd, D.; Kotiw, M.; Liu, L.; Benkendorff, K. Correlation between fatty acid profile and anti-inflammatory activity in common Australian seafood by-products. Mar. Drugs 2019, 17, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, P.D. The Scaphopoda. Adv. Mar. Biol. 2002, 42, 137–236. [Google Scholar] [PubMed]
- De Toledo-Piza, A.R.; de Oliveira, M.I.; Negri, G.; Mendonca, R.Z.; Figueiredo, C.A. Polyunsaturated fatty acids from Phyllocaulis boraceiensis mucus block the replication of influenza virus. Arch. Microbiol. 2018, 200, 961–970. [Google Scholar] [CrossRef] [PubMed]
- De Toledo-Piza, A.R.; Figueiredo, C.A.; de Oliveira, M.I.; Negri, G.; Namiyama, G.; Tonelotto, M.; Villar, K.D.; Rofatto, H.K.; Mendonca, R.Z. The antiviral effect of mollusk mucus on measles virus. Antivir. Res. 2016, 134, 172–181. [Google Scholar] [CrossRef]
- Szabó, K.; Amesbury, J.R. Molluscs in a world of islands: The use of shellfish as a food resource in the tropical island Asia-Pacific region. Quat. Int. 2011, 239, 8–18. [Google Scholar] [CrossRef]
- Dortch, C.E.; Kendrick, G.W.; Morse, K. Aboriginal mollusc exploitation in southwestern Australia. Archaeol. Ocean. 1984, 19, 81–104. [Google Scholar] [CrossRef]
- Bodeker, G.; Ong, C.-K. WHO Global Atlas of Traditional, Complementary and Alternative Medicine; World Health Organization: Kobe, Japan, 2005; Volume 1, p. 347. [Google Scholar]
- Allaertaert, F.A.; Villet, S.; Vincent, S.; Sauve, L. Observational study on the dispensing of cough syrups to children with acute cough by community pharmacists in France. Minerva Pediatr. 2018, 70, 117–126. [Google Scholar]
- Fung, F.Y.; Linn, Y.C. Developing traditional chinese medicine in the era of evidence-based medicine: Current evidences and challenges. Evid. Based Complement. Altern. Med. 2015, 2015, 425037. [Google Scholar] [CrossRef]
- Morice, A.; Kardos, P. Comprehensive evidence-based review on European antitussives. BMJ Open Respir. Res. 2016, 3, e000137. [Google Scholar] [CrossRef]
- Roch, P. Defense mechanisms and disease prevention in farmed marine invertebrates. Aquaculture 1999, 172, 125–145. [Google Scholar] [CrossRef]
- Roch, P.; Hubert, F.; van Der Knaap, W.; Noël, T. Present knowledge on the molecular basis of cytotoxicity, antibacterial activity and stress response in marine bivalves. Ital. J. Zool. 1996, 63, 311–316. [Google Scholar] [CrossRef]
- Zhang, G.; Li, X.; Xue, Z. Potential reasons and controlling strategies of mollusk dramatic death in China. Chin. Fish. 1999, 9, 34–39. [Google Scholar]
- Zannella, C.; Mosca, F.; Mariani, F.; Franci, G.; Folliero, V.; Galdiero, M.; Tiscar, P.G.; Galdiero, M. Microbial diseases of bivalve mollusks: Infections, immunology and antimicrobial defense. Mar. Drugs 2017, 15, 188. [Google Scholar] [CrossRef] [PubMed]
- Yum, H.K.; Park, I.N.; Shin, B.M.; Choi, S.J. Recurrent Pseudomonas aeruginosa infection in chronic lung diseases: Relapse or reinfection? Tuberc. Respir. Dis. 2014, 77, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Sen-Kilic, E.; Blackwood, C.B.; Boehm, D.T.; Witt, W.T.; Malkowski, A.C.; Bevere, J.R.; Wong, T.Y.; Hall, J.M.; Bradford, S.D.; Varney, M.E.; et al. Intranasal peptide-based fpva-klh conjugate vaccine protects mice from Pseudomonas aeruginosa acute murine pneumonia. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Mitta, G.; Hubert, F.; Noel, T.; Roch, P. Myticin, a novel cysteine-rich antimicrobial peptide isolated from haemocytes and plasma of the mussel Mytilus galloprovincialis. Eur. J. Biochem. 1999, 265, 71–78. [Google Scholar] [CrossRef]
- Domeneghetti, S.; Franzoi, M.; Damiano, N.; Norante, R.; El Halfawy, N.M.; Mammi, S.; Marin, O.; Bellanda, M.; Venier, P. Structural and antimicrobial features of peptides related to Myticin C, a special defense molecule from the Mediterranean mussel Mytilus galloprovincialis. J. Agric. Food Chem. 2015, 63, 9251–9259. [Google Scholar] [CrossRef]
- Elshahawi, S.I.; Trindade-Silva, A.E.; Hanora, A.; Han, A.W.; Flores, M.S.; Vizzoni, V.; Schrago, C.G.; Soares, C.A.; Concepcion, G.P.; Distel, D.L.; et al. Boronated tartrolon antibiotic produced by symbiotic cellulose-degrading bacteria in shipworm gills. Proc. Natl. Acad. Sci. USA 2013, 110, E295–E304. [Google Scholar] [CrossRef] [Green Version]
- Maselli, V.; Galdiero, E.; Salzano, A.M.; Scaloni, A.; Maione, A.; Falanga, A.; Naviglio, D.; Guida, M.; Di Cosmo, A.; Galdiero, S. OctoPartenopin: Identification and preliminary characterization of a novel antimicrobial peptide from the suckers of Octopus vulgaris. Mar. Drugs 2020, 18, 380. [Google Scholar] [CrossRef]
- Chand, S.; Karuso, P. Isolation and total synthesis of two novel metabolites from the fissurellid mollusc Scutus antipodes. Tetrahedron Lett. 2017, 58, 1020–1023. [Google Scholar] [CrossRef]
- Pani, A.; Marongiu, M.E.; Obino, P.; Gavagnin, M.; La Colla, P. Antimicrobial and antiviral activity of xylosyl-methylthio-adenosine, a naturally occurring analogue of methylthio-adenosine from Doris verrucosa. Experientia 1991, 47, 1228–1229. [Google Scholar] [CrossRef] [PubMed]
- Chellaram, C.; Edward, J. Anti-inflammatory potential of coral reef associated gastropod, Drupa Margariticola. Indian J. Sci. Technol. 2009, 2, 75–77. [Google Scholar] [CrossRef]
- Pitt, S.J.; Graham, M.A.; Dedi, C.G.; Taylor-Harris, P.M.; Gunn, A. Antimicrobial properties of mucus from the brown garden snail Helix aspersa. Br. J. Biomed. Sci. 2015, 72, 174–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Periyasamy, N.; Srinivasan, M.; Balakrishnan, S. Antimicrobial activities of the tissue extracts of Babylonia spirata Linnaeus, 1758 (Mollusca: Gastropoda) from Thazhanguda, southeast coast of India. Asian Pac. J. Trop. Biomed. 2012, 2, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Miller, B.W.; Torres, J.P.; Tun, J.O.; Flores, M.S.; Forteza, I.; Rosenberg, G.; Haygood, M.G.; Schmidt, E.W.; Concepcion, G.P. Synergistic anti-methicillin-resistant Staphylococcus aureus (MRSA) activity and absolute stereochemistry of 7,8-dideoxygriseorhodin C. J. Antibiot. 2020, 73, 290–298. [Google Scholar] [CrossRef]
- Maurice, N.M.; Bedi, B.; Sadikot, R.T. Pseudomonas aeruginosa biofilms: Host response and clinical implications in lung infections. Am. J. Respir. Cell Mol. Biol. 2018, 58, 428–439. [Google Scholar] [CrossRef]
- Gasu, E.N.; Ahor, H.S.; Borquaye, L.S. Peptide mix from Olivancillaria hiatula interferes with cell-to-cell communication in Pseudomonas aeruginosa. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Gasu, E.N.; Ahor, H.S.; Borquaye, L.S. Peptide extract from Olivancillaria hiatula exhibits broad-spectrum antibacterial activity. BioMed Res. Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ishwarya, R.; Vaseeharan, B.; Iswarya, A.; Karthikeyan, S. Haemolytic and antibiofilm properties of haemocyanin purified from the haemolymph of Indian white shrimp Fenneropenaeus indicus. Fish Shellfish Immunol. 2016, 59, 447–455. [Google Scholar] [CrossRef]
- Ishwarya, R.; Iswarya, A.; Thangaviji, V.; Sivakamavalli, J.; Esteban, M.A.; Thangaraj, M.P.; Vaseeharan, B. Immunological and antibiofilm property of haemocyanin purified from grooved tiger shrimp (Penaeus semisulcatus): An in vitro and in silico approach. Microb. Pathog. 2020, 147, 104253. [Google Scholar] [CrossRef]
- Ishwarya, R.; Vaseeharan, B.; Jayakumar, R.; Ramasubramanian, V.; Govindarajan, M.; Alharbi, N.S.; Khaled, J.M.; Al-anbr, M.N.; Benelli, G. Bio-mining drugs from the sea: High antibiofilm properties of haemocyanin purified from the haemolymph of flower crab Portunus pelagicus (L.) (Decapoda: Portunidae). Aquaculture 2018, 489, 130–140. [Google Scholar] [CrossRef]
- Van der Poll, T.; Opal, S.M. Pathogenesis, treatment, and prevention of pneumococcal pneumonia. Lancet 2009, 374, 1543–1556. [Google Scholar] [CrossRef]
- Chellaram, C.; Sreenivasan, R.S.; Jonesh, S.; Anand, T.P.; Edward, J.K.P. In vitro antibiotic bustle of coral reef associated gastropod, Drupa Margariticola (Broderip, 1832) of tuticorin coastal waters, Southeastern India. Biotechnology 2009, 8, 456–461. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, P.; Subhapradha, N.; Srinivasan, A.; Shanmugam, V.; Krishnamoorthy, J.; Shanmugam, A. In vitro evaluation of antimicrobial activity of methanolic extract from selected species of Cephalopods on clinical isolates. Afr. J. Microbiol. Res. 2011, 5, 3884–3889. [Google Scholar]
- Defres, S.; Marwick, C.; Nathwani, D. MRSA as a cause of lung infection including airway infection, community-acquired pneumonia and hospital-acquired pneumonia. Eur. Respir. J. 2009, 34, 1470. [Google Scholar] [CrossRef]
- Notariale, R.; Basile, A.; Montana, E.; Romano, N.C.; Cacciapuoti, M.G.; Aliberti, F.; Gesuele, R.; De Ruberto, F.; Sorbo, S.; Tenore, G.C.; et al. Protamine-like proteins have bactericidal activity: The first evidence in Mytilus galloprovincialis. Acta Biochim. Pol. 2018, 65, 585–594. [Google Scholar] [CrossRef]
- Wei, X.M.; Yang, D.L.; Li, H.Y.; Jiang, H.L.; Liu, X.Q.; Zhang, Q.; Yang, J.L. Sialic acid-binding lectins (SABLs) from Solen grandis function as PRRs ensuring immune recognition and bacterial clearance. Fish Shellfish Immunol. 2018, 72, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Cilia, G.; Fratini, F. Antimicrobial properties of terrestrial snail and slug mucus. J. Complement. Integr. Med. 2018, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Destoumieux-Garzon, D.; Rosa, R.D.; Schmitt, P.; Barreto, C.; Vidal-Dupiol, J.; Mitta, G.; Gueguen, Y.; Bachere, E. Antimicrobial peptides in marine invertebrate health and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [Green Version]
- Hoang, V.L.T.; Kim, S.K. Antimicrobial peptides from marine sources. Curr. Protein Pept. Sci. 2013, 14, 205–211. [Google Scholar] [CrossRef]
- Lazcano-Pérez, F.; Román-González, S.A.; Sánchez-Puig, N.; Arreguín-Espinosa, R. Bioactive peptides from marine organisms: A short overview. Protein Pept. Lett. 2012, 19, 700–707. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Sánchez-Pérez, A.; Calo-Mata, P.; Villa, T.G. Antimicrobial peptides (AMPs): Ancient compounds that represent novel weapons in the fight against bacteria. Biochem. Pharmacol. 2017, 133, 117–138. [Google Scholar] [CrossRef]
- Tincu, J.A.; Taylor, S.W. Antimicrobial peptides from marine invertebrates. Antimicrob. Agents Chemother. 2004, 48, 3645–3654. [Google Scholar] [CrossRef] [Green Version]
- Sable, R.; Parajuli, P.; Jois, S. Peptides, peptidomimetics, and polypeptides from marine sources: A wealth of natural sources for pharmaceutical applications. Mar. Drugs 2017, 15, 124. [Google Scholar] [CrossRef] [Green Version]
- Carriel-Gomes, M.C.; Kratz, J.M.; Müller, V.D.M.; Barardi, C.R.M.; Simões, C.M.O. Evaluation of antiviral activity in hemolymph from oysters Crassostrea rhizophorae and Crassostrea gigas. Aquat. Living Resour. 2006, 19, 189–193. [Google Scholar] [CrossRef]
- Dolashka-Angelova, P.; Lieb, B.; Velkova, L.; Heilen, N.; Sandra, K.; Nikolaeva-Glomb, L.; Dolashki, A.; Galabov, A.S.; Van Beeumen, J.; Stevanovic, S.; et al. Identification of glycosylated sites in Rapana hemocyanin by mass spectrometry and gene sequence, and their antiviral effect. Bioconjugate Chem. 2009, 20, 1315–1322. [Google Scholar] [CrossRef]
- Swaminathan, A.; Lucas, R.M.; Dear, K.; McMichael, A.J. Keyhole limpet haemocyanin-a model antigen for human immunotoxicological studies. Br. J. Clin. Pharmacol. 2014, 78, 1135–1142. [Google Scholar] [CrossRef]
- Gai, Z.; Matsuno, A.; Kato, K.; Kato, S.; Khan, M.R.I.; Shimizu, T.; Yoshioka, T.; Kato, Y.; Kishimura, H.; Kanno, G.; et al. Crystal Structure of the 3.8-MDa Respiratory Supermolecule Hemocyanin at 3.0 Å Resolution. Structure 2015, 23, 2204–2212. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, K.; Joy, M. High-value compounds from the molluscs of marine and estuarine ecosystems as prospective functional food ingredients: An overview. Food Res. Int. 2020, 137, 109637. [Google Scholar] [CrossRef]
- Kumar, A.; Kubota, Y.; Chernov, M.; Kasuya, H. Potential role of zinc supplementation in prophylaxis and treatment of COVID-19. Med. Hypotheses 2020, 144, 109848. [Google Scholar] [CrossRef]
- Te Velthuis, A.J.W.; van den Worm, S.H.E.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ inhibits coronavirus and arterivirus rna polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Wessels, I.; Rolles, B.; Rink, L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front. Immunol. 2020, 11, 1712. [Google Scholar] [CrossRef]
- Das, U.N.; Ramos, E.J.; Meguid, M.M. Metabolic alterations during inflammation and its modulation by central actions of omega-3 fatty acids. Curr. Opin. Clin. Nutr. Metab. Care 2003, 6, 413–419. [Google Scholar] [CrossRef]
- Black, P.N.; Sharpe, S. Dietary fat and asthma: Is there a connection? Eur. Respir. J. 1997, 10, 6–12. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Very long-chain n-3 fatty acids and human health: Fact, fiction and the future. Proc. Nutr. Soc. 2018, 77, 52–72. [Google Scholar] [CrossRef] [Green Version]
- Mickleborough, T.D.; Vaughn, C.L.; Shei, R.J.; Davis, E.M.; Wilhite, D.P. Marine lipid fraction PCSO-524™ (Lyprinol®/Omega XL®) of the New Zealand green lipped mussel attenuates hyperpnea-induced bronchoconstriction in asthma. Respir. Med. 2013, 107, 1152–1163. [Google Scholar] [CrossRef] [Green Version]
- Wood, L.G.; Hazlewood, L.C.; Foster, P.S.; Hansbro, P.M. Lyprinol reduces inflammation and improves lung function in a mouse model of allergic airways disease. Clin. Exp. Allergy 2010, 40, 1785–1793. [Google Scholar] [CrossRef]
- Lello, J.; Liang, A.; Robinson, E.; Leutenegger, D.; Wheat, A. Treatment of children’s asthma with a lipid extract of the new Zealand green lipped mussel (Perna canaliculus) (Lyprinol®)—A double blind, randomized controlled trial in children with moderate to serve chronic obstructive asthma. Internet J. Asthma Allergy Immunol. 2012, 8, 1–12. [Google Scholar]
- Emelyanov, A.; Fedoseev, G.; Krasnoschekova, O.; Abulimity, A.; Trendeleva, T.; Barnes, P.J. Treatment of asthma with lipid extract of New Zealand green-lipped mussel: A randomised clinical trial. Eur. Respir. J. 2002, 20, 596–600. [Google Scholar] [CrossRef] [Green Version]
- Thien, F.C.K.; De Luca, S.; Woods, R.K.; Abramson, M.J. Dietary marine fatty acids (fish oil) for asthma in adults and children. Cochrane Database Syst. Rev. 2000, 2000, CD001283. [Google Scholar] [CrossRef]
- Ahmad, T.B.; Rudd, D.; Smith, J.; Kotiw, M.; Mouatt, P.; Seymour, L.M.; Liu, L.; Benkendorff, K. Anti-inflammatory activity and structure-activity relationships of brominated indoles from a marine mollusc. Mar. Drugs 2017, 15, 133. [Google Scholar] [CrossRef]
- Yazbeck, R.; Lindsay, R.; Abbott, C.A.; Benkendorff, K.; Howarth, G.S. Combined effects of muricid extract and 5-fluorouracil on intestinal toxicity in rats. Evid. Based Complement. Altern. Med. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Esmaeelian, B.; Abbott, C.A.; Le Leu, R.K.; Benkendorff, K. 6-Bromoisatin found in muricid mollusc extracts inhibits colon cancer cell proliferation and induces apoptosis, preventing early stage tumor formation in a colorectal cancer rodent model. Mar. Drugs 2014, 12, 17–35. [Google Scholar] [CrossRef] [PubMed]
- Westley, C.B.; Benkendorff, K.; McIver, C.M.; Le Leu, R.K.; Abbott, C.A. Gastrointestinal and hepatotoxicity assessment of an anticancer extract from muricid molluscs. Evid. Based Complement. Altern. Med. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joung, H.J.; Kim, Y.S.; Hwang, J.W.; Han, Y.K.; Jeong, J.H.; Lee, J.S.; Moon, S.H.; Jeon, B.T.; Park, P.J. Anti-inflammatory effects of extract from Haliotis discus hannai fermented with Cordyceps militaris mycelia in RAW264.7 macrophages through TRIF-dependent signaling pathway. Fish Shellfish Immunol. 2014, 38, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.-J.; Kim, S.-A.; Lee, J.S.; Kim, H.-J.; Choi, I.L.W.; Jung, W.-K. The antioxidant and anti-inflammatory effects of abalone intestine digest, Haliotis discus hannai in RAW 264.7 macrophages. Biotechnol. Bioprocess Eng. 2012, 17, 475–484. [Google Scholar] [CrossRef]
- Chen, Z.C.; Wu, S.S.; Su, W.Y.; Lin, Y.C.; Lee, Y.H.; Wu, W.H.; Chen, C.H.; Wen, Z.H. Anti-inflammatory and burn injury wound healing properties of the shell of Haliotis diversicolor. BMC Complement. Altern. Med. 2016, 16, 487. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Chakraborty, M.; Bose, M.; Mukherjee, D.; Roychoudhury, A.; Dhar, P.; Mishra, R. Indian freshwater edible snail Bellamya bengalensis lipid extract prevents T cell mediated hypersensitivity and inhibits LPS induced macrophage activation. J. Ethnopharmacol. 2014, 157, 320–329. [Google Scholar] [CrossRef]
- Kao, Y.F.; Wu, Y.H.S.; Chou, C.H.; Fu, S.G.; Liu, C.W.; Chai, H.J.; Chen, Y.C. Manufacture and characterization of anti-inflammatory liposomes from jumbo flying squid (Dosidicus gigas) skin phospholipid extraction. Food Funct. 2018, 9, 3986–3996. [Google Scholar] [CrossRef]
- Pereira, R.B.; Taveira, M.; Valentão, P.; Sousa, C.; Andrade, P.B. Fatty acids from edible sea hares: Anti-inflammatory capacity in LPS-stimulated RAW 264.7 cells involves iNOS modulation. RSC Adv. 2015, 5, 8981–8987. [Google Scholar] [CrossRef]
- Jung, H.-J.; Nam, K.N.; Son, M.-S.; Kang, H.; Hong, J.-W.; Kim, J.W.; Lee, E.H. Indirubin-3′-oxime inhibits inflammatory activation of rat brain microglia. Neurosci. Lett. 2011, 487, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, G.M. Indirubin-3-monoxime exhibits anti-inflammatory properties by down-regulating NF-κB and JNK signaling pathways in lipopolysaccharide-treated RAW264.7 cells. Inflamm. Res. 2012, 61, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Man, Y.; Wang, Y.X.; Zhu, S.Y.; Yang, S.; Zhao, D.; Hu, F.; Li, J.Y. Indirubin inhibits ATP-induced phagocytosis attenuation, ROS production and cell death of macrophages. Yao Xue Xue Bao 2012, 47, 45–50. [Google Scholar] [PubMed]
- Salas, S.; Chakraborty, K. Polyether macrocyclic polyketide from the muricid gastropod Chicoreus ramosus attenuates pro-inflammatory 5-lipoxygenase. Med. Chem. Res. 2020, 29, 1976–1985. [Google Scholar] [CrossRef]
- Chakraborty, K.; Salas, S. First report of antioxidant 1H-benzochromenone from muricid gastropod Chicoreus ramosus as dual inhibitors of pro-inflammatory 5-lipoxygenase and carbolytic enzymes. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef]
- Chakraborty, K.; Salas, S.; Joy, M. An unreported bis-abeo cembrane-type diterpenoid with antioxidative and anti-lipoxygenase activities from the muricid gastropod mollusc Chicoreus ramosus. Nat. Prod. Res. 2018, 34, 1678–1686. [Google Scholar] [CrossRef]
- Chakraborty, K.; Krishnan, S.; Joy, M. Polygalactan from bivalve Crassostrea madrasensis attenuates nuclear factor-κB activation and cytokine production in lipopolysaccharide-activated macrophage. Carbohydr. Polym. 2020, 249, 116817. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joy, M. Characterization and bioactive potentials of secondary metabolites from mollusks Crassostrea madrasensis and Amphioctopus marginatus. Nat. Prod. Res. 2019, 33, 3190–3202. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joy, M.; Chakkalakal, S.J. Antioxidant and antiinflammatory secondary metabolites from the Asian green mussel Perna viridis. J. Food Biochem. 2019, 43, e12736. [Google Scholar] [CrossRef]
- Wu, Y.; Hu, X.; Song, L.; Zhu, J.; Yu, R. The inhibitory effect of a novel polypeptide fraction from Arca subcrenata on cancer-related inflammation in human cervical cancer HeLa cells. Sci. World J. 2014, 2014, 1–8. [Google Scholar]
- Joy, M.; Chakraborty, K. Antioxidative and anti-inflammatory pyranoids and isochromenyl analogues from Corbiculid bivalve clam, Villorita cyprinoides. Food Chem. 2018, 251, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Gomes, A.; Gomes, A. Anti-osteoarthritis, anti-nociception, anti-inflammatory activities of isolated fraction of flesh extract Viviparous bengalensis in experimental model. Int. J. Curr. Res. Acad. Rev. 2015, 3, 66–87. [Google Scholar]
- Sarkar, A.; Gomes, A.; Gomes, A. Anti-osteoporosis activity of fresh water snail (Viviparous bengalensis) flesh extracted protein fraction vb-p4 in rat models. Int. J. Curr. Res. Biosci. Plant Biol. 2002, 2, 60–72. [Google Scholar]
- Sarkar, A.; Datta, P.; Gomes, A.; Gupta, S.C.D.; Gomes, A. Anti-osteoporosis and anti-osteoarthritis activity of fresh water snail (Viviparous bengalensis) flesh extract in experimental animal model. Open J. Rheumatol. Autoimmune Dis. 2013, 3, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Akerkar, A.S.; Ponkshe, C.A.; Indap, M.M. Evaluation of immunomodulatory activity of extracts from marine animals. Indian J. Mar. Sci. 2009, 38, 22–27. [Google Scholar]
- Ponkshe, C.A.; Indap, M.M. In vivo and in vitro evaluation for immunomodulatory activity of three marine animal extracts with reference to phagocytosis. Indian J. Exp. Biol. 2002, 40, 1399–1402. [Google Scholar] [PubMed]
- Chellaram, C.; Anand, T.P.P.; Kuberan, G.; John, A.A.; Priya, G.; Kumar, B.A. Anti-inflammatory and analgesic effects of coral reef associated gastropod, Trochus tentorium from Tuticorin coastal waters, Southeastern India. Afr. J. Biotechnol. 2012, 11, 14621–14626. [Google Scholar]
- Santhi, V.; Sivakumar, V.; Thangathirupathi, A.; Thilaga, R. Analgesic, anti-pyretic and anti-inflammatory activities of chloroform extract of prosobranch mollusc Purpura Persica. Int. J. Pharm. Biol. Sci. 2011, 5, 9–15. [Google Scholar]
- Santhi, V.; Sivakumar, V.; Thilaga, R.; Thangathirupathi, A. Analgesic, antipyretic and anti inflammatory activities of column fraction of Babylonia zeylanica (Bruguiere, 1789) in albino rats. Int. J. Pharm. Biol. Sci. 2012, 2, 151–159. [Google Scholar]
- Joseph, S.M.; George, M.; Nair, J.R.; Senan, V.P.; Pillai, D.; Sherief, P. Effect of feeding cuttlefish liver oil on immune function, inflammatory response and platelet aggregation in rats. Curr. Sci. 2005, 88, 507–510. [Google Scholar]
- Fei, L.; Xu, K. Zhikang capsule ameliorates dextran sodium sulfate-induced colitis by inhibition of inflammation, apoptosis, oxidative stress and MyD88-dependent TLR4 signaling pathway. J. Ethnopharmacol. 2016, 192, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Bhattacharya, S.; Bhattacharjee, P.; Das, R.; Mishra, R. Prevention of the progression of adjuvant induced arthritis by oral supplementation of Indian fresh water mussel (Lamellidens marginalis) aqueous extract in experimental rats. J. Ethnopharmacol. 2010, 132, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Swapna, P. Anti Inflammatory, wound healing and analgesic activities of fresh water mollusc Parreysia cylindrica in albino rats. Indian J. Appl. Res. 2015, 5, 4. [Google Scholar] [CrossRef]
- Li, G.; Fu, Y.; Zheng, J.; Li, D. Anti-inflammatory activity and mechanism of a lipid extract from hard-shelled mussel (Mytilus coruscus) on chronic arthritis in rats. Mar. Drugs 2014, 12, 568–588. [Google Scholar] [CrossRef] [Green Version]
- Mimura, T.; Itoh, S.; Tsujikawa, K.; Nakajima, H.; Satake, M.; Kohama, Y.; Okabe, M. Studies on biological activities of melanin from marine animals. V. Anti-inflammatory activity of low-molecular-weight melanoprotein from squid (Fr. SM II). Chem. Pharm. Bull. 1987, 35, 1144–1150. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Liu, Z.; Wang, L.; Li, M.; Zhang, Y.; Zong, Y.; Li, Y.; Song, L. A novel tumor necrosis factor in the Pacific oyster Crassostrea gigas mediates the antibacterial response by triggering the synthesis of lysozyme and nitric oxide. Fish Shellfish Immunol. 2020, 98, 334–341. [Google Scholar] [CrossRef]
- Agrawal, S.; Chaugule, S.; More, S.; Rane, G.; Indap, M. Methanolic extract of Euchelus asper exhibits in-ovo anti-angiogenic and in vitro anti-proliferative activities. Biol. Res. 2017, 50, 41. [Google Scholar] [CrossRef] [Green Version]
- Meivelu, M.; Seedevi, P.; Vairamani, S.; Shanmugam, A. Exploring the chemical composition and anticancer potential of oil from squid (Loligo duvauceli) liver waste from fish processing industry. Waste Biomass Valorization 2019, 10, 2967–2973. [Google Scholar] [CrossRef]
- Liu, J.T.; Deng, Z.H.; Huang, J.H.; Wang, Y.; Chang, J.; Zhu, D. Apple snails polysaccharide extraction and pharmacological potential study in vitro. Appl. Mech. Mat. 2014, 651, 207–210. [Google Scholar] [CrossRef]
- Zhang, L.; Fan, X.; Han, L. Antitumor and immune regulation activities of the extracts of some Chinese marine invertebrates. Chin. J. Oceanol. Limnol. 2005, 23, 110–117. [Google Scholar]
- Wang, C.; Liu, M.; Cheng, L.; Wei, J.; Wu, N.; Zheng, L.; Lin, X. A novel polypeptide from Meretrix meretrix Linnaeus inhibits the growth of human lung adenocarcinoma. Exp. Biol. Med. 2012, 237, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Shilabin, A.G.; Hamann, M.T. In vitro and in vivo evaluation of select kahalalide F analogs with antitumor and antifungal activities. Bioorg. Med. Chem. 2007, 19, 6628–6632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalemkerian, G.P.; Ou, X.; Adil, M.R.; Rosati, R.; Khoulani, M.M.; Madan, S.K.; Pettit, G.R. Activity of dolastatin 10 against small-cell lung cancer in vitro and in vivo: Induction of apoptosis and bcl-2 modification. Cancer Chemother. Pharmacol. 1999, 43, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Natsume, T.; Tamaoki, S.; Watanabe, J.; Asano, H.; Mikami, T.; Miyasaka, K.; Miyazaki, K.; Gondo, M.; Sakakibara, K.; et al. Antitumor activity of TZT-1027, a novel dolastatin 10 derivative. Jap. J. Cancer Res. 1997, 88, 316–327. [Google Scholar] [CrossRef]
- Janmaat, M.L.; Rodriguez, J.A.; Jimeno, J.; Kruyt, F.A.E.; Giaccone, G. Kahalalide F induces necrosis-like cell death that involves depletion of ErbB3 and inhibition of Akt signaling. Mol. Pharmacol. 2005, 68, 502–510. [Google Scholar] [CrossRef] [Green Version]
- Ciavatta, M.L.; Devi, P.; Carbone, M.; Mathieu, V.; Kiss, R.; Casapullo, A.; Gavagnin, M. Kahalalide F analogues from the mucous secretion of Indian sacoglossan mollusc Elysia ornata. Tetrahedron 2016, 72, 625–631. [Google Scholar] [CrossRef]
- Teixidó, C.; Arguelaguet, E.; Pons, B.; Aracil, M.; Jimeno, J.; Somoza, R.; Marés, R.; Ramón, Y.; Cajal, S.; Hernández-Losa, J. ErbB3 expression predicts sensitivity to elisidepsin treatment: In vitro synergism with cisplatin, paclitaxel and gemcitabine in lung, breast and colon cancer cell lines. Int. J. Oncol. 2012, 41, 317–324. [Google Scholar]
- Salazar, R.; Jones, R.J.; Oaknin, A.; Crawford, D.; Cuadra, C.; Hopkins, C.; Gil, M.; Coronado, C.; Soto-Matos, A.; Cullell-Young, M.; et al. A phase I and pharmacokinetic study of elisidepsin (PM02734) in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2012, 70, 673–681. [Google Scholar] [CrossRef]
- Pardo, B.; Paz-Ares, L.; Tabernero, J.; Ciruelos, E.; Garcia, M.; Salazar, R.; Lopez, A.; Blanco, M.; Nieto, A.; Jimeno, J.; et al. Phase I clinical and pharmacokinetic study of kahalalide F administered weekly as a 1-hour infusion to patients with advanced solid tumors. Clin. Cancer Res. 2008, 14, 1116–1123. [Google Scholar] [CrossRef] [Green Version]
- Hamann, M.T. Technology evaluation: Kahalalide F, PharmaMar. Curr. Opin. Mol. Ther. 2004, 6, 657–665. [Google Scholar]
- PharmaMar. PharmaMar reports new data on Kahalalide-F and Aplidin(R) at ESMO congress. In Proceedings of the 31st European Society for Medical Oncology Congress (ESMO), Istanbul, Turkey, 29 September–3 October 2006. [Google Scholar]
- Ling, Y.H.; Aracil, M.; Jimeno, J.; Perez-Soler, R.; Zou, Y. Molecular pharmacodynamics of PM02734 (elisidepsin) as single agent and in combination with erlotinib; synergistic activity in human non-small cell lung cancer cell lines and xenograft models. Eur. J. Cancer 2009, 45, 1855–1864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poncet, J. The dolastatins, a family of promising antineoplastic agents. Curr. Pharm. Des. 1999, 5, 139–162. [Google Scholar] [PubMed]
- Salazar, R.; Cortes-Funes, H.; Casado, E.; Pardo, B.; Lopez-Martin, A.; Cuadra, C.; Tabernero, J.; Coronado, C.; Garcia, M.; Matos-Pita, A.S.; et al. Phase I study of weekly kahalalide F as prolonged infusion in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2013, 72, 75–83. [Google Scholar] [CrossRef]
- Pereira, R.B.; Andrade, P.B.; Valentao, P. Chemical diversity and biological properties of secondary metabolites from sea hares of Aplysia genus. Mar Drugs 2016, 14, 39. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-j.; Lim, H.-S. Two patients of acute liver damage following the ingestion of a sea hare eggs. J. Agric. Med. Comm. Health 2005, 30, 241–249. [Google Scholar]
- Jamil, A.; Kasi, A. Cancer: Metastasis to the Lung; NCBI, StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Riihimäki, M.; Hemminki, A.; Fallah, M.; Thomsen, H.; Sundquist, K.; Sundquist, J.; Hemminki, K. Metastatic sites and survival in lung cancer. Lung Cancer 2014, 86, 78–84. [Google Scholar] [CrossRef]
- Krishnan, K.; Khanna, C.; Helman, L.J. The molecular biology of pulmonary metastasis. Thorac. Surg. Clin. 2006, 16, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Punt, J.; Stranford, S.; Jones, P.; Owen, J. Kuby Immunology, 8th ed.; Macmillan Learning: New York, NY, USA, 2018; p. 880. [Google Scholar]
- Pizarro-Bauerle, J.; Maldonado, I.; Sosoniuk-Roche, E.; Vallejos, G.; López, M.N.; Salazar-Onfray, F.; Aguilar-Guzmán, L.; Valck, C.; Ferreira, A.; Becker, M.I. Molluskan hemocyanins activate the classical pathway of the human complement system through natural antibodies. Front. Immunol. 2017, 8, 188. [Google Scholar] [CrossRef]
- Kellar, A.; Egan, C.; Morris, D. Preclinical murine models for lung cancer: Clinical trial applications. BioMed Res. Int. 2015, 2015, 621324. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.M.; Kozlowski, E.O.; Borsig, L.; Teixeira, F.; Vlodavsky, I.; Pavao, M.S.G. Antitumor properties of a new non-anticoagulant heparin analog from the mollusk Nodipecten nodosus: Effect on P-selectin, heparanase, metastasis and cellular recruitment. Glycobiology 2015, 25, 386–393. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, C.P.; de Paiva, J.; Moraes, C.T.; Takahashi, H.K.; Porcionatto, M.A.; Nader, H.B. Isolation and characterization of a heparin with high anticoagulant activity from Anomalocardia brasiliana. Biochim. Biophys. Acta Gen. Subj. 1985, 843, 1–7. [Google Scholar] [CrossRef]
- Dietrich, C.P.; Tersariol, I.L.; Toma, L.; Moraes, C.T.; Porcionatto, M.A.; Oliveira, F.W.; Nader, H.B. Structure of heparan sulfate: Identification of variable and constant oligosaccharide domains in eight heparan sulfates of different origins. Cell Mol. Biol. 1998, 44, 417–429. [Google Scholar] [PubMed]
- Arumugam, M.; Shanmugam, A. Extraction of heparin and heparin-like substance from marine mesogastropod mollusc Turritella attenuata (Lamarck, 1779). Indian J. Exp. Biol. 2004, 42, 529–532. [Google Scholar] [PubMed]
- Pejler, G.; Danielsson, A.; Björk, I.; Lindahl, U.; Nader, H.; Dietrich, C. Structure and antithrombin-binding properties of heparin isolated from the clams Anomalocardia brasiliana and Tivela mactroides. J. Biol. Chem. 1987, 262, 11413–11421. [Google Scholar]
- Esmaeelian, B.; Benkendorff, K.; Johnston, M.R.; Abbott, C.A. Purified brominated indole derivatives from Dicathais orbita induce apoptosis and cell cycle arrest in colorectal cancer cell lines. Mar. Drugs 2013, 11, 3802–3822. [Google Scholar] [CrossRef]
- Benkendorff, K.; McIver, C.M.; Abbott, C.A. Bioactivity of the Murex homeopathic remedy and of extracts from an Australian muricid mollusc against human cancer cells. Evid. Based Complement. Altern. Med. 2011, 2011, 3802–3822. [Google Scholar] [CrossRef] [Green Version]
- Nicolaou, K.A.; Liapis, V.; Evdokiou, A.; Constantinou, C.; Magiatis, P.; Skaltsounis, A.L.; Koumas, L.; Costeas, P.A.; Constantinou, A.I. Induction of discrete apoptotic pathways by bromo-substituted indirubin derivatives in invasive breast cancer cells. Biochem. Biophys. Res. Commun. 2012, 425, 76–82. [Google Scholar] [CrossRef]
- Edwards, V.; Young, F.; Benkendorff, K. Effects of Muricidae Extracts on Estrogen-Sensitive Breast Cancer and the Steroidogenic Pathway. Flinders University, Adelaide, Australia, Unpublished Work. 2013. [Google Scholar]
- Choi, S.J.; Moon, M.J.; Lee, S.D.; Choi, S.-U.; Han, S.-Y.; Kim, Y.-C.; Letters, M.C. Indirubin derivatives as potent FLT3 inhibitors with anti-proliferative activity of acute myeloid leukemic cells. Bioorg. Med. Chem. Lett. 2010, 20, 2033–2037. [Google Scholar] [CrossRef]
- Saito, H.; Tabata, K.; Hanada, S.; Kanda, Y.; Suzuki, T.; Miyairi, S. Synthesis of methoxy-and bromo-substituted indirubins and their activities on apoptosis induction in human neuroblastoma cells. Bioorg. Med. Chem. Lett. 2011, 21, 5370–5373. [Google Scholar] [CrossRef]
- Liu, L.; Nam, S.; Tian, Y.; Yang, F.; Wu, J.; Wang, Y.; Scuto, A.; Polychronopoulos, P.; Magiatis, P.; Skaltsounis, L. 6-Bromoindirubin-3′-oxime inhibits JAK/STAT3 signaling and induces apoptosis of human melanoma cells. J. Cancer Res. 2011, 71, 3972–3979. [Google Scholar] [CrossRef] [Green Version]
- Fiebig, H.H.; Schuler, J. In vivo anti-tumor activity of indirubins. In Indirubin: The red shade of Indigo; Meijer, L., Guyard, N., Skaltsounis, A.L., Eisenbrand, G., Eds.; Life In Progress Editions: Roscoff, France, 2006. [Google Scholar]
- Westley, C.B.; McIver, C.M.; Abbott, C.A.; Le Leu, R.K.; Benkendorff, K. Enhanced acute apoptotic response to azoxymethane-induced DNA damage in the rodent colonic epithelium by Tyrian purple precursors: A potential colorectal cancer chemopreventative. Cancer Biol. Ther. 2010, 9, 371–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudd, D.A.; Benkendorff, K.; Chahal, C.; Guinan, T.; Gustafsson, O.J.R.; Esmaeelian, B.; Krysinska, H.; Pogson, L.; Voelcker, N.H.; Abbott, C.A. Mapping insoluble indole metabolites in the gastrointestinal environment of a murine colorectal cancer model using desorption/ionisation on porous silicon imaging. Sci. Rep. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Edwards, V.; Benkendorff, K.; Young, F. An in vitro high-throughput assay for screening reproductive and toxic effects of anticancer compounds. Biotechnol. Appl. Biochem. 2014, 61, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Karthik, R.; Manigandan, V.; Saravanan, R. Toxicity, teratogenicity and antibacterial activity of posterior salivary gland (PSG) toxin from the cuttlefish Sepia pharaonis (Ehrenberg, 1831). J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2017, 1064, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Karthik, R.; Saravanan, R.; Ebenezar, K.K.; Sivamalai, T. Isolation, purification and characterization of avian antimicrobial glycopeptide from the posterior salivary gland of Sepia pharaonis. Appl. Biochem. Biotechnol. 2014, 175, 1507–1518. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Wang, W.; Yang, X.; Yan, X.; Liu, R. A novel cysteine-rich antimicrobial peptide from the mucus of the snail of Achatina fulica. Peptides 2013, 39, 1–5. [Google Scholar] [CrossRef]
- Hemu, X.; Tam, J.P. Macrocyclic antimicrobial peptides engineered from ω-conotoxin. Curr. Pharm. Des. 2017, 23, 2131–2138. [Google Scholar] [CrossRef] [Green Version]
- Dolashka, P.; Moshtanska, V.; Borisova, V.; Dolashki, A.; Stevanovic, S.; Dimanov, T.; Voelter, W. Antimicrobial proline-rich peptides from the hemolymph of marine snail Rapana venosa. Peptides 2011, 32, 1477–1483. [Google Scholar] [CrossRef]
- Bitaab, M.A.; Ranaei Siadat, S.O.; Pazooki, J.; Sefidbakht, Y. Antibacterial and molecular dynamics study of the Dolabellanin B2 isolated from sea slug, Peronia peronii. Biosci. Biotechnol. Res. Asia 2015, 12, 2023–2035. [Google Scholar] [CrossRef] [Green Version]
- Borquaye, L.S.; Darko, G.; Ocansey, E.; Ankomah, E. Antimicrobial and antioxidant properties of the crude peptide extracts of Galatea paradoxa and Patella rustica. SpringerPlus 2015, 4, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Rohini, B.; Priya, C.S.; Lavanya, A.; Kalpana, K.; Karthika, V. Potential of water and methanol extracts of Lambis lambis against fish and human pathogens. Biol. Rhythm Res. 2012, 43, 205–213. [Google Scholar] [CrossRef]
- Kanchana, S.; Vennila, R.; Rajesh Kumar, K.; Arumugam, M.; Balasubramanian, T. Antagonistic and cyto-toxicity activity of mollusc methanol extracts. J. Biol. Sci. 2014, 14, 60–66. [Google Scholar] [CrossRef] [Green Version]
- Silveira-Dorta, G.; Sousa, I.J.; Fernandes, M.X.; Martín, V.S.; Padrón, J.M. Synthesis and identification of unprecedented selective inhibitors of CK1ε. Eur. J. Med. Chem. 2015, 96, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Dickler, M.N.; Ragupathi, G.; Liu, N.X.; Musselli, C.; Martino, D.J.; Miller, V.A.; Kris, M.G.; Brezicka, F.T.; Livingston, P.O.; Grant, S.C. Immunogenicity of a fucosyl-GM1-keyhole limpet hemocyanin conjugate vaccine in patients with small cell lung cancer. Clin. Cancer Res. 1999, 5, 2773–2779. [Google Scholar] [PubMed]
- Miles, D.; Roché, H.; Martin, M.; Perren, T.J.; Cameron, D.A.; Glaspy, J.; Dodwell, D.; Parker, J.; Mayordomo, J.; Tres, A.; et al. Phase III multicenter clinical trial of the sialyl-TN (STn)-keyhole limpet hemocyanin (KLH) vaccine for metastatic breast cancer. Oncologist 2011, 16, 1092–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasumi, K.; Aoki, Y.; Watanabe, R.; Hankey, K.G.; Mann, D.L. Therapeutic response in patients with advanced malignancies treated with combined dendritic cell-activated T cell based immunotherapy and intensity-modulated radiotherapy. Cancers 2011, 3, 2223–2242. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Gu, X.-X. Development of peptide mimotopes of lipooligosaccharide from nontypeable Haemophilus influenzae as vaccine candidates. J. Immunol. 2003, 170, 4373–4379. [Google Scholar] [CrossRef] [Green Version]
- Tan, H.X.; Jegaskanda, S.; Juno, J.A.; Esterbauer, R.; Wong, J.; Kelly, H.G.; Liu, Y.; Tilmanis, D.; Hurt, A.C.; Yewdell, J.W.; et al. Subdominance and poor intrinsic immunogenicity limit humoral immunity targeting influenza HA stem. J. Clin. Investig. 2019, 129, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Kyd, J.M.; Cripps, A.W.; Novotny, L.A.; Bakaletz, L.O. Efficacy of the 26-kilodalton outer membrane protein and two P5 fimbrin-derived immunogens to induce clearance of nontypeable Haemophilus influenzae from the rat middle ear and lungs as well as from the chinchilla middle ear and nasopharynx. Infect Immun. 2003, 71, 4691–4699. [Google Scholar] [CrossRef] [Green Version]
- Hughes, E.E.; Gilleland, L.B.; Gilleland, H.E., Jr. Synthetic peptides representing epitopes of outer membrane protein F of Pseudomonas aeruginosa that elicit antibodies reactive with whole cells of heterologous immunotype strains of P. aeruginosa. Infect Immun. 1992, 60, 3497–3503. [Google Scholar] [CrossRef] [Green Version]
- Hughes, E.E.; Gilleland, H.E., Jr. Ability of synthetic peptides representing epitopes of outer membrane protein F of Pseudomonas aeruginosa to afford protection against P. aeruginosa infection in a murine acute pneumonia model. Vaccine 1995, 13, 1750–1753. [Google Scholar] [CrossRef]
- Sokol, P.A.; Kooi, C.; Hodges, R.S.; Cachia, P.; Woods, D.E. Immunization with a Pseudomonas aeruginosa elastase peptide reduces severity of experimental lung infections due to P. aeruginosa or Burkholderia cepacia. J. Infect Dis. 2000, 181, 1682–1692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, H.J.; Franco, L.H.; Nair, V.R.; Collins, A.C.; Shiloh, M.U. A baculovirus-conjugated mimotope vaccine targeting Mycobacterium tuberculosis lipoarabinomannan. PLoS ONE 2017, 12, e0185945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tebbey, P.W.; Hagen, M.; Hancock, G.E. Atypical pulmonary eosinophilia is mediated by a specific amino acid sequence of the attachment (G) protein of respiratory syncytial virus. J. Exp. Med. 1998, 188, 1967–1972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallmann, J.; Epstein, M.M.; Singh, P.; Brunner, R.; Szalai, K.; El-Housseiny, L.; Pali-Schöll, I.; Jensen-Jarolim, E. Mimotope vaccination for therapy of allergic asthma: Anti-inflammatory effects in a mouse model. Clin. Exp. Allergy 2010, 40, 650–658. [Google Scholar] [CrossRef] [Green Version]
- Demoly, P.; Persi, L.; Dhivert, H.; Delire, M.; Bousquet, J. Immunotherapy with keyhole limpet hemocyanin-conjugated decapeptide vaccine in cypress pollen allergy. Clin. Exp. Allergy 2002, 32, 1071–1076. [Google Scholar] [CrossRef]
- Arancibia, S.; Campo, M.D.; Nova, E.; Salazar, F.; Becker, M.I. Enhanced structural stability of Concholepas hemocyanin increases its immunogenicity and maintains its non-specific immunostimulatory effects. Eur. J. Immunol. 2012, 42, 688–699. [Google Scholar] [CrossRef]
- Schuyler, M.; Lyons, C.R.; Masten, B.; Bice, D. Immunoglobulin response to intrapulmonary immunization of asthmatics. Immunology 1997, 91, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Jansen, H.M.; The, T.H.; de Gast, G.C.; Esselink, M.T.; Pastoor, G.; Orie, N.G. The primary immune response of patients with different stages of squamous-cell bronchial carcinoma. Thorax 1978, 33, 755–760. [Google Scholar] [CrossRef] [Green Version]
- Curtis, J.E.; Hersh, E.M.; Harris, J.E.; McBride, C.; Freireich, E.J. The human primary immune response to keyhole limpet haemocyanin: Interrelationships of delayed hypersensitivity, antibody response and in vitro blast transformation. Clin. Exp. Immunol. 1970, 6, 473–491. [Google Scholar]
- Krieger, S.M.; Boverhof, D.R.; Woolhiser, M.R.; Hotchkiss, J.A. Assessment of the respiratory sensitization potential of proteins using an enhanced mouse intranasal test (MINT). Food Chem. Toxicol. 2013, 59, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Riedl, M.A.; Landaw, E.M.; Saxon, A.; Diaz-Sanchez, D. Initial high-dose nasal allergen exposure prevents allergic sensitization to a neoantigen. J. Immunol. 2005, 174, 7440–7445. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Sanchez, D.; Garcia, M.P.; Wang, M.; Jyrala, M.; Saxon, A. Nasal challenge with diesel exhaust particles can induce sensitization to a neoallergen in the human mucosa. J. Allergy Clin. Immunol. 1999, 104, 1183–1188. [Google Scholar] [CrossRef]
- Weller, F.R.; Weller, H.H.; Kallenberg, C.G.M.; The, T.H.; Orie, N.G.M. Sensitivity to hydrocortisone is a relevant factor in the immunoendocrine relationship. I. The cell-mediated immune response in relation to blood levels and in vitro immunosuppressive effects of hydrocortisone in patients with asthma and healthy control subjects. J. Allergy Clin. Immunol. 1986, 78, 423–430. [Google Scholar]
- Gomes, A.M.; Kozlowski, E.O.; Pomin, V.H.; de Barros, C.M.; Zaganeli, J.L.; Pavão, M.S.G. Unique extracellular matrix heparan sulfate from the bivalve Nodipecten nodosus (Linnaeus, 1758) safely inhibits arterial thrombosis after photochemically induced endothelial lesion. J. Biol. Chem. 2010, 285, 7312–7323. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Baker, J.T.; Borris, R.P.; Carte, B.; Cordell, G.A.; Soejarto, D.D.; Gupta, M.P.; Iwu, M.M.; Madulid, D.A. Interactions with source countries: Guidelines for members of the American Society of Pharmacognosy. J. Nat. Prod. 1997, 60, 654–655. [Google Scholar] [CrossRef]
- Cordell, G.A.; Colvard, M.D. Some thoughts on the future of ethnopharmacology. J. Ethnopharmacol. 2005, 100, 5–14. [Google Scholar] [CrossRef]
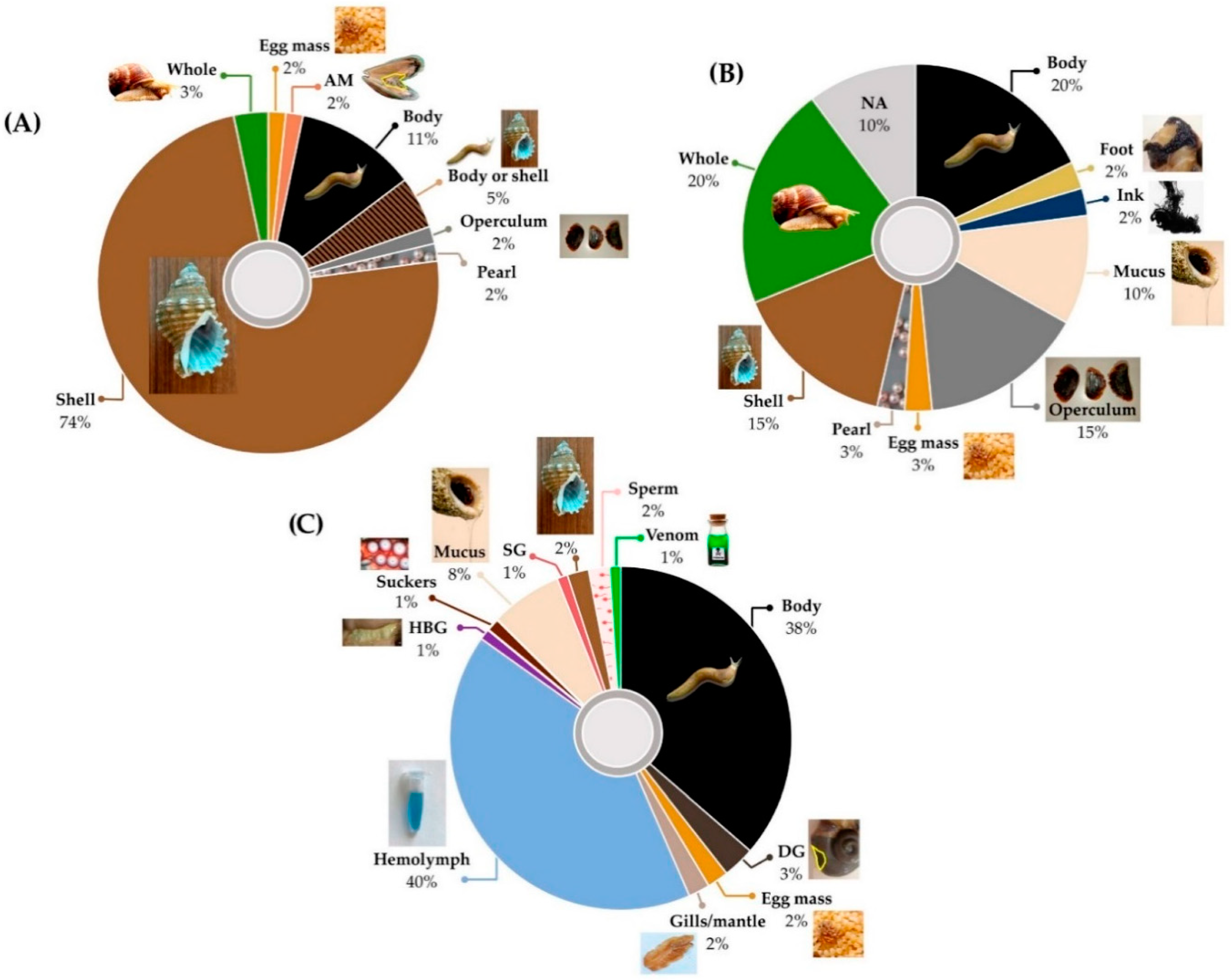

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Summer, K.; Browne, J.; Liu, L.; Benkendorff, K. Molluscan Compounds Provide Drug Leads for the Treatment and Prevention of Respiratory Disease. Mar. Drugs 2020, 18, 570. https://doi.org/10.3390/md18110570
Summer K, Browne J, Liu L, Benkendorff K. Molluscan Compounds Provide Drug Leads for the Treatment and Prevention of Respiratory Disease. Marine Drugs. 2020; 18(11):570. https://doi.org/10.3390/md18110570
Chicago/Turabian StyleSummer, Kate, Jessica Browne, Lei Liu, and Kirsten Benkendorff. 2020. "Molluscan Compounds Provide Drug Leads for the Treatment and Prevention of Respiratory Disease" Marine Drugs 18, no. 11: 570. https://doi.org/10.3390/md18110570
APA StyleSummer, K., Browne, J., Liu, L., & Benkendorff, K. (2020). Molluscan Compounds Provide Drug Leads for the Treatment and Prevention of Respiratory Disease. Marine Drugs, 18(11), 570. https://doi.org/10.3390/md18110570






