A Sulfated Polysaccharide from Saccharina japonica Suppresses LPS-Induced Inflammation Both in a Macrophage Cell Model via Blocking MAPK/NF-κB Signal Pathways In Vitro and a Zebrafish Model of Embryos and Larvae In Vivo
Abstract
1. Introduction
2. Results
2.1. Yield and Physicochemical Properties of LJPS
2.2. Structural Characterization of LJPS
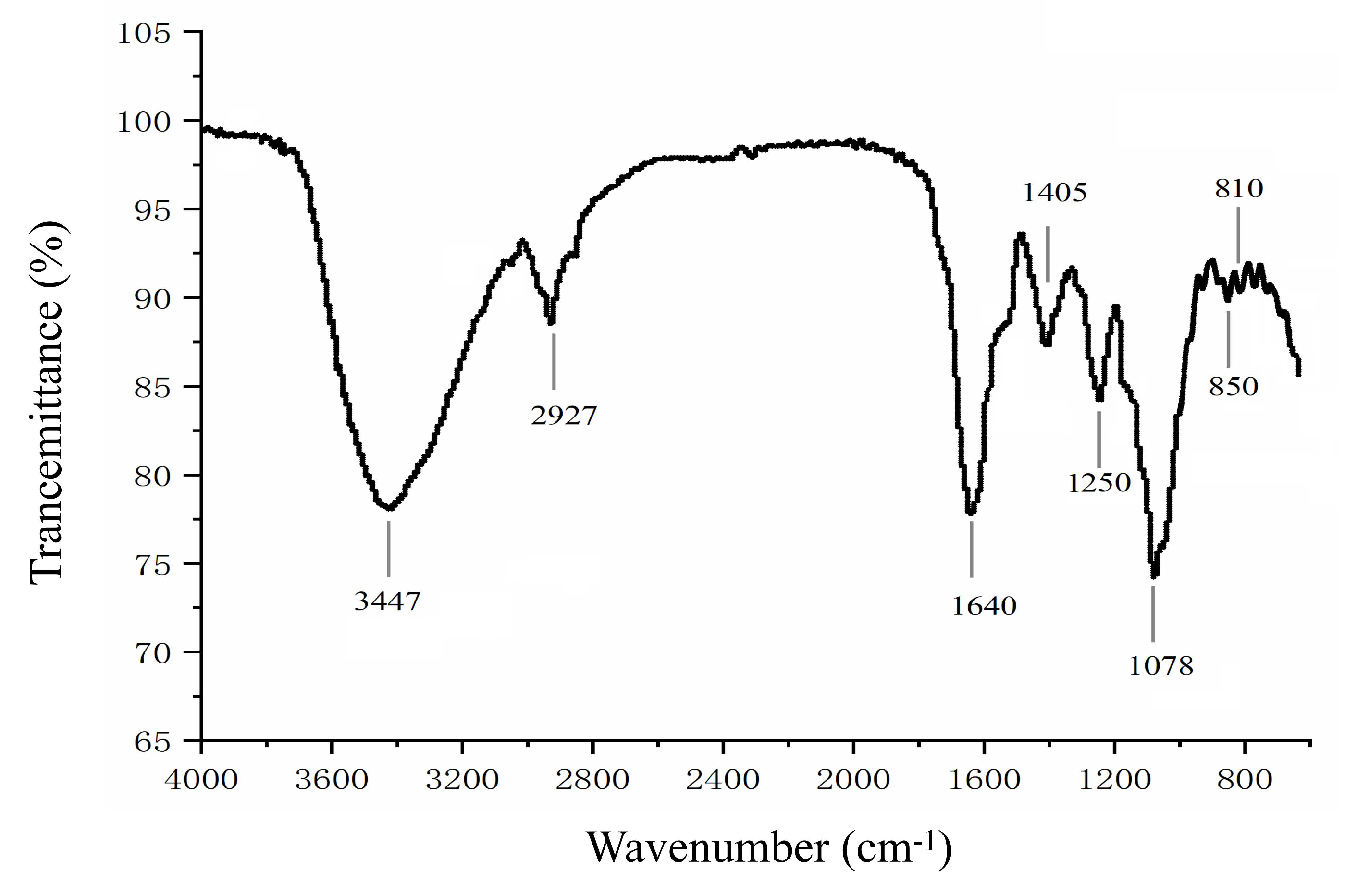
2.2.1. Fourier-Transform Infrared Spectroscopy (FTIR)
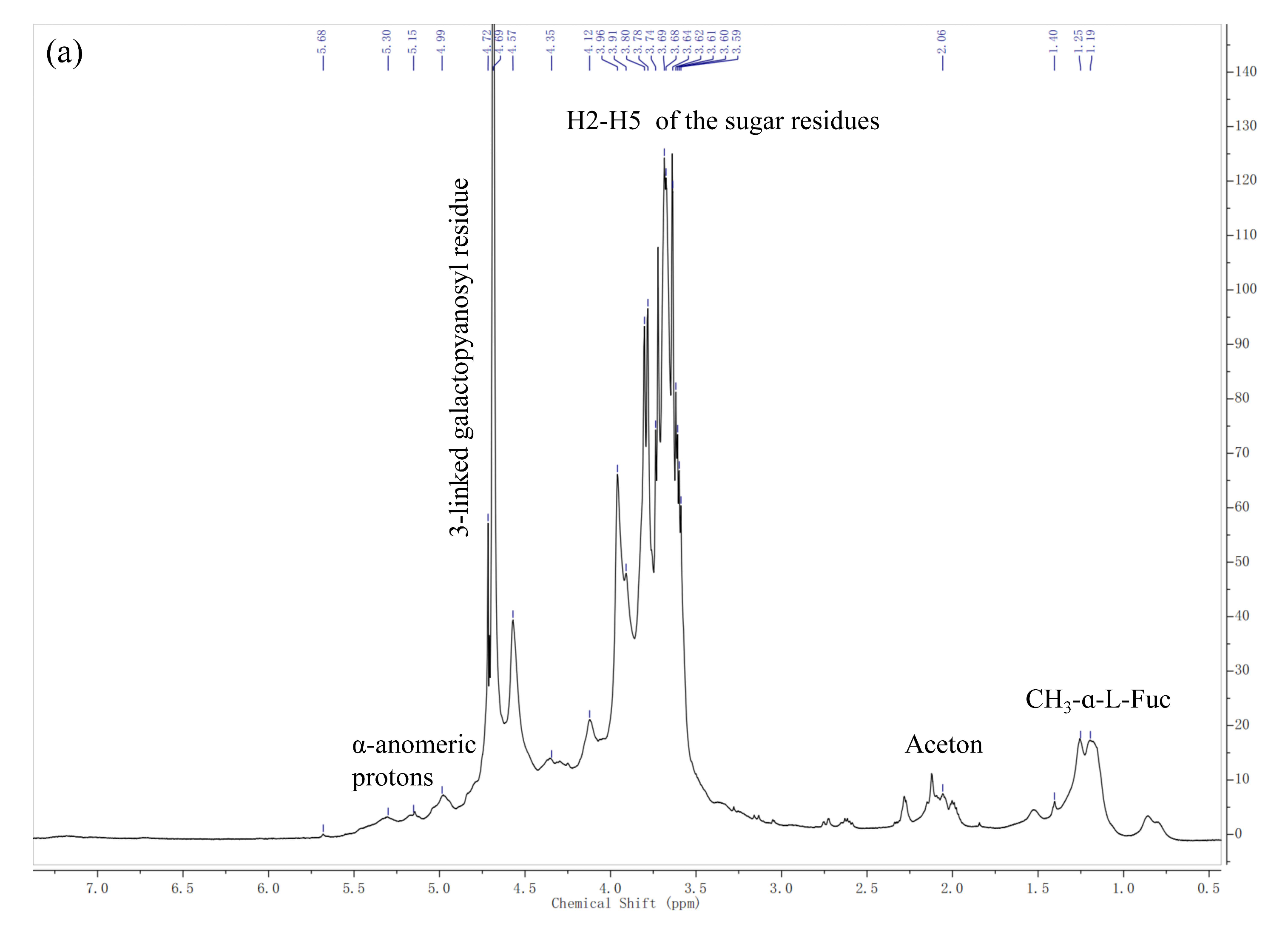
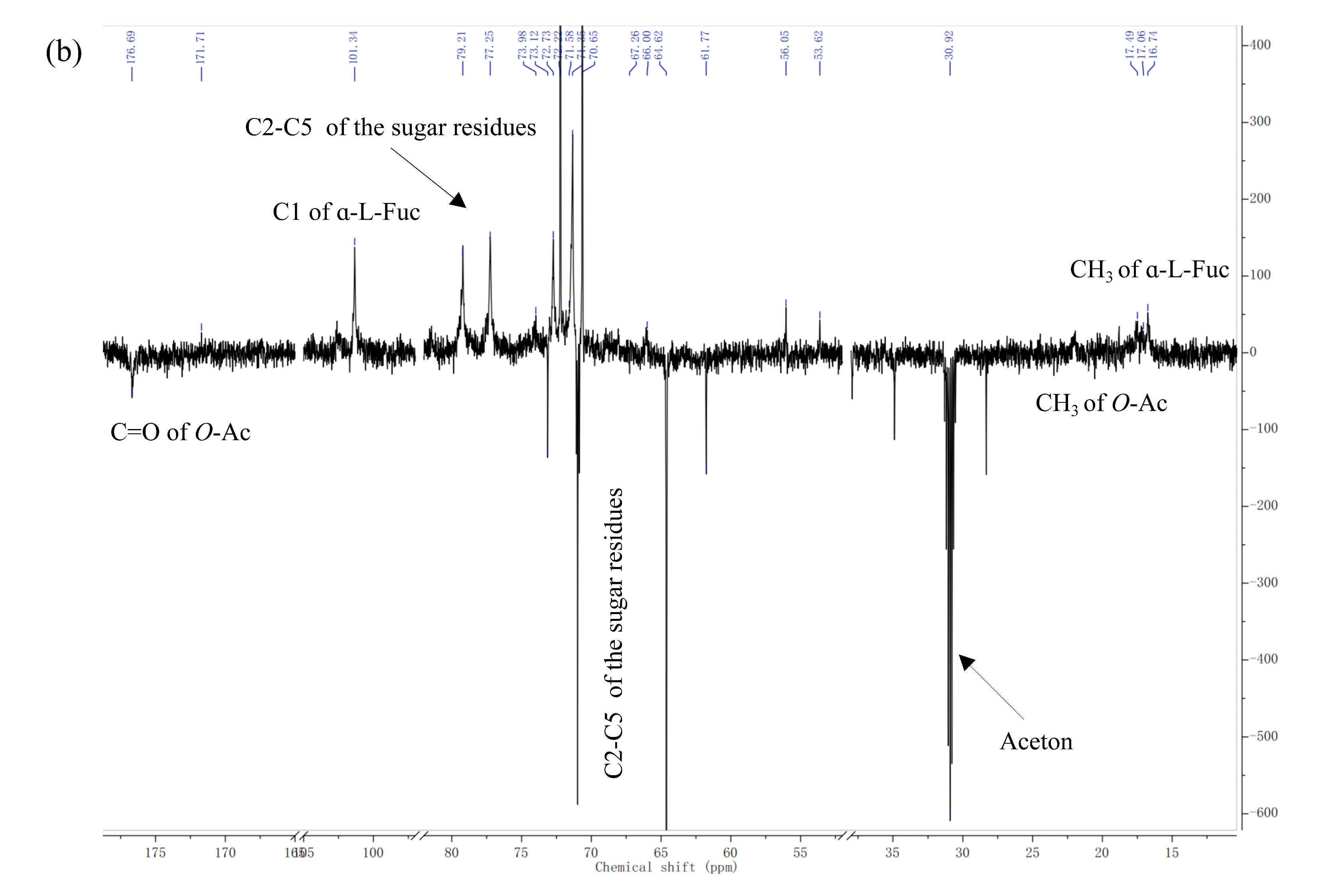
2.2.2. NMR Spectroscopy
2.3. Anti-Inflammatory Effects of LJPS In Vitro by LPS-Induced RAW 264.7 Cells
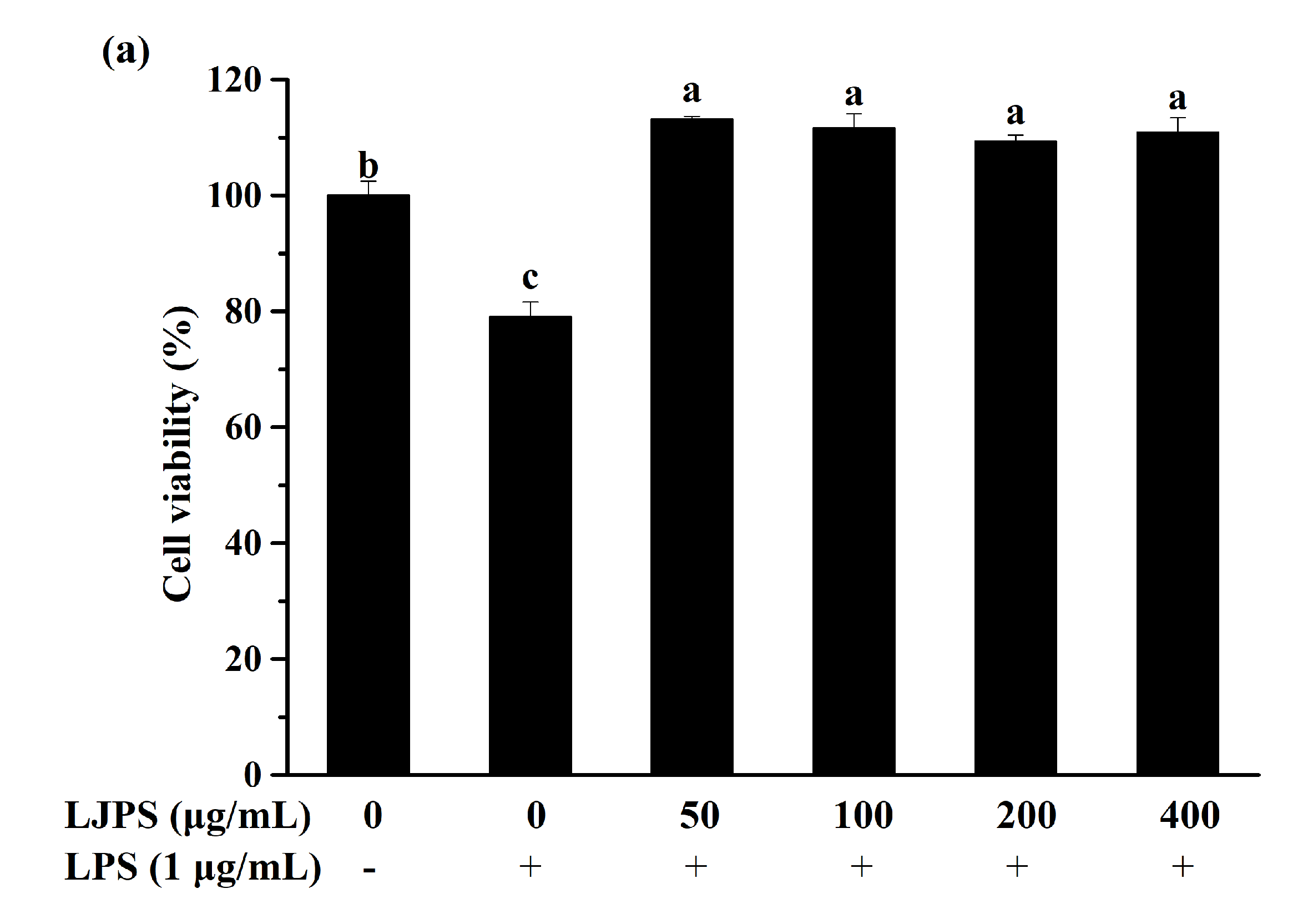
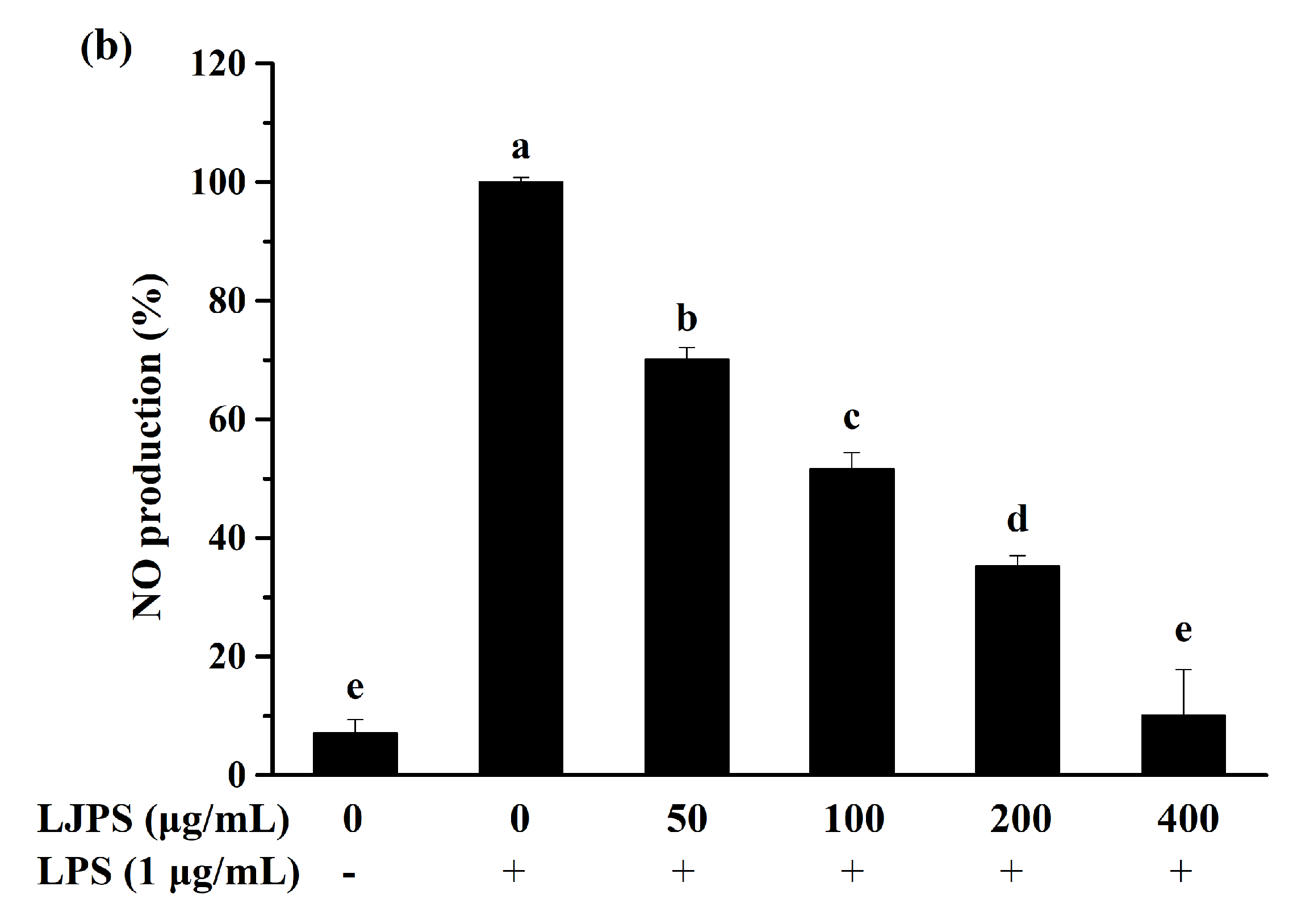
2.3.1. Effect of LJPS on Cell Viability and NO Production
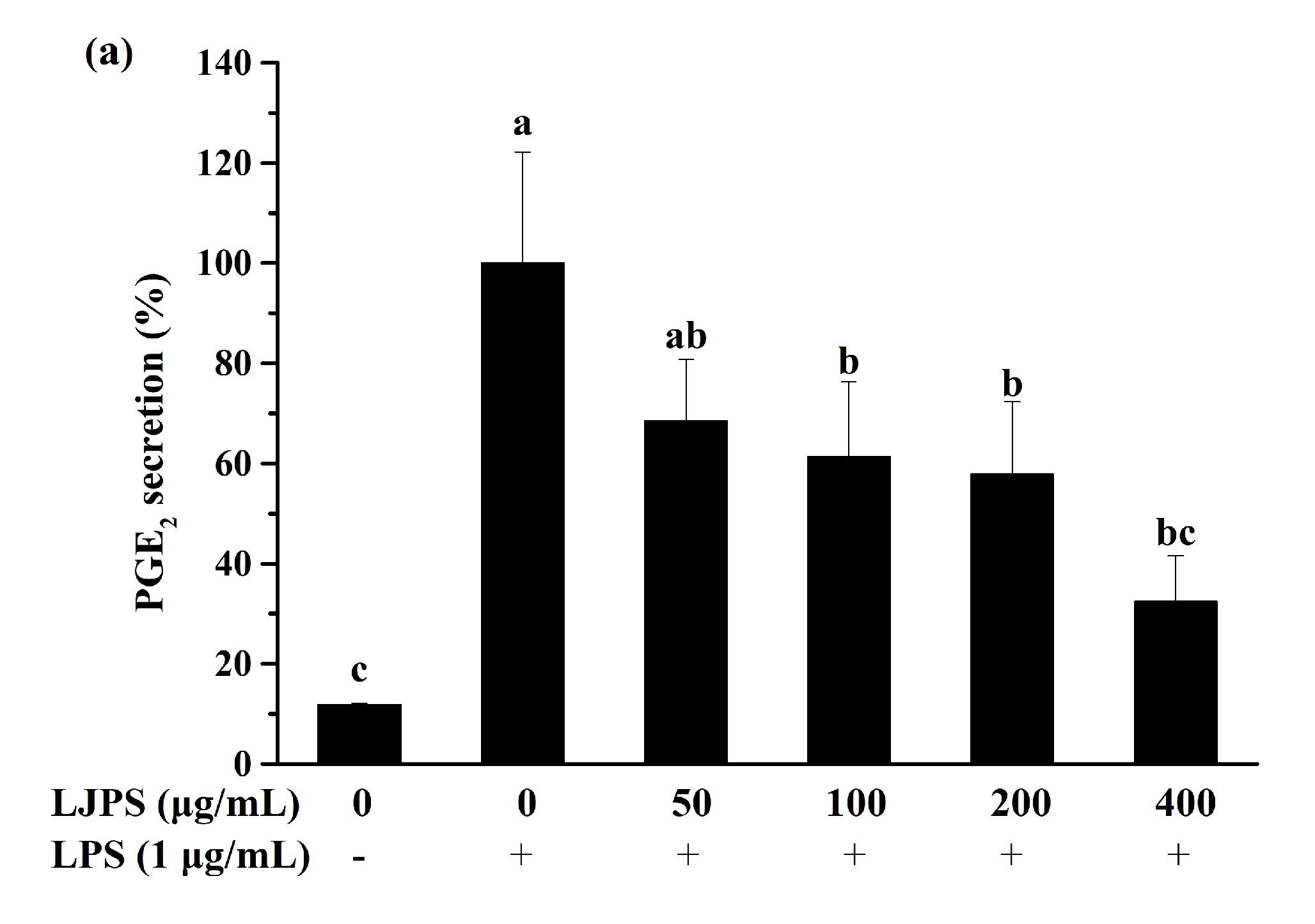
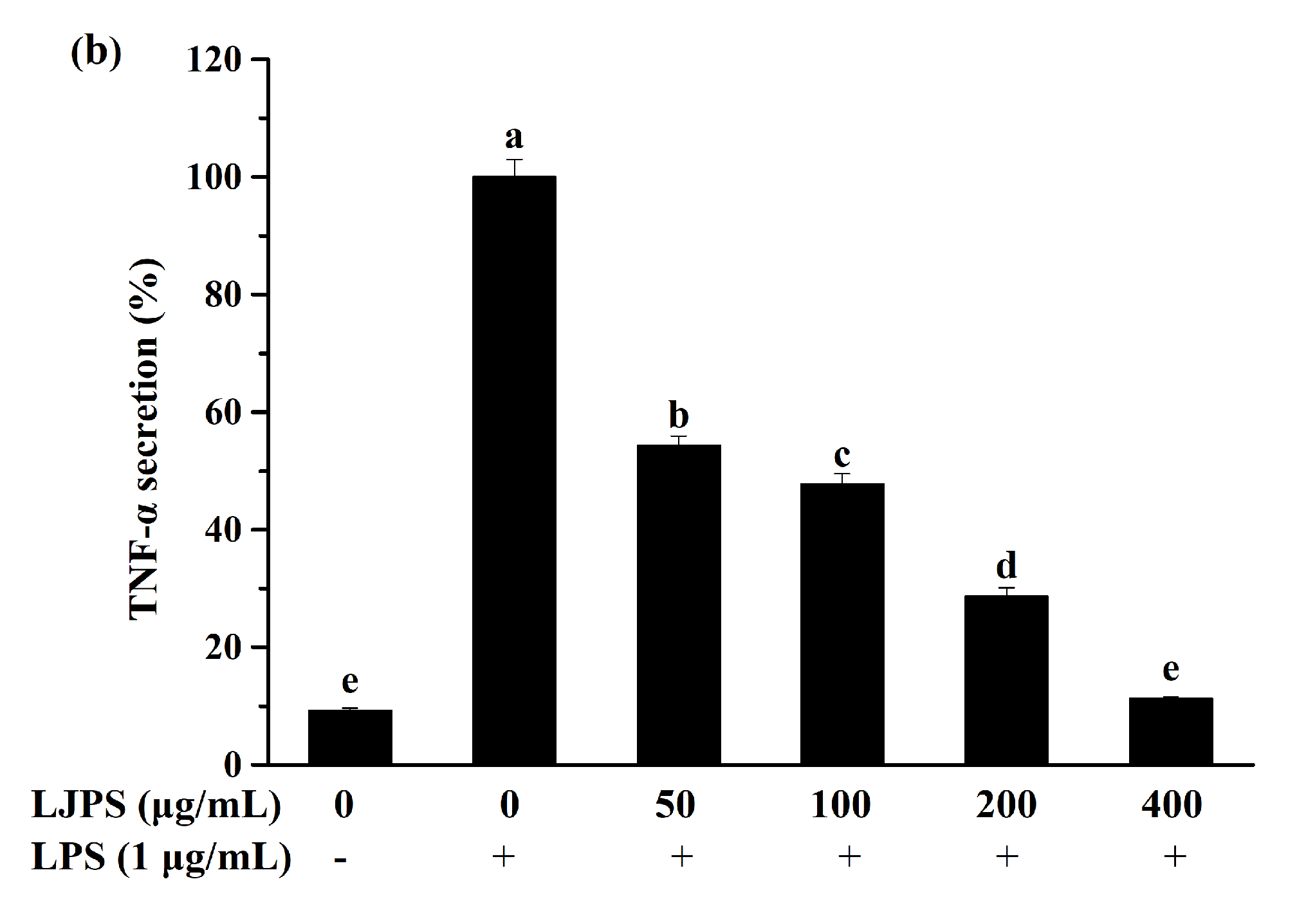
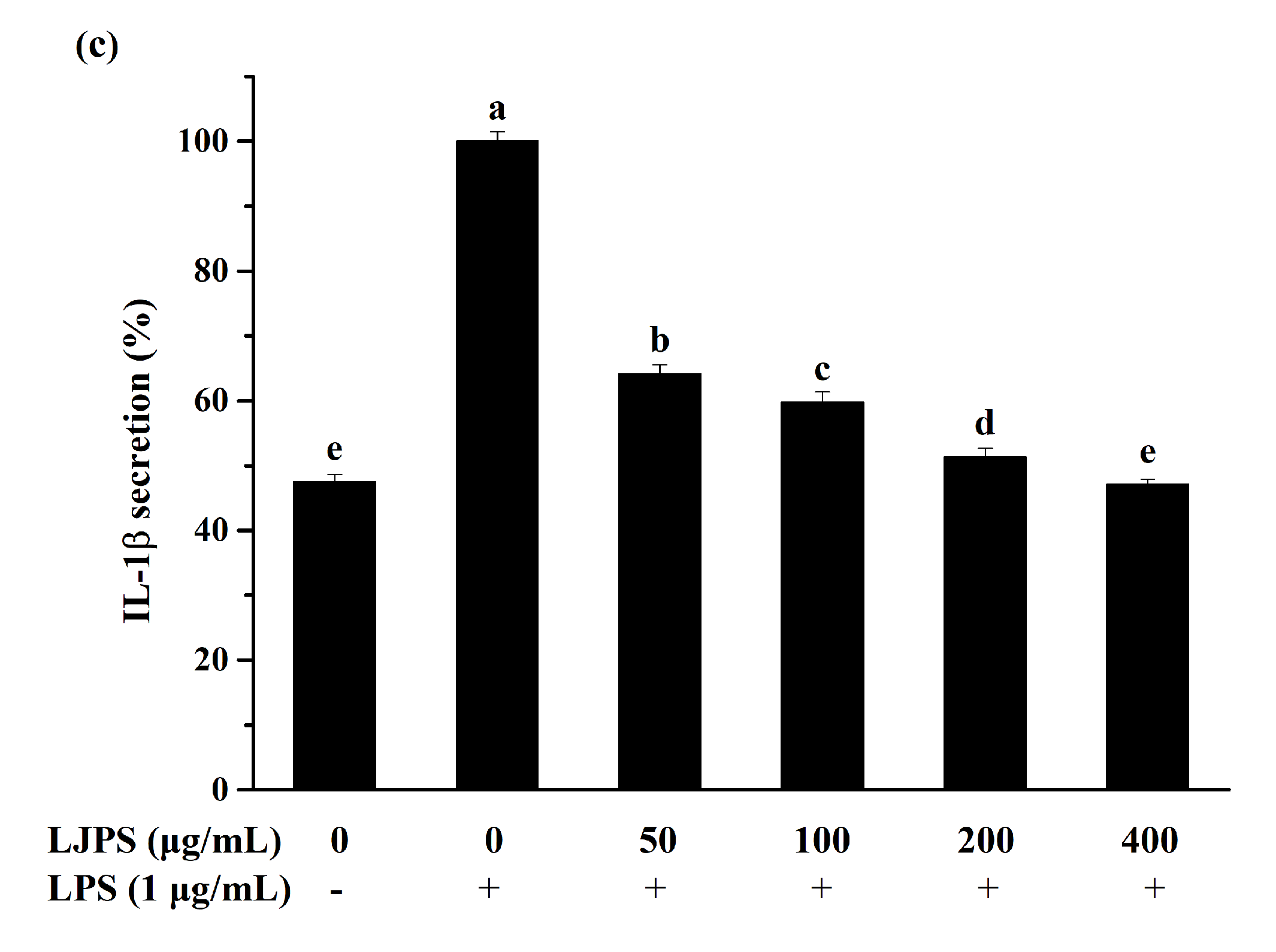
2.3.2. Effect of LJPS on PGE2 and Pro-Inflammatory Cytokines Secretion
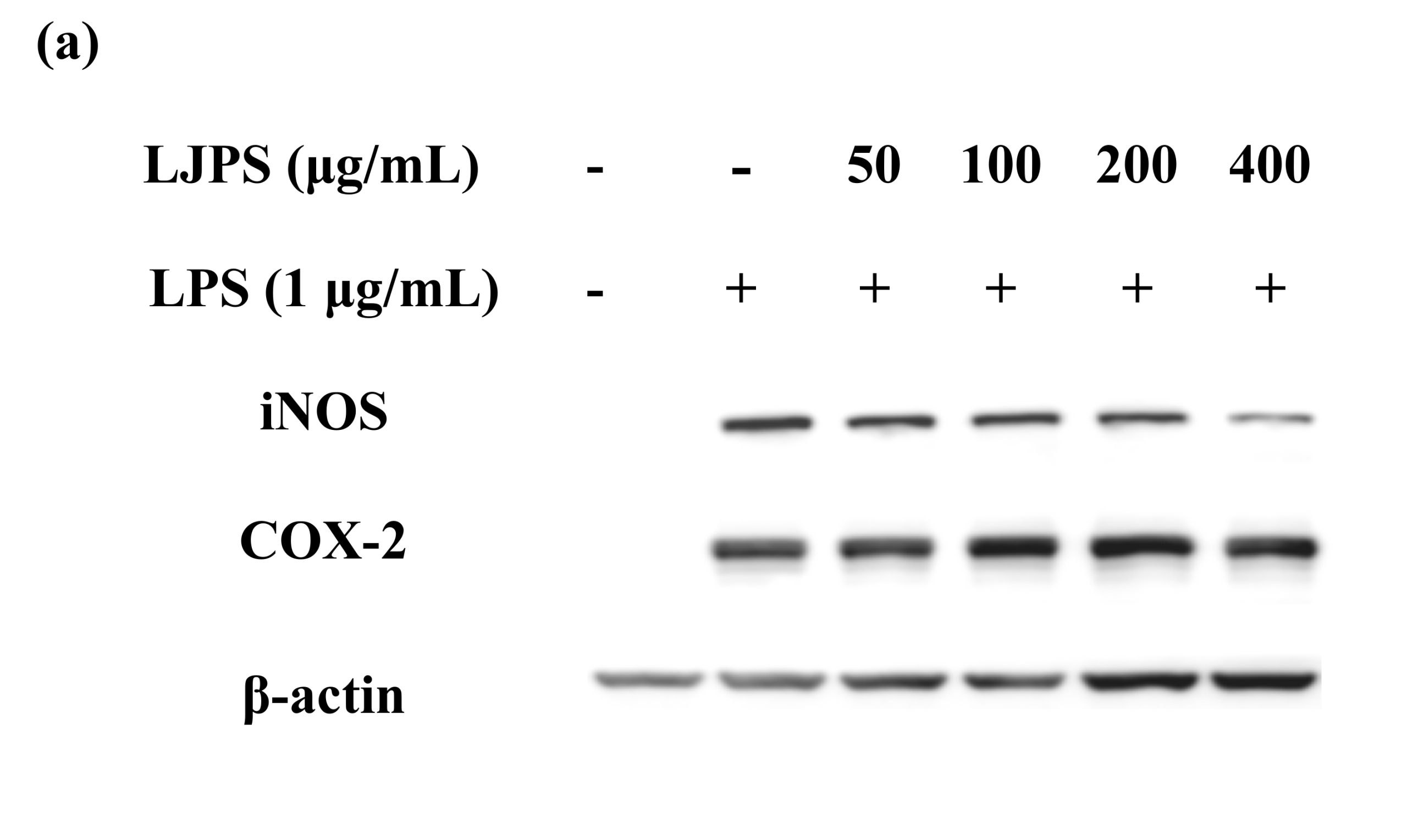
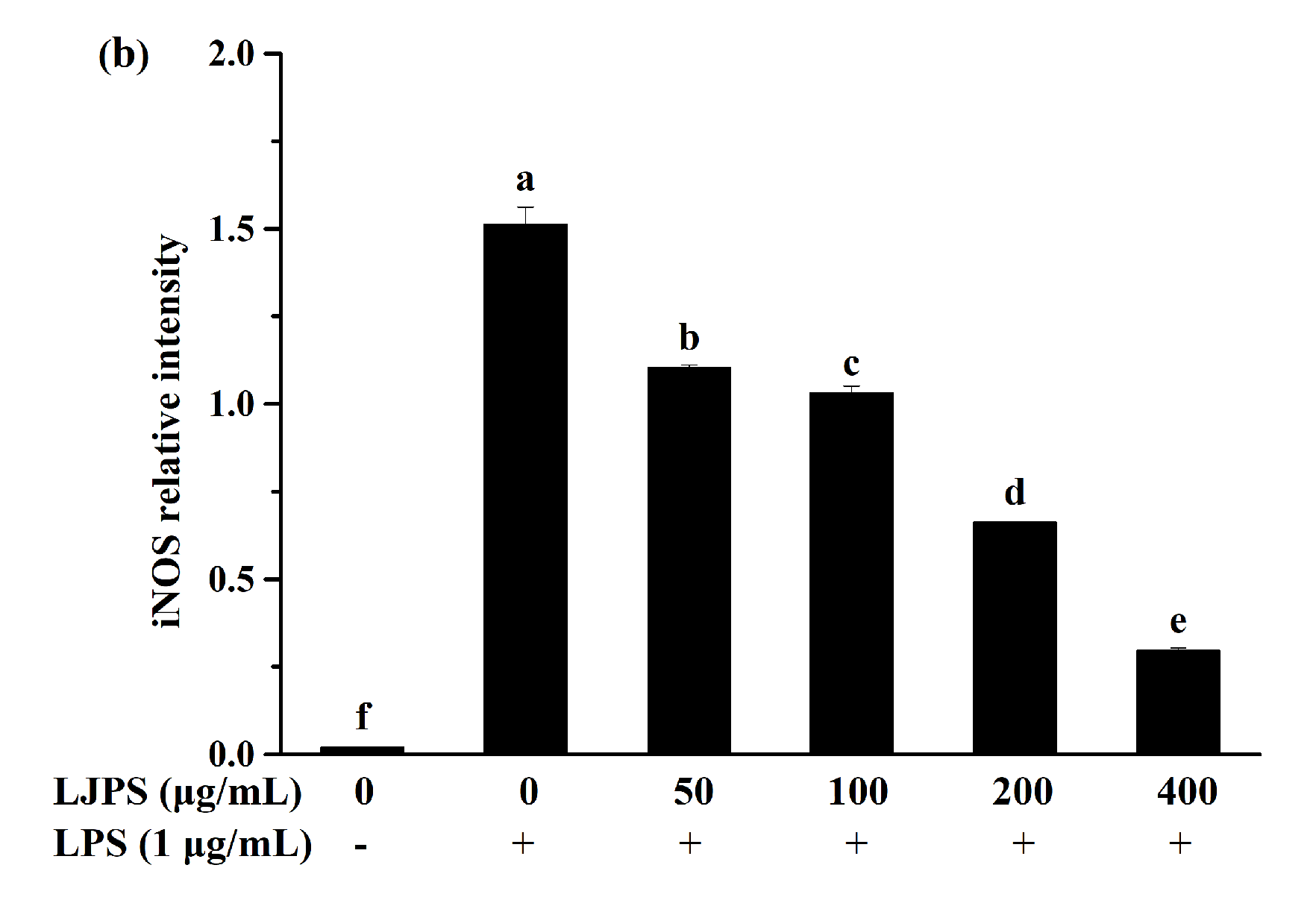
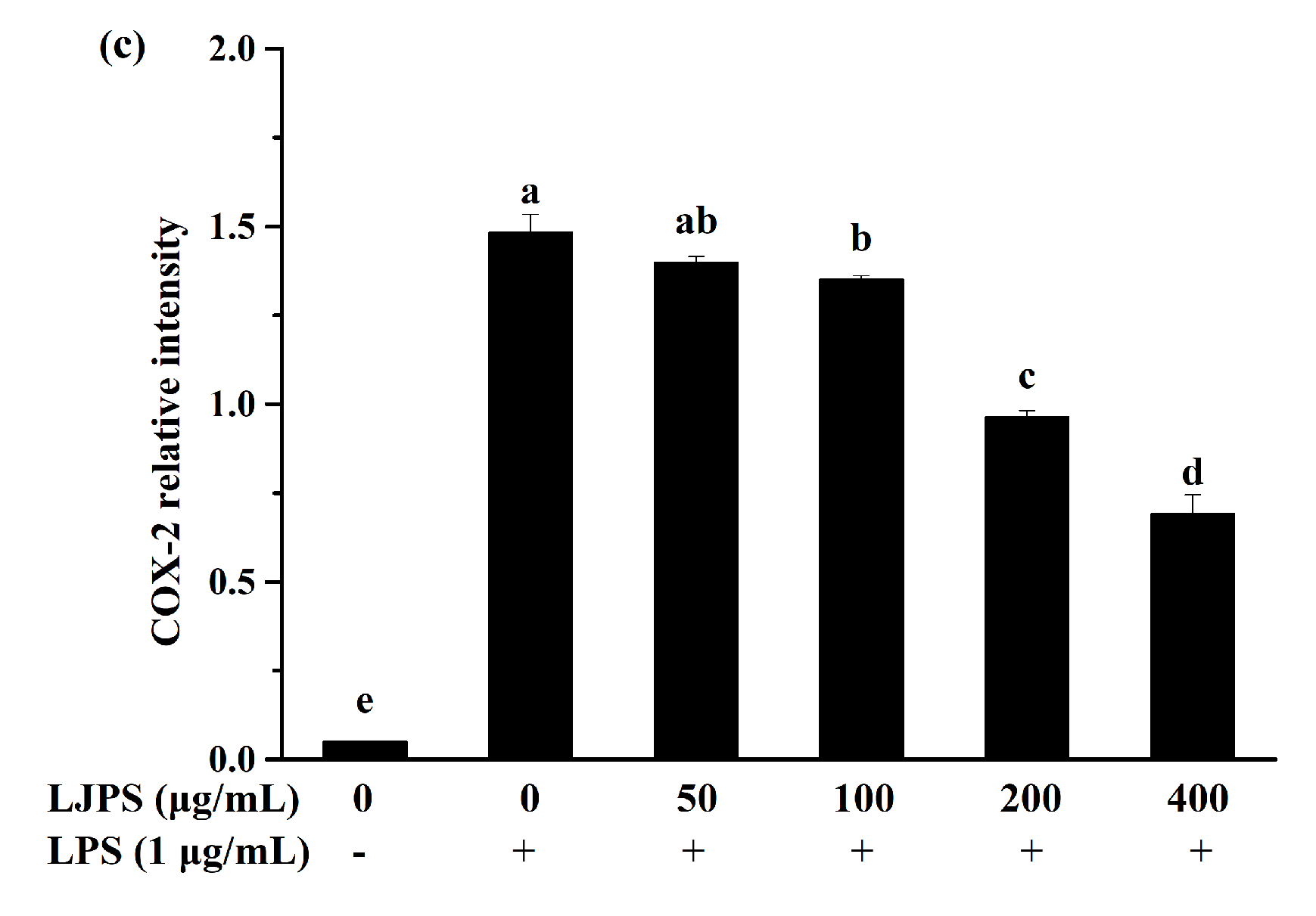
2.3.3. Effect of LJPS on iNOS and COX-2 Expression
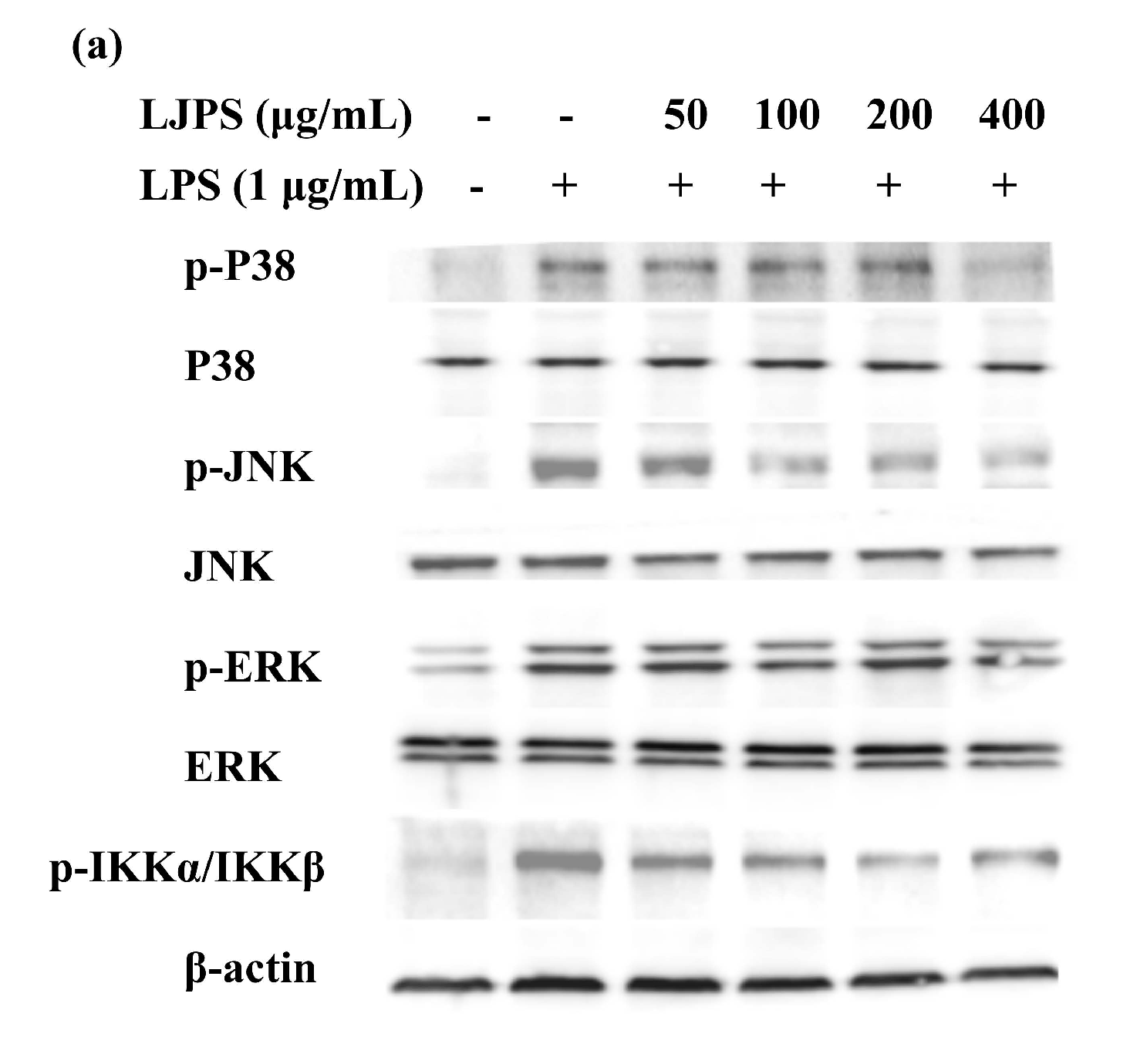
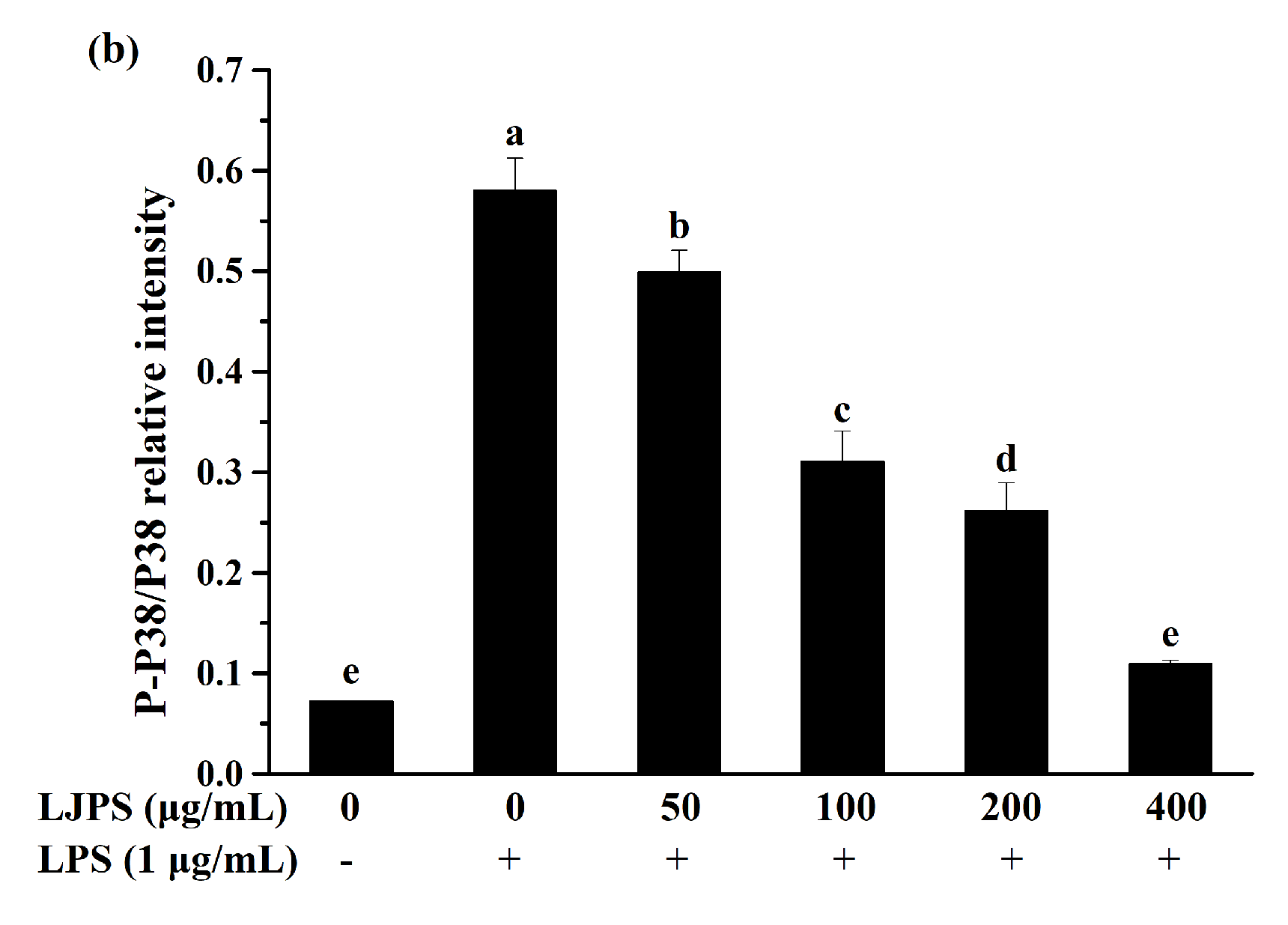
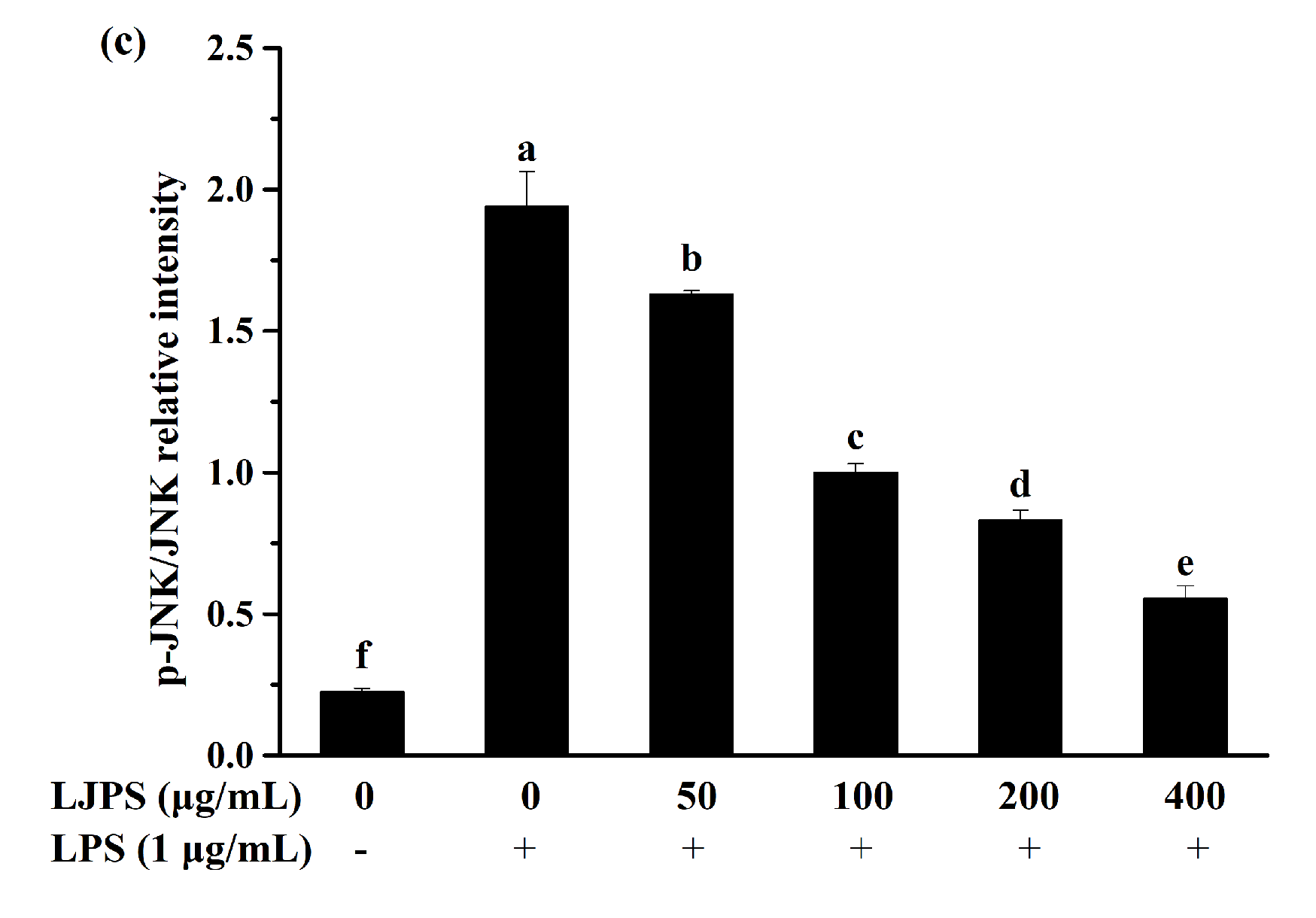
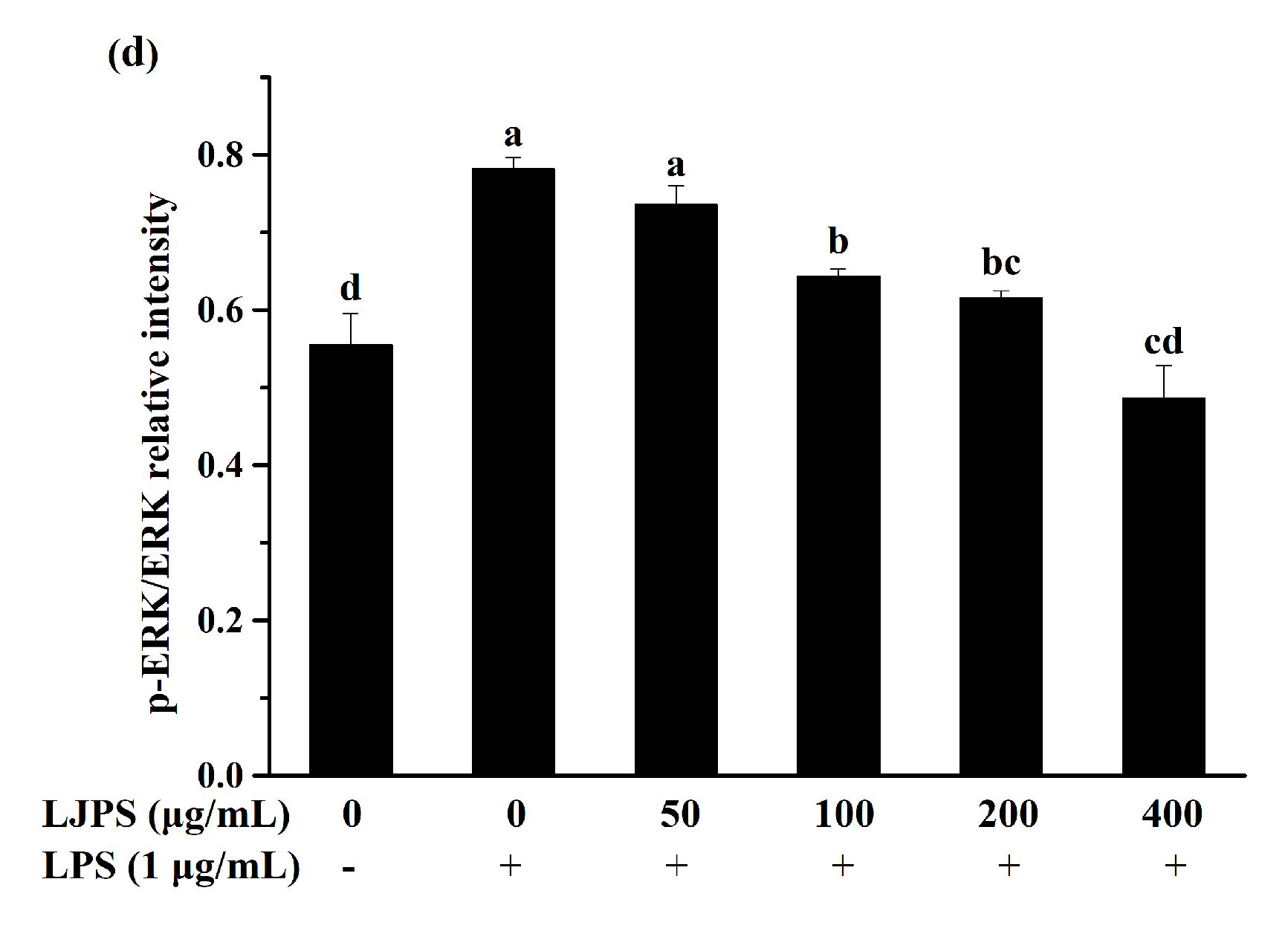
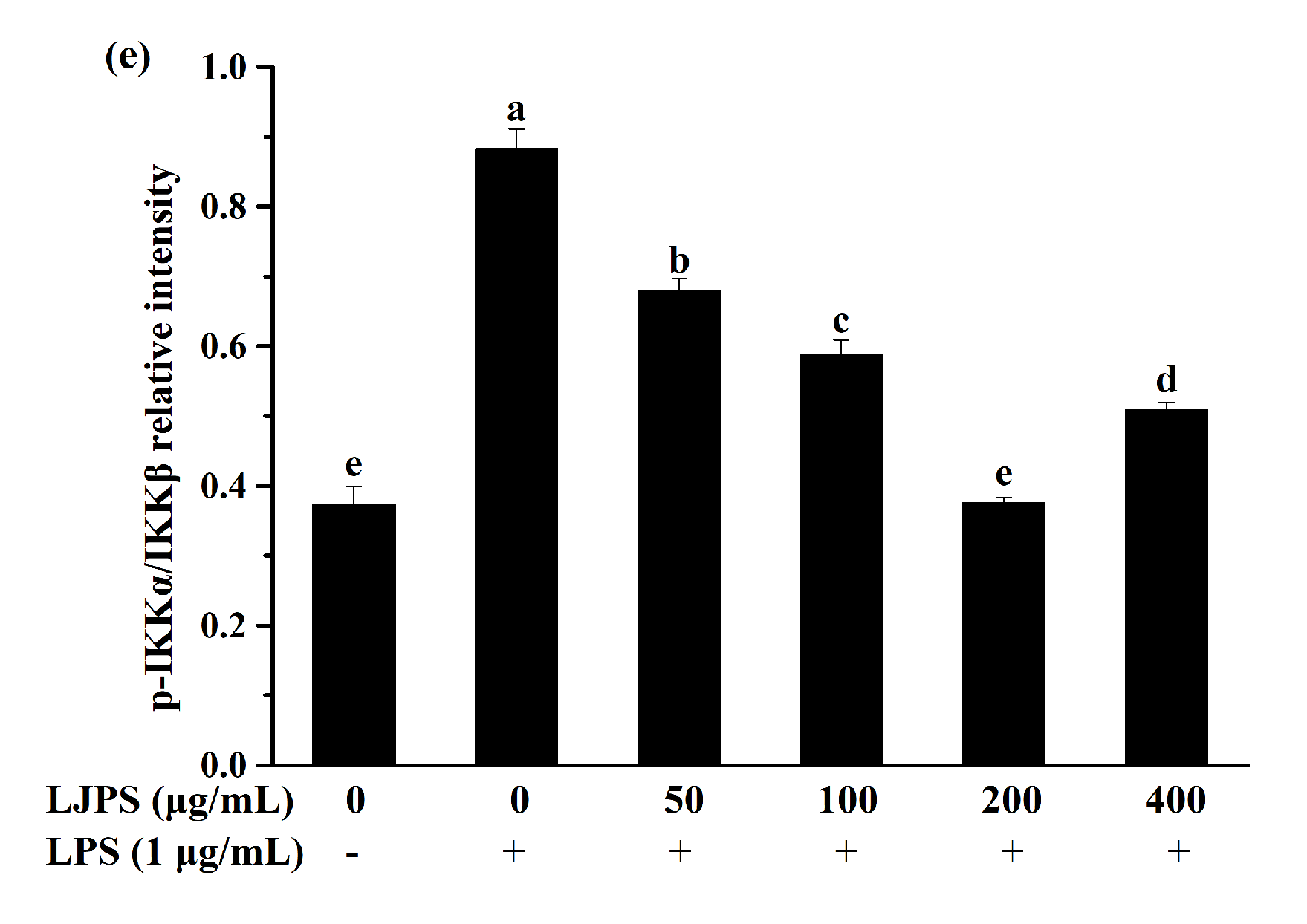
2.3.4. Effects of LJPS on Nuclear Factor-Kappa B (NF-κB) and Mitogen-Activated Protein Kinase (MAPK) Signaling Pathway
2.4. Anti-Inflammatory Effects of LJPS In Vivo by LPS-Induced Zebrafish Model
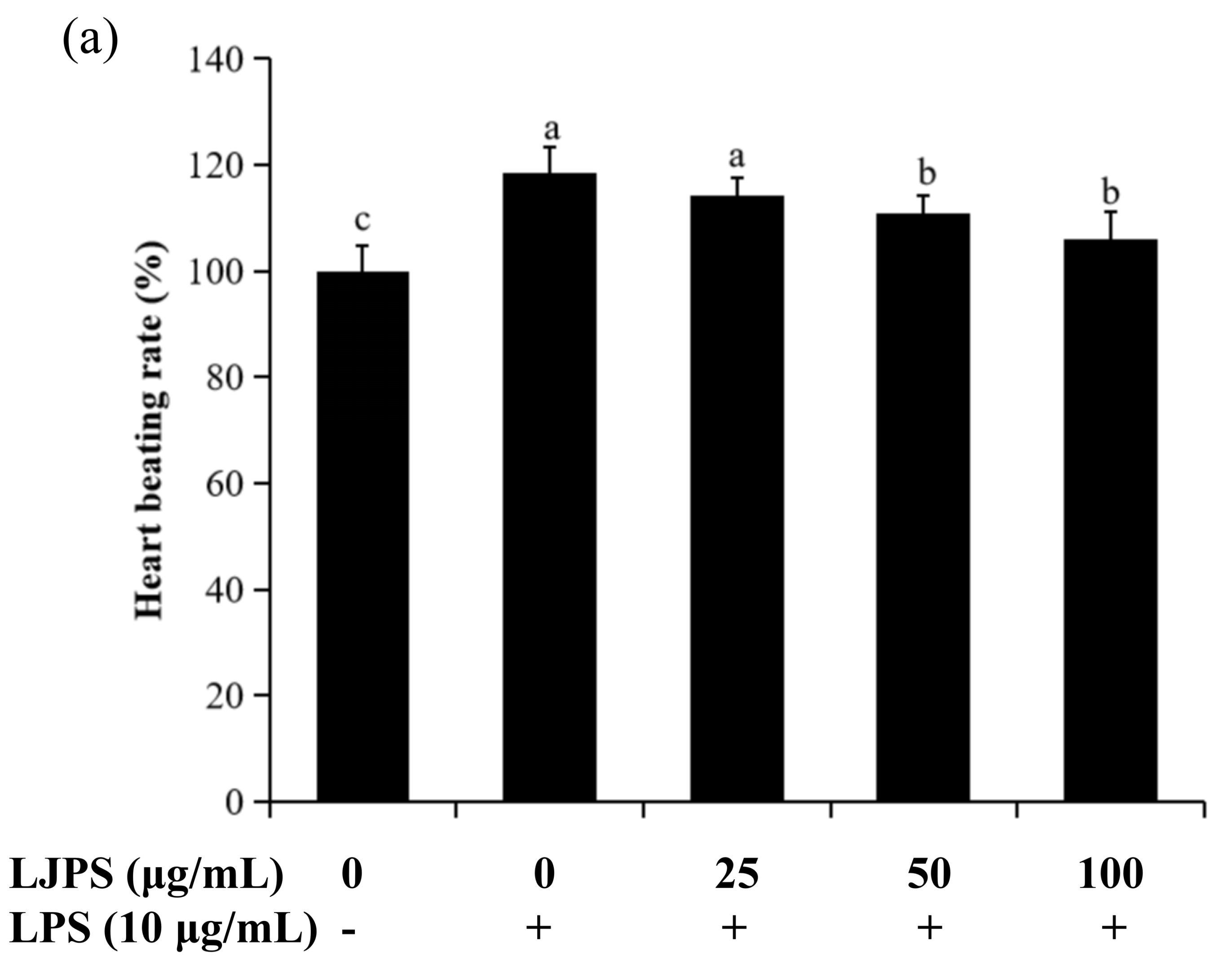
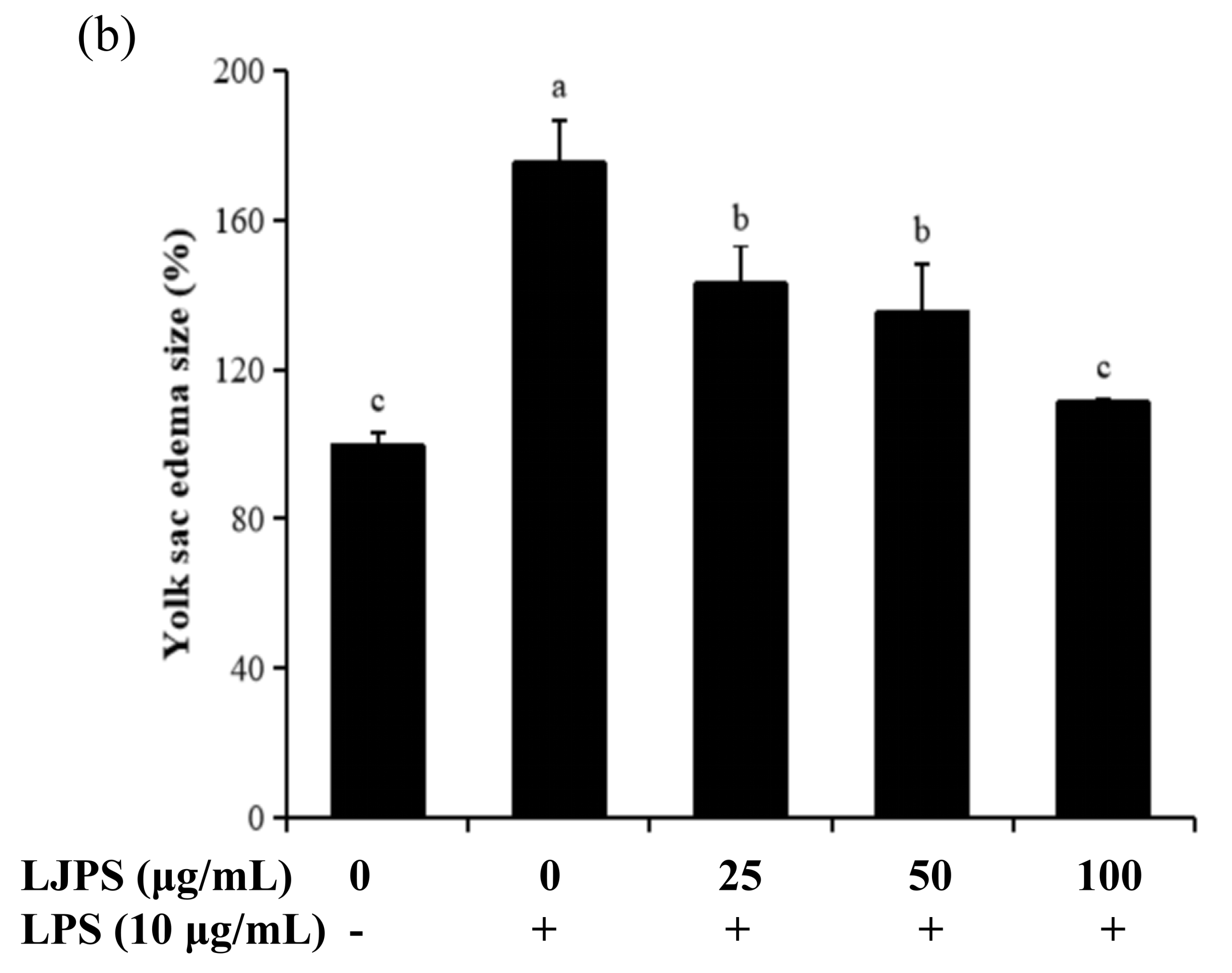
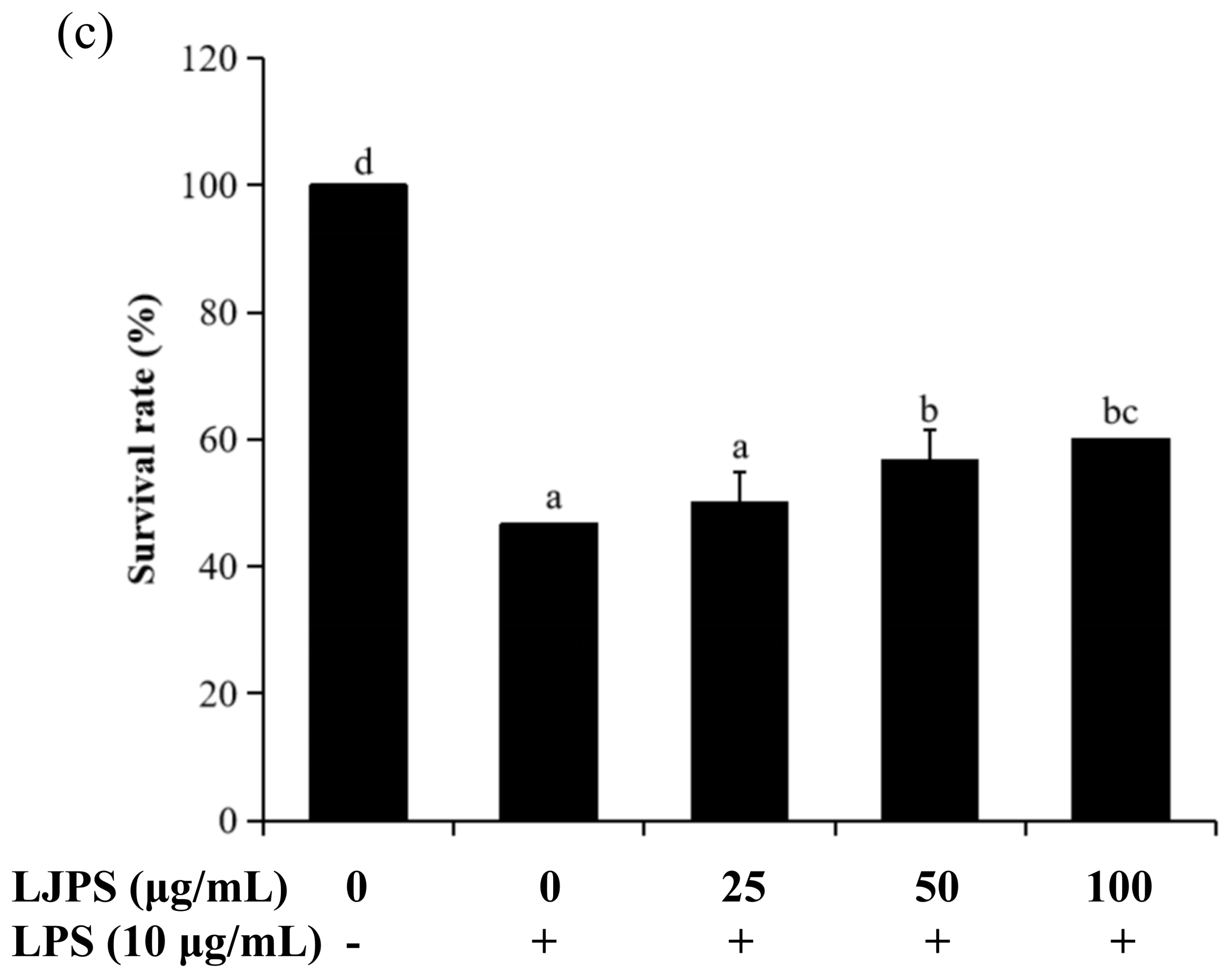
2.4.1. Effects of LJPS on Heart Rate, Yolk Sac Edema Size, and Survival Rate in LPS-Stimulated Zebrafish Embryos
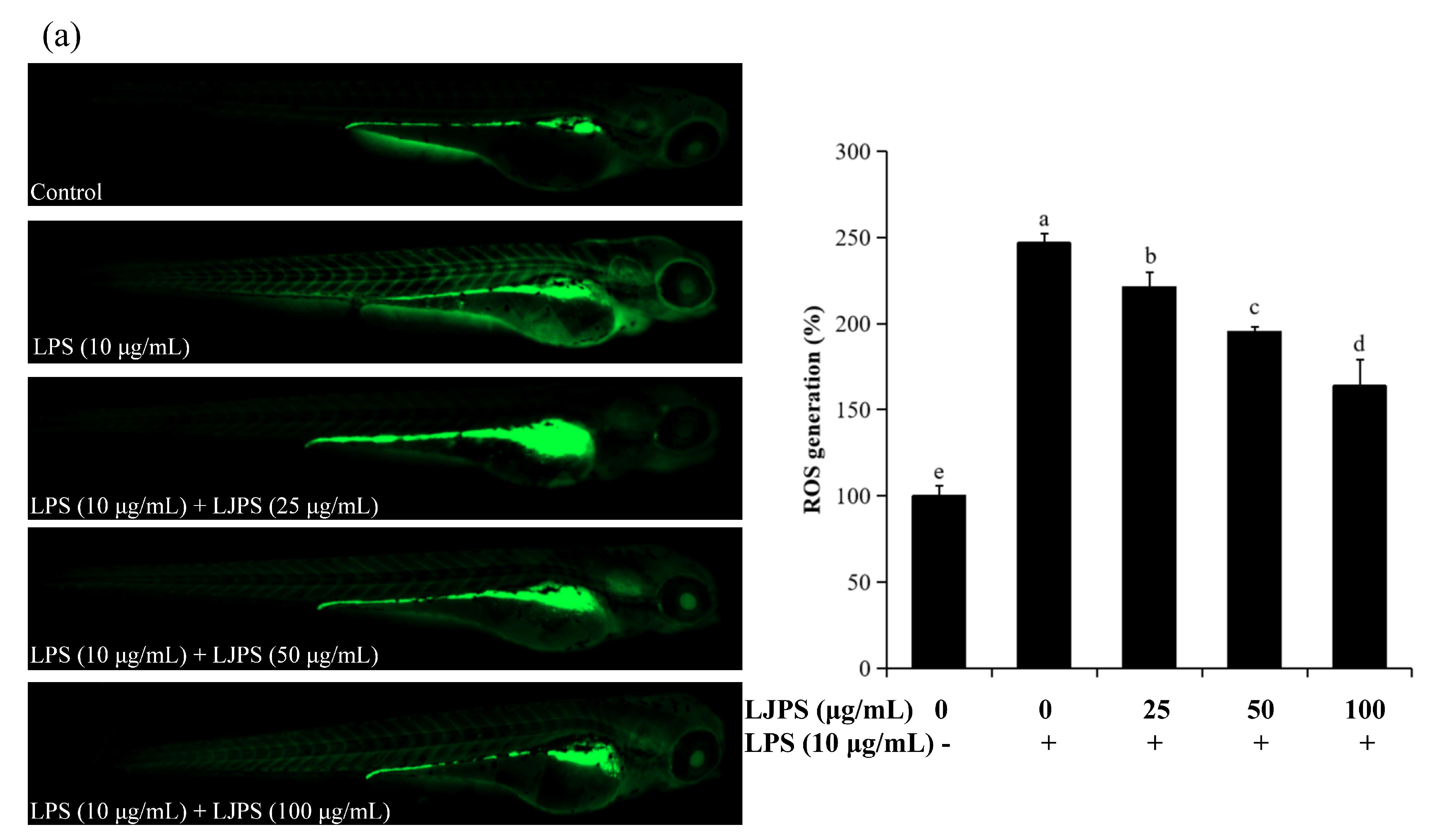
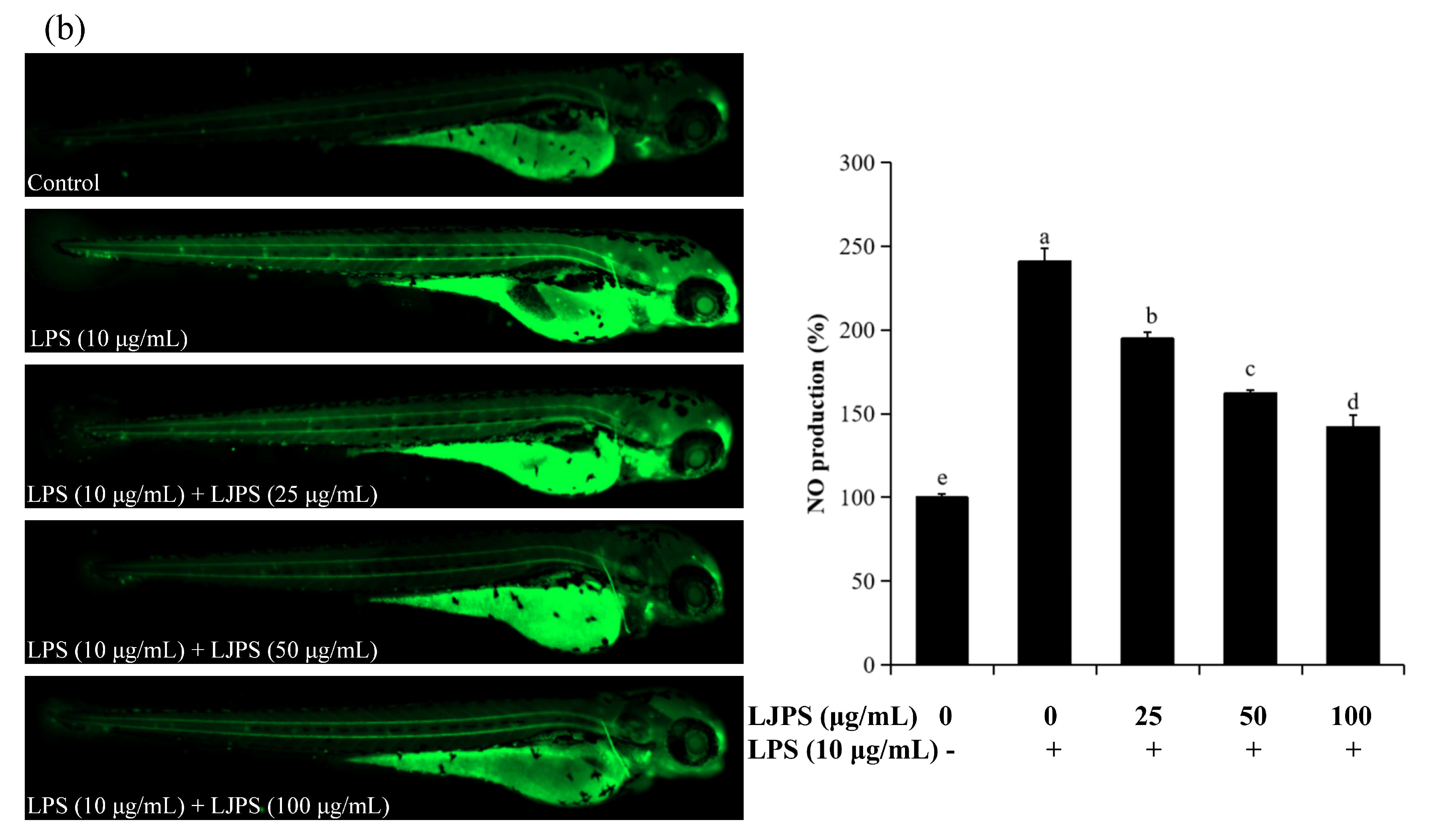
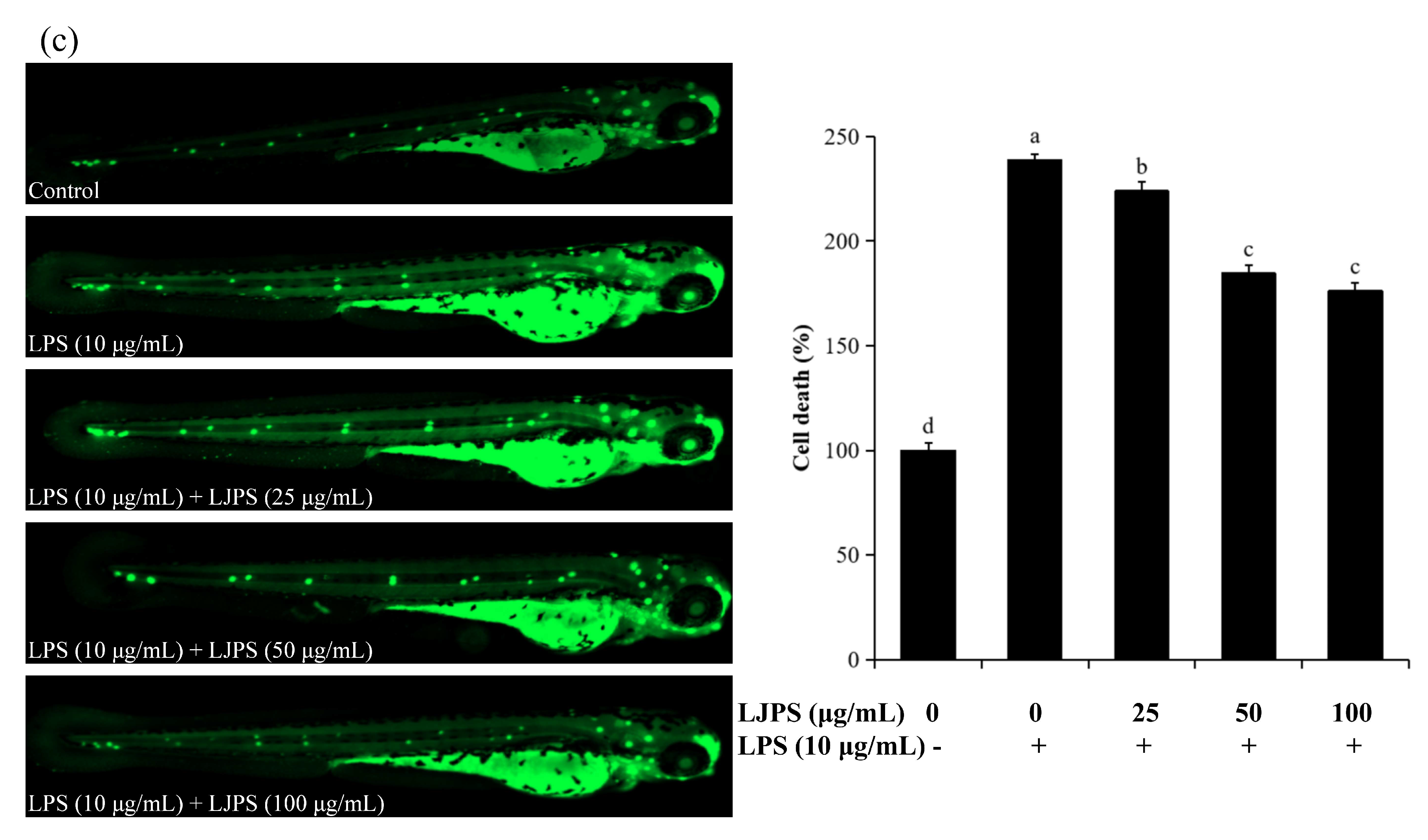
2.4.2. Effects of LJPS on Cell Death, Reactive Oxygen Species (ROS), and NO Generation in LPS-Stimulated Zebrafish Embryos
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material and Extraction
4.3. Chemical Analysis of LJPS
4.4. Structural Characterization of LJPS
4.4.1. Analysis of Monosaccharide Composition
4.4.2. Determination of MW
4.4.3. Analysis of Fourier-Transform Infrared Spectroscopy (FTIR)
4.4.4. Analysis of NMR Spectroscopy
4.5. In Vitro Cell Experiments
4.5.1. Cell Line and Culture
4.5.2. Cell Viability Measurement Using the MTT Assay
4.5.3. Measurement of NO Production, PGE2 and Cytokines Secretion
4.5.4. Western Blot Analysis
4.6. In Vivo Zebrafish Analysis
4.6.1. Origin and Maintenance of Zebrafish
4.6.2. Application of LPS and/or LJPS to Zebrafish Embryos
4.6.3. Determination of Heart Rate, Yolk Sac Edema Size, and Survival Rate
4.6.4. Production of ROS and NO, Cell Death and Image Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Paulsen, B.S. Biologically active polysaccharides as possible lead compounds. Phytochem. Rev. 2002, 1, 379–387. [Google Scholar] [CrossRef]
- Zvyagintseva, T.N.; Shevchenko, N.M.; Popivnich, I.B.; Isakov, V.V.; Scobun, A.S.; Sundukova, E.V.; Elyakova, L.A. A new procedure for the separation of water-soluble polysaccharides from brown seaweeds. Carbohydr. Res. 1999, 322, 32–39. [Google Scholar] [CrossRef]
- Okolie, C.L.; Rajendran, S.R.C.K.; Udenigwe, C.C.; Aryee, A.N.A.; Mason, B. Prospects of brown seaweed polysaccharides (BSP) as prebiotics and potential immunomodulators. J. Food Biochem. 2017, 41, e12392. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Cao, M.-J.; Liu, G.-M.; Chen, Q.; Sun, L.; Chen, H. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr. Polym. 2017, 172, 294–305. [Google Scholar] [CrossRef]
- Park, M.-K.; Jung, U.; Roh, C. Fucoidan from marine brown algae inhibits lipid accumulation. Mar. Drugs 2011, 9, 1359–1367. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, Q.; Wang, J.; Zhang, W. A comparative study of the anticoagulant activities of eleven fucoidans. Carbohydr. Polym. 2013, 91, 1–6. [Google Scholar] [CrossRef]
- Dinesh, S.; Menon, T.; Hanna, L.E.; Suresh, V.; Sathuvan, M.; Manikannan, M. In vitro anti-HIV-1 activity of fucoidan from Sargassum swartzii. Int. J. Biol. Macromol. 2016, 82, 83–88. [Google Scholar] [CrossRef]
- Geng, L.; Hu, W.; Liu, Y.; Wang, J.; Zhang, Q. A heteropolysaccharide from Saccharina japonica with immunomodulatory effect on RAW 264.7 cells. Carbohydr. Polym. 2018, 201, 557–565. [Google Scholar] [CrossRef]
- Wang, L.; Oh, J.Y.; Hwang, J.; Jeon, Y.J.; Ryu, B. In Vitro and In Vivo Antioxidant Activities of Polysaccharides Isolated from Celluclast-Assisted Extract of an Edible Brown Seaweed, Sargassum fulvellum. Antioxidants 2019, 8, 493. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.G.; Hao, Y.; Li, Z.-H.; Liu, S.-T.; Wang, L. Antiviral activity of polysaccharide extract from Laminaria japonica against respiratory syncytial virus. Biomed. Pharmacother. 2016, 84, 1705–1710. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.H.; Zha, X.Q.; Cui, S.H.; Asghar, M.N.; Pan, L.H.; Wang, J.; Luo, J.P. Purification, structure features and anti-atherosclerosis activity of a Laminaria japonica polysaccharide. Int. J. Biol. Macromol. 2015, 81, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, J.; Jiang, H.; Song, N.; Zhang, W.; Zhang, Q. The neuroprotective activities of heteropolysaccharides extracted from Saccharina japonica. Carbohydr. Polym. 2013, 97, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, Y.R.; Yoon, S.Y.; Kim, S.K.; Li, J.D.; Kang, K.W. Inhibition of acrolein-stimulated MUC5AC production by fucoidan in human bronchial epithelial cells. Pharmazie 2008, 63, 757–759. [Google Scholar]
- Li, C.; Gao, Y.; Xing, Y.; Zhu, H.; Shen, J.; Tian, J. Fucoidan, a sulfated polysaccharide from brown algae, against myocardial ischemia–reperfusion injury in rats via regulating the inflammation response. Food Chem. Toxicol. 2011, 49, 2090–2095. [Google Scholar] [CrossRef]
- Zhao, D.; Xu, J.; Xu, X. Bioactivity of fucoidan extracted from Laminaria japonica using a novel procedure with high yield. Food Chem. 2018, 245, 911–918. [Google Scholar] [CrossRef]
- Zha, X.Q.; Lu, C.Q.; Cui, S.H.; Pan, L.H.; Zhang, H.L.; Wang, J.H.; Luo, J.P. Structural identification and immunostimulating activity of a Laminaria japonica polysaccharide. Int. J. Biol. Macromol. 2015, 78, 429–438. [Google Scholar] [CrossRef]
- Ye, J.; Chen, D.; Ye, Z.; Huang, Y.; Zhang, N.; Lui, E.M.K.; Xue, C.; Xiao, M. Fucoidan Isolated from Saccharina japonica Inhibits LPS-Induced Inflammation in Macrophages via Blocking NF-κB, MAPK and JAK-STAT Pathways. Mar. Drugs 2020, 18, 328. [Google Scholar] [CrossRef]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.-J.; Xu, J.; Gao, X. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef]
- Phull, A.R.; Kim, S.J. Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Funct. Foods 2017, 38, 415–426. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.J. Cytokines and related receptor-mediated signaling pathways. Biochem. Biophys. Res. Commun. 2002, 297, 700–713. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef]
- Muralidharan, S.; Mandrekar, P. Cellular stress response and innate immune signaling: Integrating pathways in host defense and inflammation. J. Leukoc. Biol. 2013, 94, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yang, X.; Xia, B.; Yang, Z.; Wang, Z.; Wang, J.; Li, T.; Lin, P.; Song, X.; Guo, S. The fucoidan from sea cucumber Apostichopus japonicus attenuates lipopolysaccharide-challenged liver injury in C57BL/6J mice. J. Funct. Foods 2019, 61, 103493. [Google Scholar] [CrossRef]
- Wang, Y.M.; Xu, M.; Wang, D.; Yang, C.R.; Zeng, Y.; Zhang, Y.J. Anti-inflammatory compounds of “Qin-Jiao”, the roots of Gentiana dahurica (Gentianaceae). J. Ethnopharmacol. 2013, 147, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhu, J.; Cao, F.; Chen, F. Anti-inflammatory properties of extracts from Chimonanthus nitens Oliv. leaf. PLoS ONE 2017, 12, e0181094. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW 264.7 macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef]
- Kim, K.J.; Yoon, K.Y.; Lee, B.Y. Low molecular weight fucoidan from the sporophyll of Undaria pinnatifida suppresses inflammation by promoting the inhibition of mitogen-activated protein kinases and oxidative stress in RAW264.7 cells. Fitoter. 2012, 83, 1628–1635. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, C.I.; Ahn, G.; You, S.; Kim, J.S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef]
- Sanjeewa, K.; Jayawardena, T.U.; Kim, H.S.; Kim, S.Y.; Fernando, I.S.; Wang, L.; Abetunga, D.; Kim, W.S.; Lee, D.S.; Jeon, Y.J. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-κB signal pathway. Carbohydr. Polym. 2019, 224, 115195. [Google Scholar] [CrossRef]
- Jayawardena, T.U.; Fernando, I.S.; Lee, W.W.; Sanjeewa, K.; Kim, H.S.; Lee, D.S.; Jeon, Y.J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; Inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Trede, N.S.; Zapata, A.; Zon, L. Fishing for lymphoid genes. Trends Immunol. 2001, 22, 302–307. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, C.I.; Jee, Y.; Jeong, Y.; Kim, M.; Kim, J.S.; Jeon, Y.J. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr. Polym. 2013, 92, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Eisen, J.S. Zebrafish make a big splash. Cell 1996, 87, 969–977. [Google Scholar] [CrossRef]
- Terzi, M.; Altun, G.; Şen, S.; Kocaman, A.; Kaplan, A.A.; Yurt, K.K.; Kaplan, S. The use of non-steroidal anti-inflammatory drugs in neurological diseases. J. Chem. Neuroanat. 2018, 87, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Vandewalle, J.; Luypaert, A.; De Bosscher, K.; Libert, C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol. Metab. 2018, 29, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Malyarenko, O.S.; Ermakova, S.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, X.; Duan, D.; Xu, J.; Gao, X. Preparation and characterization of agar, agarose, and agaropectin from the red alga Ahnfeltia plicata. J. Oceanol. Limnol. 2019, 37, 815–824. [Google Scholar] [CrossRef]
- Bilan, M.I.; Ustyuzhanina, N.E.; Shashkov, A.S.; Thanh, T.T.T.; Bui, M.L.; Van Tran, T.T.; Bui, V.N.; Nifantiev, N.E.; Usov, A.I. A sulfated galactofucan from the brown alga Hormophysa cuneiformis (Fucales, Sargassaceae). Carbohydr. Res. 2018, 469, 48–54. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnička, F.; Čopíková, J.; Park, Y.I. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Zhang, H.; Niu, X. Structural studies on a novel fucogalactan sulfate extracted from the brown seaweed Laminaria japonica. Int. J. Biol. Macromol. 2010, 47, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Karpisheh, V.; Nikkhoo, A.; Hojjat-Farsangi, M.; Namdar, A.; Azizi, G.; Ghalamfarsa, G.; Sabz, G.; Yousefi, M.; Yousefi, B.; Jadidi-Niaragh, F. Prostaglandin E2 as a potent therapeutic target for treatment of colon cancer. Prostaglandins Oth. Lipid Mediat. 2019, 144, 106338. [Google Scholar] [CrossRef]
- Shin, E.M.; Zhou, H.Y.; Guo, L.Y.; Kim, J.A.; Lee, S.H.; Merfort, I.; Kang, S.S.; Kim, H.-S.; Kim, S.; Kim, Y.S. Anti-inflammatory effects of glycyrol isolated from Glycyrrhiza uralensis in LPS-stimulated RAW264.7 macrophages. Int. Immunopharmacol. 2008, 8, 1524–1532. [Google Scholar] [CrossRef]
- Hwang, P.A.; Chien, S.Y.; Chan, Y.L.; Lu, M.K.; Wu, C.H.; Kong, Z.L.; Wu, C.J. Inhibition of Lipopolysaccharide (LPS)-Induced Inflammatory Responses by Sargassum hemiphyllum Sulfated Polysaccharide Extract in RAW 264.7 Macrophage Cells. J. Agric. Food Chem. 2011, 59, 2062–2068. [Google Scholar] [CrossRef]
- Na, Y.R.; Seok, S.H.; Baek, M.W.; Lee, H.Y.; Kim, N.J.; Park, S.H.; Lee, H.K.; Park, J.H. Protective effects of vitamin E against 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) induced toxicity in zebrafish embryos. Ecotoxicol. Environ. Saf. 2009, 72, 714–719. [Google Scholar] [CrossRef]
- Karin, M.; Ben-Neriah, Y. Phosphorylation Meets Ubiquitination: The Control of NF-κB Activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.Y. Cinnamon subcritical water extract attenuates intestinal inflammation and enhances intestinal tight junction in a Caco-2 and RAW264.7 co-culture model. Food Funct. 2019, 10, 4350–4360. [Google Scholar] [CrossRef]
- Kim, L.; Kim, J.Y. Chondroprotective effect of curcumin and lecithin complex in human chondrocytes stimulated by IL-1β via an anti-inflammatory mechanism. Food Sci. Biotechnol. 2018, 28, 547–553. [Google Scholar] [CrossRef]
- Chiu, F.L.; Lin, J.K. Tomatidine inhibits iNOS and COX-2 through suppression of NF-κB and JNK pathways in LPS-stimulated mouse macrophages. FEBS Lett. 2008, 582, 2407–2412. [Google Scholar] [CrossRef]
- Sanjeewa, K.A.; Jayawardena, T.U.; Kim, S.Y.; Kim, H.S.; Ahn, G.; Kim, J.I.; Jeon, Y.J. Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-κB and MAPK pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Langheinrich, U. Zebrafish: A new model on the pharmaceutical catwalk. BioEssays 2003, 25, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Fu, X.T.; Liu, N.; Duan, D.; Wang, X.; Xu, J.; Gao, X. The synergistic anti-inflammatory activities of agaro-oligosaccharides with different degrees of polymerization. J. Appl. Phycol. 2019, 31, 2547–2558. [Google Scholar] [CrossRef]
- Shan, T.; Yotsukura, N.; Pang, S. Novel implications on the genetic structure of representative populations of Saccharina japonica (Phaeophyceae) in the Northwest Pacific as revealed by highly polymorphic microsatellite markers. J. Appl. Phycol. 2016, 29, 631–638. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. J. Am. Chem. Soc. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Kawai, Y.; Seno, N.; Anno, K. A modified method for chondrosulfatase assay. Anal. Biochem. 1969, 32, 314–321. [Google Scholar] [CrossRef]
- Chandler, S.F.; Dodds, J.H. The effect of phosphate, nitrogen and sucrose on the production of phenolics and solasodine in callus cultures of solanum laciniatum. Plant Cell Rep. 1983, 2, 205–208. [Google Scholar] [CrossRef]
- Winters, A.; Minchin, F.R. Modification of the Lowry assay to measure proteins and phenols in covalently bound complexes. Anal. Biochem. 2005, 346, 43–48. [Google Scholar] [CrossRef]
- Kang, M.C.; Kim, S.C.; Kim, E.A.; Lee, J.H.; Kim, Y.S.; Yu, S.K.; Chae, J.B.; Choe, I.H.; Cho, J.H.; Jeon, Y.J. Antioxidant activity of polysaccharide purified from Acanthopanax koreanum Nakai stems in vitro and in vivo zebrafish model. Carbohydr. Polym. 2015, 127, 38–46. [Google Scholar] [CrossRef]
- Choi, T.Y.; Kim, J.H.; Ko, D.H.; Kim, C.H.; Hwang, J.S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.H.; Yoon, T.J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment. Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, A.R.; Schmaldienst, S.; Stuhlmeier, K.M.; Chen, W.; Knapp, W.; Zlabinger, G.J. A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescin-diacetate. J. Immunol. Methods 1992, 156, 39–45. [Google Scholar] [CrossRef]
- Itoh, Y.; Ma, F.H.; Hoshi, H.; Oka, M.; Noda, K.; Ukai, Y.; Kojima, H.; Nagano, T.; Toda, N. Determination and bioimaging method for nitric oxide in biological specimens by diaminofluorescein fluorometry. Anal. Biochem. 2000, 287, 203–209. [Google Scholar] [CrossRef]
- Kang, M.C.; Cha, S.H.; Wijesinghe, W.; Kang, S.M.; Lee, S.H.; Kim, E.A.; Song, C.B.; Jeon, Y.J. Protective effect of marine algae phlorotannins against AAPH-induced oxidative stress in zebrafish embryo. Food Chem. 2013, 138, 950–955. [Google Scholar] [CrossRef] [PubMed]
| LJPS | |
|---|---|
| Yield (%) | 7.96 ± 0.85 |
| Molecular weight (kDa) | 131.50 |
| Chemical composition | |
| Total sugar (%) | 64.42 ± 0.59 |
| Sulfate (%) | 9.07 ± 0.19 |
| Phenol (%) | 0.05 ± 0.01 |
| Protein (%) | 0.72 ± 0.06 |
| Monosaccharide composition (%) | |
| Rhamnose | 1.18 |
| Fucose | 47.15 |
| Xylose | 5.53 |
| Mannose | 16.88 |
| Galactose | 23.02 |
| Glucose | 6.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Ni, L.; Fu, X.; Duan, D.; Xu, J.; Gao, X. A Sulfated Polysaccharide from Saccharina japonica Suppresses LPS-Induced Inflammation Both in a Macrophage Cell Model via Blocking MAPK/NF-κB Signal Pathways In Vitro and a Zebrafish Model of Embryos and Larvae In Vivo. Mar. Drugs 2020, 18, 593. https://doi.org/10.3390/md18120593
Wang S, Ni L, Fu X, Duan D, Xu J, Gao X. A Sulfated Polysaccharide from Saccharina japonica Suppresses LPS-Induced Inflammation Both in a Macrophage Cell Model via Blocking MAPK/NF-κB Signal Pathways In Vitro and a Zebrafish Model of Embryos and Larvae In Vivo. Marine Drugs. 2020; 18(12):593. https://doi.org/10.3390/md18120593
Chicago/Turabian StyleWang, Shengnan, Liying Ni, Xiaoting Fu, Delin Duan, Jiachao Xu, and Xin Gao. 2020. "A Sulfated Polysaccharide from Saccharina japonica Suppresses LPS-Induced Inflammation Both in a Macrophage Cell Model via Blocking MAPK/NF-κB Signal Pathways In Vitro and a Zebrafish Model of Embryos and Larvae In Vivo" Marine Drugs 18, no. 12: 593. https://doi.org/10.3390/md18120593
APA StyleWang, S., Ni, L., Fu, X., Duan, D., Xu, J., & Gao, X. (2020). A Sulfated Polysaccharide from Saccharina japonica Suppresses LPS-Induced Inflammation Both in a Macrophage Cell Model via Blocking MAPK/NF-κB Signal Pathways In Vitro and a Zebrafish Model of Embryos and Larvae In Vivo. Marine Drugs, 18(12), 593. https://doi.org/10.3390/md18120593






