Screening of Human CYP1A2 and CYP3A4 Inhibitors from Seaweed In Silico and In Vitro
Abstract
1. Introduction
2. Results
2.1. Prediction of CYP Inhibition In Silico
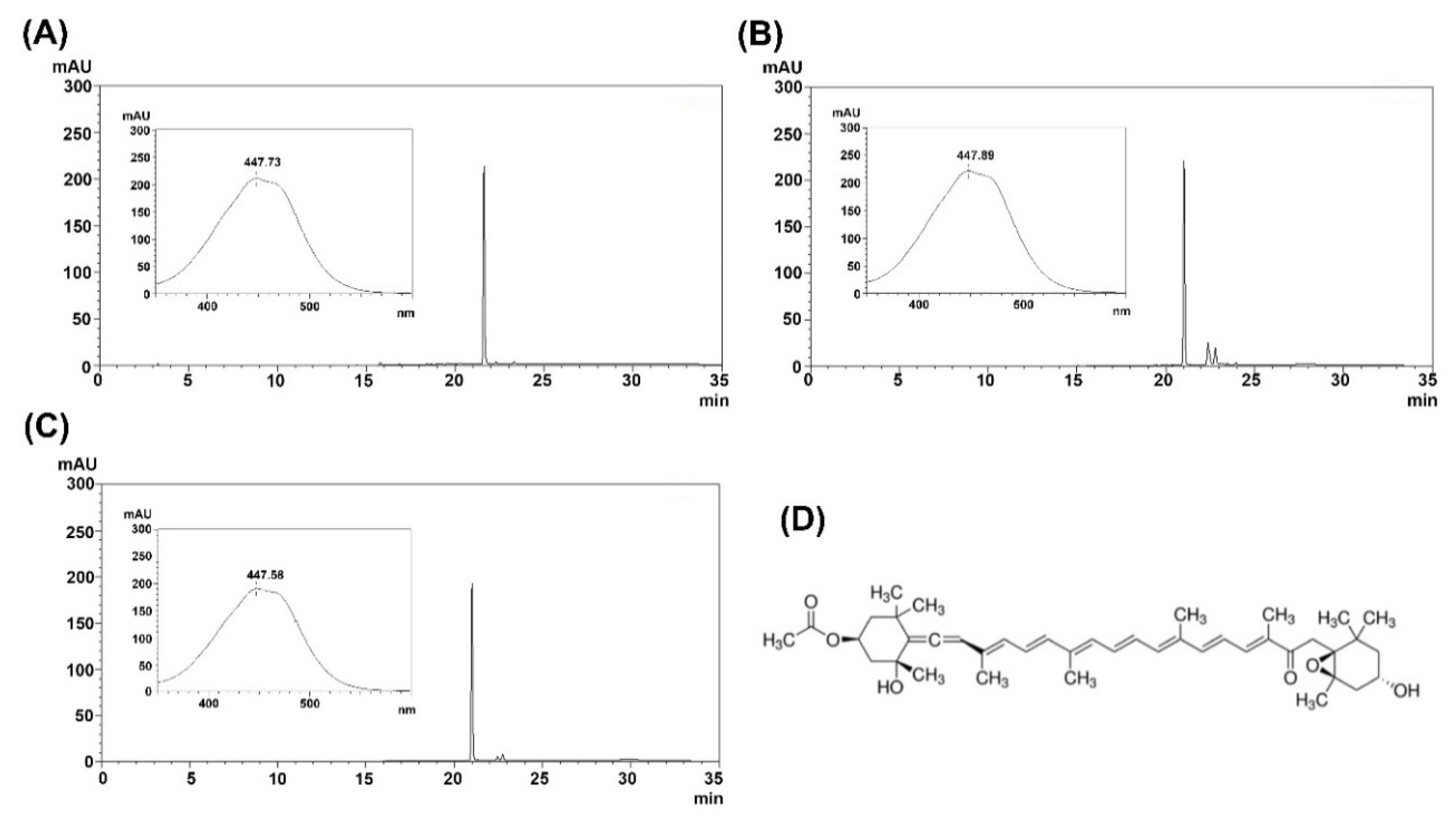
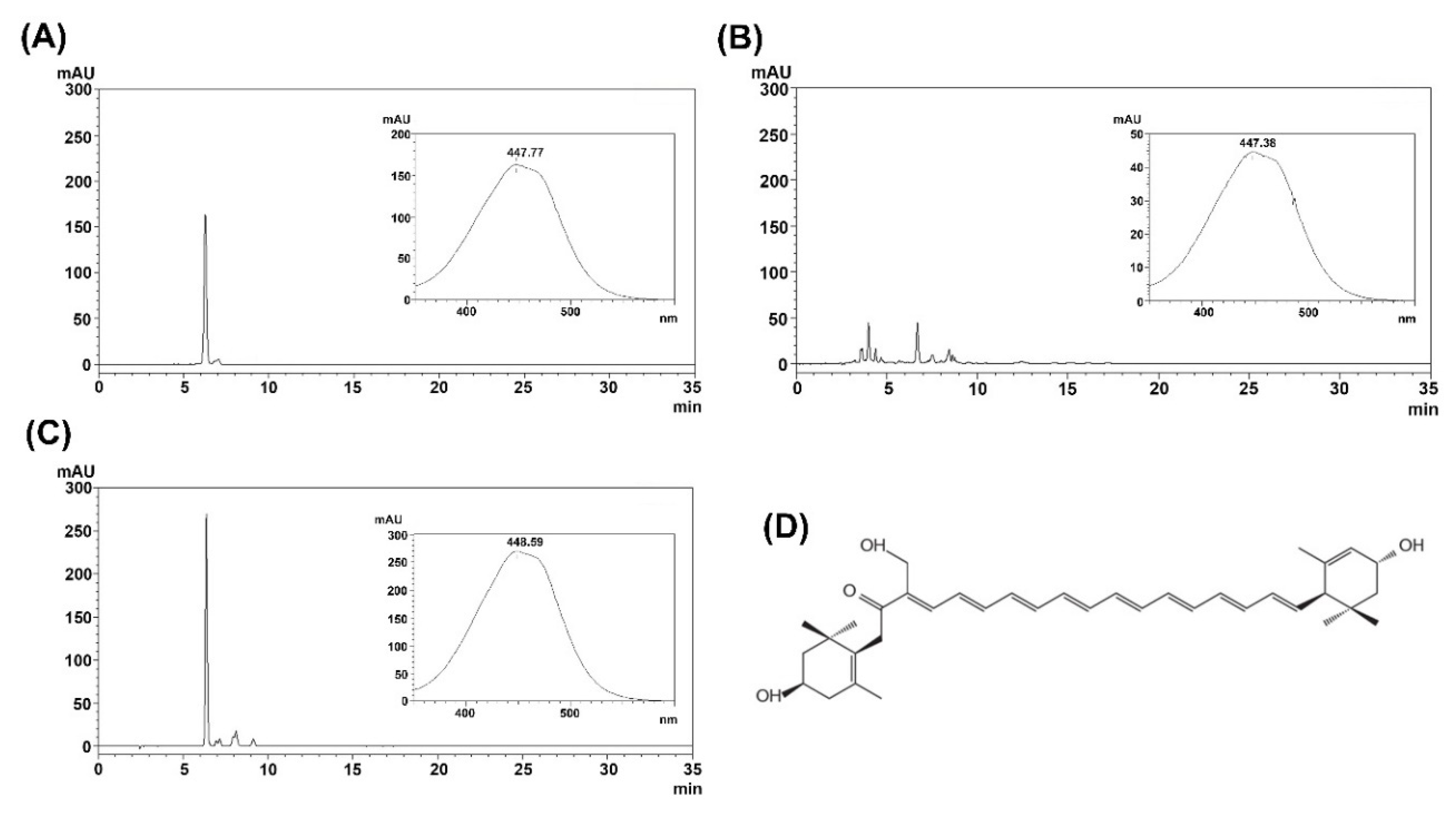
2.2. Purification of Fucoxanthin and Siphonaxanthin
2.3. Kinetics of Human CYP1A2 and CYP3A4 Inhibition by Test Compounds
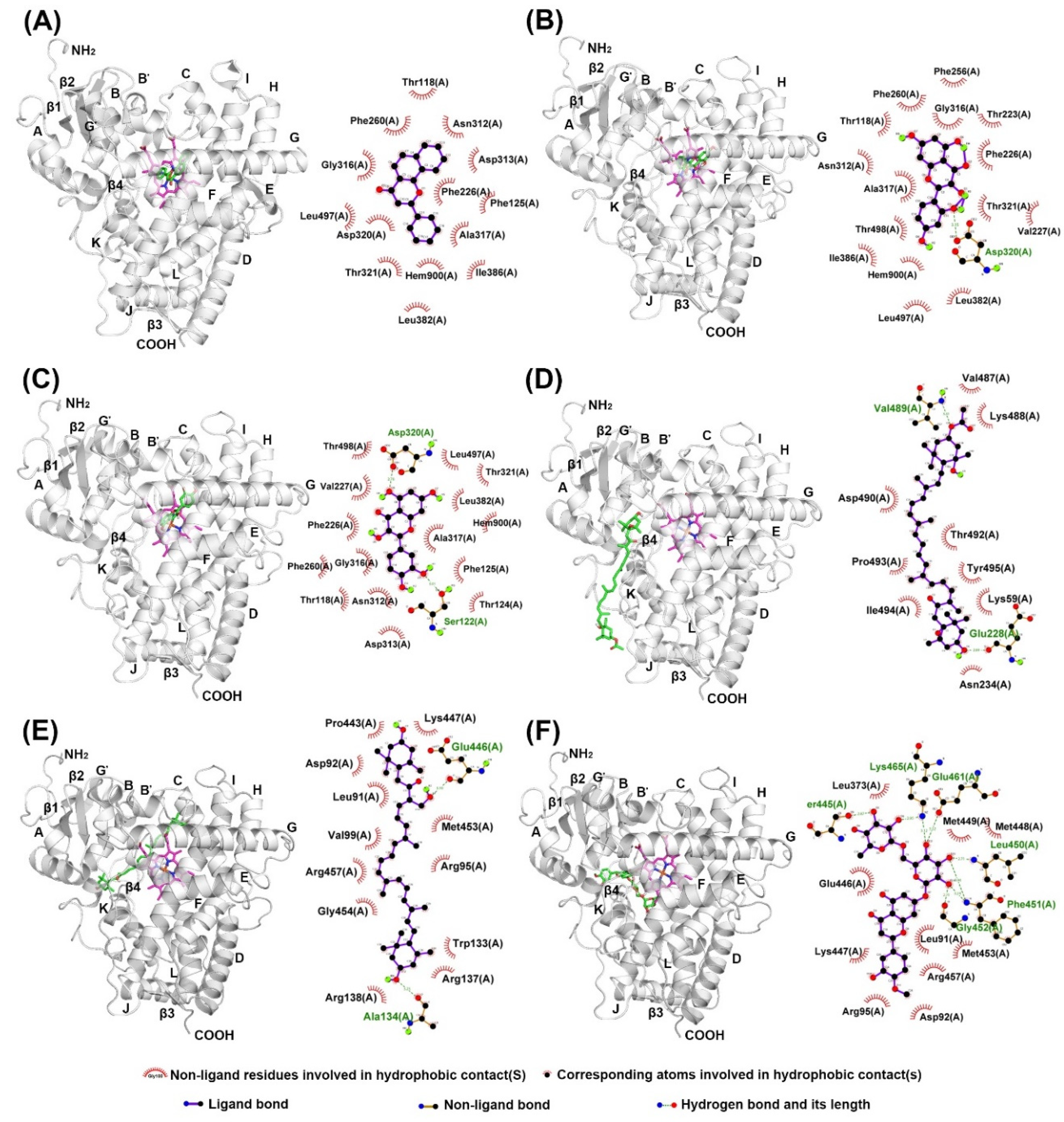
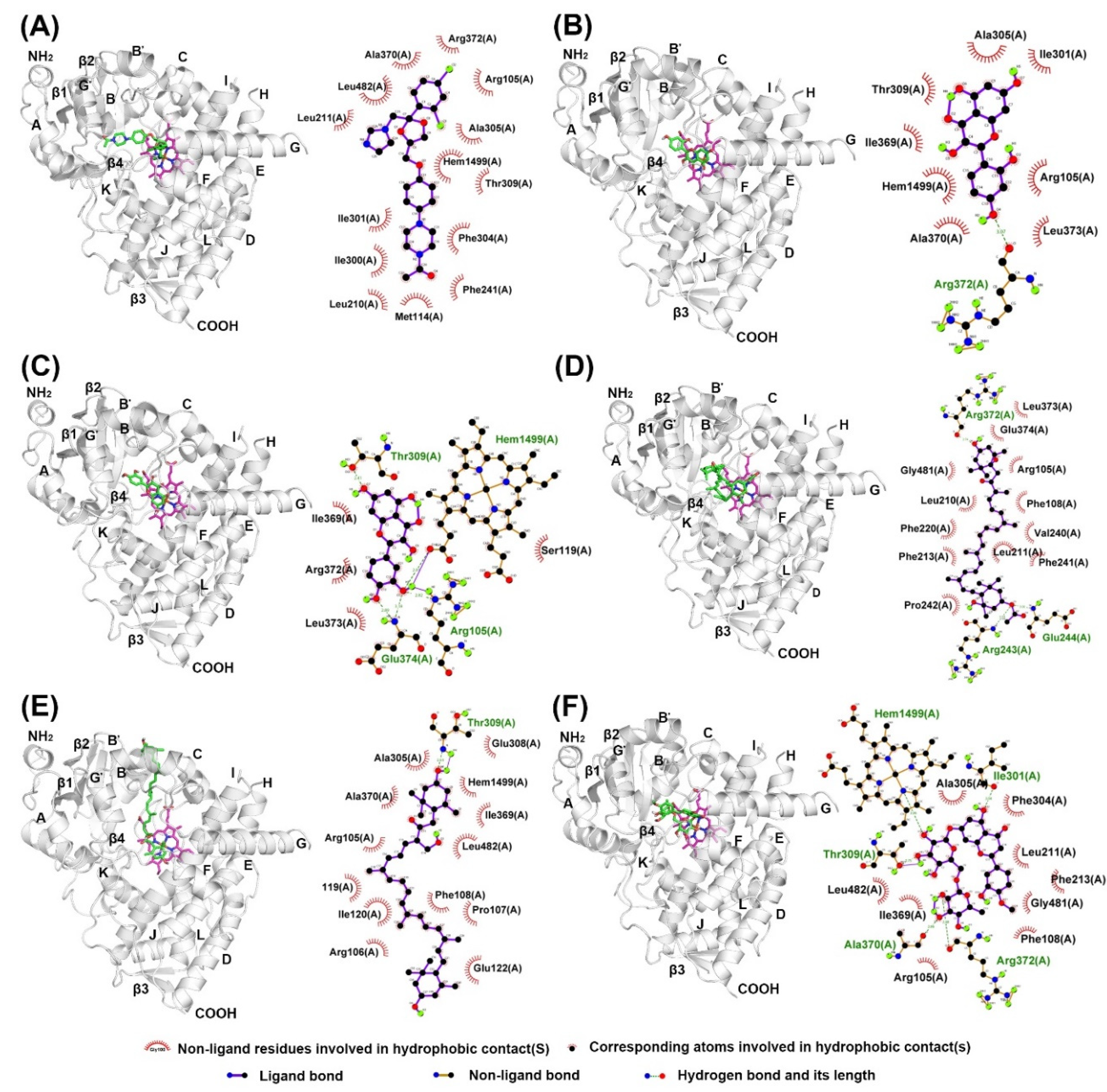
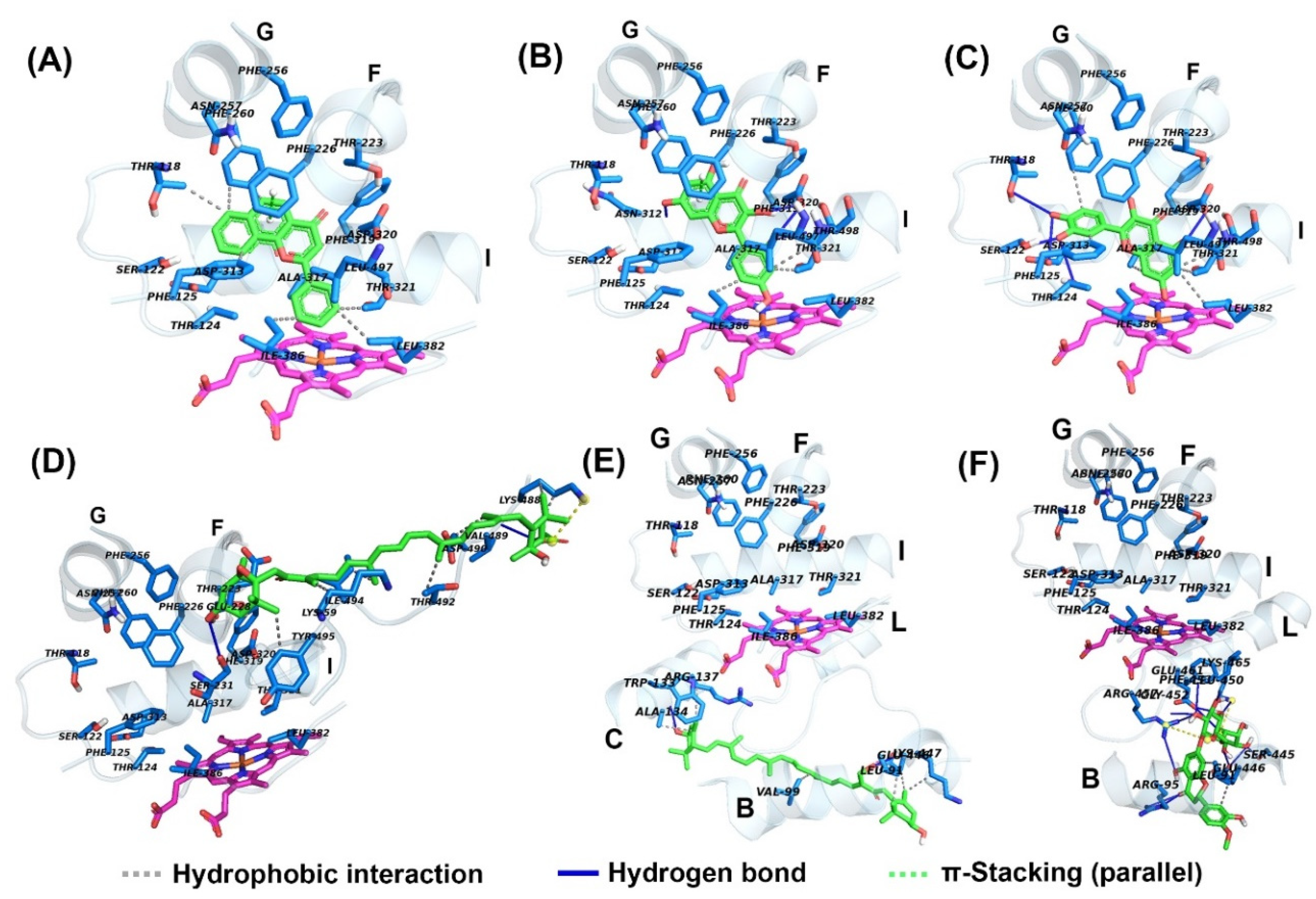
2.4. Docking of Compounds into Human CYP1A2 and CYP3A4
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Seaweed Collection
4.3. Prediction of CYP Inhibition In Silico
4.4. Supercritical CO2 Extraction
4.5. Preparative HPLC
4.6. Analytical Methods
4.7. Kinetics of Human CYP1A2 and CYP3A4 Inhibition
4.8. Docking Studies
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CYP | Cytochrome P450 |
| α-NF | α-naphthoflavone |
| KCZ | Ketoconazole |
| MR | Morin hydrate |
| QT | Quercetin |
| HSP | Hesperidin |
| FX | Fucoxanthin |
| SX | Siphonaxanthin |
| NADP | Nicotinamide adenine dinucleotide phosphate |
References
- Guengerich, F.P. Cytochrome P450 and Chemical Toxicology. Chem. Res. Toxicol. 2008, 21, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Nebert, D.W.; Wikvall, K.; Miller, W.L. Human cytochromes P450 in health and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013, 368, 20120431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Y.; Wang, Y.; Prakash, C. Xenobiotic-metabolizing enzymes in human lung. Curr. Drug Metab. 2006, 7, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T. Xenobiotic-metabolizing enzymes involved in activation and detoxification of carcinogenic polycyclic aromatic hydrocarbons. Drug Metab. Pharmacokinet. 2006, 21, 257–276. [Google Scholar] [CrossRef]
- Shimada, T.; Martin, M.V.; Uruess-Schwartz, D.; Marnett, L.J.; Guengerich, F.P. Roles of Individual Human Cytochrome P-450 Enzymes in the Bioactivation of Benzo(a)pyrene, 7,8-Dihydroxy-7,8-dihydrobenzo(a)pyrene, and Other Dihydrodiol Derivatives of Polycyclic Aromatic Hydrocarbons. Cancer Res. 1989, 49, 6304–6312. [Google Scholar]
- Tang, W.; Stearns, R.A. Heterotrophic cooperativity of cytochrome P450 3A4 and potential drug-drug interactions. Curr. Drug Metab. 2001, 2, 185–198. [Google Scholar] [CrossRef]
- Yun, C.-H.; Lee, H.S.; Lee, H.-Y.; Yim, S.-K.; Kim, K.-H.; Kim, E.; Yea, S.-S.; Guengerich, F.P. Roles of human liver cytochrome P450 3A4 and 1A2 enzymes in the oxidation of myristicin. Toxicol. Lett. 2003, 137, 143–150. [Google Scholar] [CrossRef]
- Basheer, L.; Schultz, K.; Kerem, Z. Inhibition of cytochrome P450 3A by acetoxylated analogues of resveratrol in in vitro and in silico models. Sci. Rep. 2016, 6, 31557. [Google Scholar] [CrossRef]
- Banerjee, P.; Dunkel, M.; Kemmler, E.; Preissner, R. SuperCYPsPred-a web server for the prediction of cytochrome activity. Nucleic Acids Res. 2020, 48, W580–W585. [Google Scholar] [CrossRef]
- Mabeau, S.; Fleurence, J. Seaweed in food products: Biochemical and nutritional aspects. Trends Food Sci. Technol. 1993, 4, 103–107. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Yumiko, Y.-S.; Ya-Pei, H.; Takeshi, S. Distribution of flavonoids and related compounds from seaweeds in Japan. J. Tokyo Univ. Fish. 2003, 89, 1–6. [Google Scholar]
- Xia, S.; Wang, K.; Wan, L.; Li, A.; Hu, Q.; Zhang, C. Production, characterization, and antioxidant activity of fucoxanthin from the marine diatom Odontella aurita. Mar. Drugs 2013, 11, 2667–2681. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, K.; Ozaki, Y.; Hashimoto, T.; Das, S.K.; Matsushita, S.; Hirano, M.; Okada, T.; Komoto, A.; Mori, N.; Nakatsuka, M. Commercial-scale Preparation of Biofunctional Fucoxanthin from Waste Parts of Brown Sea Algae Laminalia japonica. Food Sci. Tehnol. Res. 2008, 14, 573. [Google Scholar] [CrossRef]
- Ganesan, P.; Matsubara, K.; Ohkubo, T.; Tanaka, Y.; Noda, K.; Sugawara, T.; Hirata, T. Anti-angiogenic effect of siphonaxanthin from green alga, Codium fragile. Phytomedicine 2010, 17, 1140–1144. [Google Scholar] [CrossRef]
- Ohno, M.; Darwish, W.S.; Ikenaka, Y.; Miki, W.; Kshizuka, M. Astaxanthin can alter CYP1A-dependent activities via two different mechanisms: Induction of protein expression and inhibition of NADPH P450 reductase dependent electron transfer. Food Chem. Toxicol. 2011, 49, 1285–1291. [Google Scholar] [CrossRef]
- Satomi, Y.; Nishino, H. Inhibition of the enzyme activity of cytochrome P450 1A1, 1A2 and 3A4 by fucoxanthin, a marine carotenoid. Oncol. Lett. 2013, 6, 860–864. [Google Scholar] [CrossRef]
- Liu, C.-L.; Lim, Y.-P.; Hu, M.-L. Fucoxanthin attenuates rifampin-induced cytochrome P450 3A4 (CYP3A4) and multiple drug resistance 1 (MDR1) gene expression through pregnane X receptor (PXR)-mediated pathways in human hepatoma HepG2 and colon adenocarcinoma LS174T cells. Mar. Drugs 2012, 10, 242–257. [Google Scholar] [CrossRef]
- Firdous, A.P.; Sindhu, E.R.; Ramnath, V.; Kuttan, R. Anti-mutagenic and anti-carcinogenic potential of the carotenoid meso-zeaxanthin. Asian Pac. J. Cancer Prev. 2010, 11, 1795–1800. [Google Scholar]
- Wang, H.; Leung, L.K. The carotenoid lycopene differentially regulates phase I and II enzymes in dimethylbenz[a]anthracene-induced MCF-7 cells. Nutrition 2010, 26, 1181–1187. [Google Scholar] [CrossRef]
- Elbarbry, F.; Ung, A.; Abdelkawy, K. Studying the Inhibitory Effect of Quercetin and Thymoquinone on Human Cytochrome P450 Enzyme Activities. Pharmacogn. Mag. 2018, 13, S895–S899. [Google Scholar] [PubMed]
- Santes-Palacios, R.; Marroquín-Pérez, A.L.; Hernández-Ojeda, S.L.; Camacho-Carranza, R.; Govezensky, T.; Espinosa-Aguirre, J.J. Human CYP1A1 inhibition by flavonoids. Toxicol. In Vitro 2020, 62, 104681. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Zhang, B.; Mu, G.; Xia, J.; Xiang, Q.; Liu, A.; Du, G.; Cui, Y. Screening of cytochrome P450 3A4 inhibitors via in silico and in vitro approaches. R. Soc. Chem. 2018, 8, 34783–34792. [Google Scholar] [CrossRef]
- Lasser, K.E.; Allen, P.D.; Woolhandler, S.J.; Himmelstein, D.U.; Wolfe, S.M.; Bor, D.H. Timing of new black box warnings and withdrawals for prescription medications. JAMA 2002, 287, 2215–2220. [Google Scholar] [CrossRef]
- Wienkers, L.; Heath, T.G. Predicting in vivo drug interactions from in vitro drug discovery data. Nat. Rev. Drug Discov. 2005, 4, 825–833. [Google Scholar] [CrossRef]
- Liu, J.; Sridhar, J.; Foroozesh, M. Cytochrome P450 family 1 inhibitors and structure-activity relationships. Molecules 2013, 18, 14470–14495. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Zhang, S.-X.; Long, N.; Lin, L.-S.; Chen, T.; Zhang, F.-P.; Lv, X.-Q.; Ye, P.-Z.; Li, N.; Zhang, K.-Z. An improved substrate cocktail for assessing direct inhibition and time-dependent inhibition of multiple cytochrome P450s. Acta Pharmacol. Sin. 2016, 37, 708–718. [Google Scholar] [CrossRef]
- Bjornsson, T.D.; Callaghan, J.T.; Einolf, H.J.; Fischer, V.; Gan, L.; Grimm, S.; Kao, J.; King, S.P.; Miwa, G.; Ni, L.; et al. The conduct of in vitro and in vivo drug-drug interaction studies: A Pharmaceutical Research and Manufacturers of America (PhRMA) perspective. Drug Metab. Dispos. 2003, 31, 815–832. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Lai, L.; Pei, J. Prediction of human cytochrome P450 inhibition using a multitask deep autoencoder neural network. Mol. Pharm. 2018, 15, 4336–4345. [Google Scholar] [CrossRef]
- Sansen, S.; Yano, J.K.; Reynald, R.L.; Schoch, G.A.; Griffin, K.J.; Stout, C.D.; Johnson, E.F. Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. J. Biol. Chem. 2007, 282, 14348–14355. [Google Scholar] [CrossRef]
- Cojocaru, V.; Winn, P.J.; Wade, R.C. The ins and outs of cytochrome P450s. Biochim. Biophys. Acta 2007, 1770, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Benkaidali, L.; André, F.; Moroy, G.; Tangour, B.; Maurel, F.; Petitjean, M. Four Major Channels Detected in the Cytochrome P450 3A4: A Step toward Understanding Its Multispecificity. Int. J. Mol. Sci. 2019, 20, 987. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, G.; Nandekar, P.P.; Wade, R.C. Electron Transfer from Cytochrome P450 Reductase to Cytochrome P450: Towards a Structural and Dynamic Understanding. bioRxiv 2020. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Ekroos, M.; Sjögren, T. Structural basis for ligand promiscuity in cytochrome P450 3A4. Proc. Natl. Acad. Sci. USA 2006, 103, 13682–13687. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015, 1, W443–W447. [Google Scholar] [CrossRef]
- Wallace, A.C.; Laskowski, R.A.; Thornton, J.M. LIGPLOT: A program to generate schematic diagrams of protein-ligand interactions. Protein Eng. 1995, 8, 127–134. [Google Scholar] [CrossRef]


| Compound Names | Predicted Values of Inhibitory Effect | |
|---|---|---|
| CYP1A2 | CYP3A4 | |
| α-Naphthoflavone | 0.99 | 0.06 |
| Ketoconazole | 0.08 | 1.0 |
| Caffeic acid | 0.02 | 0.01 |
| Catechol | 0.01 | 0 |
| Dieckol | 0 | 0 |
| Difucophlorethol A | 0 | 0.06 |
| Diphlorethol | 0.1 | 0.03 |
| Eckol | 0.05 | 0.02 |
| Fucoxanthin | 0 | 0.76 |
| Hesperidin | 0 | 0.01 |
| Morin | 0.75 | 0.48 |
| Myricetin | 0.2 | 0.21 |
| Phloroglucinol | 0.02 | 0.01 |
| Quercitrin | 0 | 0.03 |
| Siphonaxanthin | 0 | 0.58 |
| Trifucol | 0.28 | 0.03 |
| Trifuhalol A | 0.01 | 0.01 |
| Rutin | 0 | 0 |
| Quercetin | 0.38 | 0.36 |
| Compounds | CYP1A2 | CYP3A4 | ||||
|---|---|---|---|---|---|---|
| In Vitro | In Silico | In Vitro | In Silico | |||
| IC50 (μM) | Binding Energy (kcal mol−1) | Interactions | IC50 (μM) | Binding Energy (kcal mol−1) | Interactions | |
| α-Naphthoflavone (α-NF) | 0.022 ± 0.003 | −10.58 | Thr118, Phe125, Phe226, Phe260, Ala317, Asp320, Thr321, Leu382, Ile386, Leu497, Thr498 | * n.d. | n.d. | n.d |
| Ketoconazole (KCZ) | n.d. | n.d. | n.d. | 0.21 ± 0.04 | −9.39 | Leu210, Ile300, Phe304, Ala305, Thr309, Ala370, Leu482 |
| Morin (MR) | 41.87 ± 2.83 | −7.22 | Phe226, Phe260, Asn312, Ala317, Asp320, Thr321, Ile386, Leu497, Thr498 | 86.6 ± 9.56 | −6.01 | Arg105, Ile301, Ala305, Thr309, Ala370, Arg372, Glu374 |
| Quercetin (QT) | 22.47 ± 3.32 | −7.28 | Thr118, Ser122, Thr124, Phe125, Phe260, Asp313, Ala317, Asp320, Thr321, Leu382, Leu497, Thr498 | 16.09 ± 7.46 | −7.57 | Arg105, Ser119, Thr309, Arg372, Glu374, Arg375 |
| Fucoxanthin (FX) | 30.26 ± 3.45 | −4.83 | Lys59, Glu228, Ser231, Lys488, Val489, Aps490, Thr492, Ile494, Tyr495 | 24.41 ± 7.42 | −7.69 | Phe108, Leu211, Phe213, Phe220, Val240, Phe241, Pro242, Arg243, Glu244, Arg372, Glu374 |
| Siphonaxanthin (SX) | ** n.i. | −5.97 | Lys59, Glu228, Leu236, Asn247, Ala249, Val489, Asp490, Leu491, Thr492, Ile494 | n.i. | −6.10 | Arg105, Phe108, Ile120, Ala305, Thr309, Ile369, Leu482 |
| Hesperidin (HSP) | n.i. | −7.30 | Val54, Gly58, Lys59, Asn60, Glu228, Ser231, Gly233, Asn234, Tyr495 | n.i. | −8.28 | Arg105, Phe108, Leu211, Phe213, Ile301, Phe304, Ala305, Thr309, Ala370 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yim, S.-K.; Kim, K.; Chun, S.; Oh, T.; Jung, W.; Jung, K.; Yun, C.-H. Screening of Human CYP1A2 and CYP3A4 Inhibitors from Seaweed In Silico and In Vitro. Mar. Drugs 2020, 18, 603. https://doi.org/10.3390/md18120603
Yim S-K, Kim K, Chun S, Oh T, Jung W, Jung K, Yun C-H. Screening of Human CYP1A2 and CYP3A4 Inhibitors from Seaweed In Silico and In Vitro. Marine Drugs. 2020; 18(12):603. https://doi.org/10.3390/md18120603
Chicago/Turabian StyleYim, Sung-Kun, Kian Kim, SangHo Chun, TaeHawn Oh, WooHuk Jung, KyooJin Jung, and Chul-Ho Yun. 2020. "Screening of Human CYP1A2 and CYP3A4 Inhibitors from Seaweed In Silico and In Vitro" Marine Drugs 18, no. 12: 603. https://doi.org/10.3390/md18120603
APA StyleYim, S.-K., Kim, K., Chun, S., Oh, T., Jung, W., Jung, K., & Yun, C.-H. (2020). Screening of Human CYP1A2 and CYP3A4 Inhibitors from Seaweed In Silico and In Vitro. Marine Drugs, 18(12), 603. https://doi.org/10.3390/md18120603





