Structure and In Vitro Bioactivity against Cancer Cells of the Capsular Polysaccharide from the Marine Bacterium Psychrobacter marincola
Abstract
:1. Introduction
2. Results
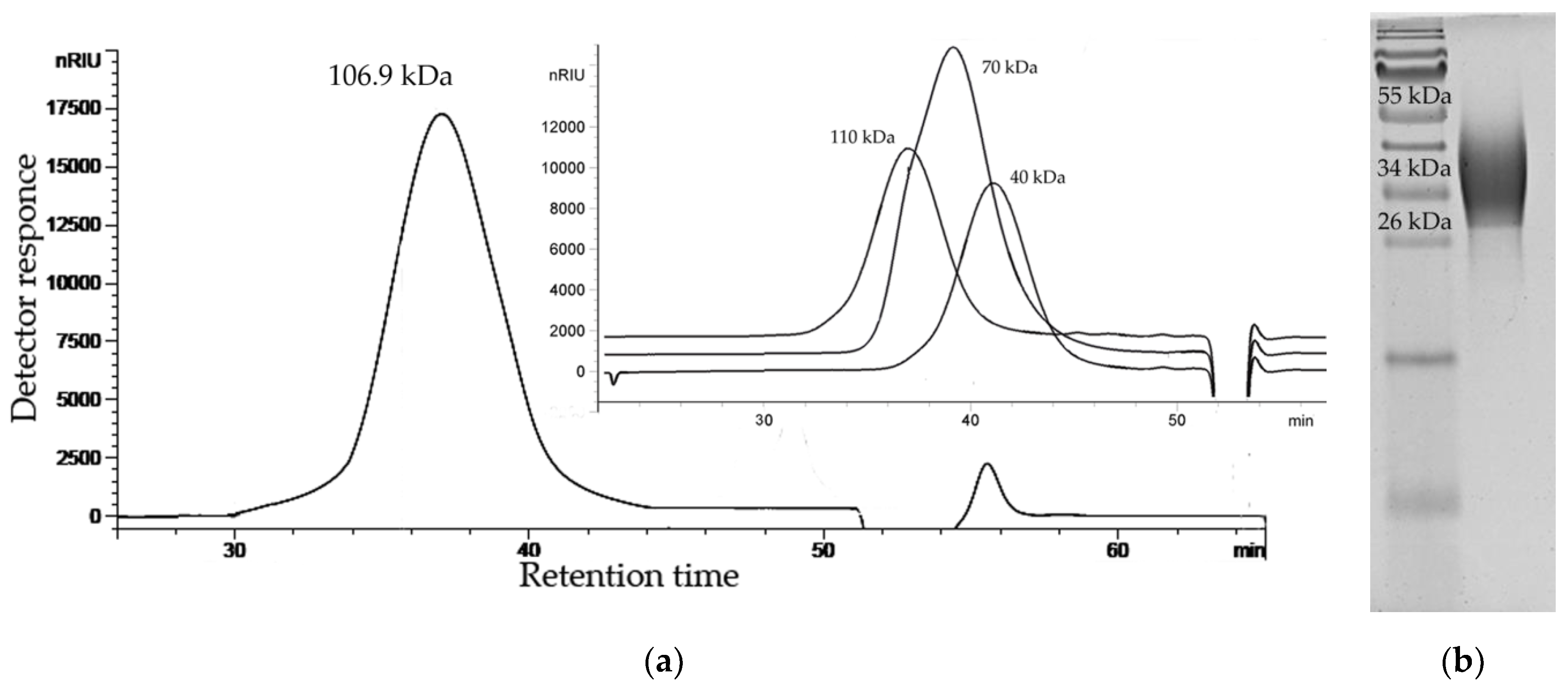
2.1. Isolation, Purification, and General Characterization of the CPS
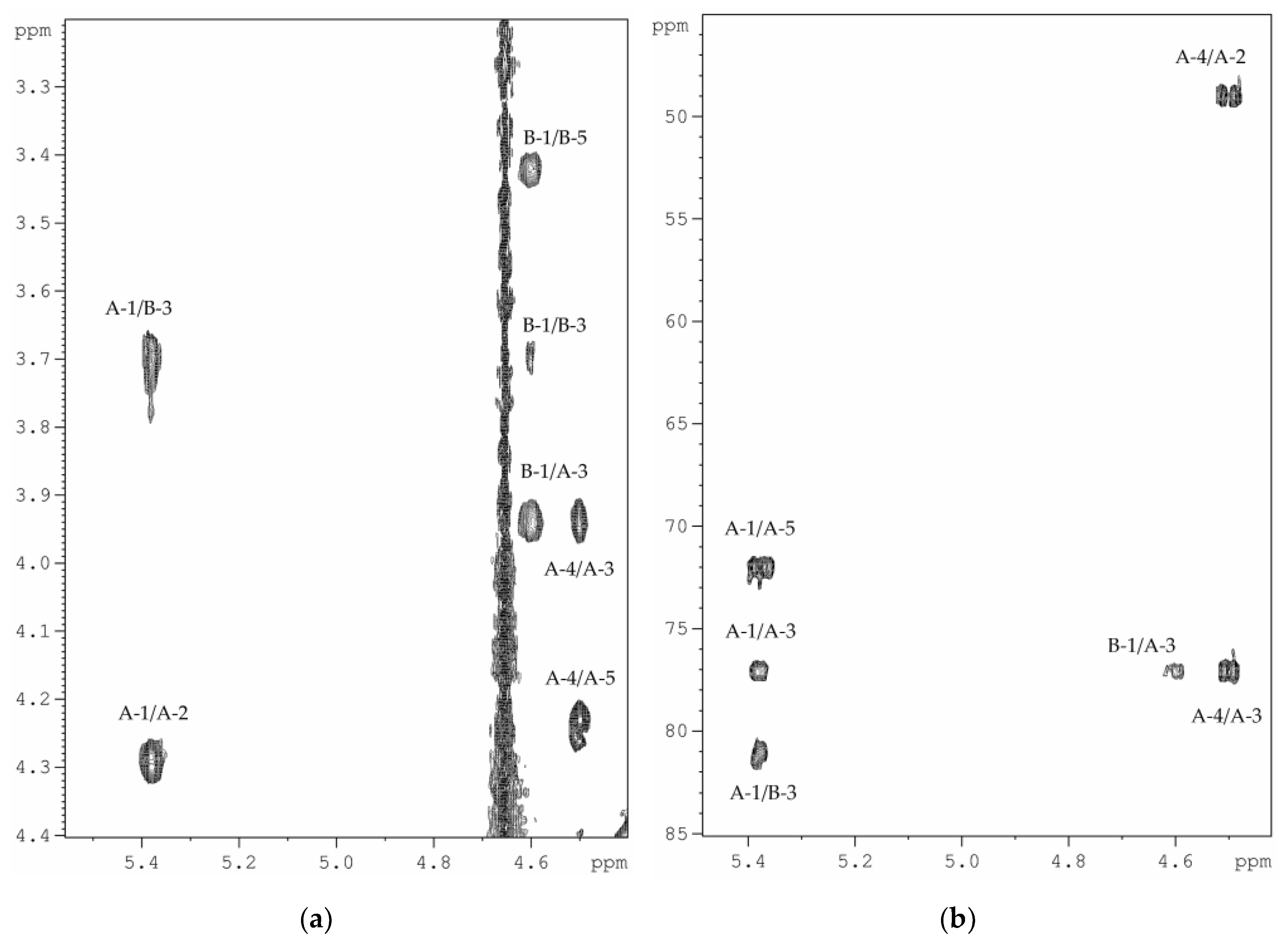
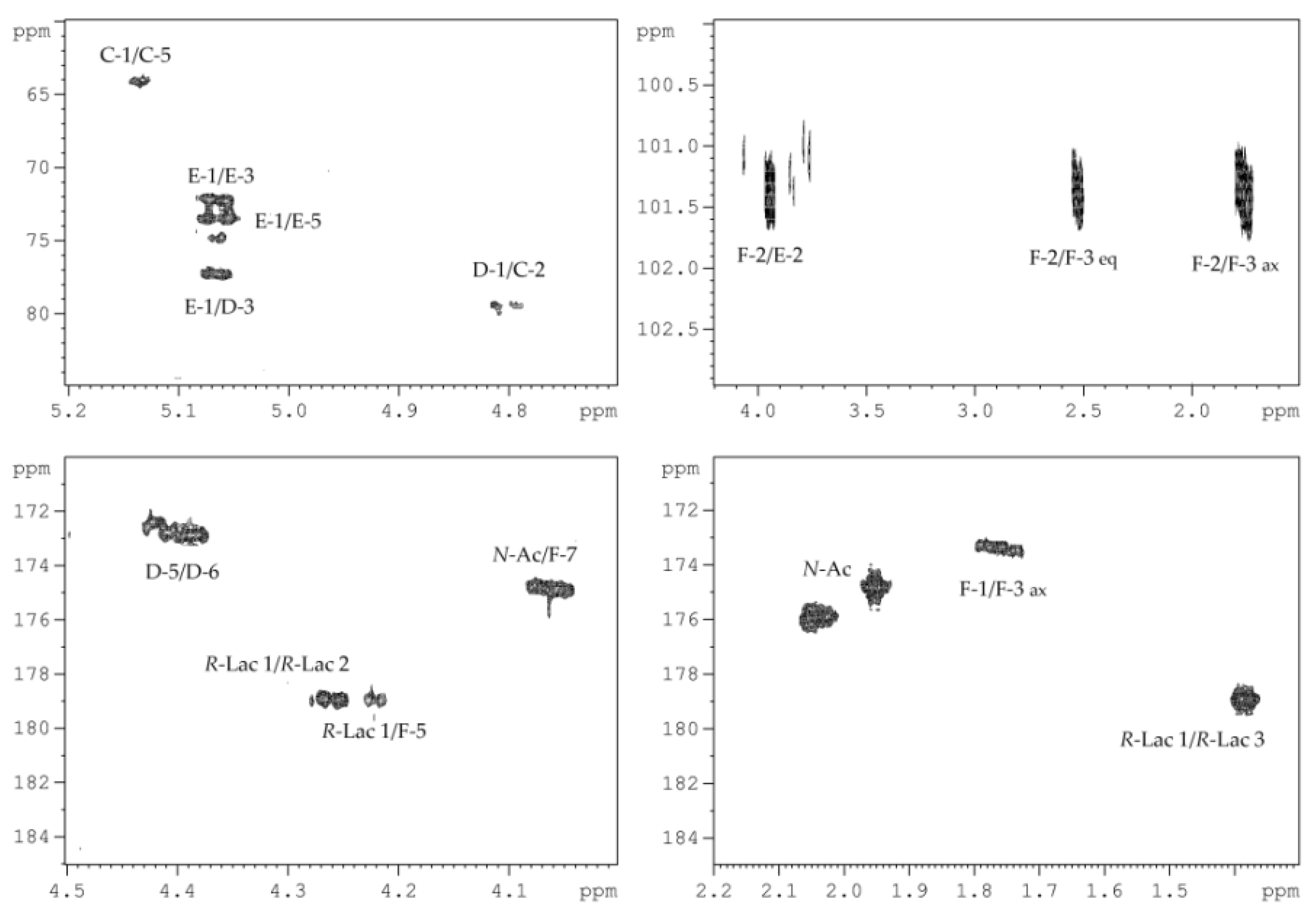
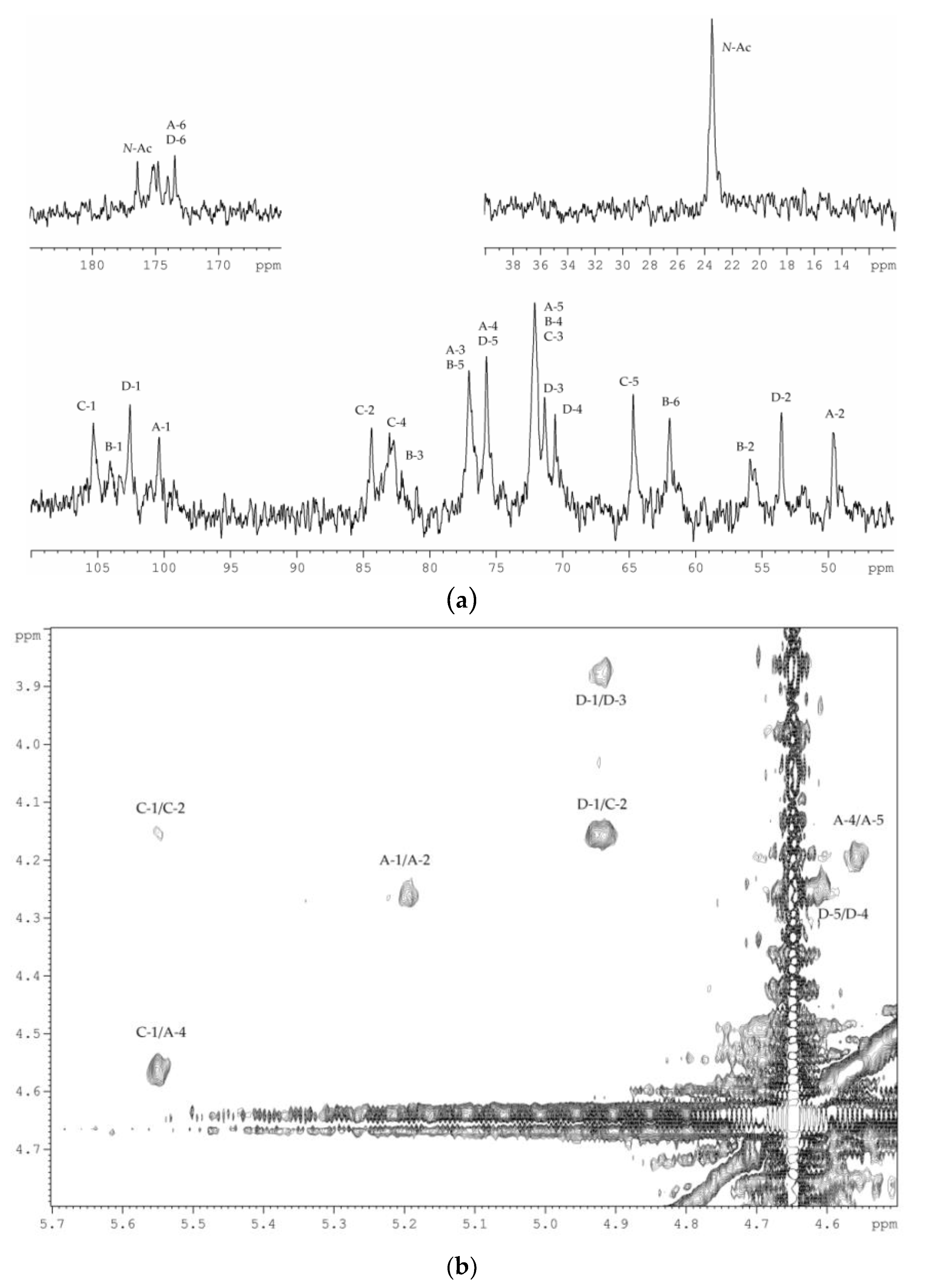
2.2. Structural Elucidation of the CPS
| →3)-β-d-GlcpNAc-(1→3)-α-d-GalpNAcA-(1→ | |||||||||||
| 4 | |||||||||||
| ↑ | |||||||||||
| 1 | |||||||||||
| β-Psep5(R-Lac)7Ac-(2→2)-α-d-Glcp-(1→3)-β-d-GalpNAcA-(1→2)-β-d-Ribf | |||||||||||
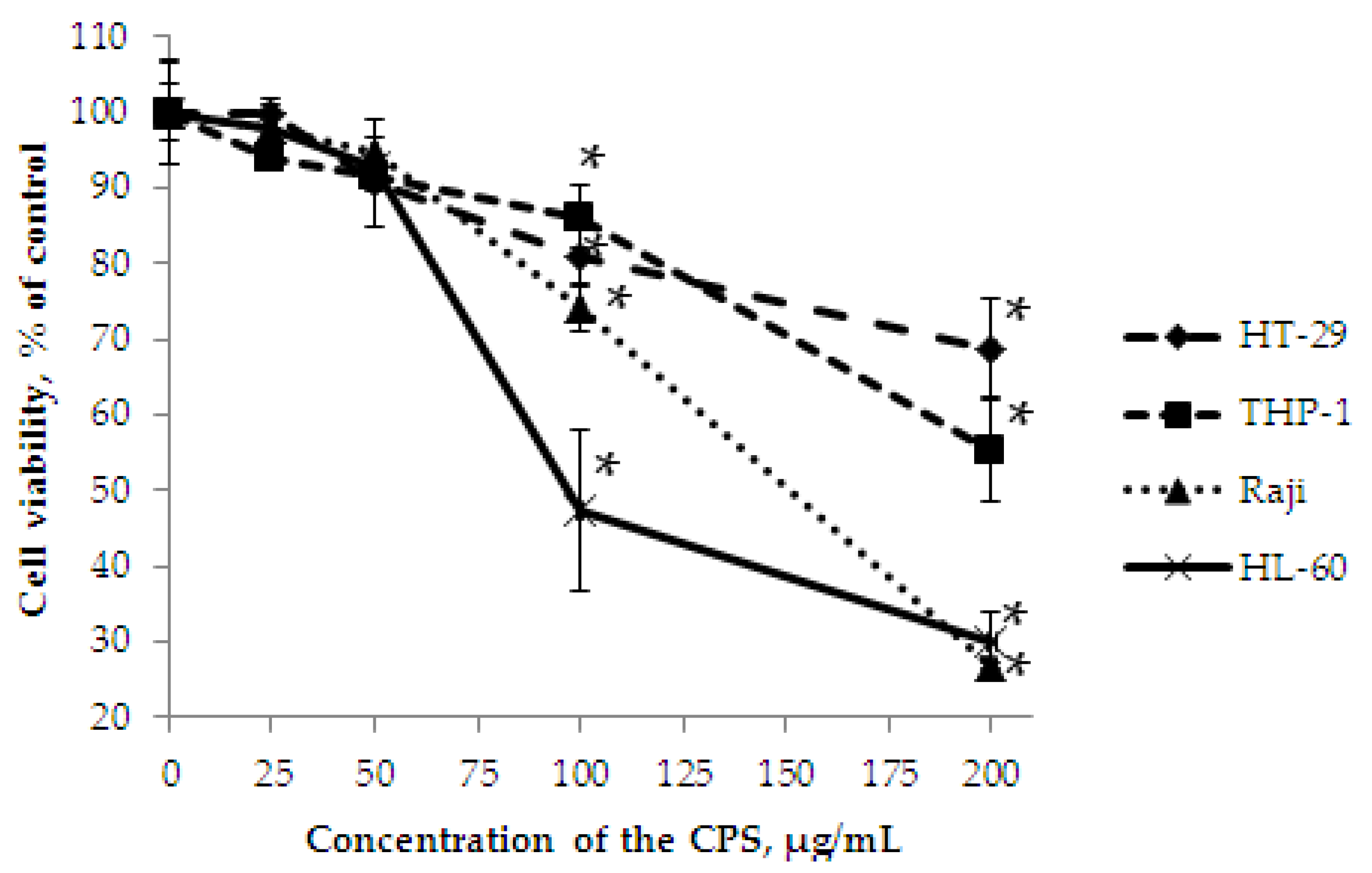
2.3. In Vitro Effect of the CPS on Cell Viability, and Colony Formation of Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Isolation and Purification of the CPS
4.2. Determination of the Molecular Weight and Electrophoretic Analysis of the CPS
4.3. Compositional Analysis of the CPS
4.4. Partial Acid Hydrolysis and Smith Degradation of the CPS
4.5. NMR Spectroscopy
4.6. Biological Activity
4.6.1. Cell Culture
4.6.2. Cell Viability Assay
4.6.3. Soft Agar Assay
4.6.4. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casillo, A.; Lanzetta, R.; Parrilli, M.; Corsaro, M.M. Exopolysaccharides from marine and marine extremophilic bacteria: Structures, properties, ecological roles and applications. Mar. Drugs 2018, 16, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poli, A.; Anzelmo, G.; Nicolaus, B. Bacterial exopolysaccharides from extreme marine habitats: Production, characterization and biological activities. Mar. Drugs 2010, 8, 1779–1802. [Google Scholar] [CrossRef] [PubMed]
- Nicolaus, B.; Kambourova, M.; Oner, E.T. Exopolysaccharides from extremophiles: From fundamentals to biotechnology. Environ. Technol. 2010, 31, 1145–1158. [Google Scholar] [CrossRef] [PubMed]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial exopolysaccharides: Functionality and prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildiz, H.; Karatas, N. Microbial exopolysaccharides: Resources and bioactive properties. Process Biochem. 2018, 72, 41–46. [Google Scholar] [CrossRef]
- Bowman, J.P.; McCammon, S.A.; Brown, M.V.; Nichols, D.S.; McMeekin, T.A. Diversity and association of psychrophilic bacteria in Antarctic sea ice. Appl. Environ. Microbiol. 1997, 63, 3068–3078. [Google Scholar] [CrossRef] [Green Version]
- Romanenko, L.A.; Tanaka, N.; Frolova, G.M.; Mikhailov, V.V. Psychrobacter fulvigenes sp. nov., isolated from a marine crustacean from the Sea of Japan. Int. J. Syst. Evol. Microbiol. 2009, 59, 1480–1486. [Google Scholar] [CrossRef] [Green Version]
- List of Prokaryotic Names with Standing in Nomenclature. Available online: https://lpsn.dsmz.de/genus/psychrobacter (accessed on 27 April 2020).
- Kondakova, A.N.; Novototskaya-Vlasova, K.A.; Drutskaya, M.S.; Senchenkova, S.N.; Shcherbakova, V.A.; Shashkov, A.S.; Gilichinsky, D.A.; Nedospasov, S.A.; Knirel, Y.A. Structure of the O-polysaccharide chain of the lipopolysaccharide of Psychrobacter muricolla 2pST isolated from overcooled water brines within permafrost. Carb. Res. 2012, 349, 78–81. [Google Scholar] [CrossRef]
- Kondakova, A.N.; Novototskaya-Vlasova, K.A.; Arbatsky, N.P.; Drutskaya, M.S.; Shcherbakova, V.A.; Shashkov, A.S.; Gilichinsky, D.A.; Nedospasov, S.A.; Knirel, Y.A. Structure of the O-specific polysaccharide from the lipopolysaccharide of Psychrobacter cryohalolentis K5T containing a 2,3,4-triacetamido-2,3,4-trideoxy-L-arabinose moiety. J. Nat. 2012, 75, 2236–2240. [Google Scholar] [CrossRef]
- Casillo, A.; Ricciardelli, A.; Parrilli, E.; Tutino, M.L.; Corsaro, M.M. Cell-wall associated polysaccharide from the psychrotolerant bacterium Psychrobacter arcticus 273-4: Isolation, purification and structural elucidation. Extremophiles 2020, 24, 63–70. [Google Scholar] [CrossRef]
- Kondakova, A.N.; Novototskaya-Vlasova, K.A.; Shashkov, A.S.; Drutskaya, M.S.; Senchenkova, S.N.; Shcherbakova, V.A.; Gilichinsky, D.A.; Nedospasov, S.A.; Knirel, Y.A. Structure of an acidic polysaccharide isolated from Psychrobacter maritimus 3pS containing a bacillosamine derivative. Carb. Res. 2012, 359, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Romanenko, L.A.; Schumann, P.; Rohde, M.; Lysenko, A.M.; Mikhailov, V.V.; Stackebrandt, E. Psychrobacter submarinus sp. nov. and Psychrobacter marincola sp. nov., psychrophilic halophiles from marine environments. Int. J. Syst. Evol. Microbiol. 2002, 52, 1291–1297. [Google Scholar] [PubMed] [Green Version]
- Leontein, K.; Lindberg, B.; Lönngren, J. Assignment of absolute configuration of sugars by G.L.C. of their acetylated glycosides formed from chiral alcohols. Carb. Res. 1978, 62, 359–362. [Google Scholar] [CrossRef]
- Gerwig, G.J.; Kamerling, J.P.; Vliegenthart, J.F.G. Determination of the absolute configuration of monosaccharides in complex carbohydrates by capillary G.L.C. Carb. Res. 1979, 77, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Sano, Y.; Kondo, S.; Isshiki, Y.; Shimada, T.; Hisatsune, K. An N-[(R)-(-)-2-hydroxypropionyl]-α-L-perosamine homopolymer constitutes the O polysaccharide chain of the lipopolysaccharide from Vibrio cholerae O144 which has antigenic factor(s) in common with V. cholerae O76. Microbiol. Immunol. 1996, 40, 735–741. [Google Scholar] [CrossRef] [Green Version]
- Knirel, Y.A.; Shashkov, A.S.; Tsvetkov, Y.E.; Jansson, P.-E.; Zähringer, U. 5,7-Diamino-3,5,7,9-tetradeoxynon-2-ulosonic acids in bacterial glycopolymers: Chemistry and biochemistry. Adv. Carbohydr. Chem. Biochem. 2003, 58, 371–417. [Google Scholar]
- Turska-Szewczuk, A.; Guz, L.; Lindner, B.; Pietras, H.; Russa, R.; Holst, O. Structural characterization of the O-specific polysaccharide from the lipopolysaccharide of the fish pathogen Aeromonas bestiarum strain P1S. Carb. Res. 2011, 346, 815–821. [Google Scholar] [CrossRef]
- Toukach, P.V. Bacterial Carbohydrate Structure Database 3: Principles and realization. J. Chem. Inf. Model. 2011, 51, 159–170. [Google Scholar] [CrossRef]
- Knirel, Y.A.; Shevelev, S.D.; Perepelov, A.V. Higher aldulosonic acids: Components of bacterial glycans. Mendeleev Commun. 2011, 21, 173–182. [Google Scholar] [CrossRef]
- Muldoon, J.; Shashkov, A.S.; Senchenkova, S.N.; Tomshich, S.V.; Komandrova, N.A.; Romanenko, L.A.; Knirel, Y.A.; Savage, A.V. Structure of an acidic polysaccharide from a marine bacterium Pseudoalteromonas distincta KMM 638 containing 5-acetamido-3,5,7,9-tetradeoxy-7-formamido-L-glycero-L-manno-nonulosonic acid. Carbohydr. Res. 2001, 330, 231–239. [Google Scholar] [CrossRef]
- Pereplov, A.V.; Shashkov, A.S.; Torgov, V.I.; Nazarenko, E.L.; Gorshkova, R.P.; Ivanova, E.P.; Gorshkova, N.M.; Widmalm, G. Structure of an acidic polysaccharide from the agar-decomposing marine bacterium Pseudoalteromonas atlantica strain IAM 14165 containing 5,7-diacetamido-3,5,7,9-tetradeoxy-L-glycero-L-manno-non-2-ulosonic acid. Carbohydr. Res. 2005, 340, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Pereplov, A.V.; Shashkov, A.S.; Tomshich, S.V.; Komandrova, N.A.; Nedashkovskaya, O.I. A pseudoaminic acid-containing O-specific polysaccharide from a marine bacterium Cellulophaga fucicola. Carbohydr. Res. 2007, 342, 1378–1381. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, G.; Ma, W.; Lu, Z.; Sun, C. Marine bacterial polysaccharide EPS11 inhibits cancer cell growth and metastasis via blocking cell adhesion and attenuating filiform structure formation. Mar. Drugs 2019, 17, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramamoorthy, S.; Gnanakan, A.; Lakshmana, S.S.; Meivelu, M.; Jeganathan, A. Structural characterization and anticancer activity of extracellular polysaccharides from ascidian symbiotic bacterium Bacillus thuringiensis. Carbohydr. Polym. 2018, 190, 113–120. [Google Scholar] [CrossRef]
- Athukorala, Y.; Ahn, G.N.; Jee, Y.-H.; Kim, G.-Y.; Kim, S.-H.; Ha, J.-H.; Kang, J.-S.; Lee, K.-W.; Jeon, Y.-J. Antiproliferative activity of sulfated polysaccharide isolated from an enzymatic digest of Ecklonia cava on the U-937 cell line. J. Appl. Phycol. 2009, 21, 307–314. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Lee, H.G.; Kim, H.-S.; Vaas, A.P.J.P.; De Silva, H.I.C.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.-S.; Lee, J.-S.; et al. Fucoidan purified from Sargassum polycystum induces apoptosis through mitochondria-mediated pathway in HL-60 and MCF-7 cells. Mar. Drugs 2020, 18, 196. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.-O.; Song, M.-G.; Kim, Y.-N.; Park, J.-I.; Kwak, J.-Y. The mechanism of fucoidan-induced apoptosis in leukemic cells: Involvement of ERK1/2, JNK, glutathione, and nitric oxide. Mol. Carcinog. 2010, 49, 771–782. [Google Scholar] [CrossRef]
- Ko, S.-C.; Lee, S.-H.; Ahn, G.; Kim, K.-N.; Cha, S.-H.; Kim, S.-K.; Jeon, B.-T.; Park, P.-J.; Lee, K.-W.; Jeon, Y.-J. Effect of enzyme-assisted extract of Sargassum coreanum on induction of apoptosis in HL-60 tumor cells. J. Appl. Phycol. 2012, 24, 675–684. [Google Scholar] [CrossRef]
- Wang, H.; Chiu, L.C.M.; Ooi, V.E.C.; Ang, P.O., Jr. A potent antitumor polysaccharide from the edible brown seaweed Hydroclathrus clathratus. Bot. Mar. 2010, 53, 265–274. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, W.-J.; Yan, M.-X.; Liu, X.; Wang, S.-Y.; Xia, Z.; Xiao, B.; Cao, S.-J.; Yang, B.-Q.; Li, J. Purification, chemical characterization, and bioactivity of an extracellular polysaccharide produced by the marine sponge endogenous fungus Alternaria sp. SP-32. Mar. Biotechnol. 2016, 18, 301–313. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Hsu, M.-L.; Hsu, H.-C.; Tzeng, C.-H.; Lee, S.-S.; Shiao, M.-S.; Ho, C.-K. The anti-tumor effect of Ganoderma Lucidum is mediated by cytokines released from activated macrophages and t lymphocytes. Int. J. Cancer 1997, 70, 699–705. [Google Scholar] [CrossRef]
- Kokoulin, M.S.; Komandrova, N.A.; Kalinovsky, A.I.; Tomshich, S.V.; Romanenko, L.A.; Vaskovsky, V.E. Structure of the O-specific polysaccharide from the deep-sea marine bacterium Idiomarina abyssalis КММ 227T containing a 2-O-sulfate-3-N-(4-hydroxybutanoyl)-3,6-dideoxy-D-glucose. Carb. Res. 2015, 413, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S.I. A rapid permethylation of glycolipid, and polysaccharide catalyzed by methylsulfinyl carbanion in dimethyl sulfoxide. J. Biochem. 1964, 55, 205–208. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Barltrop, J.A.; Owen, T.C.; Cory, A.H.; Cory, J.G. 5-(3-carboxymethoxyphenyl)-2-(4,5-dimethylthiazolyl)-3-(4-sulfophenyl)te trazolium, inner salt (MTS) and related analogs of 3-(4,5-dimethylthiazolyl)-2,5-diphenyltetrazolium bromide (MTT) reducing to purple water-soluble formazans As cell-viability indicators. Bioorg. Med. Chem. Lett. 1991, 1, 611–614. [Google Scholar]
- Kokoulin, M.S.; Kuzmich, A.S.; Kalinovsky, A.I.; Rubtsov, E.S.; Romanenko, L.A.; Mikhailov, V.V.; Komandrova, N.A. Structure and anticancer activity of sulfated O-polysaccharide from marine bacterium Poseidonocella pacifica KMM 9010T. Carbohydr. Polym. 2017, 178, 406–411. [Google Scholar] [CrossRef]





| Sugar Residue | H-1 C-1 | H-2 C-2 | H-3 C-3 | H-4 C-4 | H-5 C-5 | H-6 C-6 |
|---|---|---|---|---|---|---|
| →3)-α-d-GalpNAcA-(1→ A | 5.38 99.5 | 4.29 49.0 | 3.95 77.1 | 4.50 70.1 | 4.23 72.0 | 174.6 |
| →3)-β-d-GlcpNAc-(1→ B | 4.60 103.3 | 3.78 55.5 | 3.71 81.1 | 3.68 72.0 | 3.42 76.6 | 3.91, 3.78 61.3 |
| Sugar Residue | H-1 C-1 | H-2 C-2 | H-3eq,ax C-3 | H-4 C-4 | H-5 C-5 | H-6a,b C-6 | H-7 C-7 | H-8 C-8 | H-9 C-9 |
|---|---|---|---|---|---|---|---|---|---|
| OS-1 | |||||||||
| →2)-β-d-Ribp C | 5.13 93.8 | 3.77 79.3 | 4.17 67.8 | 3.83 68.6 | 3.87, 3.71 64.1 | ||||
| →3)-β-d-GalpNAcA-(1→ D | 4.80 101.0 | 4.05 51.9 | 3.97 77.3 | 4.55 66.6 | 4.39 74.7 | 172.8 | |||
| →2)-α-d-Glcp-(1→ E | 5.06 96.4 | 3.94 74.7 | 3.71 72.1 | 3.51 70.3 | 3.60 73.5 | 3.81, 3.78 61.5 | |||
| β-Psep5(R-Lac)7Ac -(2→ F | 173.4 | 101.4 | 2.52, 1.76 35.7 | 3.97 67.3 | 4.22 49.0 | 3.84 74.6 | 4.06 54.7 | 4.17 69.2 | 1.17 17.2 |
| R-Lac-(1→ | 178.9 | 4.26 69.3 | 1.38 20.9 | ||||||
| OS-2 | |||||||||
| →2)-β-d-Ribp C | 5.13 93.8 | 3.78 79.3 | 4.18 67.8 | 3.83 68.7 | 3.87, 3.71 64.1 | ||||
| →3)-β-d-GalpNAcA-(1→ D | 4.79 101.1 | 4.08 51.8 | 3.98 76.2 | 4.53 66.5 | 4.33 75.1 | 173.2 | |||
| →2)-α-d-Glcp-(1→ E | 5.13 96.7 | 3.56 72.4 | 3.65 74.0 | 3.44 70.4 | 3.60 73.7 | 3.81, 3.78 61.5 | |||
| Sugar Residue | H-1 C-1 | H-2 C-2 | H-3 C-3 | H-4 C-4 | H-5 C-5 | H-6 C-6 |
|---|---|---|---|---|---|---|
| →3)-α-d-GalpNAcA-(1→ A | 5.19 100.4 | 4.26 49.6 | 3.96 77.0 | 4.56 75.7 | 4.19 72.1 | n.d |
| →3)-β-d-GlcpNAc-(1→ B | n.d 104.1 | n.d 55.9 | n.d 81.9 | 3.50 72.1 | 3.38 77.0 | 3.93, 3.75 61.9 |
| →2)-β-d-Ribf-(1→ C | 5.55 105.3 | 4.16 84.4 | 4.11 72.1 | 3.98 83.0 | 3.77, 3.65 64.7 | |
| β-d-GalpNAcA-(1→ D | 4.92 102.6 | 4.03 53.5 | 3.88 71.4 | 4.25 70.6 | 4.61 75.7 | n.d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokoulin, M.S.; Kuzmich, A.S.; Romanenko, L.A.; Chikalovets, I.V.; Chernikov, O.V. Structure and In Vitro Bioactivity against Cancer Cells of the Capsular Polysaccharide from the Marine Bacterium Psychrobacter marincola. Mar. Drugs 2020, 18, 268. https://doi.org/10.3390/md18050268
Kokoulin MS, Kuzmich AS, Romanenko LA, Chikalovets IV, Chernikov OV. Structure and In Vitro Bioactivity against Cancer Cells of the Capsular Polysaccharide from the Marine Bacterium Psychrobacter marincola. Marine Drugs. 2020; 18(5):268. https://doi.org/10.3390/md18050268
Chicago/Turabian StyleKokoulin, Maxim S., Alexandra S. Kuzmich, Lyudmila A. Romanenko, Irina V. Chikalovets, and Oleg V. Chernikov. 2020. "Structure and In Vitro Bioactivity against Cancer Cells of the Capsular Polysaccharide from the Marine Bacterium Psychrobacter marincola" Marine Drugs 18, no. 5: 268. https://doi.org/10.3390/md18050268





