3D Chitin Scaffolds of Marine Demosponge Origin for Biomimetic Mollusk Hemolymph-Associated Biomineralization Ex-Vivo
Abstract
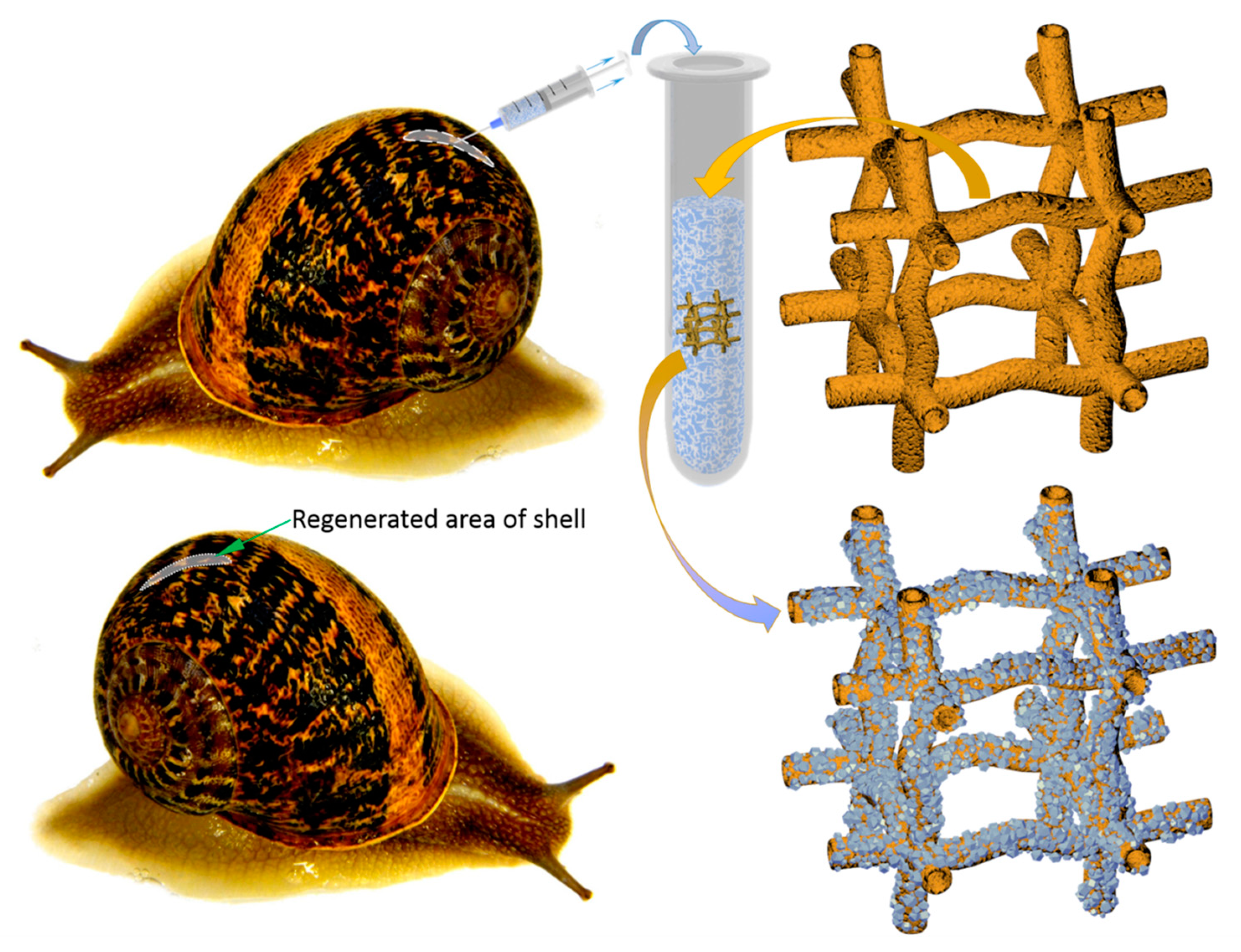
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Location and Collection
4.1.1. Aplysina archeri Sponges
4.1.2. Cornu aspersum Snails
4.2. Isolation Procedure
4.2.1. Isolation of Chitinous Scaffolds from the A. archeri Demosponge Skeleton
4.2.2. Nonlethal Isolation of the Hemolymph from the C. aspersum Snail
4.3. Ex Vivo Biomineralization of the A. archeri Chitinous Scaffolds
4.4. Short-Term Cultivation of Hemocytes on Chitinous Matrix
4.5. Characterization of Obtained Materials
4.5.1. Photography and Figures
4.5.2. Digital, Light and Fluorescence Microscopy
4.5.3. Eosin and Methylene Blue Staining
4.5.4. Alizarin Red S Staining
4.5.5. Fluorescent Microscopy Analysis
4.5.6. ATR FT-IR and Raman Spectroscopy
4.5.7. XRD
4.5.8. Scanning Electron Microscopy (SEM)
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Su, X.; Tan, M.; Duan, B.; Cai, J.; Jiang, W.; Zhang, L. Hierarchical microspheres with macropores fabricated from chitin as 3D cell culture. J. Mater. Chem. B 2019, 7, 5190–5198. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Coltelli, M.B.; Santi, S. Biobased tissues for innovative cosmetic products: Polybioskin as an EU research project. Glob. J. Nanomedicine 2018, 3, 1–6. [Google Scholar]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinger, C.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Petrenko, I.; Machałowski, T.; Ereskovsky, A.; Martinović, R.; Muzychka, L.; et al. Express Method for Isolation of Ready-to-Use 3D Chitin Scaffolds from Aplysina archeri (Aplysineidae: Verongiida) Demosponge. Mar. Drugs 2019, 17, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Vecchio, K.S. Conversion of natural marine skeletons as scaffolds for bone tissue engineering. Front. Mater. Sci. 2013, 7, 103–117. [Google Scholar] [CrossRef]
- Silva, T.H.; Moreira-Silva, J.; Marques, A.L.P.; Domingues, A.; Bayon, Y.; Reis, R.L. Marine origin collagens and its potential applications. Mar. Drugs 2014, 12, 5881–5901. [Google Scholar] [CrossRef] [Green Version]
- Nisticò, R. Aquatic-Derived Biomaterials for a Sustainable Future: A European Opportunity. Resources 2017, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Teimouri, A.; Azadi, M.; Shams Ghahfarokhi, Z.; Razavizadeh, R. Preparation and characterization of novel β-chitin/nanodiopside/nanohydroxyapatite composite scaffolds for tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2017, 28, 1–14. [Google Scholar] [CrossRef]
- Neto, A.S.; Ferreira, J.M.F. Synthetic and marine-derived porous scaffolds for bone tissue engineering. Materials 2018, 11, 1702. [Google Scholar] [CrossRef] [Green Version]
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine collagen as a promising biomaterial for biomedical applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: a source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Green, D.W.; Lai, W.F.; Jung, H.S. Evolving marine biomimetics for regenerative dentistry. Mar. Drugs 2014, 12, 2877–2912. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.H.; Ben-Nissan, B. Marine-Derived Biomaterials for Tissue Engineering Applications; Springer: Singapore, 2019; ISBN 9789811388552. [Google Scholar]
- Wysokowski, M.; Motylenko, M.; Beyer, J.; Makarova, A.; Stöcker, H.; Walter, J.; Galli, R.; Kaiser, S.; Vyalikh, D.; Bazhenov, V.V.; et al. Extreme biomimetic approach for developing novel chitin-GeO2 nanocomposites with photoluminescent properties. Nano Res. 2015, 8, 2288–2301. [Google Scholar] [CrossRef]
- Zdarta, J.; Antecka, K.; Frankowski, R.; Zgoła-Grześkowiak, A.; Ehrlich, H.; Jesionowski, T. The effect of operational parameters on the biodegradation of bisphenols by Trametes versicolor laccase immobilized on Hippospongia communis spongin scaffolds. Sci. Total Environ. 2018, 615, 784–795. [Google Scholar] [CrossRef]
- Petrenko, I.; Summers, A.P.; Simon, P.; Zółtowska-Aksamitowska, S.; Motylenko, M.; Schimpf, C.; Rafaja, D.; Roth, F.; Kummer, K.; Brendler, E.; et al. Extreme biomimetics: Preservation of molecular detail in centimeter-scale samples of biological meshes laid down by sponges. Sci. Adv. 2019, 5, eaax2805. [Google Scholar] [CrossRef] [Green Version]
- Kovalchuk, V.; Voronkina, A.; Binnewerg, B.; Schubert, M.; Muzychka, L.; Wysokowski, M.; Tsurkan, M.V.; Bechmann, N.; Petrenko, I.; Fursov, A.; et al. Naturally drug-loaded chitin: isolation and applications. Mar. Drugs 2019, 17, 574. [Google Scholar] [CrossRef] [Green Version]
- Schubert, M.; Binnewerg, B.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; Bechmann, N.; et al. Naturally prefabricated marine biomaterials: isolation and applications of flat chitinous 3D scaffolds from Ianthella labyrinthus (Demospongiae: Verongiida). Int. J. Mol. Sci. 2019, 20, 5105. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, H. Marine Biological Materials of Invertebrate Origin; Biologically-Inspired Systems; Springer International Publishing: Cham, Switzerland, 2019; Volume 13, ISBN 978-3-319-92482-3. [Google Scholar]
- Green, D.; Howard, D.; Yang, X.; Kelly, M.; Oreffo, R.O.C. Natural marine sponge fiber skeleton: A biomimetic scaffold for human osteoprogenitor cell attachment, growth, and differentiation. Tissue Eng. 2003, 9, 1159–1166. [Google Scholar] [CrossRef]
- Green, D.W. Tissue bionics: examples in biomimetic tissue engineering. Biomed. Mater. 2008, 3, 034010. [Google Scholar] [CrossRef]
- Jesionowski, T.; Norman, M.; Żółtowska-Aksamitowska, S.; Petrenko, I.; Joseph, Y.; Ehrlich, H. Marine Spongin: Naturally Prefabricated 3D Scaffold-Based Biomaterial. Mar. Drugs 2018, 16, 88. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, H.; Shaala, L.A.; Youssef, D.T.A.; Żółtowska- Aksamitowska, S.; Tsurkan, M.; Galli, R.; Meissner, H.; Wysokowski, M.; Petrenko, I.; Tabachnick, K.R.; et al. Discovery of chitin in skeletons of non-verongiid Red Sea demosponges. PLoS One 2018, 13, e0195803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehrlich, H.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; Schupp, P.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part I. Isolation and identification of chitin. Int. J. Biol. Macromol. 2010, 47, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Steck, E.; Ilan, M.; Maldonado, M.; Muricy, G.; Bavestrello, G.; Kljajic, Z.; Carballo, J.L.; Schiaparelli, S.; Ereskovsky, A.; et al. Three-dimensional chitin-based scaffolds from Verongida sponges (Demospongiae: Porifera). Part II: Biomimetic potential and applications. Int. J. Biol. Macromol. 2010, 47, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.; Bazhenov, V.V.; Debitus, C.; de Voogd, N.; Galli, R.; Tsurkan, M.V.; Wysokowski, M.; Meissner, H.; Bulut, E.; Kaya, M.; et al. Isolation and identification of chitin from heavy mineralized skeleton of Suberea clavata (Verongida: Demospongiae: Porifera) marine demosponge. Int. J. Biol. Macromol. 2017, 104, 1706–1712. [Google Scholar] [CrossRef]
- Brunner, E.; Ehrlich, H.; Schupp, P.; Hedrich, R.; Hunoldt, S.; Kammer, M.; Machill, S.; Paasch, S.; Bazhenov, V.V.; Kurek, D.V.; et al. Chitin-based scaffolds are an integral part of the skeleton of the marine demosponge Ianthella basta. J. Struct. Biol. 2009, 168, 539–547. [Google Scholar] [CrossRef] [Green Version]
- Wysokowski, M.; Bazhenov, V.V.; Tsurkan, M.V.; Galli, R.; Stelling, A.L.; Stöcker, H.; Kaiser, S.; Niederschlag, E.; Gärtner, G.; Behm, T.; et al. Isolation and identification of chitin in three-dimensional skeleton of Aplysina fistularis marine sponge. Int. J. Biol. Macromol. 2013, 62, 94–100. [Google Scholar] [CrossRef]
- Żółtowska- Aksamitowska, S.; Tsurkan, M.V.; Lim, S.; Meissner, H.; Tabachnick, K.; Shaala, L.A.; Youssef, D.T.A.; Ivanenko, V.N.; Petrenko, I.; Wysokowski, M.; et al. The demosponge Pseudoceratina purpurea as a new source of fibrous chitin. Int. J. Biol. Macromol. 2018, 112, 1021–1028. [Google Scholar] [CrossRef]
- Żółtowska-Aksamitowska, S.; Shaala, L.; Youssef, D.; Elhady, S.; Tsurkan, M.; Petrenko, I.; Wysokowski, M.; Tabachnick, K.; Meissner, H.; Ivanenko, V.; et al. First report on chitin in a non-Verongiid marine demosponge: the Mycale euplectellioides case. Mar. Drugs 2018, 16, 68. [Google Scholar] [CrossRef] [Green Version]
- Shaala, L.; Asfour, H.; Youssef, D.; Żółtowska-Aksamitowska, S.; Wysokowski, M.; Tsurkan, M.; Galli, R.; Meissner, H.; Petrenko, I.; Tabachnick, K.; et al. New source of 3D chitin scaffolds: the Red Sea demosponge Pseudoceratina arabica (Pseudoceratinidae, Verongiida). Mar. Drugs 2019, 17, 92. [Google Scholar] [CrossRef] [Green Version]
- Fromont, J.; Żółtowska-Aksamitowska, S.; Galli, R.; Meissner, H.; Erpenbeck, D.; Vacelet, J.; Diaz, C.; Tsurkan, M.V.; Petrenko, I.; Youssef, D.T.A.; et al. New family and genus of a Dendrilla-like sponge with characters of Verongiida. Part II. Discovery of chitin in the skeleton of Ernstilla lacunosa. Zool. Anz. 2019, 280, 21–29. [Google Scholar] [CrossRef]
- Vacelet, J.; Erpenbeck, D.; Diaz, C.; Ehrlich, H.; Fromont, J. New family and genus for Dendrilla-like sponges with characters of Verongiida. Part I redescription of Dendrilla lacunosa Hentschel 1912, diagnosis of the new family Ernstillidae and Ernstilla n. g. Zool. Anz. 2019, 280, 14–20. [Google Scholar] [CrossRef]
- Binnewerg, B.; Schubert, M.; Voronkina, A.; Muzychka, L.; Wysokowski, M.; Petrenko, I.; Djurović, M.; Kovalchuk, V.; Tsurkan, M.; Martinovic, R.; et al. Marine biomaterials: Biomimetic and pharmacological potential of cultivated Aplysina aerophoba marine demosponge. Mater. Sci. Eng. C 2020, 109, 110566. [Google Scholar] [CrossRef]
- Anitha, A.; Sowmya, S.; Sudheesh, K.P.T.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym.Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Morganti, P.; Del Ciotto, P.; Carezzi, F.; Nunziata, M.L.; Morganti, G. A chitin nanofibril-based non-woven tissue as medical dressing: The role of bionanotechnology. Nanomater Regen Med. 2016, 1, 123–142. [Google Scholar]
- Morganti, P.; Febo, P.; Cardillo, M.; Donnarumma, G.; Baroni, A. Chitin nanofibril and nanolignin: Natural polymers of biomedical interest. J. Clin Cosmet Dermatol 2017, 1, 1–7. [Google Scholar]
- Philibert, T.; Lee, B.H.; Fabien, N. Current status and new perspectives on chitin and chitosan as functional biopolymers. Appl. Biochem. Biotechnol. 2017, 181, 1314–1337. [Google Scholar] [CrossRef] [PubMed]
- Morganti, P.; Stoller, M. Chitin and lignin: Natural Ingredients from waste materials for making innovative and healthy products for humans and plants. Chem. Eng. Trans. 2017, 60, 319–324. [Google Scholar]
- Morganti, P.; Del Ciotto, P.; Carezzi, F.; Morganti, G.; Hong-Duo, C. From waste material a new anti-aging compound: a chitin nanofiber complex. SOFW J. 2012, 138, 30–36. [Google Scholar]
- Morganti, P.; Palombo, M.; Fabrizi, G.; Guarneri, F.; Svolacchia, F.; Cardillo, A.; Del Ciotto, P.; Francesco, C.; Gianluca, M. New insight on anti-aging activity of chitin nanofibril-hyaluronan blocks copolymers entrapping active ingredients: In vitro and in vivo study. J. Appl. Cosmetol. 2013, 31, 1–29. [Google Scholar]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; De los Llano Gandia, M.; Heras Caballer, A. Cosmetics and cosmeceutical applications of chitin, chitosan and their derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Naira, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, M.G.; Karimian, R.; Rakhshaei, R.; Pakdel, F.; Eslami, H.; Fakhrzadeh, V.; Rahimi, M.; Salehi, R.; Kafil, H.S. Chitin/silk fibroin/TiO2 bio-nanocomposite as a biocompatible wound dressing bandage with strong antimicrobial activity. Int. J. Biol. Macromol. 2018, 116, 966–976. [Google Scholar] [CrossRef] [PubMed]
- Balassa, L.L.; Prudden, J.F. Applications of chitin and chitosan in wound-healing acceleration. In Proceedings of the First International Conference on Chitin/Chitosan; Muzzarelli, R.A.A., Pariser, E.R., Eds.; MIT Sea Grant Program: Cambridge, MA, USA, 1978; pp. 296–305. [Google Scholar]
- Ohtsuki, K.; Ohnishi, M.; Nakamura, Y.; Kurokawa, E. Clinical application of “Chitin”, artificial skin material in oral mucous defects. Jpn. J. Oral Surg. 1990, 36, 2103–2110. [Google Scholar]
- Kifune, K. Clinical application of chitin artificial skin (Beschitin W). In Advances in Chitin and Chitosan; Springer: Amsterdam, The Netherlands, 1992; pp. 9–15. [Google Scholar]
- Hirota, Y.; Tanioka, S.; Tanigawa, T.; Tanaka, Y.; Ojima, R. Clinical applications of chitin and chitosan to human decubitus. In Advances in Chitin Science, 1st ed.; Domard, A., Jeuniaux, C., Muzzarelli, R., Roberts, G., Eds.; Jacques Andre Publisher: Lyon, France, 1996; Volume 2, pp. 407–413. [Google Scholar]
- Minami, S.; Okamoto, Y.; Shigemasa, Y. Intensive skin activation in veterinary clinical application; how to utilize the natural resources chitin and chitosan ZAIKAI Spec. Issue 1998, 93–97. (In Japanese) [Google Scholar]
- Gorovoj, L.; Seniouk, O.; Beketova, G.; Savichuk, N.; Amanbaeva, G. Use of the chitin-containing preparation Mycoton in pediatric gastroenterology. In Chitosan per os: From Dietary Supplement to Drug Carrier; Muzzarelli, R.A.A., Ed.; Atec: Grottammare, Italy, 2000; Volume 5, pp. 201–219. [Google Scholar]
- Fang, Y.; Hu, Y.; Wang, Z.; Zhou, W.; Yan, L.; Fan, X.; Liu, H. 3D Porous Chitin Sponge with High Absorbency, Rapid Shape Recovery, and Excellent Antibacterial Activities for Noncompressible Wound. Chem. Eng. J. 2020, 388, 124169. [Google Scholar] [CrossRef]
- Ehrlich, H. Biomimetic potential of chitin–based composite biomaterials of poriferan origin. In Biomimetic Biomaterials: Structure and Applications; Ruys, A.J., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 47–67. [Google Scholar]
- Su, C.H.; Sun, C.S.; Juan, S.W.; Hu, C.H.; Ke, W.T.; Sheu, M.T. Fungal mycelia as the source of chitin and polysaccharides and their applications as skin substitutes. Biomaterials 1997, 18, 1169–1174. [Google Scholar] [CrossRef]
- Rahman, M.A.; Halfar, J. First evidence of chitin in calcified coralline algae: New insights into the calcification process of Clathromorphum compactum. Sci. Rep. 2014, 4, 6162. [Google Scholar] [CrossRef]
- Connors, M.J.; Ehrlich, H.; Hog, M.; Godeffroy, C.; Araya, S.; Kallai, I.; Gazit, D.; Boyce, M.; Ortiz, C. Three-dimensional structure of the shell plate assembly of the chiton Tonicella marmorea and its biomechanical consequences. J. Struct. Biol. 2012, 177, 314–328. [Google Scholar] [CrossRef]
- Duan, B.; Huang, Y.; Lu, A.; Zhang, L. Recent advances in chitin based materials constructed via physical methods. Prog. Polym. Sci. 2018, 82, 1–33. [Google Scholar] [CrossRef]
- Merzendorfer, H. The cellular basis of chitin synthesis in fungi and insects: Common principles and differences. Eur. J. Cell Biol. 2011, 90, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Machałowski, T.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Bechmann, N.; Binnewerg, B.; Schubert, M.; Guan, K.; Bornstein, S.R.; Czaczyk, K.; Pokrovsky, O.; et al. Spider Chitin. The biomimetic potential and applications of Caribena versicolor tubular chitin. Carbohydr. Polym. 2019, 226, 115301. [Google Scholar] [CrossRef] [PubMed]
- Machałowski, T.; Wysokowski, M.; Tsurkan, M.V.; Galli, R.; Schimpf, C.; Rafaja, D.; Brendler, E.; Viehweger, C.; Żółtowska-Aksamitowska, S.; Petrenko, I.; et al. Spider chitin: an ultrafast microwave-assisted method for chitin isolation from Caribena versicolor spider molt cuticle. Molecules 2019, 24, 3736. [Google Scholar] [CrossRef] [Green Version]
- Rohde, S.; Schupp, P.J. Growth and regeneration of the elephant ear sponge Ianthella basta (Porifera). In Ancient Animals, New Challenges; Springer: Amsterdam, The Netherlands, 2011; pp. 219–226. [Google Scholar]
- Verdenal, B.; Vacelet, J. Sponge culture on vertical ropes in the northwestern Mediterranean Sea. In New Perspect. Sponge Biology; Rützler, K., Ed.; Smithsonian Institution Press: Washington, WA, USA, 1990; pp. 416–424. [Google Scholar]
- Pronzato, R.; Bavestrello, G.; Cerrano, C.; Magnino, G.; Manconi, R.; Pantelis, J.; Sarà, A.; Sidri, M. Sponge farming in the Mediterranean Sea: New perspectives. Mem. Queensl. Museum 1999, 44, 485–491. [Google Scholar]
- Hausmann, R.; Vitello, M.P.; Leitermann, F.; Syldatk, C. Advances in the production of sponge biomass Aplysina aerophoba- a model sponge for ex situ sponge biomass production. J. Biotechnol. 2006, 124, 117–127. [Google Scholar] [CrossRef]
- Klöppel, A.; Pfannkunchen, M.; Putz, A.; Proksch, P.; Brümmer, F. Ex situ cultivation of Aplysina aerophoba close to in situ conditions: ecological, biochemical and histological aspects. Mar. Ecol. 2008, 29, 259–272. [Google Scholar]
- Ehrlich, H.; Maldonado, M.; Parker, A.R.; Kulchin, Y.N.; Schilling, J.; Köhler, B.; Skrzypczak, U.; Simon, P.; Reiswig, H.M.; Tsurkan, M.V.; et al. Supercontinuum generation in naturally occurring glass sponges spicules. Adv. Opt. Mater. 2016, 4, 1608–1613. [Google Scholar] [CrossRef] [Green Version]
- Ehrlich, H.; Maldonado, M.; Spindler, K.D.; Eckert, C.; Hanke, T.; Born, R.; Goebel, C.; Simon, P.; Heinemann, S.; Worch, H. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part, I. Verongidae (demospongia: Porifera). J. Exp. Zool. B Mol. Dev. Evol. 2007, 308B, 347–356. [Google Scholar] [CrossRef]
- Ehrlich, H.; Krautter, M.; Hanke, T.; Simon, P.; Knieb, C.; Heinemann, S.; Worch, H. First evidence of the presence of chitin in skeletons of marine sponges. Part II. Glass sponges (Hexactinellida: Porifera). J. Exp. Zool. 2007, 308B, 473–483. [Google Scholar] [CrossRef]
- Steck, E.; Burkhardt, M.; Ehrlich, H.; Richter, W. Discrimination between cells of murine and human origin in xenotransplants by species specific genomic in situ hybridization. Xenotransplantation 2010, 17, 153–159. [Google Scholar] [CrossRef]
- Ehrlich, H. Chapter 26: Chitin of Poriferan Origin as a Unique Biological Material. In Blue Biotechnol. Prod. Use Mar. Mol.; Bates, S.S., La Barre, S., Eds.; Wiley: Weinheim, Germany, 2019; Volume 1, pp. 821–854. [Google Scholar]
- Taylor, D.A.; Sampaio, L.C.; Ferdous, Z.; Gobin, A.S.; Taite, L.J. Decellularized matrices in regenerative medicine. Acta Biomater. 2018, 74, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Rogulska, O.Y.; Mutsenko, V.V.; Revenko, E.B.; Petrenko, Y.A.; Ehrlich, H.; Petrenko, A.Y. Culture and differentiation of human adipose tissue mesenchymal stromal cells within carriers based on sea sponge chitin skeletons. Probl. Cryobiol. Cryomedicine 2013, 23, 267–270. [Google Scholar]
- Rogulska, O.Y.; Revenko, O.B.; Petrenko, Y.O.; Ehrlich, H.; Petrenko, O.Y. Prospects for the application of Aplysinidae family marine sponge skeletons and mesenchymal stromal cells in tissue engineering. Biotechnologia Acta 2013, 6, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Mutsenko, V.V.; Bazhenov, V.V.; Rogulska, O.; Tarusin, D.N.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; Meissner, H.; Lode, A.; et al. 3D chitinous scaffolds derived from cultivated marine demosponge Aplysina aerophoba for tissue engineering approaches based on human mesenchymal stromal cells. Int. J. Biol Macromol. 2017, 104, 1966–1974. [Google Scholar] [CrossRef]
- Mutsenko, V.V.; Gryshkov, O.; Lauterboeck, L.; Rogulska, O.; Tarusin, D.N.; Bazhenov, V.V.; Schütz, K.; Brüggemeier, S.; Gossla, E.; Akkineni, A.R.; et al. Novel chitin scaffolds derived from marine sponge Ianthella basta for tissue engineering approaches based on human mesenchymal stromal cells: Biocompatibility and cryopreservation. Int. J. Biol. Macromol. 2017, 104, 1955–1965. [Google Scholar] [CrossRef]
- Mutsenko, V.; Gryshkov, O.; Rogulska, O.; Lode, A.; Petrenko, A.Y.; Gelinsky, M.; Glasmacher, B.; Ehrlich, H. Chitinous scaffolds from marine sponges for tissue engineering. In Marine-Derived Biomaterials for Tissue Engineering Applications; Choi, A., Ben-Nissan, B., Eds.; Springer Series in Biomaterials Science and Engineering; Springer: Singapore, 2019; Volume 14, pp. 285–307. [Google Scholar]
- Wagge, L.E. The activity of amoebocytes and of alkaline phosphatases during the regeneration of the shell in the snail, Helix aspersa. Q. J. Microsc. Sci. 1951, 92, 307–320. [Google Scholar]
- Abolins-Krogis, A. Ultrastructural study of the shell-repair membrane in the snail Helix pomatia L. Cell Tissue Res. 1976, 172, 455–476. [Google Scholar] [CrossRef]
- Mount, A.S.; Wheeler, A.P.; Paradkar, R.P.; Snider, D. Hemocyte-mediated shell mineralization in the eastern oyster. Science 2004, 304, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Chan, V.B.S.; Johnstone, M.B.; Wheeler, A.P.; Mount, A.S. Chitin facilitated mineralization in the Eastern oyster. Front. Mar. Sci. 2018, 5, 347. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Liu, Y.; Liu, C.; Huang, J.; Zheng, G.; Xie, L.; Zhang, R. Hemocytes participate in calcium carbonate crystal formation, transportation Pinctada fucata. Fish. Shellfish Immunol. 2016, 51, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Mount, A.S.; Gohad, N.V.; Hansen, D.C.; Muller, K.; Johnstone, M.B. Deposition of Nanocrystalline Calcite on Surfaces by A Tissue and Cellular Biomineralization. U.S. Patent US8541031B2, 24 September 2013. [Google Scholar]
- Mount, A.S.; Gohad, N.V.; Hansen, D.C.; Hansen, K.M.; Johnstone, M.B. Deposition of nanocrystalline calcite on surfaces by a tissue and cellular biomineralization. U.S. Patent US009371451B2, 21 June 2016. [Google Scholar]
- Johnstone, M.B.; Gohad, N.V.; Falwell, E.P.; Hansen, D.C.; Hansen, K.M.; Mount, A.S. Cellular orchestrated biomineralization of crystalline composites on implant surfaces by the eastern oyster, Crassostrea virginica (Gmelin, 1791). J. Exp. Mar. Bio. Ecol. 2015, 463, 8–16. [Google Scholar] [CrossRef]
- Szkucik, K.; Ziomek, M.; Paszkiewicz, W.; Drozd, Ł.; Gondek, M.; Knysz, P. Fatty acid profile in fat obtained from edible part of land snails harvested in Poland. J. Vet. Res. 2018, 62, 519–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biradar, S.; Ravichandran, P.; Gopikrishnan, R.; Goornavar, V.; Hall, J.C.; Ramesh, V.; Baluchamy, S.; Jeffers, R.B.; Ramesh, G.T. Calcium carbonate nanoparticles: Synthesis, characterization and biocompatibility. J. Nanosci. Nanotechnol. 2011, 11, 6868–6874. [Google Scholar] [CrossRef] [PubMed]
- Poologasundarampillai, G.; Boix-Alberich, M.; Clarke, D.; Smith, A.; Martin, R.; Lee, P.D.; Jones, J.R. Hydroxyapatite or calcite: How does physiological proteins influence the type, mechanism and kinetics of their formation on bioactive glasses? Front. Bioeng. Biotechnol. 2016, 4. [Google Scholar] [CrossRef]
- Bahrom, H.; Goncharenko, A.A.; Fatkhutdinova, L.I.; Peltek, O.O.; Muslimov, A.R.; Koval, O.Y.; Eliseev, I.E.; Manchev, A.; Gorin, D.; Shishkin, I.I.; et al. Controllable Synthesis of Calcium Carbonate with Different Geometry: Comprehensive Analysis of Particle Formation, Cellular Uptake, and Biocompatibility. ACS Sustain. Chem. Eng. 2019, 7, 19142–19156. [Google Scholar] [CrossRef]
- Fujita, Y.; Yamamuro, T.; Nakamura, T.; Kotani, S.; Ohtsuki, C.; Kokubo, T. The bonding behavior of calcite to bone. J. Biomed. Mater. Res. 1991, 25, 991–1003. [Google Scholar] [CrossRef]
- Geblinger, D.; Geiger, B.; Addadi, L. Surface-induced regulation of podosome organization and dynamics in cultured osteoclasts. ChemBioChem 2009, 10, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Geblinger, D.; Addadi, L.; Geiger, B. Nano-topography sensing by osteoclasts. J. Cell Sci. 2010, 123, 1503–1510. [Google Scholar] [CrossRef] [Green Version]
- Lopez, E.; Vidal, B.; Berland, S.; Camprasse, S.; Camprasse, G.; Silve, C. Demonstration of the capacity of nacre to induce bone formation by human osteoblasts maintained in vitro. Tissue Cell 1992, 24, 667–679. [Google Scholar] [CrossRef]
- Fricain, J.C.; Bareille, R.; Ulysse, F.; Dupuy, B.; Amedee, J. Evaluation of proliferation and protein expression of human bone marrow cells cultured on coral crystallized in the aragonite of calcite form. J. Biomed. Mater. Res. 1998, 42, 96–102. [Google Scholar] [CrossRef]
- Kreklau, B.; Sittinger, M.; Mensing, M.B.; Voigt, C.; Berger, G.; Burmester, G.R.; Rahmanzadeh, R.; Gross, U. Tissue engineering of biphasic joint cartilage transplants. Biomaterials 1999, 20, 1743–1749. [Google Scholar] [CrossRef]
- Demers, C.; Hamdy, C.R.; Corsi, K.; Chellat, F.; Tabrizian, M.; Yahia, L. Natural coral exoskeleton as a bone graft substitute: a review. Biomed. Mater. Eng. 2002, 12, 15–35. [Google Scholar] [PubMed]
- Michalowski, S.; Jaegermann, Z.; Karas, J. Properties of calcite materials for cell culture scaffolds. EngBiomater. 2004, 38–42, 94–96. [Google Scholar]
- Rocha, J.H.; Lemos, A.F.; Agathopoulos, S.; Valerio, P.; Kannan, S.; Oktar, F.N.; Ferreira, J.M. Scaffolds for bone restoration from cuttlefish. Bone 2005, 37, 850–857. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, T.; Zhou, Y.; Zhang, Z.; Wang, Z.J.; Tong, H.; Shen, X.Y.; Wang, Y.N. Evaluation of the attachment, proliferation, and differentiation of osteoblast on a calcium carbonate coating on titanium surface. Mater. Sci. Eng. C. 2011, 31, 1055–1061. [Google Scholar] [CrossRef]
- Monchau, F.; Hivart, P.; Genestie, B.; Chai, F.; Descamps, M.; Hildebrand, H.F. Calcite as a bone substitute. Comparison with hydroxyapatite and tricalcium phosphate with regard to the osteoblastic activity. Mater. Sci. Eng. C 2013, 33, 490–498. [Google Scholar] [CrossRef]
- Kamba, A.S.; Zakaria, Z.A.B. Osteoblasts growth behaviour on bio-based calcium carbonate aragonite nanocrystal. BioMed Res. Int. 2014, 2014, 215097. [Google Scholar]
- Tolba, E.; Müller, W.E.G.; Abd El-Hady, B.M.; Neufurth, M.; Wurm, F.; Wang, S.; Schröder, H.C.; Wang, X. High biocompatibility and improved osteogenic potential of amorphous calcium carbonate/vaterite. J. Mater. Chem. B 2016, 4, 376–386. [Google Scholar] [CrossRef] [Green Version]
- Chróścicka, A.; Jaegermann, Z.; Wychowański, P.; Ratajska, A.; Sadło, J.; Hoser, G.; Michałowski, S.; Lewandowska-Szumiel, M. Synthetic Calcite as a Scaffold for Osteoinductive Bone Substitutes. Ann. Biomed. Eng. 2016, 44, 2145–2157. [Google Scholar] [CrossRef] [Green Version]
- Woldetsadik, A.D.; Sharma, S.K.; Khapli, S.; Jagannathan, R.; Magzoub, M. Hierarchically Porous Calcium Carbonate Scaffolds for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2017, 3, 2457–2469. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Łapa, A.; Samal, S.K.; Declercq, H.A.; Schaubroeck, D.; Mendes, A.C.L.; Van der Voort, P.; Dokupil, A.; Plis, A.; De Schamphelaere, K.; et al. Enzymatic, urease-mediated mineralization of gellan gum hydrogel with calcium carbonate, magnesium-enriched calcium carbonate and magnesium carbonate for bone regeneration applications. J. Tiss. Eng. Regen. Med. 2017, 11, 3556–3566. [Google Scholar] [CrossRef] [PubMed]
- Higgin, T. On a new sponge of the genus Luffaria, from Yucatan, in the Liverpool Free Museum. Ann. Mag. Nat. Hist. 1875, 16, 223–227. [Google Scholar] [CrossRef]
- Villamizar, E.; Laughlin, R.A. Fauna Associated with the Sponges Aplysina archeri and Aplysina lacunosa in a Coral Reef of the Archipiélago de Los Roques, National Park, Venezuela. In Fossil and Recent Sponges; Springer: Berlin, Germany, 1991; pp. 522–542. [Google Scholar]
- Humann, P.; DeLoach, N. Reef Creature Identification: Florida, Caribbean, Bahamas; New World Publications: Jacksonville, FL, USA, 2002; p. 420. [Google Scholar]
- Alcolado, P.M.; Busutil, L. Inventaire des spongiaires néritiques du Parc National de La Guadeloupe. Serie Oceanológica 2012, 10, 62–76. [Google Scholar]
- Yoshino, T.P.; Bickham, U.; Bayne, C.J. Molluscan cells in culture: Primary cell cultures and cell lines. Can. J. Zool. 2013, 91, 391–404. [Google Scholar] [CrossRef]
- Grandiosa, R.; Mérien, F.; Pillay, K.; Alfaro, A. Innovative application of classic and newer techniques for the characterization of haemocytes in the New Zealand black-footed abalone (Haliotis iris). Fish. Shellfish Immunol. 2016, 48, 175–184. [Google Scholar] [CrossRef]
- Fernández, M.S.; Valenzuela, F.; Arias, J.I.; Neira-Carrillo, A.; Arias, J.L. Is the snail shell repair process really influenced by eggshell membrane as a template of foreign scaffold? J. Struct. Biol. 2016, 196, 187–196. [Google Scholar] [CrossRef]
- Huang, J.; Li, S.; Liu, Y.; Liu, C.; Xie, L.; Zhang, R. Hemocytes in the extrapallial space of Pinctada fucata are involved in immunity and biomineralization. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef]
- Modreski, P.J.; Aumente-Modreski, R. Fluorescent minerals, a review. Rocks Miner. Mag. 1996, 71, 14–22. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [Green Version]
- Dement, J. Handbook of Fluorescent Gems and Minerals—An Exposition and Catalog of the Fluorescent and Phosphorescent Gems and Minerals, Including the Use of Ultraviolet Light in the Earth Sciences, 2nd ed.; Brunauer Press: Oxford, UK, 2013. [Google Scholar]
- Reig, F.B.; Adelantado, J.V.G.; Moya Moreno, M.C.M. FTIR quantitative analysis of calcium carbonate (calcite) and silica (quartz) mixtures using the constant ratio method. Application to geological samples. Talanta 2002, 58, 811–821. [Google Scholar] [CrossRef]
- Coleyshaw, E.E.; Crump, G.; Griffith, W.P. Vibrational spectra of the hydrated carbonate minerals ikaite, monohydrocalcite, lansfordite and nesquehonite. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2003, 59, 2231–2239. [Google Scholar] [CrossRef]
- Prinsloo, L.C. Rock hyraces: A cause of San rock art deterioration? J. Raman Spectrosc. 2007, 38, 496–503. [Google Scholar] [CrossRef]
- Señorale-Pose, M.; Chalar, C.; Dauphin, Y.; Massard, P.; Pradel, P.; Marín, M. Monohydrocalcite in calcareous corpuscles of Mesocestoides corti. Exp. Parasitol. 2008, 118, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Iordanidis, A.; Garcia-Guinea, J.; Strati, A.; Gkimourtzina, A.; Papoulidou, A. Thermal, mineralogical and spectroscopic study of plasters from three post-Byzantine churches from Kastoria (northern Greece). J. Therm. Anal. Calorim. 2011, 103, 577–586. [Google Scholar] [CrossRef]
- Singh, N.; Koziol, K.K.K.; Chen, J.; Patil, A.J.; Gilman, J.W.; Trulove, P.C.; Kafienah, W.; Rahatekar, S.S. Ionic liquids-based processing of electrically conducting chitin nanocomposite scaffolds for stem cell growth. Green Chem. 2013, 15, 1192–1202. [Google Scholar] [CrossRef] [Green Version]
- Wehrmeister, U.; Jacob, D.E.; Soldati, A.L.; Häger, T.; Hofmeister, W. Vaterite in freshwater cultured pearls from China and Japan. J. Gemmol. 2007, 30, 399–412. [Google Scholar] [CrossRef]
- Bischoff, W.D.; Sharma, S.K.; MacKenzie, F.T. Carbonate ion disorder in synthetic and biogenic magnesian calcites: a Raman spectral study. Am. Mineral. 1985, 70, 581–589. [Google Scholar]
- Kadokawa, J.I. Fabrication of nanostructured and microstructured chitin materials through gelation with suitable dispersion media. RSC Adv. 2015, 5, 12736–12746. [Google Scholar] [CrossRef]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications. Green Chem. 2017, 19, 1208–1220. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.A. Biomedical exploitation of chitin and chitosan via mechano-chemical disassembly, electrospinning, dissolution in imidazolium ionic liquids, and supercritical drying. Mar. Drugs 2011, 9, 1510–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzzarelli, R.A.A.; El Mehtedi, M.; Mattioli-Belmonte, M. Emerging biomedical applications of nano-chitins and nano-chitosans obtained via advanced eco-friendly technologies from marine resources. Mar. Drugs 2014, 12, 5468–5502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coltelli, M.B.; Gigante, V.; Panariello, L.; Morganti, P.; Cinelli, P.; Danti, S.; Lazzeri, A. Chitin nanofibrils in renewable materials for packaging and personal care applications. Adv. Mater. Lett. 2018, 10, 425–430. [Google Scholar]
- Coltelli, M.-B.; Cinelli, P.; Gigante, V.; Aliotta, L.; Morganti, P.; Panariello, L.; Lazzeri, A. Chitin Nanofibrils in Poly (Lactic Acid) (PLA) Nanocomposites: Dispersion and Thermo-Mechanical Properties. Int. J. Mol. Sci. 2019, 20, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [Green Version]
- Chocholata, P.; Kulda, V.; Babuska, V. Fabrication of scaffolds for bone-tissue regeneration. Materials 2019, 12, 568. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.C. A Classification of Molluscan Hemocytes Based on Functional Evidences. In Invertebrate Blood; Springer: New York, NY, USA, 1984; pp. 111–146. [Google Scholar]
- Cheng, T.C. Functional morphology and biochemistry of molluscan phagocytes. Ann. N. Y. Acad. Sci. 1975, 266, 343–379. [Google Scholar] [CrossRef]
- Cheng, T.C.; Guida, V.G. Hemocytes of Bulinus truncatus rohlfsi (Mollusca: Gastropoda). J. Invertebr. Pathol. 1980, 35, 158–167. [Google Scholar] [CrossRef]
- Adema, C.M.; Harris, R.A.; van Deutekom-Mulder, E.C. A comparative study of hemocytes from six different snails. J. Invertabrates Pathol. 1992, 59, 24. [Google Scholar] [CrossRef]
- Adamowicz, A.; Bolaczek, M. Blood cells morphology of the snail Helix aspersa maxima (Helicidae). Zool. Pol. 2003, 48, 93–101. [Google Scholar]
- Bayne, C.J.; Moore, M.N.; Carefoot, T.H.; Thompson, R.J. Hemolymph functions in Mytilus californianus: The cytochemistry of hemocytes and their responses to foreign implants and hemolymph factors in phagocytosis. J. Invertebr. Pathol. 1979, 34, 1–20. [Google Scholar] [CrossRef]
- Pila, E.A.; Sullivan, J.T.; Wu, X.Z.; Fang, J.; Rudko, S.P.; Gordy, M.A.; Hanington, P.C. Haematopoiesis in molluscs: a review of haemocyte development and function in gastropods, cephalopods and bivalves. Dev. Comp. Immunol. 2016, 58, 119–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sminia, T.; Van der Knaap, W.P.W.; Van Asselt, L.A. Blood cell types and blood cell formation in gastropod molluscs. Dev. Comp. Immunol. 1983, 7, 665–668. [Google Scholar] [CrossRef]
- Sminia, T.; Barendsen, L. A comparative morphological and enzyme histochemical study on blood cells of the freshwater snails Lymnae astagnalis, Biomphalaria glabrata, and Bulinus truncatus. J. Morphol. 1980, 165, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk shell formation: a source of new concepts for understanding biomineralization processes. Chem. - A Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Fan, G.; Jiao, Y.; Zhang, H.; Guo, X.; Huang, R.; Zheng, Z.; Bian, C.; Deng, Y.; Wang, Q.; et al. The pearl oyster Pinctada fucata martensii genome and multi-omic analyses provide insights into biomineralization. Gigascience 2017, 6, 1–12. [Google Scholar] [CrossRef]
- Abolins-Krogis, A. Fluorescence and histochemical studies of the calcification-initiating lipofuscin type pigment granules in the shell-repair membrane of the snail, Helix pomatia L. Zeitschrift fur Zellforsch. und mikroskopische Anat. 1973, 142, 205–221. [Google Scholar] [CrossRef]
- Xiang, L.; Kong, W.; Su, J.; Liang, J.; Zhang, G.; Xie, L.; Zhang, R. Amorphous calcium carbonate precipitation by cellular biomineralization in mantle cell cultures of Pinctada fucata. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Kapur, S.P.; Sen Gupta, A. The role of amoebocytes in the regeneration of shell in the land pulmonate, Euplecta indica (Pfieffer). Biol. Bull. 1970, 139, 502–509. [Google Scholar] [CrossRef]
- Abolins-Krogis, A. Shell regeneration in Helix pomatia with special reference to the elementary calcifying particles. Symp. Zool. Soc. London 1968, 22, 75–92. [Google Scholar]
- Abolins-Krogis, A. The histochemistry of the mantle of Helix pomatia (L.) in relation to the repair of the damaged shell. Ark. för Zool. 1963, 15, 461–474. [Google Scholar]
- Munro, N.H.; McGrath, K.M. Biomimetic approach to forming chitin/aragonite composites. Chem. Commun. 2012, 48, 4716–4718. [Google Scholar] [CrossRef] [PubMed]
- Falini, G.; Fermani, S.; Ripamonti, A. Crystallization of calcium carbonate salts into beta-chitin scaffold. J. Inorg. Biochem. 2002, 91, 475–480. [Google Scholar] [CrossRef]
- Sommerdijk, N.A.J.M.; With, G. de Biomimetic CaCO3 Mineralization using Designer Molecules and Interfaces. Chem. Rev. 2008, 108, 4499–4550. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tijssen, K.C.H.; Bomans, P.H.H.; Akiva, A.; Friedrich, H.; Kentgens, A.P.M.; Sommerdijk, N.A.J.M. Microscopic structure of the polymer-induced liquid precursor for calcium carbonate. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayano, K.; Saruwatari, K.; Kogure, T.; Shiraiwa, Y. Effect of Coccolith Polysaccharides Isolated from the Coccolithophorid, Emiliania huxleyi, on Calcite Crystal Formation in in vitro CaCO3 Crystallization. Mar. Biotechnol. 2011, 13, 83–92. [Google Scholar] [CrossRef]
- Matsumura, S.; Kajiyama, S.; Nishimura, T.; Kato, T. Formation of Helically Structured Chitin/CaCO3 Hybrids through an Approach Inspired by the Biomineralization Processes of Crustacean Cuticles. Small 2015, 11, 5127–5133. [Google Scholar] [CrossRef]
- Gal, A.; Wirth, R.; Barkay, Z.; Eliaz, N.; Scheffel, A.; Faivre, D. Templated and self-limiting calcite formation directed by coccolith organic macromolecules. Chem. Commun. 2017, 53, 7740–7743. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.F.; Lu, Z.; Zhang, Z.; Xiao, C.; Li, M.; Huang, Y.X.; Liu, X.Y.; Jiang, Y. Correlations of crystal shape and lateral orientation in bioinspired CaCO3 mineralization. CrystEngComm 2018, 20, 5241–5248. [Google Scholar] [CrossRef]
- Kruppke, B.; Farack, J.; Weil, S.; Aflalo, E.D.; Poláková, D.; Sagi, A.; Hanke, T. Crayfish hemocyanin on chitin bone substitute scaffolds promotes the proliferation and osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. 2019, 108, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Astachov, L.; Nevo, Z.; Vago, R. Calcite Biohybrids as Microenvironment for Stem Cells. Polymers 2012, 4, 1065–1083. [Google Scholar] [CrossRef]
- Ziętek, J.; Guz, L.; Panasiuk, K.; Winiarczyk, S.; Adaszek, Ł. Nowa metoda przyżyciowego pobierania hemolimfy wraz z ustaleniem norm fizjologicznych wybranych parametrów biochemicznych hemolimfy ślimaków Cornu aspersum. Med. Weter. 2017, 73, 366–369. [Google Scholar]
- Saleuddin, A.S.M.; Chan, W. Shell regeneration in Helix: shell matrix composition and crystal formation. Can. J. Zool. 1969, 47, 1107–1111. [Google Scholar] [CrossRef]
- Abolins-Krogis, A. The morphological and chemical characteristics of organic crystals in the regenerating shell of Helix pomatia L. Acta Zool. 1958, 39, 19–38. [Google Scholar] [CrossRef]
- De Waele, A. Le sang d’Anodonta cygnea et la formation de la coquille. Bull. l’Académie R. des Sci. Belgique Cl. des Sci. 1930, 10, 1–52. [Google Scholar]
- Lebel, J.-M.; Giard, W.; Favrel, P.; Boucaud-Camou, E. Effects of different vertebrate growth factors on primary cultures of hemocytes from the gastropod mollusc, Haliotis tuberculata. Biol Cell 1996, 86, 67–72. [Google Scholar] [CrossRef]
- Donaghy, L.; Hong, H.K.; Lambert, C.; Park, H.S.; Shim, W.J.; Choi, K.S. First characterisation of the populations and immune-related activities of hemocytes from two edible gastropod species, the disk abalone, Haliotis discus discus and the spiny top shell, Turbo. cornutus. Fish. Shellfish Immunol. 2010, 28, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; He, P.; Wu, Y.; Zhang, Y.; Xia, H.; Zheng, Y.; Han, Y. Stimulatory effects of the degradation products from Mg-Ca-Sr alloy on the osteogenesis through regulating ERK signaling pathway. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]













© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysokowski, M.; Machałowski, T.; Petrenko, I.; Schimpf, C.; Rafaja, D.; Galli, R.; Ziętek, J.; Pantović, S.; Voronkina, A.; Kovalchuk, V.; et al. 3D Chitin Scaffolds of Marine Demosponge Origin for Biomimetic Mollusk Hemolymph-Associated Biomineralization Ex-Vivo. Mar. Drugs 2020, 18, 123. https://doi.org/10.3390/md18020123
Wysokowski M, Machałowski T, Petrenko I, Schimpf C, Rafaja D, Galli R, Ziętek J, Pantović S, Voronkina A, Kovalchuk V, et al. 3D Chitin Scaffolds of Marine Demosponge Origin for Biomimetic Mollusk Hemolymph-Associated Biomineralization Ex-Vivo. Marine Drugs. 2020; 18(2):123. https://doi.org/10.3390/md18020123
Chicago/Turabian StyleWysokowski, Marcin, Tomasz Machałowski, Iaroslav Petrenko, Christian Schimpf, David Rafaja, Roberta Galli, Jerzy Ziętek, Snežana Pantović, Alona Voronkina, Valentine Kovalchuk, and et al. 2020. "3D Chitin Scaffolds of Marine Demosponge Origin for Biomimetic Mollusk Hemolymph-Associated Biomineralization Ex-Vivo" Marine Drugs 18, no. 2: 123. https://doi.org/10.3390/md18020123
APA StyleWysokowski, M., Machałowski, T., Petrenko, I., Schimpf, C., Rafaja, D., Galli, R., Ziętek, J., Pantović, S., Voronkina, A., Kovalchuk, V., Ivanenko, V. N., Hoeksema, B. W., Diaz, C., Khrunyk, Y., Stelling, A. L., Giovine, M., Jesionowski, T., & Ehrlich, H. (2020). 3D Chitin Scaffolds of Marine Demosponge Origin for Biomimetic Mollusk Hemolymph-Associated Biomineralization Ex-Vivo. Marine Drugs, 18(2), 123. https://doi.org/10.3390/md18020123











