Biosynthesis of Saxitoxin in Marine Dinoflagellates: An Omics Perspective
Abstract
:1. Introduction
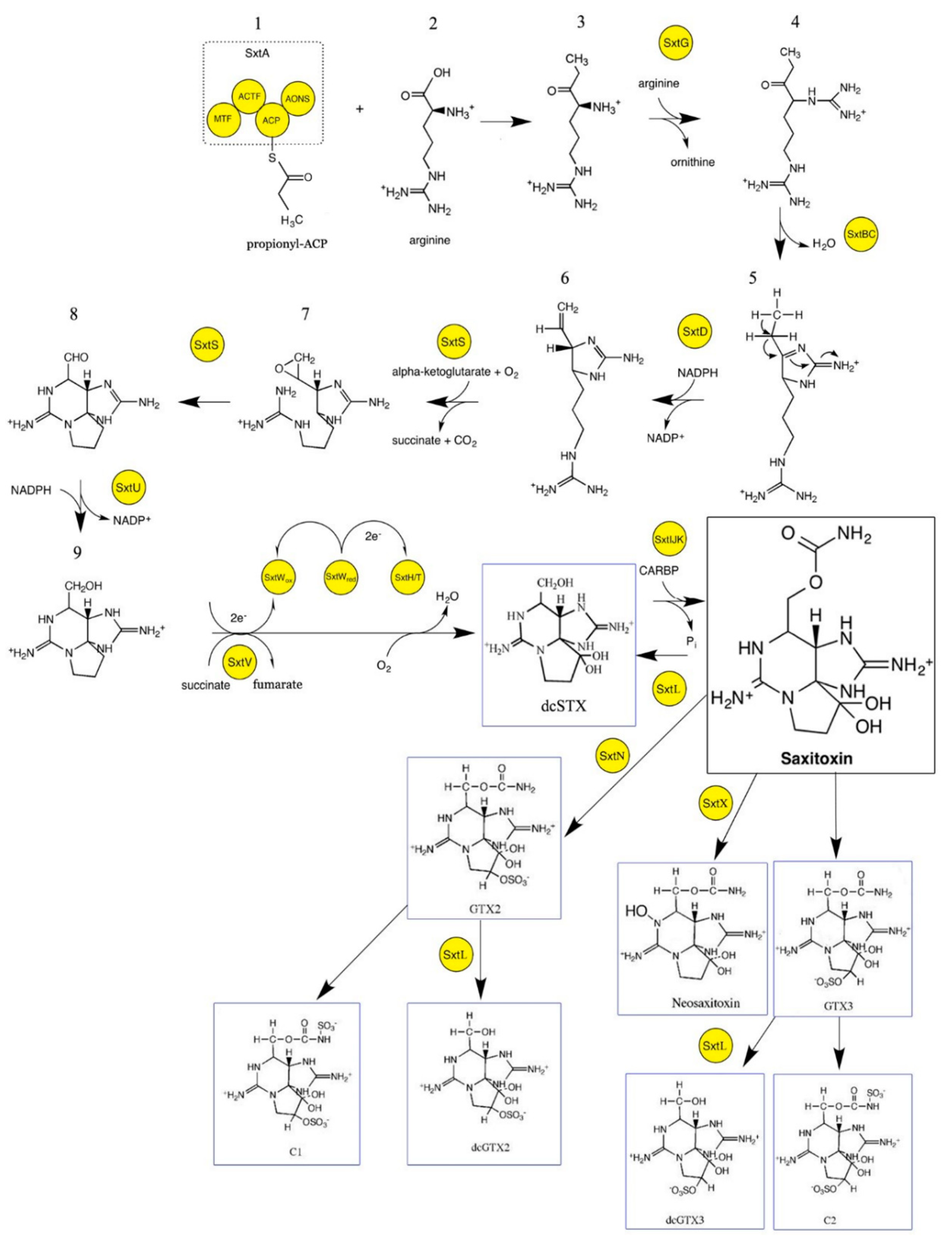
2. Overview of Saxitoxin Molecular Biosynthesis and Gene Cluster
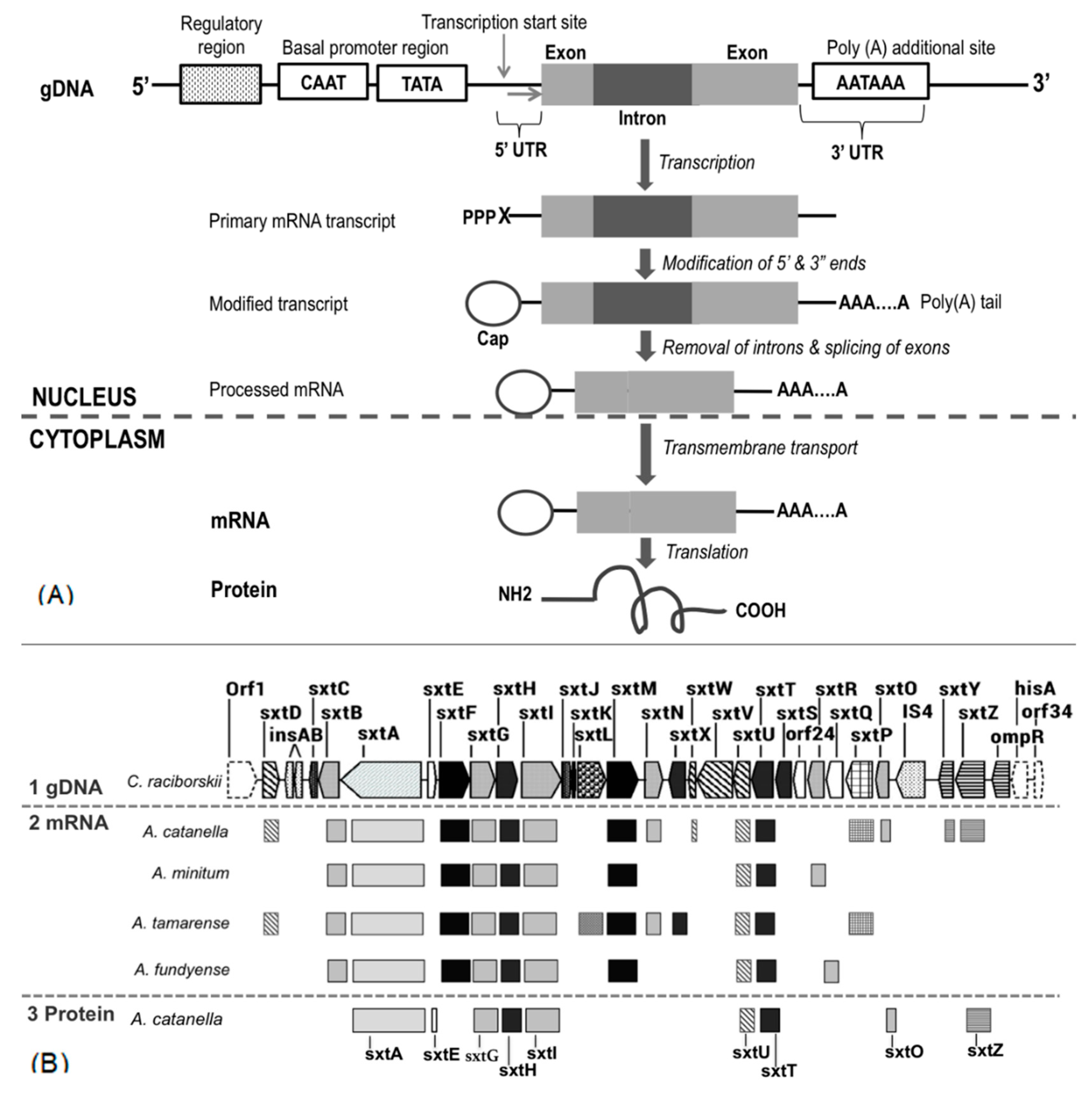
3. Recent Insight into Saxitoxin Biosynthesis through Transcriptomic Analysis
4. Translational Control in Dinoflagellates and Its Implication on Saxitoxin Biosynthesis
5. Proteomics Insight into Saxitoxin Biosynthesis
6. Metabolomics within the Context of Saxitoxin Biosynthesis: An Unexplored Approach?
7. Future Directions and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wiese, M.; D’agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [Green Version]
- Cusick, K.; Sayler, G. An overview on the marine neurotoxin, saxitoxin: Genetics, molecular targets, methods of detection and ecological functions. Mar. Drugs 2013, 11, 991–1018. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.Z.; Zhang, S.F.; Zhang, Y.; Lin, L. Paralytic shellfish toxin biosynthesis in cyanobacteria and dinoflagellates: A molecular overview. J. Proteom. 2016, 135, 132–140. [Google Scholar] [CrossRef]
- Schantz, E.J.; Mold, J.D.; Stanger, D.W.; Shavel, J.; Riel, F.J.; Bowden, J.P.; Sommer, H. Paralytic shellfish poison. VI. A procedure for the isolation and purification of the poison from toxic clam and mussel tissues. J. Am. Chem. Soc. 1957, 79, 5230–5235. [Google Scholar] [CrossRef]
- Walker, J.R.; Merit, J.E.; Thomas-Tran, R.; Tang, D.T.; Du Bois, J. Divergent Synthesis of Natural Derivatives of (+)-Saxitoxin Including 11-Saxitoxinethanoic Acid. Angew. Chem. Int. Ed. 2019, 58, 1689–1693. [Google Scholar] [CrossRef]
- Orr, R.; Stüken, A.; Murray, S.; Jakobsen, K. Evolution and distribution of saxitoxin biosynthesis in dinoflagellates. Mar. Drugs 2013, 11, 2814–2828. [Google Scholar] [CrossRef] [Green Version]
- Ciminiello, P.; Fattorusso, E.; Forino, M.; Montresor, M. Saxitoxin and neosaxitoxin as toxic principles of Alexandrium andersoni (Dinophyceae) from the Gulf of Naples, Italy. Toxicon 2000, 38, 1871–1877. [Google Scholar] [CrossRef]
- Krock, B.; Seguel, C.G.; Cembella, A.D. Toxin profile of Alexandrium catenella from the Chilean coast as determined by liquid chromatography with fluorescence detection and liquid chromatography coupled with tandem mass spectrometry. Harmful Algae 2007, 6, 734–744. [Google Scholar] [CrossRef] [Green Version]
- Poulton, N.J.; Keafer, B.A.; Anderson, D.M. Toxin variability in natural populations of Alexandrium fundyense in Casco Bay, Maine—Evidence of nitrogen limitation. Deep Sea Res. PT II 2005, 52, 2501–2521. [Google Scholar] [CrossRef]
- Ichimi, K.; Suzuki, T.; Ito, A. Variety of PSP toxin profiles in various culture strains of Alexandrium tamarense and change of toxin profile in natural A. tamarense population. J. Exp. Mar. Biol. Ecol. 2002, 273, 51–60. [Google Scholar] [CrossRef]
- Usup, G.; Kulis, D.M.; Anderson, D.M. Growth and toxin production of the toxic dinoflagellate Pyrodinium bahamense var. compressum in laboratory cultures. Nat. Toxins 1994, 2, 254–262. [Google Scholar] [CrossRef]
- Gárate-Lizárraga, I.; Bustillos-Guzmán, J.J.; Morquecho, L.; Band-Schmidt, C.J.; Alonso-Rodríguez, R.; Erler, K.; Luckas, B.; Reyes-Salinas, A.; Góngora-González, D.T. Comparative paralytic shellfish toxin profiles in the strains of Gymnodinium catenatum Graham from the Gulf of California, Mexico. Mar. Pollut. Bull. 2005, 50, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, G.C.; Cembella, A.D.; Joyce, L.B.; Larsen, J.; Probyn, T.A.; Ruiz Sebastián, C. The dinoflagellate Alexandrium minutum in Cape Town harbour (South Africa): Bloom characteristics, phylogenetic analysis and toxin composition. Harmful Algae 2007, 6, 823–836. [Google Scholar] [CrossRef]
- Sebastián, C.R.; Etheridge, S.M.; Cook, P.A.; O’Ryan, C.; Pitcher, G.C. Phylogenetic analysis of toxic Alexandrium (Dinophyceae) isolates from South Africa: Implications for the global phylogeography of the Alexandrium tamarense species complex. Phycologia 2005, 44, 49–60. [Google Scholar] [CrossRef]
- Hansen, P.J.; Cembella, A.D.; Moestrup, Ø. The marine dinoflagellate Alexandrium ostenfeldii: Paralytic shellfish toxin concentration, composition and toxicity to a tintinnid cilliate. J. Phycol. 1992, 28, 597–603. [Google Scholar] [CrossRef]
- Holmes, M.J.; Bolch, C.J.S.; Green, D.H.; Cembella, A.D.; Teo, S.L.M. Singapore isolates of the dinoflagellate Gymnodinium catenatum (Dinophyceae) produce a unique profile of paralytic shellfish poisoning toxins. J. Phycol. 2002, 38, 96–106. [Google Scholar] [CrossRef]
- Jaime, E.; Gerdts, G.; Luckas, B. In vitro transformation of PSP toxins by different shellfish tissues. Harmful Algae 2007, 6, 308–316. [Google Scholar] [CrossRef]
- Yu, R.; Hummert, C.; Luckas, B.; Qian, P.; Li, J.; Zhou, M. A modified HPLC method for analysis of PSP toxins in algae and shellfish from china. Chromatographia 1998, 48, 671–676. [Google Scholar] [CrossRef]
- Vale, P. Metabolites of saxitoxin analogues in bivalves contaminated by Gymnodinium catenatum. Toxicon 2010, 55, 162–165. [Google Scholar] [CrossRef]
- Cho, Y.; Tsuchiya, S.; Yoshioka, R.; Omura, T.; Konoki, K.; Oshima, Y.; Yotsu-Yamashita, M. The presence of 12β-deoxydecarbamoylsaxitoxin in the Japanese toxic dinoflagellate Alexandrium determined by simultaneous analysis for paralytic shellfish toxins using HILIC-LC–MS/MS. Harmful Algae 2015, 49, 58–67. [Google Scholar] [CrossRef]
- Negri, A.; Stirling, D.; Quilliam, M.; Blackburn, S.; Bolch, C.; Burton, I.; Eaglesham, G.; Thomas, K.; Walter, J.; Willis, R. Three novel hydroxybenzoate saxitoxin analogues isolated from the dinoflagellate Gymnodinium catenatum. Chem. Res. Toxicol. 2003, 16, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Oshima, Y.; Sugino, K.; Itakura, H.; Hirota, M.; Yasumoto, T.; Grane’li, E.; Sundstrom, B.; Edler, L.; Anderson, D.M. Comparative studies on paralytic shellfish toxin profile of dinoflagellates and bivalves. In Toxic Marine Phytoplankton; Elsevier Science Publishing: New York, NY, USA, 1990; pp. 391–396. [Google Scholar]
- Mohana, N.C.; Rao, H.Y.; Rakshith, D.; Mithun, P.R.; Nuthan, B.R.; Satish, S. Omics based approach for biodiscovery of microbial natural products in antibiotic resistance era. J. Genet. Eng. Biotechnol. 2018, 16, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Palazzotto, E.; Weber, T. Omics and multi-omics approaches to study the biosynthesis of secondary metabolites in microorganisms. Curr. Opin. Microbiol. 2018, 45, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, F.; Nie, L. Integrating multiple ‘omics’ analysis for microbial biology: Application and methodologies. Microbiology 2010, 156, 287–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weckwerth, W.; Wenzel, K.; Fiehn, O. Process for the integrated extraction, identification and quantification of metabolites, proteins and RNA to reveal their co-regulation in biochemical networks. Proteomics 2004, 4, 78–83. [Google Scholar] [CrossRef]
- Shimizu, Y.; Norte, M.; Hori, A.; Genenah, A.; Kobayashi, M. Biosynthesis of saxitoxin analogs: The unexpected pathway. J. Am. Chem. Soc. 1984, 106, 6433–6434. [Google Scholar] [CrossRef]
- Kellmann, R.; Mihali, T.K.; Jeon, Y.J.; Pickford, R.; Pomati, F.; Neilan, B.A. Biosynthetic intermediate analysis and functional homology reveal a saxitoxin gene cluster in cyanobacteria. Appl. Environ. Microbiol. 2008, 74, 4044–4053. [Google Scholar] [CrossRef] [Green Version]
- Mihali, T.K.; Kellmann, R.; Neilan, B.A. Characterisation of the paralytic shellfish toxin biosynthesis gene clusters in Anabaena circinalis AWQC131C and Aphanizomenon sp. NH-5. BMC Biochem. 2009, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Mihali, T.K.; Carmichael, W.W.; Neilan, B.A. A putative gene cluster from a Lyngbya wollei bloom that encodes paralytic shellfish toxin biosynthesis. PLoS ONE 2011, 6, e14657. [Google Scholar] [CrossRef]
- Moustafa, A.; Loram, J.E.; Hackett, J.D.; Anderson, D.M.; Plumley, F.G.; Bhattacharya, D. Origin of saxitoxin biosynthetic genes in cyanobacteria. PLoS ONE 2009, 4, e5758. [Google Scholar] [CrossRef] [Green Version]
- Akbar, M.A.; Ahmad, A.; Usup, G.; Bunawan, H. Current Knowledge and Recent Advances in Marine Dinoflagellate Transcriptomic Research. J. Mar. Sci. Eng. 2018, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Akbar, M.A.; Ahmad, A.; Usup, G.; Bunawan, H. RNA-Seq as an Emerging Tool for Marine Dinoflagellate Transcriptome Analysis: Process and Challenges. Processes 2018, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, S.; Cho, Y.; Konoki, K.; Nagasawa, K.; Oshima, Y.; Yotsu-Yamashita, M. Synthesis of a tricyclic bisguanidine compound structurally related to saxitoxin and its identification in paralytic shellfish toxin-producing microorganisms. Chem.: Eur. J 2015, 21, 7835–7840. [Google Scholar] [CrossRef]
- Cho, Y.; Tsuchiya, S.; Yoshioka, R.; Omura, T.; Konoki, K.; Oshima, Y.; Yotsu-Yamashita, M. Column switching combined with hydrophilic interaction chromatography-tandem mass spectrometry for the analysis of saxitoxin analogues, and their biosynthetic intermediates in dinoflagellates. J. Chromatogr. A 2016, 1474, 109–120. [Google Scholar] [CrossRef]
- Yang, I.; John, U.; Beszteri, S.; Glöckner, G.; Krock, B.; Goesmann, A.; Cembella, A.D. Comparative gene expression in toxic versus non-toxic strains of the marine dinoflagellate Alexandrium minutum. BMC Genom. 2010, 11, 248. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef]
- Hii, K.S.; Lim, P.T.; Tan, T.H.; Leaw, C.P. Characterization of the saxitoxin biosynthetic starting gene, sxtA in the toxic dinoflagellate Alexandrium tamiyavanichii. In Proceedings of the 12th Symposium of the Malaysian Society of Applied Biology: Solutions to Global Challenges and Issues, Kuala Terengganu, Malaysia, 1–3 June 2012; pp. 196–202. [Google Scholar]
- Le Gac, M.; Metegnier, G.; Chomérat, N.; Malestroit, P.; Quéré, J.; Bouchez, O.; Siano, R.; Destombe, C.; Guillou, L.; Chapelle, A. Evolutionary processes and cellular functions underlying divergence in Alexandrium minutum. Mol. Ecol. 2016, 25, 5129–5143. [Google Scholar] [CrossRef] [Green Version]
- Hii, K.S.; Lim, P.T.; Kon, N.F.; Takata, Y.; Usup, G.; Leaw, C.P. Physiological and transcriptional responses to inorganic nutrition in a tropical Pacific strain of Alexandrium minutum: Implications for the saxitoxin genes and toxin production. Harmful Algae 2016, 56, 9–21. [Google Scholar] [CrossRef]
- Zhuang, Y.; Zhang, H.; Hannick, L.; Lin, S. Metatranscriptome profiling reveals versatile N-nutrient utilization, CO 2 limitation, oxidative stress, and active toxin production in an Alexandrium fundyense bloom. Harmful Algae. 2015, 42, 60–70. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.F.; Lin, L.; Wang, D.Z. Comparative transcriptome analysis of a toxin-producing dinoflagellate Alexandrium catenella and its non-toxic mutant. Mar. Drugs 2014, 12, 5698–5718. [Google Scholar] [CrossRef] [Green Version]
- Stüken, A.; Orr, R.J.S.; Kellmann, R.; Murray, S.A.; Neilan, B.A.; Jakobsen, K.S. Discovery of nuclear-encoded genes for the neurotoxin saxitoxin in dinoflagellates. PLoS ONE 2011, 6, e20096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orr, R.J.; Stüken, A.; Murray, S.A.; Jakobsen, K.S. Evolutionary acquisition and loss of saxitoxin biosynthesis in dinoflagellates: The second “core” gene-sxtG. Appl. Environ. Microbiol. 2013, 79, 2128–2136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiese, M.; Murray, S.A.; Alvin, A.; Neilan, B.A. Gene expression and molecular evolution of sxtA4 in a saxitoxin producing dinoflagellate Alexandrium catenella. Toxicon 2014, 92, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.F.; Lin, L.; Wang, D.Z. Whole transcriptomic analysis provides insights into molecular mechanisms for toxin biosynthesis in a toxic dinoflagellate Alexandrium catenella (ACHK-T). Toxins 2017, 9, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suikkanen, S.; Kremp, A.; Hautala, H.; Krock, B. Paralytic shellfish toxins or spirolides? The role of environmental and genetic factors in toxin production of the Alexandrium ostenfeldii complex. Harmful Algae 2013, 26, 52–59. [Google Scholar] [CrossRef]
- Hackett, J.D.; Wisecaver, J.H.; Brosnahan, M.L.; Kulis, D.M.; Anderson, D.M.; Bhattacharya, D.; Gerald Plumley, F.; Erdner, D.L. Evolution of saxitoxin synthesis in cyanobacteria and dinoflagellates. Mol. Biol. Evol. 2012, 30, 70–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perini, F.; Galluzzi, L.; Dell’Aversano, C.; Iacovo, E.D.; Tartaglione, L.; Ricci, F.; Forino, M.; Ciminiello, P.; Penna, A. SxtA and sxtG gene expression and toxin production in the Mediterranean Alexandrium minutum (Dinophyceae). Mar. Drugs 2014, 12, 5258–5276. [Google Scholar] [CrossRef]
- Stüken, A.; Riobó, P.; Franco, J.; Jakobsen, K.S.; Guillou, L.; Figueroa, R.I. Paralytic shellfish toxin content is related to genomic sxtA4 copy number in Alexandrium minutum strains. Front. Microbiol. 2015, 6, 404. [Google Scholar] [CrossRef]
- Savela, H.; Harju, K.; Spoof, L.; Lindehoff, E.; Meriluoto, J.; Vehniäinen, M.; Kremp, A. Quantity of the dinoflagellate sxtA4 gene and cell density correlates with paralytic shellfish toxin production in Alexandrium ostenfeldii blooms. Harmful Algae 2016, 52, 1–10. [Google Scholar] [CrossRef]
- Stüken, A.; Dittami, S.M.; Eikrem, W.; McNamee, S.; Campbell, K.; Jakobsen, K.S.; Edvardsen, B. Novel hydrolysis-probe based qPCR assay to detect saxitoxin transcripts of dinoflagellates in environmental samples. Harmful Algae 2013, 28, 108–117. [Google Scholar] [CrossRef]
- Murray, S.A.; Wiese, M.; Stüken, A.; Brett, S.; Kellmann, R.; Hallegraeff, G.; Neilan, B.A. A quantitative molecular assay based on the gene sxtA to identify saxitoxin-producing harmful algal blooms in marine waters. Appl. Environ. Microbiol 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, S.A.; Diwan, R.; Orr, R.J.; Kohli, G.S.; John, U. Gene duplication, loss and selection in the evolution of saxitoxin biosynthesis in alveolates. Mol. Phylogenet. Evol. 2015, 92, 165–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, U.; Fensome, R.A.; Medlin, L.K. The application of a molecular clock based on molecular sequences and the fossil record to explain biogeographic distributions within the Alexandrium tamarense “species complex” (Dinophyceae). Mol. Biol. Evol. 2003, 20, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Chen, S.; Chen, W. Dinoflagellates, a unique lineage for retrogene research. Front. Microbiol. 2018, 9, 1556. [Google Scholar] [CrossRef] [Green Version]
- Kaessmann, H.; Vinckenbosch, N.; Long, M. RNA-based gene duplication: Mechanistic and evolutionary insights. Nat. Rev. Genet. 2009, 10, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Song, B.; Morse, D.; Song, Y.; Fu, Y.; Lin, X.; Wang, W.; Lin, S. Comparative genomics reveals two major bouts of gene retroposition coinciding with crucial periods of Symbiodinium evolution. Genome Biol. Evol. 2017, 9, 2037–2047. [Google Scholar] [CrossRef] [Green Version]
- Reen, F.J.; Romano, S.; Dobson, A.D.; O’Gara, F. The sound of silence: Activating silent biosynthetic gene clusters in marine microorganisms. Mar. Drugs 2015, 13, 4754–4783. [Google Scholar] [CrossRef] [Green Version]
- Cooper, J.T.; Sinclair, G.A.; Wawrik, B. Transcriptome analysis of Scrippsiella trochoidea CCMP 3099 reveals physiological changes related to nitrate depletion. Front. Microbiol. 2016, 7, 639. [Google Scholar] [CrossRef] [Green Version]
- Biecker, A.L.; Liu, X.; Thorson, J.S.; Yang, Z.; Van Lanen, S.G. Biosynthetic and synthetic strategies for assembling capuramycin-type antituberculosis antibiotics. Molecules 2019, 24, 433. [Google Scholar] [CrossRef] [Green Version]
- Yang, I.; Beszteri, S.; Tillmann, U.; Cembella, A.; John, U. Growth-and nutrient-dependent gene expression in the toxigenic marine dinoflagellate Alexandrium minutum. Harmful Algae 2011, 12, 55–69. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Lee, H.; Anderson, D.M.; Kim, B. Paralytic shellfish toxin production by the dinoflagellate Alexandrium pacificum (Chinhae Bay, Korea) in axenic, nutrient-limited chemostat cultures and nutrient-enriched batch cultures. Mar. Pollut. Bull. 2016, 104, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erdner, D.L.; Anderson, D.M. Global transcriptional profiling of the toxic dinoflagellate Alexandrium fundyense using massively parallel signature sequencing. BMC Genom. 2006, 7, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moustafa, A.; Evans, A.N.; Kulis, D.M.; Hackett, J.D.; Erdner, D.L.; Anderson, D.M.; Bhattacharya, D. Transcriptome profiling of a toxic dinoflagellate reveals a gene-rich protist and a potential impact on gene expression due to bacterial presence. PLoS ONE 2010, 5, e9688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauritano, C.; De Luca, D.; Ferrarini, A.; Avanzato, C.; Minio, A.; Esposito, F.; Ianora, A. De novo transcriptome of the cosmopolitan dinoflagellate Amphidinium carterae to identify enzymes with biotechnological potential. Sci. Rep. 2017, 7, 11701. [Google Scholar] [CrossRef] [Green Version]
- Lin, S. Genomic understanding of dinoflagellates. Res. Microbiol. 2011, 16, 551–569. [Google Scholar] [CrossRef]
- Gacek-Matthews, A.; Berger, H.; Sasaki, T.; Wittstein, K.; Gruber, C.; Lewis, Z.A.; Strauss, J. KdmB, a Jumonji histone H3 demethylase, regulates genome-wide H3K4 trimethylation and is required for normal induction of secondary metabolism in Aspergillus nidulans. PLoS Genet. 2016, 12, e1006222. [Google Scholar] [CrossRef]
- Pidroni, A.; Faber, B.; Brosch, G.; Bauer, I.; Graessle, S. A Class 1 Histone Deacetylase as Major Regulator of Secondary Metabolite Production in Aspergillus nidulans. Front. Microbiol. 2018, 9, 2212. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Morse, D. A full suite of histone and histone modifying genes are transcribed in the dinoflagellate Lingulodinium. PLoS ONE 2012, 7, e34340. [Google Scholar] [CrossRef]
- Yang, I.; Selander, E.; Pavia, H.; John, U. Grazer-induced toxin formation in dinoflagellates: A transcriptomic model study. Eur. J. Phycol. 2011, 46, 66–73. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Hou, Y.; Miranda, L.; Campbell, D.A.; Sturm, N.R.; Gaasterland, T.; Lin, S. Spliced leader RNA trans-splicing in dinoflagellates. Proc. Natl. Acad. Sci. USA 2007, 104, 4618–4623. [Google Scholar] [CrossRef] [Green Version]
- Lidie, K.B.; Van Dolah, F.M. Spliced leader RNA-mediated trans-splicing in a dinoflagellate, Karenia brevis. J. Eukaryot Microbiol. 2007, 54, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Danks, G.B.; Raasholm, M.; Campsteijn, C.; Long, A.M.; Manak, J.R.; Lenhard, B.; Thompson, E.M. Trans-splicing and operons in metazoans: Translational control in maternally regulated development and recovery from growth arrest. Mol. Biol. Evol. 2015, 32, 585–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitar, M.; Boroni, M.; Macedo, A.M.; Machado, C.R.; Franco, G.R. The spliced leader trans-splicing mechanism in different organisms: Molecular details and possible biological roles. Front. Genet. 2013, 4, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgarten, S.; Bayer, T.; Aranda, M.; Liew, Y.J.; Carr, A.; Micklem, G.; Voolstra, C.R. Integrating microRNA and mRNA expression profiling in Symbiodinium microadriaticum, a dinoflagellate symbiont of reef-building corals. BMC Genom. 2013, 14, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.; Qiu, L.; Hou, Z.; Zhang, Q.; Wu, J.; Gao, Q.; Song, L. Computational identification of microRNAs from the expressed sequence tags of toxic dinoflagellate Alexandrium tamarense. Evol. Bioinform. 2013, 9, 479–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, H.; Sui, Z.; Zhang, S.; Du, Q.; Ren, Y.; Liu, Y.; Kong, F.; Zhong, J.; Ma, Q. Identification of microRNAs in the toxigenic Dinoflagellate Alexandrium catenella by high-throughput illumina sequencing and bioinformatic analysis. PLoS ONE 2015, 10, e0138709. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Cheng, S.; Song, B.; Zhong, X.; Lin, X.; Li, W.; Li, L.; Zhang, Y.; Zhang, H.; Ji, Z.; et al. The Symbiodinium kawagutii genome illuminates dinoflagellate gene expression and coral symbiosis. Science 2015, 350, 691–694. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Lin, X.; Li, L.; Li, M.; Palenik, B.; Lin, S. Transcriptomic and microRNAomic profiling reveals multi-faceted mechanisms to cope with phosphate stress in a dinoflagellate. ISME J. 2017, 11, 2209–2218. [Google Scholar] [CrossRef] [Green Version]
- Jagus, R.; Bachvaroff, T.R.; Joshi, B.; Place, A.R. Diversity of eukaryotic translational initiation factor eIF4E in protists. Comp. Funct. Genom. 2012, 2012, 134839. [Google Scholar] [CrossRef] [Green Version]
- Herdy, B.; Jaramillo, M.; Svitkin, Y.V.; Rosenfeld, A.B.; Kobayashi, M.; Walsh, D.; Alain, T.; Sean, P.; Robichaud, N.; Topisirovic, I.; et al. Translational control of the activation of transcription factor NF-κB and production of type I interferon by phosphorylation of the translation factor eIF4E. Nat. Immunol. 2012, 13, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Jones, G.D.; Williams, E.P.; Place, A.R.; Jagus, R.; Bachvaroff, T.R. The alveolate translation initiation factor 4E family reveals a custom toolkit for translational control in core dinoflagellates. BMC Evol. Biol. 2015, 15, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akimoto, H.; Kinumi, T.; Ohmiya, Y. Circadian rhythm of a TCA cycle enzyme is apparently regulated at the translational level in the dinoflagellate Lingulodinium polyedrum. J. Biol. Rhythm 2005, 20, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Brunelle, S.A.; Van Dolah, F.M. Post-transcriptional regulation of S-phase genes in the dinoflagellate, Karenia brevis. J. Eukaryot Microbiol. 2011, 58, 373–382. [Google Scholar] [CrossRef]
- Roy, S.; Beauchemin, M.; Dagenais-Bellefeuille, S.; Letourneau, L.; Cappadocia, M.; Morse, D. The Lingulodinium circadian system lacks rhythmic changes in transcript abundance. BMC Biol. 2014, 12, 107. [Google Scholar] [CrossRef] [Green Version]
- Tse, S.P.; Beauchemin, M.; Morse, D.; Lo, S.C. Refining Transcriptome Gene Catalogs by MS-Validation of Expressed Proteins. Proteomics 2018, 18, 1700271. [Google Scholar] [CrossRef]
- Morse, D.; Sirius, P.K.; Lo, S.C. Exploring dinoflagellate biology with high-throughput proteomics. Harmful Algae 2018, 75, 16–26. [Google Scholar] [CrossRef]
- Erdjument-Bromage, H.; Huang, F.K.; Neubert, T.A. Sample Preparation for Relative Quantitation of Proteins Using Tandem Mass Tags (TMT) and Mass Spectrometry (MS). Methods Mol. Biol. 2018, 1741, 135–149. [Google Scholar] [CrossRef]
- Wang, D.Z.; Zhang, H.; Zhang, Y.; Zhang, S.F. Marine dinoflagellate proteomics: Current status and future perspectives. J. Proteom. 2014, 105, 121–132. [Google Scholar] [CrossRef]
- Chan, L.L.; Sit, W.H.; Lam, P.K.S.; Hsieh, D.P.H.; Hodgkiss, I.J.; Wan, J.M.F.; Ho, A.Y.; Choi, N.M.; Wang, D.Z.; Dudgeon, D. Identification and characterization of a “biomarker of toxicity” from the proteome of the paralytic shellfish toxin-producing dinoflagellate Alexandrium tamarense (Dinophyceae). Proteomics 2006, 6, 654–666. [Google Scholar] [CrossRef]
- Chan, L.L.; Hodgkiss, I.J.; Lam, P.K.S.; Wan, J.M.F.; Chou, H.N.; Lum, J.H.; Lo, M.G.; Mak, A.S.; Sit, W.H.; Lo, S.C. Use of two-dimensional gel electrophoresis to differentiate morphospecies of Alexandrium minutum, a paralytic shellfish poisoning toxin-producing dinoflagellate of harmful algal blooms. Proteomics 2005, 5, 1580–1593. [Google Scholar] [CrossRef]
- Wang, D.Z.; Li, C.; Zhang, Y.; Wang, Y.Y.; He, Z.P.; Lin, L.; Hong, H.S. Quantitative proteomic analysis of differentially expressed proteins in the toxicity-lost mutant of Alexandrium catenella (Dinophyceae) in the exponential phase. J. Proteom. 2012, 75, 5564–5577. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.Z.; Gao, Y.; Lin, L.; Hong, H.S. Comparative proteomic analysis reveals proteins putatively involved in toxin biosynthesis in the marine dinoflagellate Alexandrium catenella. Mar. Drugs 2013, 11, 213–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.F.; Zhang, Y.; Xie, Z.X.; Zhang, H.; Lin, L.; Wang, D.Z. iTRAQ-based quantitative proteomic analysis of a toxigenic dinoflagellate Alexandrium catenella and its non-toxic mutant. Proteomics 2015, 15, 4041–4050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.F.; Zhang, Y.; Lin, L.; Wang, D.Z. iTRAQ-based quantitative proteomic analysis of a toxigenic dinoflagellate Alexandrium catenella and its non-toxigenic mutant exposed to a cell cycle inhibitor colchicine. Front. Microbiol. 2018, 9, 650. [Google Scholar] [CrossRef]
- Jiang, X.W.; Wang, J.; Gao, Y.; Chan, L.L.; Lam, P.K.S.; Gu, J.D. Relationship of proteomic variation and toxin synthesis in the dinoflagellate Alexandrium tamarense CI01 under phosphorus and inorganic nitrogen limitation. Ecotoxicology 2015, 24, 1744–1753. [Google Scholar] [CrossRef]
- Etheridge, S.M.; Roesler, C.S. Effects of temperature, irradiance, and salinity on photosynthesis, growth rates, total toxicity, and toxin composition for Alexandrium fundyense isolates from the Gulf of Maine and Bay of Fundy. Deep Sea Res. Pt II 2005, 52, 2491–2500. [Google Scholar] [CrossRef]
- Cho, Y.; Hiramatsu, K.; Ogawa, M.; Omura, T.; Ishimaru, T.; Oshima, Y. Non-toxic and toxic subclones obtained from a toxic clonal culture of Alexandrium tamarense (Dinophyceae): Toxicity and molecular biological feature. Harmful Algae 2008, 7, 740–751. [Google Scholar] [CrossRef]
- Martins, C.A.; Kulis, D.; Franca, S.; Anderson, D.M. The loss of PSP toxin production in a formerly toxic Alexandrium lusitanicum clone. Toxicon 2004, 43, 195–205. [Google Scholar] [CrossRef]
- Fritz, C.; Palacios-Rojas, N.; Feil, R.; Stitt, M. Regulation of secondary metabolism by the carbon–nitrogen status in tobacco: Nitrate inhibits large sectors of phenylpropanoid metabolism. Plant J. 2006, 46, 533–548. [Google Scholar] [CrossRef]
- Twumasi-Boateng, K.; Yu, Y.; Chen, D.; Gravelat, F.N.; Nierman, W.C.; Sheppard, D.C. Transcriptional profiling identifies a role for BrlA in the response to nitrogen depletion and for StuA in the regulation of secondary metabolite clusters in Aspergillus fumigatus. Eukaryot. Cell 2009, 8, 104–115. [Google Scholar] [CrossRef] [Green Version]
- Allen, A.E.; Vardi, A.; Bowler, C. An ecological and evolutionary context for integrated nitrogen metabolism and related signaling pathways in marine diatoms. Curr. Opin. Plant Biol. 2006, 9, 264–273. [Google Scholar] [CrossRef]
- Goodacre, R.; Vaidyanathan, S.; Dunn, W.B.; Harrigan, G.G.; Kell, D.B. Metabolomics by numbers: Acquiring and understanding global metabolite data. Trends Biotechnol. 2004, 22, 245–252. [Google Scholar] [CrossRef]
- Stringer, K.A.; McKay, R.T.; Karnovsky, A.; Quémerais, B.; Lacy, P. Metabolomics and its application to acute lung diseases. Front. Immunol. 2016, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- van der Greef, J.; van Wietmarschen, H.; van Ommen, B.; Verheij, E. Looking back into the future: 30 years of metabolomics at TNO. Mass Spectrom. Rev. 2013, 32, 399–415. [Google Scholar] [CrossRef]
- Suárez-Ulloa, V.; Fernández-Tajes, J.; Manfrin, C.; Gerdol, M.; Venier, P.; Eirín-López, J. Bivalve omics: State of the art and potential applications for the biomonitoring of harmful marine compounds. Mar. Drugs 2013, 11, 4370–4389. [Google Scholar] [CrossRef] [Green Version]
- Fiehn, O. Combining genomics, metabolome analysis, and biochemical modelling to understand metabolic networks. Comp. Funct. Genom. 2001, 2, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Jamers, A.; Blust, R.; De Coen, W. Omics in algae: Paving the way for a systems biological understanding of algal stress phenomena? Aquat. Toxicol. 2009, 92, 114–121. [Google Scholar] [CrossRef]
- Guasch-Ferre, M.; Bhupathiraju, S.N.; Hu, F.B. Use of metabolomics in improving assessment of dietary intake. Clin. Chem. 2018, 64, 82–98. [Google Scholar] [CrossRef] [Green Version]
- Goulitquer, S.; Potin, P.; Tonon, T. Mass spectrometry-based metabolomics to elucidate functions in marine organisms and ecosystems. Mar. Drugs 2012, 10, 849–880. [Google Scholar] [CrossRef]
- Chun, S.W.; Hinze, M.E.; Skiba, M.A.; Narayan, A.R. Chemistry of a unique polyketide-like synthase. J. Am. Chem. Soc. 2018, 140, 2430–2433. [Google Scholar] [CrossRef]
- Cho, Y.; Tsuchiya, S.; Omura, T.; Koike, K.; Oikawa, H.; Konoki, K.; Yotsu-Yamashita, M. Metabolomic study of saxitoxin analogues and biosynthetic intermediates in dinoflagellates using 15 N-labelled sodium nitrate as a nitrogen source. Sci. Rep. 2019, 9, 3460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, B.R.; Leggat, W. Symbiodinium—Invertebrate symbioses and the role of metabolomics. Mar. Drugs 2010, 8, 2546–2568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillyer, K.E.; Tumanov, S.; Villas-Bôas, S.; Davy, S.K. Metabolite profiling of symbiont and host during thermal stress and bleaching in a model cnidarian–dinoflagellate symbiosis. J. Exp. Biol. 2016, 219, 516–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selander, E.; Kubanek, J.; Hamberg, M.; Andersson, M.X.; Cervin, G.; Pavia, H. Predator lipids induce paralytic shellfish toxins in bloom-forming algae. Proc. Natl. Acad. Sci. USA 2015, 112, 6395–6400. [Google Scholar] [CrossRef] [Green Version]
- Lundholm, N.; Krock, B.; John, U.; Skov, J.; Cheng, J.; Pančić, M.; Harðardóttir, S. Induction of domoic acid production in diatoms—Types of grazers and diatoms are important. Harmful Algae 2018, 79, 64–73. [Google Scholar] [CrossRef]
- Albinsson, M.E.; Negri, A.P.; Blackburn, S.I.; Bolch, C.J. Bacterial community affects toxin production by Gymnodinium catenatum. PLoS ONE 2014, 9, e104623. [Google Scholar] [CrossRef]
- Sörenson, E.; Bertos-Fortis, M.; Farnelid, H.; Kremp, A.; Krüger, K.; Lindehoff, E.; Legrand, C. Consistency in microbiomes in cultures of Alexandrium species isolated from brackish and marine waters. Environ. Microbiol. Rep. 2019, 11, 425–433. [Google Scholar] [CrossRef]
- Wang, H.; Tomasch, J.; Jarek, M.; Wagner-Döbler, I. A dual-species co-cultivation system to study the interactions between Roseobacters and dinoflagellates. Front. Microbiol 2014, 5, 311. [Google Scholar] [CrossRef]
- Verma, A.; Barua, A.; Ruvindy, R.; Savela, H.; Ajani, P.A.; Murray, S.A. The genetic basis of toxin biosynthesis in dinoflagellates. Microorganisms 2019, 7, 222. [Google Scholar] [CrossRef] [Green Version]
- Bachvaroff, T.R.; Place, A.R. From stop to start: Tandem gene arrangement, copy number and trans-splicing sites in the dinoflagellate Amphidinium carterae. PLoS ONE 2008, 3, e2929. [Google Scholar] [CrossRef] [Green Version]
- Jaeckisch, N.; Yang, I.; Wohlrab, S.; Glöckner, G.; Kroymann, J.; Vogel, H.; John, U. Comparative genomic and transcriptomic characterization of the toxigenic marine dinoflagellate Alexandrium ostenfeldii. PLoS ONE 2011, 6, e28012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, J.M.; Rödelsperger, C.; Eichholz, K.; Tillmann, U.; Cembella, A.; McGaughran, A.; John, U. Transcriptomic characterisation and genomic glimps into the toxigenic dinoflagellate Azadinium spinosum, with emphasis on polykeitde synthase genes. BMC Genom. 2015, 16, 27. [Google Scholar] [CrossRef] [Green Version]
- McEwan, M.; Humayun, R.; Slamovits, C.H.; Keeling, P.J. Nuclear genome sequence survey of the dinoflagellate Heterocapsa triquetra. J. Eukaryot Microbiol. 2008, 55, 530–535. [Google Scholar] [CrossRef] [PubMed]
- Shoguchi, E.; Shinzato, C.; Kawashima, T.; Gyoja, F.; Mungpakdee, S.; Koyanagi, R.; Hamada, M. Draft assembly of the Symbiodinium minutum nuclear genome reveals dinoflagellate gene structure. Curr. Biol. 2013, 23, 1399–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranda, M.; Li, Y.; Liew, Y.J.; Baumgarten, S.; Simakov, O.; Wilson, M.C.; Ryu, T. Genomes of coral dinoflagellate symbionts highlight evolutionary adaptations conducive to a symbiotic lifestyle. Sci. Rep. 2016, 6, 39734. [Google Scholar] [CrossRef] [Green Version]
- Simam, J.; Rono, M.; Ngoi, J.; Nyonda, M.; Mok, S.; Marsh, K.; Mackinnon, M. Gene copy number variation in natural populations of Plasmodium falciparum in Eastern Africa. BMC Genom. 2018, 19, 372. [Google Scholar] [CrossRef]
- López-Legentil, S.; Song, B.; DeTure, M.; Baden, D.G. Characterization and localization of a hybrid non-ribosomal peptide synthetase and polyketide synthase gene from the toxic dinoflagellate Karenia brevis. Mar. Biotechnol. 2010, 12, 32–41. [Google Scholar] [CrossRef]
- Shoguchi, E.; Beedessee, G.; Tada, I.; Hisata, K.; Kawashima, T.; Takeuchi, T.; Kawachi, M. Two divergent Symbiodinium genomes reveal conservation of a gene cluster for sunscreen biosynthesis and recently lost genes. BMC Genom. 2018, 19, 458. [Google Scholar] [CrossRef] [Green Version]
- Nowoshilow, S.; Schloissnig, S.; Fei, J.F.; Dahl, A.; Pang, A.W.; Pippel, M.; Winkler, S.; Hastie, A.R.; Young, G.; Roscito, J.G.; et al. The axolotl genome and the evolution of key tissue formation regulators. Nature 2018, 554, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Jansen, H.J.; Liem, M.; Jong-Raadsen, S.A.; Dufour, S.; Weltzien, F.A.; Swinkels, W.; Koelewijn, A.; Palstra, A.P.; Pelster, B.; Spaink, H.P.; et al. Rapid de novo assembly of the European eel genome from nanopore sequencing reads. Sci. Rep. 2017, 7, 7213. [Google Scholar] [CrossRef] [Green Version]
- Bleidorn, C. Third generation sequencing: Technology and its potential impact on evolutionary biodiversity research. Syst. Biodivers. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Valledor, L.; Escandón, M.; Meijón, M.; Nukarinen, E.; Cañal, M.J.; Weckwerth, W. A universal protocol for the combined isolation of metabolites, DNA, long RNAs, small RNAs, and proteins from plants and microorganisms. Plant Pathol. J. 2014, 79, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.B.; Langefeld, C.; Olivier, M.; Cox, L.A. Integrated omics: Tools, advances and future approaches. J. Mol. Endocrinol. 2019, 62, R21–R45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valledor, L.; Furuhashi, T.; Recuenco-Muñoz, L.; Wienkoop, S.; Weckwerth, W. System-level network analysis of nitrogen starvation and recovery in Chlamydomonas reinhardtii reveals potential new targets for increased lipid accumulation. Biotechnol. Biofuels 2014, 7, 171. [Google Scholar] [CrossRef] [Green Version]
| Structure of Saxitoxin (STX) | |||||||
|---|---|---|---|---|---|---|---|
 | |||||||
| Analogues | R1 | R2 | R3 | R4 | R5 | Sources | Reference |
| STX | H | H | H | OCONH2 | OH | Alexandrium andersoni, Alexandrium catenella, Alexandrium fundyense, Alexandrium tamarense, Gymnodinium catenatum, Pyrodinium bahamense | [7,8,9,10,11,12] |
| neoSTX | OH | H | H | OCONH2 | OH | A. andersoni, A. catenella, A. fundyense, A. tamarense, G. catenatum, P. bahamense | [7,8,9,10,11,12] |
| Mono-sulfated | |||||||
| GTX1 | OH | H | OSO3− | OCONH2 | OH | A. catenella, A. fundyense, A. minutum, A. tamarense, G. catenatum | [8,9,10,12,13] |
| GTX2 | H | H | OSO3− | OCONH2 | OH | A. catenella, A. fundyense, A. minutum, Alexandrium ostenfeldii, A. tamarense, G. catenatum | [8,10,12,13,14,15] |
| GTX3 | H | OSO3− | H | OCONH2 | OH | A. catenella, A. fundyense, A. minutum, A. ostenfeldii, A. tamarense, G. catenatum | [8,9,12,13,14,15] |
| GTX4 | OH | OSO3− | H | OCONH2 | OH | A. catenella, A. fundyense, A. minutum, A. tamarense, G. catenatum | [8,9,10,13,16] |
| GTX5 (B1) | H | H | H | OCONHSO3− | OH | A. catenella, A. fundyense, A. tamarense, G. catenatum, P. bahamense | [8,9,10,11,12] |
| GTX6 (B2) | OH | H | H | OCONHSO3− | OH | A. catenella, A. fundyense, A. ostenfeldii, A. tamarense, G. catenatum, P. bahamense | [8,9,10,11,12] |
| Di-sulfated | |||||||
| C1 | H | H | OSO3− | OCONHSO3− | OH | A. catenella, A. fundyense, A. ostenfeldii, A. tamarense, G. catenatum | [8,9,10,12,15,17,18] |
| C2 | H | OSO3− | H | OCONHSO3− | OH | A. catenella, A. fundyense, A. ostenfeldii, A. tamarense, G. catenatum | [8,9,10,12,15,18] |
| C3 | OH | H | OSO3− | OCONHSO3− | OH | A. catenella, G. catenatum | [14,19] |
| C4 | OH | OSO3− | H | OCONHSO3− | OH | A. catenella, G. catenatum | [14,19] |
| Decarbamoylated | |||||||
| dcSTX | H | H | H | OH | OH | A. catenella, G. catenatum, P. bahamense | [8,11,12] |
| dcneoSTX | OH | H | H | OH | OH | A. tamarense | [20] |
| dcGTX1 | OH | H | OSO3− | OH | OH | G. catenatum | [21] |
| dcGTX2 | H | H | OSO3− | OH | OH | A. catenella, A. fundyense, G. catenatum | [8,12,17] |
| dcGTX3 | H | OSO3− | H | OH | OH | A. catenella, A. fundyense, G. catenatum | [8,12,17] |
| dcGTX4 | OH | OSO3− | H | OH | OH | G. catenatum | [21] |
| Deoxy-decarbamoylated | |||||||
| doSTX | H | H | H | H | OH | G. catenatum | [22] |
| doGTX1 | OH | H | OSO3− | H | OH | G. catenatum | [22] |
| doGTX2 | H | H | OSO3− | H | OH | G. catenatum | [22] |
| Mono-hydroxybenzoate Analogues | |||||||
| GC1 | H | H | OSO3− | OCOPhOH | OH | G. catenatum | [21] |
| GC2 | H | OSO3− | H | OCOPhOH | OH | G. catenatum | [21] |
| GC3 | H | H | H | OCOPhOH | OH | G. catenatum | [21] |
| * GC4 | OH | H | OSO3− | OCOPhOH | OH | G. catenatum | [19] |
| * GC5 | OH | OSO3− | H | OCOPhOH | OH | G. catenatum | [19] |
| * GC6 | OH | H | H | OCOPhOH | OH | G. catenatum | [19] |
| Di-hydroxybenzoate Analogues | |||||||
| + GC1a | H | H | OSO3− | DHB | OH | G. catenatum | [19] |
| + GC2a | H | OSO3− | H | DHB | OH | G. catenatum | [19] |
| + GC3a | H | H | H | DHB | OH | G. catenatum | [19] |
| + GC4a | OH | H | OSO3− | DHB | OH | G. catenatum | [19] |
| + GC5a | OH | OSO3− | H | DHB | OH | G. catenatum | [19] |
| + GC6a | OH | H | H | DHB | OH | G. catenatum | [19] |
| Sulfated Benzoate Analogues | |||||||
| + GC1b | H | H | OSO3− | SB | OH | G. catenatum | [19] |
| + GC2b | H | OSO3− | H | SB | OH | G. catenatum | [19] |
| + GC3b | H | H | H | SB | OH | G. catenatum | [19] |
| + GC4b | OH | H | OSO3− | SB | OH | G. catenatum | [19] |
| + GC5b | OH | OSO3− | H | SB | OH | G. catenatum | [19] |
| + GC6b | OH | H | H | SB | OH | G. catenatum | [19] |
| Role | Genes | Size (bp) | Putative Function | Species | Reference |
|---|---|---|---|---|---|
| Core genes | sxtA | 3702–3735 | Methylation, loading of ACP, Claisen condensation | A. minutum (AIN34673.1), A. catanella (AIR95660.1), A. tamarense (AIL29903.1), A. fundyense (ADY62525.1), A. ostenfeldii, Alexandrium tamiyavanichii, G. catenatum (AVV62437.1), P. bahamense (QEX95300.1) | [38,39,40,41,42,43,44,45,46,47,48,49] |
| sxtB | 954–975 | Cyclization | A. catenella, A. fundyense, A. minutum, A. tamarense | [42,43,46,48] | |
| sxtC | 282–351 | Regulatory | - | - | |
| sxtD | 756–798 | Desaturation | A. catenella, A. tamarense | [42,46,48] | |
| sxtG | 1131 | Amidinotransfer | A. minutum (AGC84341.1), A. catenella (AGC84338.1), A. fundyense (AGC84339.1), A. tamarense (AGC84356.1), G. catenatum (AGC84343.1), P. bahamense (JAG92740.1) | [40,42,43,44,46,48,49] | |
| sxtH/T | 1002–1059 | C-12 hydroxylation | A. catenella, A. fundyense, A. minutum, A. tamarense | [42,43,46,48] | |
| sxtI | 1836–1923 | Carbamoylation | A. catenella, A. fundyense, A. minutum, A. tamarense | [40,42,43,46,48] | |
| sxtJ | 399–441 | Regulatory | - | - | |
| sxtK | 162 | Regulatory | - | - | |
| sxtS | 723–798 | Ring formation | A. minutum, G. catenatum, P. bahamense | [43,48] | |
| sxtU | 774–777 | Short-chain alcohol dehydrogenase | A. catenella, A. fundyense, A. minutum, A. tamarense, P. bahamense | [42,43,46,48] | |
| sxtV | 1650–1677 | Dioxygenase reductase | - | - | |
| sxtW | 324–327 | Ferredoxin | A. catenella | [46] | |
| Tailoring genes | sxtL | 1269–1296 | Decarbamoylation | A. tamarense | [48] |
| sxtN | 825–906 | Sulfotransferase | A. catenella, A. tamarense | [42,48] | |
| sxtO | 495–600 | PAPS biosynthesis | A. catenella | [42,46] | |
| sxtX | 753–771 | N-1 hydroxylation | A. catenella, A. tamarense, P. bahamense | [42,46,48] | |
| Regulator genes | sxtY | 663 | Signal transduction | - | - |
| sxtZ | 1350 | Signal transduction | A. catenella | [42,46] | |
| Transporter genes | sxtF/M | 1413–1455 | Export of PSTs | A. catenella, A. fundyense, A. minutum, A. tamarense | [42,43,46,48] |
| sxtP | 1125–1479 | Binding of PSTs | A. catenella, A. tamarense | [46,48] | |
| Unknown | sxtE | 360–474 | Unknown | - | - |
| sxtQ | 774 | Unknown | - | - | |
| sxtR | 744–879 | Unknown | A. fundyense, A. minutum | [43] |
| Studied Species | Experimental Design | Summary of Findings | Reference |
|---|---|---|---|
| A. minutum | Construction of EST library for A. minutum | In silico search against EST library failed to identify any homologues of cyanobacteria saxitoxin genes | [36] |
| A. minutum | Microarray-based analysis of differentially expressed nutrient and toxin-related genes | Two unannotated genes were expressed during toxin production | [62] |
| A. fundyense and A. minutum | Gene survey study using 454 sequencing | Two different sxtA transcripts were present in dinoflagellates transcriptome with the longer transcript (sxtA4) exclusive to saxitoxin-producing dinoflagellates, which are likely involved in saxitoxin biosynthesis | [43] |
| A. tamarense, G. catenatum and P. bahamense | Gene survey study using 454 sequencing and Illumina Hiseq | Several sxt genes present in the transcriptome; however, phylogenetic analysis of these genes indicated that saxitoxin biosynthesis in dinoflagellates and cyanobacteria is acquired independently | [48] |
| A. minutum | Microarray-based analysis of transcriptome response toward grazer-induced induction | Two unannotated genes showed consistent regulation pattern with saxitoxin content in dinoflagellates | [71] |
| A. catenella | Comparison of transcriptome profile obtained using Illumina Hiseq between toxic and non-toxic dinoflagellates | Long isoform of sxtA was highly downregulated in the non-toxic strain; sxtO and sxtZ were discovered in dinoflagellates for the first time | [42] |
| Alexandrium spp. | Screening and analysis of EST library for 36 dinoflagellate species for occurrence of sxtA1, sxtA4, and sxtG genes | SxtA4 gene is highly conserved and exclusive to saxitoxin-producing dinoflagellates | [54] |
| A. fundyense | Metatranscriptome profiling during A. fundyense bloom in Northport/Huntington Bay complex, Long Island | SxtA expression was upregulated in the presence of low dissolved inorganic nitrogen in the environment | [41] |
| A. minutum | Transcriptome profiling and gene expression studies of several toxin-related genes under different nutritional conditions | SxtA4, sxtI, and sxtG gene expression patterns were consistent with toxin production | [40] |
| S. trochoidea | Transcriptome profiling under nitrate depletion | A total of 113 transcripts were recognized as homologues for sxt genes despite the fact that no saxitoxin is produced by this species; low transcriptional changes during nitrogen depletion detected | [60] |
| A. catenella | Transcriptome profiling at different toxin biosynthesis stages within cell cycle | 138 homologues of sxt genes were identified; however, their expression patterns were inconsistent with toxin level, suggesting that saxitoxin regulation occurs at the post-transcriptional level | [46] |
| Findings | Reference |
|---|---|
| Enzyme in the TCA cycle exhibited circadian changes in accordance with protein abundance, whereas its messenger RNA (mRNA) level remained constant throughout the cycle | [84] |
| Presence of unique splice leader at 5’ of dinoflagellates mRNA might provide translational regulation in dinoflagellates via trans-splicing | [72,73] |
| Expression of conserved S-phase genes in Karenia brevis remains unchanged throughout cell cycle, but other protein expression level was observed | [85] |
| Presence of dinoflagellate spliced leader sequence at 5’ of sxtA and sxtG genes might indicate that saxitoxin biosynthesis is regulated at the translational level | [43,44] |
| Daily circadian system in dinoflagellate Lingulodinium showed lack of regulation at the transcript level using RNA-sequencing approach, suggesting the involvement of translational or post-translational control of this system | [86] |
| Identification of microRNAs (miRNAs) in several species of dinoflagellates, including saxitoxin-producing dinoflagellates, indicates regulation of several genes in dinoflagellates at post-transcriptional level via a small RNA gene silencing mechanism | [76,77,78,79,80] |
| Characterization of extensive transcript encoding protein elF4E family in dinoflagellates | [83] |
| Genome sequence of Symbiodinium kawagutii revealed substantial translational control by miRNA in biological processes involving carbohydrate metabolism, transcription regulation, and biosynthesis of amino acids and antibiotics | [79] |
| Poor correlation between protein and mRNA level in dinoflagellate Lingulodinium | [87] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbar, M.A.; Mohd Yusof, N.Y.; Tahir, N.I.; Ahmad, A.; Usup, G.; Sahrani, F.K.; Bunawan, H. Biosynthesis of Saxitoxin in Marine Dinoflagellates: An Omics Perspective. Mar. Drugs 2020, 18, 103. https://doi.org/10.3390/md18020103
Akbar MA, Mohd Yusof NY, Tahir NI, Ahmad A, Usup G, Sahrani FK, Bunawan H. Biosynthesis of Saxitoxin in Marine Dinoflagellates: An Omics Perspective. Marine Drugs. 2020; 18(2):103. https://doi.org/10.3390/md18020103
Chicago/Turabian StyleAkbar, Muhamad Afiq, Nurul Yuziana Mohd Yusof, Noor Idayu Tahir, Asmat Ahmad, Gires Usup, Fathul Karim Sahrani, and Hamidun Bunawan. 2020. "Biosynthesis of Saxitoxin in Marine Dinoflagellates: An Omics Perspective" Marine Drugs 18, no. 2: 103. https://doi.org/10.3390/md18020103
APA StyleAkbar, M. A., Mohd Yusof, N. Y., Tahir, N. I., Ahmad, A., Usup, G., Sahrani, F. K., & Bunawan, H. (2020). Biosynthesis of Saxitoxin in Marine Dinoflagellates: An Omics Perspective. Marine Drugs, 18(2), 103. https://doi.org/10.3390/md18020103





