Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector
Abstract
:1. Introduction
2. Description, Growth, and Distribution
3. Canadian Landings, Processing, Commercial Products, and Trade Market
4. Proximate Composition
5. Bioactive Compounds and Methods of their Extraction and Isolation
5.1. Polysaccharides
5.2. Fucoidan
5.3. Collagen
5.4. Saponins
5.5. Phenolic Compounds
6. Potential Biological Activities and Medicinal Effects
6.1. Anticancer Activities
6.2. Antitumor Activities
6.3. Antithrombotic and Anticoagulant Activities
6.4. Anti-Hyperglycemic Activities
6.5. Immunomodulatory Activities
6.6. Anti-Inflammatory Activities
6.7. Antioxidant Activities
6.8. Antimicrobial Activities
6.9. Anti-Angiogenic Activities
6.10. Antihypertensive Activities
6.11. Other Activities
7. Prospects, Challenges, and Future Aspects of Atlantic Sea Cucumber
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wen, J.; Hu, C.; Fan, S. Chemical composition and nutritional quality of sea cucumbers. J. Sci. Food Agric. 2010, 90, 2469–2474. [Google Scholar] [CrossRef] [PubMed]
- Eckert, G.L. Sea cucumbers. In Encyclopedia of Tidepools and Rocky Shores; Denny, M.W., Gaines, S.D., Eds.; University of California Press: London, UK, 2007; pp. 491–492. [Google Scholar]
- Purcell, S.W.; Lovatelli, A.; Vasconcellos, M.; Ye, Y. Managing Sea Cucumber Fisheries with an Ecosystem Approach; FAO: Rome, Italy, 2010. [Google Scholar]
- Aminin, D.L.; Menchinskaya, E.S.; Pisliagin, E.A.; Silchenko, A.S.; Avilov, S.A.; Kalinin, V.I. Anticancer activity of sea cucumber triterpene glycosides. Mar. Drugs 2015, 13, 1202–1223. [Google Scholar] [CrossRef] [PubMed]
- Bordbar, S.; Anwar, F.; Saari, N. High-value components and bioactives from sea cucumbers for functional foods -A review. Mar. Drugs 2011, 9, 1761–1805. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.X.; Zhou, D.Y.; Ma, D.D.; Liu, Z.Q.; Liu, Y.F.; Song, L.; Dong, X.P.; Li, D.M.; Zhu, B.W.; Konno, K.; et al. Effects of endogenous cysteine proteinases on structures of collagen fibres from dermis of sea cucumber (Stichopus japonicus). Food Chem. 2017, 232, 10–18. [Google Scholar] [CrossRef]
- Trotter, J.A.; Lyons-Levy, G.; Chino, K.; Koob, T.J.; Keene, D.R.; Atkinson, M.A.L. Collagen fibril aggregation-inhibitor from sea cucumber dermis. Matrix Biol. 1999, 18, 569–578. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, D.-Y.; Yin, F.-W.; Song, L.; Liu, Y.-X.; Xie, H.-K.; Gang, K.-Q.; Zhu, B.-W.; Shahidi, F. Glycerophospholipids in sea cucumber (Stichopus japonicus) and its processing by-products serve as bioactives and functional food ingredients. J. Food Bioact. 2018, 1, 134–142. [Google Scholar] [CrossRef]
- Aminin, D.L.; Chaykina, E.L.; Agafonova, I.G.; Avilov, S.A.; Kalinin, V.I.; Stonik, V.A. Antitumor activity of the immunomodulatory lead Cumaside. Int. Immunopharmacol. 2010, 10, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Nagase, B.H.; Enjyoji, K.; Minamiguchi, K.; Kitazato, K.T.; Kitazato, K.; Saito, H.; Kato, H. Depolymerized holothurian glycosaminoglycan with novel anticoagulant actions: Antithrombin 111- and heparin cofactor 11-independent inhibition of factor x activation by factor ixa-factor viiia complex and heparin cofactor II-dependent inhibition of thrombin. Blood 1995, 1527–1534. [Google Scholar]
- Vieira, R.P.; Mulloy, B.; Mourão, P.P. Structure of a fucose-branched chondroitin sulfate from sea cucumber. J. Biol. Chem. 1991, 266, 13530–13536. [Google Scholar] [PubMed]
- Mourao, P.A.S.; Bastos, G.I. Highly acidic glycans from sea cucumbers. Eur. J. Biochem. 1987, 645, 639–645. [Google Scholar] [CrossRef]
- Mourao, P.A.S.; Pereira, M.S.; Pavao, M.S.G. Structure and anticoagulant activity of a fucosylated chondroitin sulfate from echinoderm. J. Biol. Chem. 1996, 271, 23973–23984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mamelona, J.; Pelletier, É.; Girard-Lalancette, K.; Legault, J.; Karboune, S.; Kermasha, S. Quantification of phenolic contents and antioxidant capacity of Atlantic sea cucumber, Cucumaria frondosa. Food Chem. 2007, 104, 1040–1047. [Google Scholar] [CrossRef]
- Ahmed, M.R.; Venkateshwarlu, U.; Jayakumar, R. Multilayered peptide incorporated collagen tubules for peripheral nerve repair. Biomaterials 2004, 25, 2585–2594. [Google Scholar] [CrossRef] [PubMed]
- Mojica, E.R.E.; Merca, F. Lectin from the body walls of black sea cucumber (Holothuria atra Jäger). Philipp. J. Sci. 2004, 133, 77–85. [Google Scholar]
- Ming-Ping, L.A.; Jun-Jie, S.; Jian, J.; Yang-Hua, Y.I. Three cerebrosides from the sea cucumber Cucumaria frondosa. Chin. J. Nat. Med. 2012, 10, 105–109. [Google Scholar]
- Sugawara, T.; Zaima, N.; Yamamoto, A.; Sakai, S.; Noguchi, R.; Hirata, T. Isolation of sphingoid bases of sea cucumber cerebrosides and their cytotoxicity against human colon cancer cells. Biosci. Biotechnol. Biochem. 2006, 70, 2906–2912. [Google Scholar] [CrossRef]
- Findlay, J.A.; Daljeet, A.; Moharir, Y.E. Some constituents of the sea cucumber Cucumaria frondosa. Mar. Chem. 1983, 12, 228. [Google Scholar]
- Yahyavi, M.; Afkhami, M.; Javadi, A.; Ehsanpour, M.; Khazaali, A.; Khoshnood, R.; Mokhlesi, A. Fatty acid composition in two sea cucumber species, Holothuria scabra and Holothuria leucospilata from Qeshm Island (Persian Gulf). Afr. J. Biotechnol. 2012, 11, 2862–2868. [Google Scholar]
- Chen, J. Overview of sea cucumber farming and sea ranching practices in China. SPC Beche-mer Inf. 18 Bull. 2003, 18, 18–23. [Google Scholar]
- Janakiram, N.B.; Mohammed, A.; Rao, C.V. Sea cucumbers metabolites as potent anti-cancer agents. Mar. Drugs 2015, 13, 2909–2923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Liu, Y.; Hao, J.; Zhao, X.; Lang, Y.; Fan, F.; Cai, C.; Li, G.; Zhang, L.; Yu, G. In Vivo anti-cancer mechanism of low-molecular-weight fucosylated chondroitin sulfate (LFCS) from sea cucumber Cucumaria frondosa. Molecules 2016, 21, 625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, F.; Zhang, X.; Tong, Y.; Yi, Y.; Zhang, S.; Li, L.; Sun, P.; Lin, L.; Ding, J. PE, a new sulfated saponin from sea cucumber, exhibits anti-angiogenic and anti-tumor activities in vitro and in vivo. Cancer Biol. Ther. 2005, 4, 874–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Xue, C.; Yin, L.; Tang, Q.; Yu, G.; Chai, W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011, 83, 688–696. [Google Scholar] [CrossRef]
- Mourão, P.A.S.; Boisson-Vidal, C.; Tapon-Bretaudière, J.; Drouet, B.; Bros, A.; Fischer, A.M. Inactivation of thrombin by a fucosylated chondroitin sulfate from echinoderm. Thromb. Res. 2001, 102, 167–176. [Google Scholar] [CrossRef]
- Borsig, L.; Wang, L.; Cavalcante, M.C.M.; Cardilo-Reis, L.; Ferreira, P.L.; Mourão, P.A.S.; Esko, J.D.; Pavão, M.S.G. Selectin blocking activity of a fucosylated chondroitin sulfate glycosaminoglycan from sea cucumber: Effect on tumor metastasis and neutrophil recruitment. J. Biol. Chem. 2007, 282, 14984–14991. [Google Scholar] [CrossRef] [Green Version]
- Whitehouse, M.W.; Fairlie, D.P. Anti-Inflammatory activity of a holothurian (sea cucumber) food supplement in rats. Inflammopharmacology 1994, 2, 411–417. [Google Scholar] [CrossRef]
- Kiew, P.L.; Don, M.M. Jewel of the seabed: Sea cucumbers as nutritional and drug candidates. Int. J. Food Sci. Nutr. 2012, 63, 616–636. [Google Scholar] [CrossRef]
- Beauregard, K.A.; Truong, N.T.; Zhang, H.; Lin, W.; Beck, G. The detection and isolation of a novel antimicrobial peptide from the echinoderm, Cucumaria frondosa. Adv. Exp. Med. Biol. 2001, 484, 55–62. [Google Scholar]
- Hing, H.; Ambia, K.M.; Azraul-Mumtazah, R.; Hamidah, S.; Sahalan, A.; Shamsudin, N.; Shamsudin, M.; Hashim, R. Effect of methanol extracts from sea cucumbers Holothuria edulis and Stichopus chloronotus on Candida albicans. Microsc. Microanal. 2007, 13, 48–50. [Google Scholar] [CrossRef]
- Althunibat, O.Y.; Hashim, R.B.; Taher, M.; Daud, J.M.; Ikeda, M.-A.; Zali, B.I. In vitro antioxidant and antiproliferative activities of three Malaysian sea cucumber species. Eur. J. Sci. Res. 2009, 37, 376–387. [Google Scholar]
- Zou, S.; Pan, R.; Dong, X.; He, M.; Wang, C. Physicochemical properties and antioxidant activities of two fucosylated chondroitin sulfate from sea cucumber Acaudina molpadioidea and Holothuria nobilis. Process Biochem. 2016, 51, 650–658. [Google Scholar] [CrossRef]
- San Miguel-Ruiz, J.E.; García-Arrarás, J.E. Common cellular events occur during wound healing and organ regeneration in the sea cucumber Holothuria glaberrima. BMC Dev. Biol. 2007, 7, 115. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Pan, R.; Deng, X.; Chen, Y.; Zhao, G.; Wang, C. Separation, purification, anticoagulant activity and preliminary structural characterization of two sulfated polysaccharides from sea cucumber Acaudina molpadioidea and Holothuria nobilis. Process Biochem. 2014, 49, 1352–1361. [Google Scholar] [CrossRef]
- Siahaan, E.; Pangestuti, R.; Munandar, H.; Kim, S.-K. Cosmeceuticals properties of sea cucumbers: Prospects and trends. Cosmetics 2017, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Taiyeb-Ali, T.B.; Zainuddin, S.L.A.; Swaminathan, D.; Yaacob, H. Efficacy of “Gamadent” toothpaste on the healing of gingival tissues: A preliminary report. J. Oral Sci. 2003, 45, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Fredalina, B.D.; Ridzwan, B.H.; Abidin, A.A.Z.; Kaswandi, M.A.; Zaiton, H.; Zali, I.; Kittakoop, P.; Mat Jais, A.M. Fatty acid compositions in local sea cucumber, Stichopus chloronotus, for wound healing. Gen. Pharmacol. 1999, 33, 337–340. [Google Scholar] [CrossRef]
- Hamel, J.F.; Mercier, A. Diet and feeding behaviour of the sea cucumber Cucumaria frondosa in the St. Lawrence estuary, eastern Canada. Can. J. Zool. 1998, 76, 1194–1198. [Google Scholar] [CrossRef]
- Pantin, J.R.; Coughlan, E.J.; Hynick, E.M.; Skanes, K.R. An assessment of the sea cucumber (Cucumaria frondosa) resource on the St. Pierre Bank (NAFO subdivision 3Ps) in 2016. DFO Can. Sci. Advis. Secr. 2018, 024, 23. [Google Scholar]
- Koob, T.J.; Koob-Emunds, M.M.; Trotter, J.A. Cell-derived stiffening and plasticizing factors in sea cucumber (Cucumaria frondosa) dermis. J. Exp. Biol. 1999, 202, 2291–2301. [Google Scholar] [PubMed]
- Mo, J.; Prévost, S.F.; Blowes, L.M.; Egertová, M.; Terrill, N.J.; Wang, W.; Elphick, M.R.; Gupta, H.S. Interfibrillar stiffening of echinoderm mutable collagenous tissue demonstrated at the nanoscale. Proc. Natl. Acad. Sci. USA 2016, 113, E6362–E6371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DFO. An assessment of the Sea Cucumber (Cucumaria frondosa) Resource on the St. Pierre Bank (NAFO subdivision 3PS) in 2016. Newfoundland and Labrador Region, Science Advisory Report; Fisheries and Oceans, Science, Newfoundland and Labrador Region: St. John’s, NL, Canada, 2017; pp. 1–8. [Google Scholar]
- Hamel, J.; Mercier, A. Early development, settlement, growth, and spatial distribution of the sea cucumber Cucumaria frondosa (Echinodermata: Holothuroidea). Can. J. Fish. Aquat. Sci. 1996, 53, 253–271. [Google Scholar] [CrossRef]
- Bruckner, A.W. The recent status of sea cucumber fisheries in the continental United States of America. SPC Beche-mer Inf. Bull. 2005, 22, 39–46. [Google Scholar]
- Hamel, J.-F.; Mercier, A. Precautionary Management of Cucumaria frondosa in Newfoundland and Labrador, Canada. In A Global Review of Fisheries and Trade, FAO Fisheries and Aquaculture Technical Paper, No. 516; FAO: Rome, Italy, 2008; pp. 293–306. [Google Scholar]
- Nelson, E.J.; MacDonald, B.A.; Robinson, S.M.C. A review of the northern sea cucumber Cucumaria frondosa (Gunnerus, 1767) as a potential aquaculture species. Rev. Fish. Sci. 2012, 20, 212–219. [Google Scholar] [CrossRef]
- Gianasi, B.L.; Parrish, C.C.; Hamel, J.-F.; Mercier, A. Influence of diet on growth, reproduction and lipid and fatty acid composition in the sea cucumber Cucumaria frondosa. Aquac. Res. 2017, 48, 3413–3432. [Google Scholar] [CrossRef]
- DFO. Seafisheries landings, Fisheries and Oceans Canada. 2018. Available online: http://www.dfo-mpo.gc.ca/stats/commercial/sea-maritimes-eng.htm (accessed on 20 May 2020).
- Gianasi, B.L.; Hamel, J.F.; Mercier, A. Experimental test of optimal holding conditions for live transport of temperate sea cucumbers. Fish. Res. 2016, 174, 298–308. [Google Scholar] [CrossRef]
- Yan, L.J.; Zhan, C.L.; Cai, Q.F.; Weng, L.; Du, C.H.; Liu, G.M.; Su, W.J.; Cao, M.J. Purification, characterization, cdna cloning and in vitro expression of a serine proteinase from the intestinal tract of sea cucumber (stichopus japonicus) with collagen degradation activity. J. Agric. Food Chem. 2014, 62, 4769–4777. [Google Scholar] [CrossRef]
- Zhong, Y.; Khan, M.A.; Shahidi, F. Compositional Characteristics and Antioxidant Properties of Fresh and Processed Sea Cucumber (Cucumaria frondosa). J. Agric. Food Chem. 2007, 1188–1192. [Google Scholar] [CrossRef]
- Mamelona, J.; Saint-Louis, R.; Pelletier, É. Proximate composition and nutritional profile of by-products from green urchin and Atlantic sea cucumber processing plants. Int. J. Food Sci. Technol. 2010, 45, 2119–2126. [Google Scholar] [CrossRef]
- Kale, V.; Freysdottir, J.; Paulsen, B.S.; Friojónsson, Ó.H.; Óli Hreggviosson, G.; Omarsdottir, S. Sulphated polysaccharide from the sea cucumber Cucumaria frondosa affect maturation of human dendritic cells and their activation of allogeneic CD4(+) T cells in vitro. Bioact. Carbohydrates Diet. Fibre 2013, 2, 108–117. [Google Scholar] [CrossRef]
- Ustyuzhanina, N.E.; Bilan, M.I.; Dmitrenok, A.S.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. The structure of a fucosylated chondroitin sulfate from the sea cucumber Cucumaria frondosa. Carbohydr. Polym. 2017, 165, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Li, S.; Song, W.; Ji, L.; Cai, L.; Wang, Y.; Jiang, W. Fucoidan from Cucumaria frondosa inhibits pancreatic islets apoptosi through mitochondrial signaling pathway in insulin resistant mice. Food Sci. Technol. Res. 2016, 22, 507–517. [Google Scholar] [CrossRef] [Green Version]
- Malavaki, C.; Mizumoto, S.; Karamanos, N.; Sugahara, K. Recent advances in the structural study of functional chondroitin sulfate and dermatan sulfate in health and disease. Connect. Tissue Res. 2008, 49, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Meng, Y.-C.; Li, J.; Chen, J.; Liu, Y.; Bai, X. Chondroitin sulfate: Extraction, purification, microbial and chemical synthesis. J. Chem. Technol. Biotechnol. 2014, 89, 1445–1465. [Google Scholar] [CrossRef]
- Kim, C.-T.; Gujral, N.; Ganguly, A.; Suh, J.-W.; Sunwoo, H.H. Chondroitin sulphate extracted from antler cartilage using high hydrostatic pressure and enzymatic hydrolysis. Biotechnol. Rep. 2014, 4, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.A.C.; Barcellos, P.G.; Queiroz, I.N.L.; Santos, G.R.C.; Soares, P.A.G.; Pomin, V.H.; Mourao, P.A.S.; Ribeiro, K.A.; Pereira, M.S. Depolymerization of fucosylated chondroitin sulfate from sea cucumber using hydrogen peroxide and copper ions. In Proceedings of the 44th Annual Meeting of the Brazilian Society for Biochemistry and Molecular Biology, Foz do Iguacu, Brazil, 24–28 August 2015. [Google Scholar]
- Srichamroen, A.; Nakano, T.; Pietrasik, Z.; Ozimek, L.; Betti, M. Chondroitin sulfate extraction from broiler chicken cartilage by tissue autolysis. LWT Food Sci. Technol. 2013, 50, 607–612. [Google Scholar] [CrossRef]
- Wu, N.; Ye, X.; Guo, X.; Liao, N.; Yin, X.; Hu, Y.; Sun, Y.; Liu, D.; Chen, S. Depolymerization of fucosylated chondroitin sulfate from sea cucumber, Pearsonothuria graeffei, via 60Co irradiation. Carbohydr. Polym. 2013, 93, 604–614. [Google Scholar] [CrossRef]
- Falch, B.H.; Espevik, T.; Ryan, L.; Stokke, B.T. The cytokine stimulating activity of (1→3)-β-D-glucans is dependent on the triple helix conformation. Carbohydr. Res. 2000, 329, 587–596. [Google Scholar] [CrossRef]
- Morya, V.K.; Kim, J.; Kim, E.K. Algal fucoidan: Structural and size-dependent bioactivities and their perspectives. Appl. Microbiol. Biotechnol. 2012, 93, 71–82. [Google Scholar] [CrossRef]
- Chen, S.; Hu, Y.; Ye, X.; Li, G.; Yu, G.; Xue, C.; Chai, W. Sequence determination and anticoagulant and antithrombotic activities of a novel sulfated fucan isolated from the sea cucumber Isostichopus badionotus. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 989–1000. [Google Scholar] [CrossRef] [PubMed]
- Kariya, Y.; Mulloy, B.; Imai, K.; Tominaga, A.; Kaneko, T.; Asari, A.; Suzuki, K.; Masuda, H.; Kyogashima, M.; Ishii, T. Isolation and partial characterization of fucan sulfates from the body wall of sea cucumber Stichopus japonicus and their ability to inhibit osteoclastogenesis. Carbohydr. Res. 2004, 339, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Su, W.; Zhang, C.; Xue, C.; Chang, Y.; Wu, X.; Tang, Q.; Wang, J. Protective effect of sea cucumber (Acaudina molpadioides) fucoidan against ethanol-induced gastric damage. Food Chem. 2012, 133, 1414–1419. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhao, Y.; Hu, S.; Shi, D.; Xue, C. Fucoidan from sea cucumber Cucumaria frondosa exhibits anti-hyperglycemic effects in insulin resistant mice via activating the PI3K/PKB pathway and GLUT4. J. Biosci. Bioeng. 2016, 121, 36–42. [Google Scholar] [CrossRef]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Sherman, V.R.; Yang, W.; Meyers, M.A. The materials science of collagen. J. Mech. Behav. Biomed. Mater. 2015, 52, 22–50. [Google Scholar] [CrossRef]
- Dave, D.; Liu, Y.; Clark, L.; Dave, N.; Trenholm, S.; Westcott, J. Availability of marine collagen from Newfoundland fisheries and aquaculture waste resources. Bioresour. Technol. Rep. 2019, 7, 100271. [Google Scholar] [CrossRef]
- Liu, D.; Nikoo, M.; Boran, G.; Zhou, P.; Regenstein, J.M. Collagen and Gelatin. Annu. Rev. Food Sci. Technol. 2015, 6, 527–557. [Google Scholar] [CrossRef]
- Wood, A.; Ogawa, M.; Portier, R.J.; Schexnayder, M.; Shirley, M.; Losso, J.N. Biochemical properties of alligator (Alligator mississippiensis) bone collagen. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 246–249. [Google Scholar] [CrossRef]
- Silvipriya, K.S.; Krishna Kumar, K.; Bhat, A.R.; Dinesh Kumar, B.; John, A.; Lakshmanan, P. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Saito, M.; Kunisaki, N.; Urano, N.; Kimura, S. Collagen as the major edible component of sea cucumber (Stichopus japonicus). J. Food Sci. 2002, 67, 1319–1322. [Google Scholar] [CrossRef]
- Liu, Y.X.; Liu, Z.Q.; Song, L.; Ma, Q.R.; Zhou, D.Y.; Zhu, B.W.; Shahidi, F. Effects of collagenase type I on the structural features of collagen fibres from sea cucumber (Stichopus japonicus) body wall. Food Chem. 2019, 301, 125302. [Google Scholar] [CrossRef] [PubMed]
- Tipper, J.P.; Lyons-Levy, G.; Atkinson, M.A.L.; Trotter, J.A. Purification, characterization and cloning of tensilin, the collagen-fibril binding and tissue-stiffening factor from Cucumaria frondosa dermis. Matrix Biol. 2002, 21, 625–635. [Google Scholar] [CrossRef]
- Trotter, J.A.; Lyons-Levy, G.; Thurmond, F.A.; Koob, T.J. Covalent composition of collagen fibrils from the dermis of the sea cucumber, Cucumaria frondosa, a tissue with mutable mechanical properties. Comp. Biochem. Physiol. Part Physiol. 1995, 112, 463–478. [Google Scholar] [CrossRef]
- Nigrelli, R.F.; Jakowska, S. Effects of Holothurin, a steroid saponin from the Bahamian sea cucumber (Actinopyga agassizi), on various biological systems. Ann. N. Y. Acad. Sci. 1960, 90, 884–892. [Google Scholar] [CrossRef]
- Mondol, M.A.M.; Shin, H.J.; Rahman, M.A.; Islam, M.T. Sea cucumber glycosides: Chemical structures, producing species and important biological properties. Mar. Drugs 2017, 15, 317. [Google Scholar] [CrossRef] [Green Version]
- Avilov, S.A.; Drozdova, O.A.; Kalinin, V.I.; Kalinovsky, A.I.; Stonik, V.A.; Gudimova, E.N.; Riguera, R.; Jimenez, C.; Frondoside, C. A new nonholostane triterpene glycoside from the sea cucumber Cucumaria frondosa: Structure and cytotoxicity of its desulfated derivative. Can. J. Chem. 1998, 76, 137–141. [Google Scholar]
- Findlay, J.A.; Daljeet, A.; Matsoukas, J.; Moharir, Y.E. Constituents of the sea cucumber cucumaria frondosa. J. Nat. Prod. 1984, 47, 560. [Google Scholar] [CrossRef]
- Findlay, J.A.; Yayli, N.; Radics, L. Novel sulfated oligosaccharides from the sea cucumber cucumaria frondosa. J. Nat. Prod. 1992, 55, 93–101. [Google Scholar] [CrossRef]
- Yayli, N. Minor saponins from the sea cucumber Cucumaria frondosa. Indian J. Chem. Sect. B Org. Med. Chem. 2001, 40, 399–404. [Google Scholar]
- Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Smirnov, A.V. Sea cucumbers triterpene glycosides, the recent progress in structural elucidation and chemotaxonomy. Phytochem. Rev. 2005, 4, 221–236. [Google Scholar] [CrossRef]
- Girard, M.; Bélanger, J.; ApSimon, J.W.; Garneau, F.-X.; Harvey, C.; Brisson, J.-R. Frondoside A. A novel triterpene glycoside from the holothurian Cucumaria frondosa. Can. J. Chem. 1990, 68, 11–18. [Google Scholar] [CrossRef]
- Yayli, N.; Findlay, J.A. A triterpenoid saponin from Cucumaria frondosa. Phytochemistry 1999, 50, 135–138. [Google Scholar] [CrossRef]
- Tian, F.; Zhu, C.; Zhang, X.; Xie, X.; Xin, X.; Yi, Y.; Lin, L.; Geng, M.; Ding, J. Philinopside E, A new sulfated saponin from sea cucumber, blocks the interaction between kinase insert domain-containing receptor (KDR) and avb3 integrin via binding to the extracellular domain of KDR. Mol. Pharmacol. 2007, 72, 545–552. [Google Scholar] [CrossRef] [Green Version]
- Macheix, J.J.; Fleuriet, A.; Billot, J. Fruit Phenolics; CRC Press: Boca Raton, FL, USA, 1990; pp. 101–126. [Google Scholar]
- Hossain, A.; Moon, H.K.; Kim, J.-K. Antioxidant properties of Korean major persimmon (Diospyros kaki) leaves. Food Sci. Biotechnol. 2018, 27, 177–184. [Google Scholar] [CrossRef]
- Mamelona, J.; Saint-Louis, R.; Pelletier, É. Nutritional composition and antioxidant properties of protein hydrolysates prepared from echinoderm byproducts. Int. J. Food Sci. Technol. 2009, 45, 147–154. [Google Scholar] [CrossRef]
- Stefaniak-Vidarsson, M.M.; Kale, V.A.; Gudjónsdóttir, M.; Marteinsdottir, G.; Fridjonsson, O.; Hreggvidsson, G.O.; Sigurjonsson, O.E.; Omarsdottir, S.; Kristbergsson, K. Bioactive effect of sulphated polysaccharides derived from orange-footed sea cucumber (Cucumaria frondosa) toward THP-1 macrophages. Bioact. Carbohydr. Diet. Fibre 2017, 12, 14–19. [Google Scholar] [CrossRef]
- Yan, M.; Tao, H.; Qin, S. Effect of enzyme type on the antioxidant activities and functional properties of enzymatic hydrolysates from sea cucumber (Cucumaria frondosa) viscera. J. Aquat. Food Prod. Technol. 2016, 25, 940–952. [Google Scholar] [CrossRef]
- Mamelona, J.; Pelletier, E. Producing high antioxidant activity extracts from echinoderm by products by using pressured liquid extraction. Biotechnology 2010, 9, 523–528. [Google Scholar] [CrossRef]
- Hossain, A.; Dave, D.; Shahidi, F. Phenolic compounds and antioxidant activity of Atlantic sea cucumber (Cucumaria frondosa). In Proceedings of the International Society for Nutraceuticals and Functional Foods (ISNFF), Vancouver, BC, Canada, 14–17 October 2018. [Google Scholar]
- Xu, C.; Zhang, R.; Wen, Z. Bioactive compounds and biological functions of sea cucumbers as potential functional foods. J. Funct. Foods 2018, 49, 73–84. [Google Scholar] [CrossRef]
- Liu, X.; Hao, J.; Shan, X.; Zhang, X.; Zhao, X.; Li, Q.; Wang, X.; Cai, C.; Li, G.; Yu, G. Antithrombotic activities of fucosylated chondroitin sulfates and their depolymerized fragments from two sea cucumbers. Carbohydr. Polym. 2016, 152, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Aminin, D.L.; Agafonova, I.G.; Kalinin, V.I.; Silchenko, A.S.; Avilov, S.A.; Stonik, V.A.; Collin, P.D.; Woodward, C. Immunomodulatory properties of frondoside A, a major triterpene glycoside from the North Atlantic commercially harvested sea cucumber Cucumaria frondosa. J. Med. Food 2008, 11, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Al Shemaili, J.; Mensah-Brown, E.; Parekh, K.; Thomas, S.A.; Attoub, S.; Hellman, B.; Nyberg, F.; Adem, A.; Collin, P.; Adrian, T.E. Frondoside A enhances the antiproliferative effects of gemcitabine in pancreatic cancer. Eur. J. Cancer 2014, 50, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Guo, S.; Du, L.; Wang, Y.M.; Sugawara, T.; Hirata, T.; Xue, C.H. Isolation of cytotoxic glucoerebrosides and long-chain bases from sea cucumber Cucumaria frondosa using high speed counter-current chromatography. J. Oleo Sci. 2013, 62, 133–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Wang, F.; Wang, J.; Xu, J.; Wang, Y.; Xue, C. The WNT/β-catenin pathway is involved in the anti-adipogenic activity of cerebrosides from the sea cucumber Cucumaria frondosa. Food Funct. 2015, 6, 2396–2404. [Google Scholar] [CrossRef]
- Hu, S.; Xu, L.; Shi, D.; Wang, J.; Wang, Y.; Lou, Q.; Xue, C. Eicosapentaenoic acid-enriched phosphatidylcholine isolated from Cucumaria frondosa exhibits anti-hyperglycemic effects via activating phosphoinositide 3-kinase/protein kinase B signal pathway. J. Biosci. Bioeng. 2014, 117, 457–463. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M.; Snader, K.M. Natural products as sources of new drugs over the period 1981–2002. J. Nat. Prod. 2003, 66, 1022–1037. [Google Scholar] [CrossRef]
- Adrian, T.E. Novel Marine-Derived Anti-Cancer Agents. Curr. Pharm. Des. 2007, 13, 3417–3426. [Google Scholar] [CrossRef]
- Nigrelli, R.F. The effects of holothurin on fish, and mice with Sarcoma 180. Zoologica Sci. Contrib. N. Y. Zool. Soc. 1952, 37, 89–90. [Google Scholar]
- Li, X.; Roginsky, A.B.; Ding, X.Z.; Woodward, C.; Collin, P.; Newman, R.A.; Bell, R.H.; Adrian, T.E. Review of the apoptosis pathways in pancreatic cancer and the anti-apoptotic effects of the novel sea cucumber compound, frondoside A. Ann. N. Y. Acad. Sci. 2008, 1138, 181–198. [Google Scholar] [CrossRef]
- Al Shemaili, J.; Parekh, K.A.; Newman, R.A.; Hellman, B.; Woodward, C.; Adem, A.; Collin, P.; Adrian, T.E. Pharmacokinetics in mouse and comparative effects of frondosides in pancreatic cancer. Mar. Drugs 2016, 14, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janakiram, N.B.; Mohammed, A.; Zhang, Y.; Choi, C.I.; Woodward, C.; Collin, P.; Steele, V.E.; Rao, C.V. Chemopreventive effects of Frondanol A5, a Cucumaria frondosa extract, against rat colon carcinogenesis and inhibition of human colon cancer cell growth. Cancer Prev. Res. 2010, 3, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Roginsky, A.B.; Ding, X.Z.; Woodward, C.; Ujiki, M.B.; Singh, B.; Bell, R.H.; Collin, P.; Adrian, T.E. Anti-pancreatic cancer effects of a polar extract from the edible sea cucumber, cucumaria frondosa. Pancreas 2010, 39, 646–652. [Google Scholar] [CrossRef]
- Al Marzouqi, N.; Iratni, R.; Nemmar, A.; Arafat, K.; Ahmed Al Sultan, M.; Yasin, J.; Collin, P.; Mester, J.; Adrian, T.E.; Attoub, S. Frondoside A inhibits human breast cancer cell survival, migration, invasion and the growth of breast tumor xenografts. Eur. J. Pharmacol. 2011, 668, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Kundu, N.; Collin, P.D.; Goloubeva, O.; Fulton, A.M. Frondoside A inhibits breast cancer metastasis and antagonizes prostaglandin E receptors EP4 and EP2. Breast Cancer Res. Treat. 2012, 132, 1001–1008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.Y.; Kim, Y.H.; Kim, Y.; Lee, S.J. Frondoside A has an anti-invasive effect by inhibiting TPA-induced MMP-9 activation via NF-κB and AP-1 signaling in human breast cancer cells. Int. J. Oncol. 2012, 41, 933–940. [Google Scholar] [CrossRef]
- Attoub, S.; Arafat, K.; Gélaude, A.; Al Sultan, M.A.; Bracke, M.; Collin, P.; Takahashi, T.; Adrian, T.E.; de Wever, O. Frondoside A suppressive effects on lung cancer survival, tumor growth, angiogenesis, invasion, and metastasis. PLoS ONE 2013, 8, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.O.; Shastina, V.V.; Shin, S.W.; Xu, Q.; Park, J.I.; Rasskazov, V.A.; Avilov, S.A.; Fedorov, S.N.; Stonik, V.A.; Kwak, J.Y. Differential effects of triterpene glycosides, frondoside A and cucumarioside A2-2 isolated from sea cucumbers on caspase activation and apoptosis of human leukemia cells. FEBS Lett. 2009, 583, 697–702. [Google Scholar] [CrossRef] [Green Version]
- Adrian, T.E.; Collin, P. The anti-cancer effects of frondoside A. Mar. Drugs 2018, 16, 64. [Google Scholar] [CrossRef] [Green Version]
- Janakiram, N.B.; Mohammed, A.; Bryant, T.; Lightfoot, S.; Collin, P.D.; Steele, V.E.; Rao, C.V. Improved innate immune responses by Frondanol A5, a sea cucumber extract, prevent intestinal tumorigenesis. Cancer Prev. Res. 2015, 8, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Han, H.; Chen, X.; Yi, Y.; Sun, H. Cytotoxic and apoptosis-inducing activity of triterpene glycosides from Holothuria scabra and Cucumaria frondosa against HepG2 Cells. Mar. Drugs 2014, 12, 4274–4290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, L.; Yang, Y.H.; Wang, Y.M.; Xue, C.H.; Kurihara, H.; Takahashi, K. Antitumour activity of EPA-enriched phospholipids liposomes against S180 ascitic tumour-bearing mice. J. Funct. Foods 2015, 19, 970–982. [Google Scholar] [CrossRef]
- Melo, F.R.; Pereira, M.S.; Foguel, D.; Mourão, P.A.S. Antithrombin-mediated anticoagulant activity of sulfated polysaccharides: Different mechanisms for heparin and sulfated galactans. J. Biol. Chem. 2004, 279, 20824–20835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Chang, Y.; Wang, J.; Xue, C.; Li, Z.; Wang, Y. Fucosylated chondroitin sulfate from sea cucumber in combination with rosiglitazone improved glucose metabolism in the liver of the insulin-resistant mice. Biosci. Biotechnol. Biochem. 2013, 77, 2263–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Wang, J.; Xu, H.; Wang, Y.; Li, Z.; Xue, C. Fucosylated chondroitin sulphate from sea cucumber inhibits high-fat-sucrose diet-induced apoptosis in mouse pancreatic islets via down-regulating mitochondrial signaling pathway. J. Funct. Foods 2014, 7, 517–526. [Google Scholar] [CrossRef]
- Subramanya, S.B.; Chandran, S.; Almarzooqi, S.; Raj, V.; Salem Al Zahmi, A.; Ahmed Al Katheeri, R.; Ali Al Zadjali, S.; Collin, P.D.; Adrian, T.E. Frondanol, a nutraceutical extract from Cucumaria frondosa, attenuates colonic inflammation in a DSS-induced colitis model in mice. Mar. Drugs 2018, 16, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, F.; Hossain, A. Bioactives in spices, and spice oleoresins: Phytochemicals and their beneficial effects in food preservation and health promotion. J. Food Bioact. 2018, 3, 8–75. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.; Huang, J.; Xu, X.; Jin, Z. Antioxidant activity of peptides isolated from alfalfa leaf protein hydrolysate. Food Chem. 2008, 111, 370–376. [Google Scholar] [CrossRef]
- Xia, Y.; Bamdad, F.; Gänzle, M.; Chen, L. Fractionation and characterization of antioxidant peptides derived from barley glutelin by enzymatic hydrolysis. Food Chem. 2012, 134, 1509–1518. [Google Scholar] [CrossRef]
- Wu, F.J.; Xue, Y.; Liu, X.F.; Xue, C.H.; Wang, J.F.; Du, L.; Takahashi, K.; Wang, Y.M. The protective effect of eicosapentaenoic acid-enriched phospholipids from sea cucumber Cucumaria frondosa on oxidative stress in PC12 cells and SAMP8 mice. Neurochem. Int. 2014, 64, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Haug, T.; Kjuul, A.K.; Styrvold, O.B.; Sandsdalen, E.; Olsen, Ø.M.; Stensvåg, K. Antibacterial activity in Strongylocentrotus droebachiensis (Echinoidea), Cucumaria frondosa (Holothuroidea), and Asterias rubens (Asteroidea). J. Invertebr. Pathol. 2002, 81, 94–102. [Google Scholar] [CrossRef]
- Tripoteau, L.; Bedoux, G.; Gagnon, J.; Bourgougnon, N. In vitro antiviral activities of enzymatic hydrolysates extracted from byproducts of the Atlantic holothurian Cucumaria frondosa. Process Biochem. 2015, 50, 867–875. [Google Scholar] [CrossRef]
- Kalinin, V.I.; Aminin, D.L.; Avilov, S.A.; Silchenko, A.S.; Stonik, V.A. Triterpene glycosides from sea cucucmbers (holothurioidea, echinodermata). Biological activities and functions. Stud. Nat. Prod. Chem. 2008, 35, 135–196. [Google Scholar]
- Collin, P.D. Inhibition of Angiogenesis by Sea Cucumber Fractions. U.S. Patent 5,985, 330, 16 November 1999. [Google Scholar]
- Hamaguchi, P.; Geirsdottir, M.; Vrac, A.; Kristinsson, H.G.; Sveinsdottir, H.; Fridjonsson, O.H.; Hreggvidsson, G.O. In vitroantioxidant and antihypertensiveproperties of Icelandic sea cucumber (Cucumaria frondosa). In Proceedings of the IFT 10 Annual Meeting & Food Expo, Chicago, IL, USA, 17–20 July 2010. presentation no. 282-04. [Google Scholar]
- Zhang, L.; Wang, D.; Wen, M.; Du, L.; Xue, C.; Wang, J.; Xu, J.; Wang, Y. Rapid modulation of lipid metabolism in C57BL/6J mice induced by eicosapentaenoic acid-enriched phospholipid from Cucumaria frondosa. J. Funct. Foods 2017, 28, 28–35. [Google Scholar] [CrossRef]
- Hui, X.; Jie, X.; Yanlei, Z.; Zhe, Y.; Jingfeng, W. Studies on the inhibitory effect of sphingolipid from the sea cucumber Cucumaria frondosa gunnerus on proliferation and differentiation of 3T3-L1 cells and the structure-function relationship. J. Chin. Inst. Food Sci. Technol. 2017, 17, 17–22. [Google Scholar]
- Jia, Z.; Song, Y.; Tao, S.; Cong, P.; Wang, X.; Xue, C.; Xu, J. Structure of sphingolipids from sea cucumber cucumaria frondosa and structure-specific cytotoxicity against human hepg2 cells. Lipids 2016, 51, 321–334. [Google Scholar] [CrossRef]
- Tian, Y.; Hu, S.; Xu, H.; Wang, J.; Xue, C.; Wang, Y. Long-chain bases from Cucumaria frondosa inhibit adipogenesis and regulate lipid metabolism in 3T3-L1 adipocytes. Food Sci. Biotechnol. 2016, 25, 1753–1760. [Google Scholar] [CrossRef]
- Lin, L.; Yang, K.; Zheng, L.; Zhao, M.; Sun, W.; Zhu, Q.; Liu, S. Anti-aging effect of sea cucumber (Cucumaria frondosa) hydrolysate on fruit flies and D-galactose-induced aging mice. J. Funct. Foods 2018, 47, 11–18. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, M.; Mujumdar, A.S. Study on a combination drying technique of sea cucumber. Dry. Technol. 2007, 25, 2011–2019. [Google Scholar] [CrossRef]
- Stonik, V.A.; Kalinin, V.I.; Avilov, S.A. Toxins from sea cucumbers (holothuroids): Chemical structures, properties, taxonomic distribution, biosynthesis and evolution. J. Nat. Toxins. 1999, 8, 235–248. [Google Scholar]










| Product Name | Brand/Manufacturer | Price (US$) |
|---|---|---|
| PEACE PAVILION dried sea cucumber | Peace Pavilion | $150.00/LB |
| Canadian wild freshly dried sea cucumber | NorthBay Foods | $135.00/LB |
| Dried Canada wild sea cucumber | Arctica Food | $98.00/LB |
| Dried sea cucumber- butterfly cut/whole cut | Atlantic Sea Cucumber | $55.00–75.00/LB |
| Arctic dried sea cucumber | Naturally North | $65.00/LB |
| Sea cucumber liquid extract | Hawaii Pharm | $20.00/oz. |
| Dried Cucumaria frondosa flower | NorthBay Foods | $15.00/LB |
| Canadian sea cucumber (whole/split east coast) | SEACOO | N/A |
| Amino acids | Sea cucumber with viscera (mg/g) a | Body wall (mg/g) a | Viscera (%) b | Fatty Acids | Sea cucumber with viscera (%) a | Body wall (%) a | Viscera (%) b |
|---|---|---|---|---|---|---|---|
| Valine | 16.8 | 19.7 | 5.4 | 14:00 | 1.8 | 1.88 | 10.1 |
| Methionine | 9.4 | 10.3 | 2.3 | 15:00 | 4.03 | 2.18 | 0.3 |
| Isoleucine | 12.6 | 13.9 | 4.7 | 16:00 | 2.83 | 2.33 | 13.3 |
| Leucine | 28.3 | 30.8 | 7.2 | 16:1 n-7 | 7.36 | 5.75 | 6 |
| Phenylalanine | 13.1 | 17.8 | 3.5 | 17:1 n-7 | 2.44 | 3.87 | N/A |
| Histidine | 3.3 | 2.8 | 2.3 | 18:00 | 4.2 | 5.41 | 2.1 |
| Threonine | 10.9 | 12.9 | 5 | 18:1 n-9 | 3.72 | 2.43 | 4.9 |
| Lysine | 30.6 | 29.1 | 6.6 | 18:1 n-7 | 3.37 | 3.52 | N/A |
| Aspartic acid | 27.8 | 39.1 | 10 | 20:1 n-9 | 1.66 | 4 | N/A |
| Glutamic acid | 57.5 | 66.4 | 14.3 | 20:3 n-3 | 2.54 | 5 | 2.5 |
| Serine | 15.5 | 19.3 | 4.3 | 20:5 n-3 | 43.2 | 46.1 | 17.1 |
| Glycine | 29.8 | 56.1 | 8 | 22:00 | 2.09 | 1.95 | 0 |
| Alanine | 23.2 | 32 | 6.6 | 22:1 n-9 | 3.34 | 2.25 | 2.5 |
| Arginine | 24.7 | 30.1 | 9.1 | 22:6 n-3 | 5.81 | 4.96 | 0.3 |
| Proline | 17.3 | 24 | 4 | 20:2 n-6 | N/A | N/A | 2.2 |
| Tyrosine | 13.4 | 15.6 | 5 | 20:4 n-6 | N/A | N/A | 10.4 |
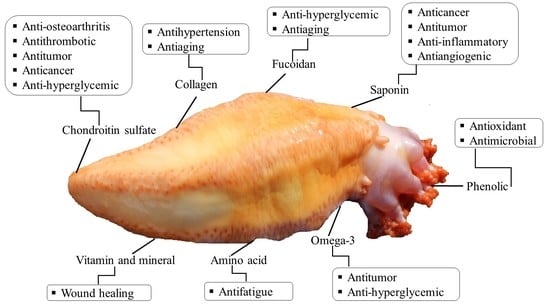
| Bioactives | Body Parts | Biological and Medicinal Effects | Extraction and Isolation Method | References |
|---|---|---|---|---|
| Fucosylated chondroitin sulfate | Body wall | Antithrombotic, anticoagulant, anticancer, anti-inflammatory, antitumor, antidiabetic, anti-osteoarthritis, alleviates inflammation, alleviates pain, and improve immune system | Enzymatic (papain/ Alcalase) hydrolysis followed by precipitation (cetylpyridinium chloride/ ethanol/ sodium hydroxide/ tricholoracetic acid) | [57,93,99] |
| Collagen | Body wall | Antihypertension, antiaging, anti-wrinkle, alleviates skin problems, and wound healing | A divalent cation chelator (EDTA) followed by extraction in water | [79,80] |
| glycosides (saponins) | Body wall | Antibacterial, antifungal, antiviral, antitumor, anticancer, antiangiogenic, and photo-protective | Isopropyl alcohol/ water extraction and refluxing with chloroform/ methanol/ethanol | [83,100,101] |
| Fucoidan | Body wall | Anticoagulant, antibacterial, antiaging, anti-hyperglycemic, lowering blood glucose level, and photo-protective | Hydrolysis with papain and precipitation with cetylpyridinium chloride | [58,70] |
| Phenolic compounds | Body wall, tentacles, and viscera | Antioxidants and antibacterial | Solvent extraction (methanol), water, organic solvent (ethyl acetate) and a mixture of water/ miscible organic solvent (acetonitrile) | [16,54] |
| Cerebrosides | Body wall | Anticancer, anti-inflammatory, and anti-adipogenic activity | Solvent extraction (65% ethanol) and isolated by High-performance liquid chromatography (HPLC), extracted by chloroform/ methanol using high speed counter-current chromatography | [19,102,103] |
| Amino acid | Body wall, tentacles, and viscera | Anti-fatigue, repairing tissue, nutritional storage, and wound healing | Reversed phase HPLC | [54,55] |
| Protein (bioactive peptide) | Body wall | Antimicrobial | Fractionated utilizing ammonium sulfate precipitation and analyzed by size exclusion chromatography | [32] |
| Vitamin and minerals | Body wall, tentacles, and viscera | Cosmeceutical properties, promote healthy growth and metabolism, lower the blood sugar level | Association of Official Analytical Chemists (AOAC)-and inductively coupled plasma mass spectrometry (ICP-MS) | [55,93] |
| Omega-3 (EPA) | Body wall, tentacles, and viscera | Anti-hyperglycemic, decrease cholesterol, and protect the heart | Solvent extraction (methanol: chloroform: water) and analyzed by gas chromatography (GC)/ HPLC | [50,54,55,104] |
| Compounds | Activities | Type of Cancer | References |
|---|---|---|---|
| Frondoside A | Anti-proliferation, antitumor, anti-anti-metastatic, apoptosis, p21 increase, increased caspase 3and 7, migration and invasion, increase in p53, caspase 3/7, decrease ERK ½, decrease cell viability, cell cycle arrest, inhibit colony formation, angiogenesis, and inhibit pro-survival autophagy | Pancreatic cancer cells, xenografts, lung and breast cancer, lung cancer xenografts, breast cancer, breast cancer xenografts, non-small lung carcinoma, leukemia, cervical cancer, and urothelial carcinoma (HT-1197, RT112, and RT4) | [56,101,108,109,110,111,112,113,114,115,116,117] |
| Frondanol A5 | Anti-proliferation, inhibition of cell cycle, induce apoptosis, aberrant crypt inhibition, p21 increase, DNA fragmentation, apoptosis, G2/M inhibition, inhibition of small intestinal and colon tumors, increase in GILT expression, macrophage phagocytosis, decrease expression of cyclin A, cyclin B, and cdc25c | Pancreatic cancer cells, AOM-induced rat colon cancer model, human colon cancer cells HCT116, ApcMin/+ colon cancer model, colon cancer, and pancreatic cancer (AsPC-1 and S2013) | [110,111,118] |
| Frondoside A and B | Induce apoptosis, inhibition of cell cycle, antitumor, anti-anti-metastatic, decrease cell viability, cell cycle arrest, and inhibit colony formation | Pancreatic cancer (AsPC-1 and S2013), non-small lung carcinoma, hepatocellular liver carcinoma, leukemia, breast adenocarcinoma, Luc-2 breast cancer, and breast cancer | [101,108,109,112,115,116] |
| Frondoside A + gemcitabine | Tumor inhibition, apoptosis, necrosis, increased caspase 3,7 and 9 | Pancreatic cancer xenografts | [115] |
| Frondoside A + cisplatin | Antitumor | Lung cancer xenografts | [113] |
| Frondoside A + paclitaxel | Cytotoxic | Breast cancer cells | [111] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hossain, A.; Dave, D.; Shahidi, F. Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector. Mar. Drugs 2020, 18, 274. https://doi.org/10.3390/md18050274
Hossain A, Dave D, Shahidi F. Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector. Marine Drugs. 2020; 18(5):274. https://doi.org/10.3390/md18050274
Chicago/Turabian StyleHossain, Abul, Deepika Dave, and Fereidoon Shahidi. 2020. "Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector" Marine Drugs 18, no. 5: 274. https://doi.org/10.3390/md18050274
APA StyleHossain, A., Dave, D., & Shahidi, F. (2020). Northern Sea Cucumber (Cucumaria frondosa): A Potential Candidate for Functional Food, Nutraceutical, and Pharmaceutical Sector. Marine Drugs, 18(5), 274. https://doi.org/10.3390/md18050274