Algae-Derived Bioactive Compounds with Anti-Lung Cancer Potential
Abstract
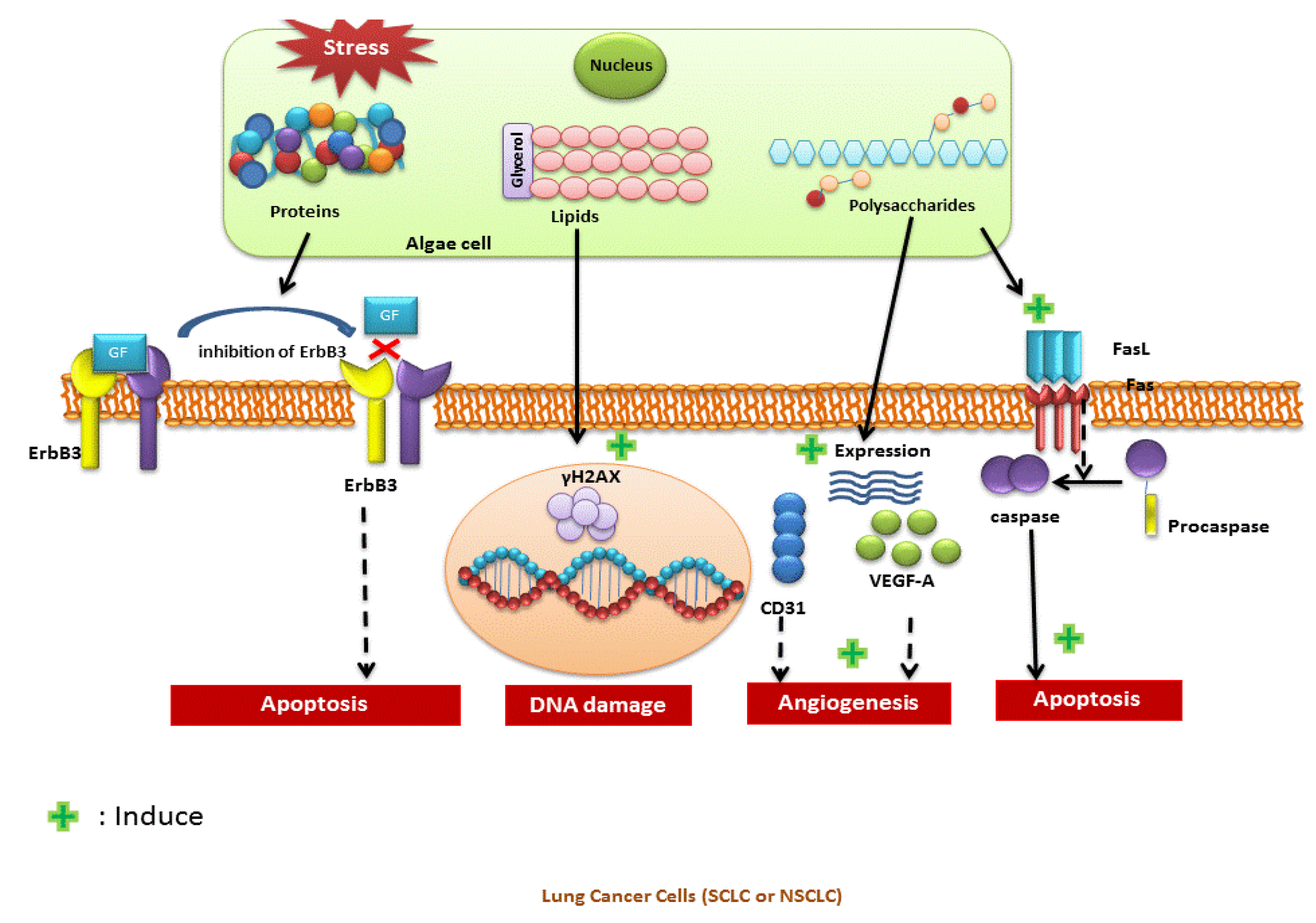
:1. Introduction
2. Molecular Origin and Signaling Pathways Associated to Lung Cancer
3. Photosynthetic Microorganisms as a Natural and Renewable Source of Anti-Lung Cancer Agents
3.1. Macroalgae
3.2. Microalgae
3.3. Cyanobacteria
4. Bioactive Compounds from Algae
4.1. Derivatives of Carbohydrates
4.1.1. Polysaccharides
4.1.2. Fucoidan
4.2. Derivatives of Protein
4.2.1. Phycobiliprotein
4.2.2. Glycoprotein
4.2.3. The Cyclo Depsipeptides
- Kahalalides F
- Other cyclo depsipeptide from cyanobacteria
4.3. Derivatives of Lipids
4.3.1. Carotenoids
4.3.2. Omega Fatty Acids
4.4. Tuberatolide B (TTB, C27H34O4)
5. Anticancer Mode of Action and Putative Targets
6. Potential to Combine Natural Products with Existing Drug Regimens in the Treatment of Lung Cancer
7. Economic Feasibility and Challenges in Using Microalgae for Lung Cancer
8. Future Perspective
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Agency for Research on Cancer, WHO. World Cancer Report 2014. Available online: http://publichealthwell.ie/node/725845 (accessed on 28 March 2020).
- Curran, W.J., Jr. Evolving chemoradiation treatment strategies for locally advanced non-small-cell lung cancer. Oncology 2003, 17, 7–14. [Google Scholar] [PubMed]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Samet, J.M.; Avila-Tang, E.; Boffetta, P.; Hannan, L.M.; Olivo-Marston, S.; Thun, M.J.; Rudin, C.M. Lung cancer in never smokers: Clinical epidemiology and environmental risk factors. Clin. Cancer Res. 2009, 15, 5626–5645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, P.; Erehman, J.; Gohlke, B.O.; Wilhelm, T.; Preissner, R.; Dunkel, M. Super Natural II-a database of natural products. Nucleic Acids Res. 2015, 43, D935–D939. [Google Scholar] [CrossRef] [Green Version]
- Richardson, M.A.; Sanders, T.; Palmer, J.L.; Greisinger, A.; Singletary, S.E. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J. Clin. Oncol. 2000, 18, 2505–2514. [Google Scholar] [CrossRef]
- Guiry, M.D. How many species of algae are there? J. Phycol. 2012, 48, 1057–1063. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2015, 32, 116–211. [Google Scholar] [CrossRef] [Green Version]
- Tseng, C.Y.; Lin, C.H.; Wu, L.Y.; Wang, J.S.; Chung, M.C.; Chang, J.F.; Chao, M.W. Potential combinational anticancer therapy in non-small cell lung cancer with traditional Chinese medicine Sun-Bai-Pi extract and cisplatin. PLoS ONE 2016, 11, e0155469. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Review Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [Green Version]
- Panov, S.Z. Molecular biology of the lung cancer. Radiol. Oncol. 2005, 39, 197. [Google Scholar]
- Larsen, J.E.; Minna, J.D. Molecular Biology of Lung Cancer: Clinical Implications. Clin. Chest Med. 2011, 32, 703–740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z. ErbB receptors and cancer. Methods Mol. Biol. 2017, 1652, 3–35. [Google Scholar] [PubMed]
- Janmaat, M.L.; Kruyt, F.A.E.; Rodriguez, J.A.; Giaccone, G. Response to epidermal growth factor receptor inhibitors in non-small cell lung cancer cells: Limited antiproliferative effects and absence of apoptosis associated with persistent activity of extracellular signal-regulated kinase or Akt kinase pathways. Clin. Cancer Res. 2003, 9, 2316–2326. [Google Scholar] [PubMed]
- Gridelli, C.; Bareschino, M.A.; Schettino, C.; Rossi, A.; Maione, P.; Ciardiello, F. Erlotinib in non-small cell lung cancer treatment: Current status and future development. Oncologist 2007, 12, 840–849. [Google Scholar] [CrossRef] [Green Version]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Joon, O.P.; Lindeman, N.; Gale, C.M.; Zhao, X.; Christensen, J.; et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007, 316, 1039–1043. [Google Scholar] [CrossRef]
- Morgillo, F.; Della Corte, C.M.; Fasano, M.; Ciardiello, F. Mechanisms of resistance to EGFR-targeted drugs: Lung cancer. ESMO Open 2016, 1, e000060. [Google Scholar] [CrossRef]
- Iwamoto, C.; Minoura, K.; Hagishita, S.; Nomoto, K.; Numata, A. Penostatins F–I, novel cytotoxic metabolites from a Penicillium species separated from an Enteromorpha marine alga. J. Chem. Soc. Perkin Trans. 1998, 3, 449–456. [Google Scholar] [CrossRef]
- Iwamoto, C.; Minoura, K.; Oka, T.; Ohta, T.; Hagishita, S.; Numata, A. Absolute stereostructures of novel cytotoxic metabolites, penostatins A-E, from a Penicillium species separated from an Enteromorpha alga. Tetrahedron 1999, 55, 14353–14368. [Google Scholar] [CrossRef]
- Shimizu, Y. Microalgal metabolites: A new perspective. Annu. Rev. Microbiol. 1996, 50, 431–465. [Google Scholar] [CrossRef]
- Dhargalkar, V.K.; Pereira, N. Seaweed: Promising Plant of the Millennium. Sci. Cult. 2005, 71, 60–66. [Google Scholar]
- Chengkui, Z.; Tseng, C.K.; Junfu, Z.; Chang, C.F. Chinese seaweeds in herbal Source medicine. Hydrobiologia 1984, 116–117, 152–154. [Google Scholar] [CrossRef]
- Nabti, E. Biotechnological Applications of Seaweeds; Categories: Agricultural Engineering, Agriculture, Science and Technology; Nova: New York, NY, USA, 2017; ISBN 978-1-53610-968-9. [Google Scholar]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, P.; González-García, S.; Ulloa, R.G.; Sineiro, J.; Feijoo, G.; Moreira, M.T. Life cycle assessment of the production of bioactive compounds from Tetraselmis suecica at pilot scale. J. Clean. Prod. 2014, 64, 323–331. [Google Scholar] [CrossRef]
- Somasekharan, S.P.; El-Naggar, A.; Sorensen, P.H.; Wang, Y.; Cheng, H. An Aqueous Extract of Marine Microalgae Exhibits Antimetastatic Activity through Preferential Killing of Suspended Cancer Cells and Anticolony Forming Activity. Evid. Based Complement Altern. Med. 2016, 2016, 9730654. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.M.; Pan, J.L.; Chen, C.Y.; Chiu, C.C.; Yang, M.H.; Chang, H.W.; Chang, J.S. Identification of anti-lung cancer extract from Chlorella vulgaris C-C by antioxidant property using supercritical carbon dioxide extraction. Process Biochem. 2010, 45, 1865–1872. [Google Scholar] [CrossRef]
- Guedes, É.A.C.; da Silva, T.G.; Aguiar, J.S.; de Barros, L.D.; Pinotti, L.M.; Sant’Ana, A.E.G. Cytotoxic activity of marine algae against cancerous cells. Rev. Bras. Farmacogn. 2013, 23, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.D.; Zhang, X.; Mooberry, S.L.; Patterson, G.M.L.; Moore, R.E. Cryptophycin: A New Antimicrotubule Agent Active against Drug-resistant Cells. Cancer Res. 1994, 54, 3779–3784. [Google Scholar]
- Deniz, I.; Ozen, M.O.; Yesil-Celiktas, O. Supercritical fluid extraction of phycocyanin and investigation of cytotoxicity on human lung cancer cells. J. Supercrit. Fluids 2016, 108, 13–18. [Google Scholar] [CrossRef]
- Tan, L.T. Bioactive natural products from marine cyanobacteria for drug discovery. Phytochemistry 2007, 68, 954–979. [Google Scholar] [CrossRef]
- Cancer Chemotherapy National Service Center. Protocols for screening chemical agents and natural products against animal tumors and other biological systems. Cancer Chemother. Rep. 1962, 25, 1–66. [Google Scholar]
- Boo, H.-J.; Hyun, J.-H.; Kim, S.-C.; Kang, J.-I.; Kim, M.-K.; Kim, S.-Y.; Cho, H.; Yoo, E.-S.; Kang, H.-K. Fucoidan from Undaria pinnatifida induces apoptosis in A549 human lung carcinoma cells. Phytother. Res. 2011, 25, 1082–1086. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Chiu, Y.H.; Chan, Y.L.; Chiu, Y.H.; Wang, H.; Huang, K.C.; Li, T.L.; Hsu, K.H.; Wu, C.J. Prophylactic administration of fucoidan represses cancer metastasis by inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in Lewis tumor-bearing mice. Mar. Drugs 2015, 13, 1882–1900. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, Z.J.; Xie, D.; Sun, X.; Yang, W.; Zhao, X.; Xu, N. Characterization and potential antitumor activity of polysaccharide from gracilariopsis lemaneiformis. Mar. Drugs 2017, 15, 100. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, L.; Long, X.; Li, P.; Chen, S.; Kuang, W.; Guo, J. Sargassum fusiforme polysaccharides inhibit VEGF-A-related angiogenesis and proliferation of lung cancer in vitro and in vivo. Biomed. Pharmacother. 2017, 85, 22–27. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, J.; Lee, K.M.; Choi, Y.J.; Ye, B.R.; Kim, M.S.; Ko, S.G.; Lee, S.H.; Kang, D.H.; Heo, S.J. Tuberatolide B suppresses cancer progression by promoting ROS-mediated inhibition of STAT3 signaling. Mar. Drugs 2017, 15, 55. [Google Scholar] [CrossRef] [Green Version]
- Sabry, O.M.M.; Goeger, D.E.; Valeriote, F.A.; Gerwick, W.H. Cytotoxic halogenated monoterpenes from Plocamium cartilagineum. Nat. Prod. Res. 2017, 31, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Senthilkumar, D.; Jayanthi, S. Partial characterization and anticancer activities of purified glycoprotein extracted from green seaweed Codium decorticatum. J. Funct. Foods 2016, 25, 323–332. [Google Scholar] [CrossRef]
- Sakthivel, R.; Muniasamy, S.; Archunan, G.; Devi, K.P. Gracilaria edulis exhibit antiproliferative activity against human lung adenocarcinoma cell line A549 without causing adverse toxic effect in vitro and in vivo. Food Funct. 2016, 7, 1155–1165. [Google Scholar] [CrossRef]
- Zhang, D.; Wan, M.; del Rio-Chanona, E.A.; Huang, J.; Wang, W.; Li, Y.; Vassiliadis, V.S. Dynamic modelling of Haematococcus pluvialis photoinduction for astaxanthin production in both attached and suspended photobioreactors. Algal Res. 2016, 13, 69–78. [Google Scholar] [CrossRef]
- Levy, J.; Bosin, E.; Feldman, B.; Giat, Y.; Miinster, A.; Danilenko, M.; Sharoni, Y. Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or beta-carotene. Nutr. Cancer 1995, 24, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Cordero, B.F.; Couso, I.; Leon, R.; Rodriguez, H.; Vargas, M.A. Isolation and characterization of a lycopene ε-cyclase gene of Chlorella (Chromochloris) zofingiensis. Regulation of the carotenogenic pathway by nitrogen and light. Mar. Drugs 2012, 10, 2069–2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mein, J.R.; Lian, F.; Wang, X.D. Biological activity of lycopene metabolites: Implications for cancer prevention. Nutr. Rev. 2008, 66, 667–683. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.-J.; Huang, G.-J.; Wu, C.-H.; Chen, J.-S.; Chang, H.-Y.; Chang, S.-J.; Chung, J.-G. Ethanol extract of Dunaliella salina induces cell cycle arrest and apoptosis in A549 human non-small cell lung cancer cells. In Vivo 2008, 22, 369–378. [Google Scholar]
- Yam, D.; Peled, A.; Shinitzky, M. Suppression of tumor growth and metastasis by dietary fish oil combined with vitamins E and C and cisplatin. Cancer Chemother. Pharmacol. 2001, 47, 34–40. [Google Scholar] [CrossRef]
- Yang, X.; Xie, P.; Yu, Y.; Shen, H.; Deng, X.; Ma, Z.; Wang, P.; Tao, M.; Niu, Y. Microcystis aeruginosa/Pseudomonas pseudoalcaligenes interaction effects on off-flavors in algae/bacteria co-culture system under different temperatures. J. Environ. Sci. 2015, 31, 38–43. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Thangam, R.; Suresh, V.; Asenath Princy, W.; Rajkumar, M.; Senthilkumar, N.; Gunasekaran, P.; Rengasamy, R.; Anbazhagan, C.; Kaveri, K.; Kannan, S. C-Phycocyanin from Oscillatoria tenuis exhibited an antioxidant and in vitro antiproliferative activity through induction of apoptosis and G 0/G1 cell cycle arrest. Food Chem. 2013, 140, 262–272. [Google Scholar] [CrossRef]
- Soria-Mercado, I.E.; Pereira, A.; Cao, Z.; Murray, T.F.; Gerwick, W.H. Alotamide A, a novel neuropharmacological agent from the marine cyanobacterium Lyngbya bouillonii. Org. Lett. 2009, 11, 4704–4707. [Google Scholar] [CrossRef] [Green Version]
- Medina-Gundrum, L.; Cerna, C.; Gomez, L.R.; Yochmowitz, M.; Weitman, S. AMD473 (ZD0473) exhibits marked in vitro anticancer activity in human tumor specimens taken directly from patients. Anticancer. Drugs 2003, 14, 275–280. [Google Scholar] [CrossRef]
- Umemura, K.; Yanase, K.; Suzuki, M.; Okutani, K.; Yamori, T.; Andoh, T. Inhibition of DNA topoisomerases I and II, and growth inhibition of human cancer cell lines by a marine microalgal polysaccharide. Biochem. Pharmacol. 2003, 66, 481–487. [Google Scholar] [CrossRef]
- Samarakoon, K.W.; Ko, J.Y.; Lee, J.H.; Kwon, O.N.; Kim, S.W.; Jeon, Y.J. Apoptotic anticancer activity of a novel fatty alcohol ester isolated from cultured marine diatom, Phaeodactylum tricornutum. J. Funct. Foods 2014, 6, 231–240. [Google Scholar] [CrossRef]
- Zorofchian Moghadamtousi, S.; Karimian, H.; Khanabdali, R.; Razavi, M.; Firoozinia, M.; Zandi, K.; Abdul Kadir, H. Anticancer and antitumor potential of fucoidan and fucoxanthin, two main metabolites isolated from brown algae. Sci. World J. 2014, 2014, 768323. [Google Scholar] [CrossRef] [PubMed]
- Usoltseva (Menshova), R.V.; Anastyuk, S.D.; Shevchenko, N.M.; Zvyagintseva, T.N.; Ermakova, S.P. The comparison of structure and anticancer activity in vitro of polysaccharides from brown algae Alaria marginata and A. angusta. Carbohydr. Polym. 2016, 153, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Noda, H.; Amano, H.; Ito, H. Immunological analysis of inhibition of lung metastases by fucoidan (GIV-A) prepared from brown seaweed Sargassum thunbergii. Anticancer Res. 1995, 15, 1937–1947. [Google Scholar]
- Riou, D.; Colliec-Jouault, S.; Pinczon Du Sel, D.; Bôsch, S.; Siavoshian, S.; Le Bert, V.; Tomasoni, C.; Sinquin, C.; Durand, P.; Roussakis, C. Antitumor and antiproliferative effects of a fucan extracted from ascophyllum nodosum against a non-small-cell bronchopulmonary carcinoma line. Anticancer Res. 1996, 16, 1213–1218. [Google Scholar]
- Aisa, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human HS-Sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef]
- Yoon, S.J.; Pyun, Y.R.; Hwang, J.K.; Mourão, P.A.S. A sulfated fucan from the brown alga Laminaria cichorioides has mainly heparin cofactor II-dependent anticoagulant activity. Carbohydr. Res. 2007, 342, 2326–2330. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anticancer potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, N.T. Production of phycocyanin-A pigment with applications in biology, biotechnology, foods and medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef]
- Madamwar, D.; Kaushal, A.; Patel, D.K.; Desai, S.N.; Upadhyay, K.; Devkar, R.V. Cyanobacterial phycoerythrin purified from marine Lyngbya sp. Induces apoptosis in lung carcinoma cells. Bangladesh J. Pharmacol. 2015, 10, 770–778. [Google Scholar] [CrossRef] [Green Version]
- Hamann, M.T.; Scheuer, P. Kahalalide F: A bioactive depsipeptide from the sacoglossan mollusk Elysia rufescens and the green alga Bryopsis sp. J. Am. Chem. Soc. 1993, 115, 5825–5826. [Google Scholar] [CrossRef]
- Hamann, M.T. Technology evaluation: Kahalalide F, PharmaMar. Curr. Opin. Mol. Ther. 2004, 6, 657–665. [Google Scholar] [PubMed]
- López-Macià, A.; Jiménez, J.C.; Royo, M.; Giralt, E.; Albericio, F. Synthesis and structure determination of kahalalide F (1,2). J. Am. Chem. Soc. 2001, 123, 11398–11401. [Google Scholar]
- Burja, A.M.; Banaigs, B.; Abou-Mansour, E.; Grant Burgess, J.; Wright, P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Taniguchi, M.; Nunnery, J.K.; Engene, N.; Esquenazi, E.; Byrum, T.; Dorrestein, P.C.; Gerwick, W.H. Palmyramide a, a cyclic depsipeptide from a palmyra atoll collection of the marine cyanobacterium lyngbya majuscula. J. Nat. Prod. 2010, 73, 393–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mevers, E.; Liu, W.T.; Engene, N.; Mohimani, H.; Byrum, T.; Pevzner, P.A.; Dorrestein, P.C.; Spadafora, C.; Gerwick, W.H. Cytotoxic veraguamides, alkynyl bromide-containing cyclic depsipeptides from the marine cyanobacterium cf. Oscillatoria margaritifera. J. Nat. Prod. 2011, 74, 928–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasso, S.; Pohnert, G.; Lohr, M.; Mittag, M.; Hertweck, C. Microalgae in the postgenomic era: A blooming reservoir for new natural products. FEMS Microbiol. Rev. 2012, 36, 761–785. [Google Scholar]
- Varela, J.C.; Pereira, H.; Vila, M.; León, R. Production of carotenoids by microalgae: Achievements and challenges. Photosynth. Res. 2015, 125, 423–436. [Google Scholar] [CrossRef]
- Bhosale, P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biotechnol. 2004, 63, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sevilla, J.M.; Acién Fernández, F.G.; Molina Grima, E. Biotechnological production of lutein and its applications. Appl. Microbiol. Biotechnol. 2010, 86, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.C.; Chen, J.C.; Wang, T.J.; Zheng, H.Y.; Chen, W.C.; Chang, P.Y.; Lin, Y.W. Astaxanthin down-regulates Rad51 expression via inactivation of AKT kinase to enhance mitomycin C-induced cytotoxicity in human non-small cell lung cancer cells. Biochem. Pharmacol. 2016, 105, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.A.; Choi, J.S.; Lee, M.C.; Kim, E.; Nam, T.J.; Fujii, H.; Hong, Y.K. Anti-inflammatory activities of methanol extracts from various seaweed species. J. Environ. Biol. 2008, 29, 465–469. [Google Scholar] [PubMed]
- Panis, G.; Carreon, J.R. Commercial astaxanthin production derived by green alga Haematococcus pluvialis: A microalgae process model and a techno-economic assessment all through production line. Algal Res. 2016, 18, 175–190. [Google Scholar] [CrossRef] [Green Version]
- Serini, S.; Fasano, E.; Piccioni, E.; Cittadini, A.R.; Calviello, G. Differential anticancer effects of purified EPA and DHA and possible mechanisms involved. Curr. Med. Chem. 2011, 18, 4065–4075. [Google Scholar] [CrossRef]
- Mernitz, H.; Lian, F.; Smith, D.E.; Meydani, S.N.; Wang, X.D. Fish oil supplementation inhibits NNK-induced lung carcinogenesis in the A/J mouse. Nutr. Cancer 2009, 61, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.-H.; Zhang, X.-C.; Fu, T.; Gu, J.-Z.; Wang, L.; Wang, Y.; Lai, Y.-B.; Wang, Y.-Q.; Guo, Y. ω-3 polyunsaturated fatty acids inhibit the proliferation of the lung adenocarcinoma cell line A549 in vitro. Mol. Med. Rep. 2014, 9, 401–406. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.; Hwang, H.; Chin, J.; Kim, E.; Lee, J.; Nam, S.J.; Lee, B.C.; Rho, B.J.; Kang, H. Tuberatolides, potent FXR antagonists from the Korean marine tunicate Botryllus tuberatus. J. Nat. Prod. 2011, 74, 90–94. [Google Scholar] [CrossRef]
- Kanadaswami, C.; Lee, L.T.; Lee, P.P.H.; Hwang, J.J.; Ke, F.C.; Huang, Y.T.; Lee, M.T. The antitumor activities of flavonoids. In Vivo 2005, 19, 895–910. [Google Scholar]
- Moosavi, M.A.; Yazdanparast, R.; Sanati, M.H.; Nejad, A.S. 3-Hydrogenkwadaphnin targets inosine 5′-monophosphate dehydrogenase and triggers post-G1 arrest apoptosis in human leukemia cell lines. Int. J. Biochem. Cell Biol. 2005, 37, 2366–2379. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Guo, J.; Liu, D.; Zhang, S. Aloe-emodin induces in vitro G2/M arrest and alkaline phosphatase activation in human oral cancer KB cells. Oral Oncol. 2007, 43, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Lee, K.W.; Kim, J.Y.; Kim, K.H.; Lee, H.J. Induction of apoptosis in a human lymphoma cell line by hydrophobic peptide fraction separated from anchovy sauce. Biofactors 2004, 21, 63–67. [Google Scholar] [CrossRef]
- Tzianabos, A.O. Polysaccharide immunomodulators as therapeutic agents: Structural aspects and biologic function. Clin. Microbiol. Rev. 2000, 13, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Janmaat, M.L.; Rodriguez, J.A.; Jimeno, J.M.; Kruyt, F.A.E.; Giaccone, G. Kahalalide F induces necrosis-like cell death that involves depletion of ErbB3 and inhibition of Akt signaling. Mol. Pharmacol. 2005, 68, 502–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.; Hamann, M.T. Chemistry and biology of kahalalides. Chem. Rev. 2011, 111, 3208–3235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotter, T.G. Apoptosis and cancer: The genesis of a research field. Nat. Rev. Cancer 2009, 9, 501–507. [Google Scholar] [CrossRef]
- Heimli, H.; Giske, C.; Naderi, S.; Drevon, C.A.; Hollung, K. Eicosapentaenoic acid promotes apoptosis in Ramos cells via activation of caspase-3 and -9. Lipids 2002, 37, 797–802. [Google Scholar] [CrossRef]
- Rovito, D.; Giordano, C.; Vizza, D.; Plastina, P.; Barone, I.; Casaburi, I.; Lanzino, M.; De Amicis, F.; Sisci, D.; Mauro, L.; et al. Omega-3 PUFA ethanolamides DHEA and EPEA induce autophagy through PPARγ activation in MCF-7 breast cancer cells. J. Cell. Physiol. 2013, 228, 1314–1322. [Google Scholar] [CrossRef]
- Gravaghi, C.; La Perle, K.M.D.; Ogrodwski, P.; Kang, J.X.; Quimby, F.; Lipkin, M.; Lamprecht, S.A. Cox-2 expression, PGE2and cytokines production are inhibited by endogenously synthesized n-3 PUFAs in inflamed colon of fat-1 mice. J. Nutr. Biochem. 2011, 22, 360–365. [Google Scholar] [CrossRef]
- Liu, G.; Bibus, D.M.; Bode, A.M.; Ma, W.Y.; Holman, R.T.; Dong, Z. Omega 3 but not omega 6 fatty acids inhibit AP-1 activity and cell transformation in JB6 cells. Proc. Natl. Acad. Sci. USA 2001, 98, 7510–7515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Dor, A.; Nahum, A.; Danilenko, M.; Giat, Y.; Stahl, W.; Martin, H.D.; Emmerich, T.; Noy, N.; Levy, J.; Sharoni, Y. Effects of acyclo-retinoic acid and lycopene on activation of the retinoic acid receptor and proliferation of mammary cancer cells. Arch. Biochem. Biophys. 2001, 391, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Judé, S.; Martel, E.; Vincent, F.; Besson, P.; Couet, C.; Ogilvie, G.K.; Pinault, M.; De Chalendar, C.; Bougnoux, P.; Richard, S.; et al. Dietary long-chain n-3 fatty acids modify blood and cardiac phospholipids and reduce protein kinase-C-δ and protein kinase-C-ε translocation. Br. J. Nutr. 2007, 98, 1143–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Gao, M.H.; Chu, X.M.; Teng, L.; Lv, C.Y.; Yang, P.; Yin, Q.F. The synergistic antitumor effects of all-trans retinoic acid and C-phycocyanin on the lung cancer A549 cells in vitro and in vivo. Eur. J. Pharmacol. 2015, 749, 107–114. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Qu, D.; Liu, H.B.; Gu, X.; Jiao, G.Y.; Zhao, L. Combined anticancer activity of osthole and cisplatin in NCI-H460 lung cancer cells in vitro. Exp. Ther. Med. 2013, 5, 707–710. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wang, P.; Wang, H.; Li, Q.; Teng, H.; Liu, Z.; Yang, W.; Hou, L.; Zou, X. Fucoidan derived from Undaria pinnatifida induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar. Drugs 2013, 11, 1961–1976. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Sheng, W.; Yao, W.; Wang, C. Effect of low molecular λ-carrageenan from Chondrus ocellatus on antitumor H-22 activity of 5-Fu. Pharmacol. Res. 2006, 53, 129–134. [Google Scholar] [CrossRef]
- Lins, K.O.A.L.; Bezerra, D.P.; Alves, A.P.N.N.; Alencar, N.M.N.; Lima, M.W.; Torres, V.M.; Farias, W.R.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V. Antitumor properties of a sulfated polysaccharide from the red seaweed Champia feldmannii (Diaz-Pifferer). J. Appl. Toxicol. 2009, 29, 20–26. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z. The antitumor activity of a red alga polysaccharide complexes carrying 5-fluorouracil. Int. J. Biol. Macromol. 2014, 69, 542–545. [Google Scholar] [CrossRef]
- Charkie, J. Psammaplin A: A Putative Adjuvant for DNA Damaging Therapies. J. Cancer Sci. Ther. 2014, 6, 505–509. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Abdelnour, S.; Alagawany, M.; Abdo, M.; Sakr, M.A.; Khafaga, A.F.; Mahgoub, S.A.; Elnesr, S.S.; Gebriel, M.G. Microalgae in modern cancer therapy: Current knowledge. Biomed. Pharmacother. 2019, 111, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Mata, T.M.; Martins, A.A.; Caetano, N.S. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010, 14, 217–232. [Google Scholar] [CrossRef] [Green Version]
- Mulders, K.J.M.; Lamers, P.P.; Martens, D.E.; Wijffels, R.H. Phototrophic pigment production with microalgae: Biological constraints and opportunities. J. Phycol. 2014, 50, 229–242. [Google Scholar] [CrossRef]
- González, L.E.; Díaz, G.C.; Aranda, D.A.G.; Cruz, Y.R.; Fortes, M.M. Biodiesel Production Based in Microalgae: A Biorefinery Approach. Nat. Sci. 2015, 7, 358–369. [Google Scholar] [CrossRef] [Green Version]
- Tredici, M.R.; Rodolfi, L.; Biondi, N.; Bassi, N.; Sampietro, G. Techno-economic analysis of microalgal biomass production in a 1-ha Green Wall Panel (GWP®) plant. Algal Res. 2016, 19, 253–263. [Google Scholar] [CrossRef] [Green Version]
- Ummalyma, S.B.; Gnansounou, E.; Sukumaran, R.K.; Sindhu, R.; Pandey, A.; Sahoo, D. Bioflocculation: An alternative strategy for harvesting of microalgae–An overview. Bioresour. Technol. 2017, 242, 227–235. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Vervaeren, H.; Desmet, S.; Boon, N. Bioflocculation of microalgae and bacteria combined with flue gas to improve sewage treatment. New Biotechnol. 2011, 29, 23–31. [Google Scholar] [CrossRef]
- Ndikubwimana, T.; Zeng, X.; Murwanashyaka, T.; Manirafasha, E.; He, N.; Shao, W.; Lu, Y. Harvesting of freshwater microalgae with microbial bioflocculant: A pilot-scale study. Biotechnol. Biofuels 2016, 9, 47. [Google Scholar] [CrossRef] [Green Version]
- Alam, A.; Vandamme, D.; Chun, W.; Zhao, X.; Foubert, I.; Wang, Z.; Muylaert, K.; Yuan, Z. Bioflocculation as an innovative harvesting strategy for microalgae. Rev. Environ. Sci. Biotechnol. 2016, 15, 573–583. [Google Scholar] [CrossRef]
- Ventura, S.P.M.; Nobre, B.P.; Ertekin, F.; Hayes, M.; Garciá-Vaquero, M.; Vieira, F.; Koc, M.; Gouveia, L.; Aires-Barros, M.R.; Palavra, A.M.F. Extraction of value-added compounds from microalgae. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Woodhead Publishing: Sawston, Cambridge, UK, 2017; pp. 461–483. [Google Scholar]
- El-Sheekh, M.M.; El-Kassas, H.Y. Application of biosynthesized silver nanoparticles against a cancer promoter cyanobacterium, Microcystis aeruginosa. Asian Pac. J. Cancer Prev. 2014, 15, 6773–6779. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Algae Species | Active Molecule | Lung Cancer Cells | Dose IC50 | Inhibition Pathways | Ref. |
|---|---|---|---|---|---|
| U. pinnatifid algae | Fucoidan | Human lung cancer A549 cells | 50, 100, 200 μg mL−1 | ↓ procaspase 3 PARP cleavage Caspase-9 activation ↓ procaspase 3 ↓ Bcl-2, ↑ Bax | [33] |
| F. vesiculosus | Fucoidan | lung cancer A549 cells | 50–400 μg mL−1 | inhibited the MMP-2 and MMP-9 protein expression, cell migration, and invasion activities of LLC cells | [34] |
| Bryopsis | Kahalalide F | lung cancer A549 cells | 2.5 µg L−1 | Targets the lysosomes, | [35] |
| Hypnea musciformis | Chloroform extract | NCI-H292 (human lung mucoepidermoid carcinoma) | 15.0 ± 1.3 μg mL−1 | - | [28] |
| Ethanol extract | NCI-H292 | 22.0 ± 3.5 μg mL−1 | - | [28] | |
| Gracilariopsis lemaneiformis | Polysaccharide | NSCLC cell line, A549 | 50 μg mL−1 | - | [36] |
| Sargassum fusiforme | Ploysaccharide | lung adenocarcinoma SPC-A-1 cells and its xenograft model | - | inhibit VEGF-A-related angiogenesis and proliferation | [37] |
| Sargassum macrocarpum | Tuberatolide B (TTB, C27H34O4) | (A549 and H1299) | - | promotes ROS, mediated inhibition of STAT3 signaling, decreased the expression of Bcl2, increases the cleavage of caspase-3 and PARP, enhances the percentage of annexin V, positive apoptotic cells, induces ROS generation, induces DNA Damage, induces the phosphorylation of Chk2 and H2AX. | [38] |
| Plocamium cartilagineum | halogenated monoterpene 1 (5E,7Z)-3 48-trichloro-7-dichloromethyl-3-methyl-157-octatriene) | (NCI-H460) | 4 μg mL−1 | - | [39] |
| Codium decorticatum | glycoprotein | A549 lung cancer | 40 ± 0.41 μg mL−1 | Induction of apoptosis | [40] |
| Gracilaria edulis | phytol, a diterpene | A549 lung cancer | 24.5 ± 19.1 μg mL−1 at 48 h | - | [41] |
| Algae Species | Active Molecule | Lung Cancer Cells | Dose IC50 | Inhibition Pathways | Ref. |
|---|---|---|---|---|---|
| Haematococcus sp. | Astaxanthin | NSCLC | 5–25 μg mL−1 | ↓ MKK1/2-ERK1/2 inducing cytotoxicity against cancer | [42] |
| Chlorella zofingiensis | Lycopene | A549 | 3–5 mM | ↓ mRNA and protein levels of cyclin E | [43,44,45] |
| Dunaliella salina | Beta-carotene | A549 | 25–100 μg mL−1 | ↓cell proliferation, induce apoptosis and cell cycle G0/G1 arrest | [46] |
| Tetraselmis; Nannochloropsis | EPA and DHA | A549 H1299 | EPA = 6.05 μM 50% inhibition | Generate PGE3 through COX-2 enzyme ↓ Of Akt phosphorylation by PGE3. | [47,48] |
| Chlorella Vulgaris | Polyphenols, Flavonoid | H1299, A549, and H1437 | 13.40 and 0.46 (μg Gallic acid/g lyophilized extract), 3.18 μg quercetin/g lyophilized extract | Affects migration of cells, inhibits metastasis | [27] |
| Lyngbya sp. | Phycoerythrin | A549 | 100–200 µg mL−1 | Cell arrest at G0/G1 phase, ↓ cell viability and mitochondrial membrane potential, an increment in lactate dehydrogenase release | [49] |
| Arthrospira platensis, Oscillatoria tenuise | Phycocyanin | A549 | 26.82 μg mL−1 | Cell apoptosis/blebbing necrosis, | [30,50] |
| Lyngbya majuscule, Lyngbya sordida | Alotmide A, Aurilide | H-460 NCI–H460 | - | Cell apoptosis | [51] |
| Leptolyngbya | Coibamide | NCI-H460 | LC50 < 23 nM | Inhibits cell proliferation through novel mechanism | [52] |
| Caldora penicillata | Laucysteinamide A, Curacin. | H-460 | 11 uM, | Anti-tubulin formation | [42] |
| Gymnodinium | GA3P, d-galactan sulfate | NCI-H23 | 2.8 | Inhibits topo I and topo II | [53] |
| NCI-H226 | 2.2 | ||||
| NCI-H522 | 1.3 | ||||
| NCI-H460 | 3.8 | ||||
| A54 | 11 | ||||
| DMS273 | 2.0 | ||||
| DMS11 | 2.7 | ||||
| P. tricornutum | Nonyl 8-acetoxy-6-methyloctanoate (NAMO) | A549 | 35% inhibition at 50 μg mL−1 | p53 and caspace-3 mediated cell apoptotic pathway | [54] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saadaoui, I.; Rasheed, R.; Abdulrahman, N.; Bounnit, T.; Cherif, M.; Al Jabri, H.; Mraiche, F. Algae-Derived Bioactive Compounds with Anti-Lung Cancer Potential. Mar. Drugs 2020, 18, 197. https://doi.org/10.3390/md18040197
Saadaoui I, Rasheed R, Abdulrahman N, Bounnit T, Cherif M, Al Jabri H, Mraiche F. Algae-Derived Bioactive Compounds with Anti-Lung Cancer Potential. Marine Drugs. 2020; 18(4):197. https://doi.org/10.3390/md18040197
Chicago/Turabian StyleSaadaoui, Imen, Rihab Rasheed, Nabeel Abdulrahman, Touria Bounnit, Maroua Cherif, Hareb Al Jabri, and Fatima Mraiche. 2020. "Algae-Derived Bioactive Compounds with Anti-Lung Cancer Potential" Marine Drugs 18, no. 4: 197. https://doi.org/10.3390/md18040197
APA StyleSaadaoui, I., Rasheed, R., Abdulrahman, N., Bounnit, T., Cherif, M., Al Jabri, H., & Mraiche, F. (2020). Algae-Derived Bioactive Compounds with Anti-Lung Cancer Potential. Marine Drugs, 18(4), 197. https://doi.org/10.3390/md18040197






