Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances
Abstract
:1. Introduction
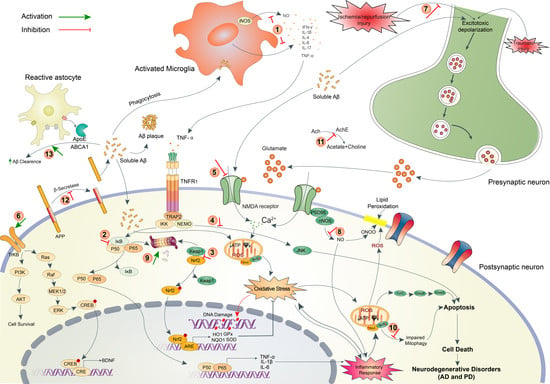
2. Pathophysiology of Brain Disorders
2.1. Neurodegenerative Disorders (AD and PD)
2.2. Ischemic Stroke
2.3. Traumatic Brain Injury
3. Neuropharmacological Potentials of Marine Algae and Their Metabolites: Evidence from In Vitro Studies
3.1. Antioxidant Activity
3.2. Anti-Inflammatory Activity
3.3. Anticholinesterase Activity
3.4. Anti-Amyloidogenic and Aggregation Inhibition Activity
3.5. Cholesterol Homeostasis and Aβ Clearance Activity
3.6. Monoamine Oxidase Inhibition and Affinity to Dopaminergic Receptors
3.7. Anti-Aging
3.8. Neurotrophic Activity
3.9. Neuroprotective Activity
4. Neuropharmacological Potentials of Marine Algae and Their Metabolites: Evidence from In Vivo Studies
5. Recent Progress on the Development of Marine Algae-Based Neurotherapeutics
6. Algal Metabolites-Based Drug Discovery and Design
7. Safety Issues on Marine Algae-Derived Compounds
8. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chi, H.; Chang, H.Y.; Sang, T.K. Neuronal Cell Death Mechanisms in Major Neurodegenerative Diseases. Int. J. Mol. Sci. 2018, 19, 3082. [Google Scholar] [CrossRef] [Green Version]
- Vasili, E.; Dominguez-Meijide, A.; Outeiro, T.F. Spreading of α-Synuclein and Tau: A Systematic Comparison of the Mechanisms Involved. Front. Mol. Neurosci. 2019, 12, 107. [Google Scholar] [CrossRef] [Green Version]
- Zhong, X.; Wang, J.; Carlsson, C.; Okonkwo, O.; Zetterberg, H.; Li, L. A Strategy for Discovery and Verification of Candidate Biomarkers in Cerebrospinal Fluid of Preclinical Alzheimer’s Disease. Front. Mol. Neurosci. 2018, 11, 483. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, G.; Chakrabarti, S.; Chatterjee, U.; Saso, L. Proteinopathy, oxidative stress and mitochondrial dysfunction: Cross talk in Alzheimer’s disease and Parkinson’s disease. Drug Des. Dev. Ther. 2017, 11, 797–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verri, M.; Pastoris, O.; Dossena, M.; Aquilani, R.; Guerriero, F.; Cuzzoni, G.; Venturini, L.; Ricevuti, G.; Bongiorno, A.I. Mitochondrial Alterations, Oxidative Stress and Neuroinflammation in Alzheimer’s Disease. Int. J. Immunopathol. Pharmacol. 2012, 25, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leon, R.; Garcia, A.G.; Marco-Contelles, J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer’s disease. Med. Res. Rev. 2013, 33, 139–189. [Google Scholar] [CrossRef]
- Angeloni, C.; Vauzour, D. Natural Products and Neuroprotection. Int. J. Mol. Sci. 2019, 20, 5570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, M.U.; Wali, A.F.; Ahmad, A.; Shakeel, S.; Rasool, S.; Ali, R.; Rashid, S.M.; Madkhali, H.; Ganaie, M.A.; Khan, R. Neuroprotective Strategies for Neurological Disorders by Natural Products: An update. Curr. Neuropharmacol. 2019, 17, 247–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, H.J.; Lee, K.W. Naturally occurring phytochemicals for the prevention of Alzheimer’s disease. J. Neurochem. 2010, 112, 1415–1430. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharifi-Rad, M.; Anil Kumar, N.V.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of curcumin: A review of clinical trials. Eur. J. Med. Chem. 2019, 163, 527–545. [Google Scholar] [CrossRef] [PubMed]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse Applications of Marine Macroalgae. Mar. Drugs 2020, 18, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.P.; Wu, C.F.; Wang, J.H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, I.; Trincone, A.; Mishra, A.P.; Nigam, M.; Zam, W.; Martins, N. Current Trends on Seaweeds: Looking at Chemical Composition, Phytopharmacology, and Cosmetic Applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Kim, S.K. Biological activities and health benefit effects of natural pigments derived from marine algae. J. Funct. Foods 2011, 3, 255–266. [Google Scholar] [CrossRef]
- Alghazwi, M.; Kan, Y.Q.; Zhang, W.; Gai, W.P.; Garson, M.J.; Smid, S. Neuroprotective activities of natural products from marine macroalgae during 1999–2015. J. Appl. Phycol. 2016, 28, 3599–3616. [Google Scholar] [CrossRef]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-Inflammatory Activities of Marine Algae in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, M.; Valentão, P.; Andrade, P.B. Bioactive compounds from macroalgae in the new millennium: Implications for neurodegenerative diseases. Mar. Drugs 2014, 12, 4934–4972. [Google Scholar] [CrossRef]
- Cornish, M.L.; Critchley, A.T.; Mouritsen, O.G. Consumption of seaweeds and the human brain. J. Appl. Phycol. 2017, 29, 2377–2398. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Mahomoodally, M.F.; Aumeeruddy, M.Z.; Zengin, G.; Xiao, J.; Kim, D.H. Bioactive compounds in seaweeds: An overview of their biological properties and safety. Food Chem. Toxicol. 2020, 135, 111013. [Google Scholar] [CrossRef]
- Rajeswari, R.; Jeyaprakash, K. Biopotential effects of seaweeds for neurological disorders mini review. J. Pharm. Pharm. Sci. 2017, 6, 427–436. [Google Scholar]
- Lauritzen, L.; Brambilla, P.; Mazzocchi, A.; Harsløf, L.B.S.; Ciappolino, V.; Agostoni, C. DHA Effects in Brain Development and Function. Nutrients 2016, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Caltagirone, C.; Ferrannini, L.; Marchionni, N.; Nappi, G.; Scapagnini, G.; Trabucchi, M. The potential protective effect of tramiprosate (homotaurine) against Alzheimer’s disease: A review. Aging Clin. Exp. Res. 2012, 24, 580–587. [Google Scholar]
- Schepers, M.; Martens, N.; Tiane, A.; Vanbrabant, K.; Liu, H.B.; Lütjohann, D.; Mulder, M.; Vanmierlo, T. Edible seaweed-derived constituents: An undisclosed source of neuroprotective compounds. Neural Regen. Res. 2020, 15, 790–795. [Google Scholar]
- Williams, L.L. Marine algae as a source of prevention and relief in those with depression and dementia. World J. Pharm. Pharm. Sci. 2017, 6, 26–38. [Google Scholar] [CrossRef]
- Kim, S.K.; Pangestuti, R. Biological activities and potential health benefits of fucoxanthin derived from marine brown algae. Adv. Food Nutr. Res. 2011, 64, 111–128. [Google Scholar]
- Syed, Y.Y. Sodium Oligomannate: First Approval. Drugs 2020, 80, 441–444. [Google Scholar] [CrossRef] [PubMed]
- Pangestuti, R.; Kim, S.K. Neuroprotective effects of marine algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Z.; Cui, W. Marine-Derived Natural Compounds for the Treatment of Parkinson’s Disease. Mar. Drugs 2019, 17, 221. [Google Scholar] [CrossRef] [Green Version]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Macroalgae as a Valuable Source of Naturally Occurring Bioactive Compounds for the Treatment of Alzheimer’s Disease. Mar. Drugs 2019, 17, 609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pangestuti, R.; Vo, T.S.; Ngo, D.H.; Kim, S.K. Fucoxanthin Ameliorates Inflammation and Oxidative Reponses in Microglia. J. Agric. Food Chem. 2013, 61, 3876–3883. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uddin, M.S.; Kabir, M.T.; Mamun, A.A.; Barreto, G.E.; Rashid, M.; Perveen, A.; Ashraf, G.M. Pharmacological approaches to mitigate neuroinflammation in Alzheimer’s disease. Int. Immunopharmacol. 2020, 84, 106479. [Google Scholar] [CrossRef]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection against oxidative stress: Phytochemicals targeting TrkB signaling and the Nrf2-ARE antioxidant system. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef] [PubMed]
- Castelli, V.; Benedetti, E.; Antonosante, A.; Catanesi, M.; Pitari, G.; Ippoliti, R.; Cimini, A.; d’Angelo, M. Neuronal Cells Rearrangement During Aging and Neurodegenerative Disease: Metabolism, Oxidative Stress and Organelles Dynamic. Front. Mol. Neurosci. 2019, 12, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kandlur, A.; Satyamoorthy, K.; Gangadharan, G. Oxidative Stress in Cognitive and Epigenetic Aging: A Retrospective Glance. Front. Mol. Neurosci. 2020, 13, 41. [Google Scholar] [CrossRef] [Green Version]
- Chaudhari, N.; Talwar, P.; Parimisetty, A.; Lefebvre d’Hellencourt, C.; Ravanan, P. A Molecular Web: Endoplasmic Reticulum Stress, Inflammation, and Oxidative Stress. Front. Mol. Neurosci. 2014, 8, 213. [Google Scholar] [CrossRef]
- Gerakis, Y.; Hetz, C. Emerging roles of ER stress in the etiology and pathogenesis of Alzheimer’s disease. FEBS J. 2018, 285, 995–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a Common Feature of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef] [Green Version]
- Yanuck, S.F. Microglial Phagocytosis of Neurons: Diminishing Neuronal Loss in Traumatic, Infectious, Inflammatory, and Autoimmune CNS Disorders. Front. Psychiatry 2019, 10, 712. [Google Scholar] [CrossRef] [Green Version]
- Sofroniew, M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef] [Green Version]
- Dash, R.; Mitra, S.; Ali, M.C.; Oktaviani, D.F.O.; Hannan, M.A.; Choi, S.M.; Moon, I.S. Phytosterols: Targeting Neuroinflammation in Neurodegeneration. Curr. Pharm. Des. 2020, 26, 1–23. [Google Scholar]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative Stress in Neurodegenerative Diseases: From Molecular Mechanisms to Clinical Applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef]
- Mouzat, K.; Chudinova, A.; Polge, A.; Kantar, J.; Camu, W.; Raoul, C.; Lumbroso, S. Regulation of Brain Cholesterol: What Role Do Liver X Receptors Play in Neurodegenerative Diseases? Int. J. Mol. Sci. 2019, 20, 3858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, A.; Hong, C.; Rong, X.; Zhu, X.; Tarling, E.J.; Hedde, P.N.; Gratton, E.; Parks, J.; Tontonoz, P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. eLife 2015, 4, e08009. [Google Scholar] [CrossRef]
- Xu, P.; Li, D.; Tang, X.; Bao, X.; Huang, J.; Tang, Y.; Yang, Y.; Xu, H.; Fan, X. LXR agonists: New potential therapeutic drug for neurodegenerative diseases. Mol. Neurobiol. 2013, 48, 715–728. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.B.; Tan, X.J.; Wu, W.F.; Warner, M.; Gustafsson, J.A. Liver X receptor beta protects dopaminergic neurons in a mouse model of Parkinson disease. Proc. Natl. Acad. Sci. USA 2012, 109, 13112–13117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Futter, M.; Diekmann, H.; Schoenmakers, E.; Sadiq, O.; Chatterjee, K.; Rubinsztein, D.C. Wild-type but not mutant huntingtin modulates the transcriptional activity of liver X receptors. J. Med. Genet. 2009, 46, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Wolf, A.; Bauer, B.; Hartz, A.M. ABC Transporters and the Alzheimer’s Disease Enigma. Front. Psychiatry 2012, 3, 54. [Google Scholar] [CrossRef] [Green Version]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Évora, P.; Castroesilva, O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef] [Green Version]
- Tschoe, C.; Bushnell, C.D.; Duncan, P.W.; Alexander-Miller, M.A.; Wolfe, S.Q. Neuroinflammation after Intracerebral Hemorrhage and Potential Therapeutic Targets. J. Stroke 2020, 22, 29–46. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Lu, J.; Manaenko, A.; Tang, J.; Hu, Q. Mitochondria in Ischemic Stroke: New Insight and Implications. Aging Dis. 2018, 9, 924–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.L.; Mukda, S.; Chen, S.D. Diverse roles of mitochondria in ischemic stroke. Redox Biol. 2018, 16, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Gadani, S.P.; Walsh, J.T.; Lukens, J.R.; Kipnis, J. Dealing with Danger in the CNS: The Response of the Immune System to Injury. Neuron 2015, 87, 47–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mracsko, E.; Veltkamp, R. Neuroinflammation after intracerebral hemorrhage. Front. Cell. Neurosci. 2014, 8, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malekahmadi, M.; Moradi Moghaddam, O.; Islam, S.M.S.; Tanha, K.; Nematy, M.; Pahlavani, N.; Firouzi, S.; Zali, M.R.; Norouzy, A. Evaluation of the effects of pycnogenol (French maritime pine bark extract) supplementation on inflammatory biomarkers and nutritional and clinical status in traumatic brain injury patients in an intensive care unit: A randomized clinical trial protocol. Trials 2020, 21, 162. [Google Scholar] [CrossRef] [Green Version]
- Beauchamp, K.; Mutlak, H.; Smith, W.R.; Shohami, E.; Stahel, P.F. Pharmacology of traumatic brain injury: Where is the “golden bullet”? Mol. Med. 2008, 14, 731–740. [Google Scholar] [CrossRef]
- Khatri, N.; Thakur, M.; Pareek, V.; Kumar, S.; Sharma, S.; Datusalia, A.K.J.C.; Targets, N.D.-D. Oxidative stress: Major threat in traumatic brain injury. CNS Neurol. Disord. Drug Targets 2018, 17, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, A.; Jose Egea-Guerrero, J.; Murillo-Cabezas, F.; Carrillo-Vico, A. Oxidative stress in traumatic brain injury. Curr. Med. Chem. 2014, 21, 1201–1211. [Google Scholar] [CrossRef]
- Heo, S.J.; Ko, S.C.; Kang, S.M.; Kang, H.S.; Kim, J.P.; Kim, S.H.; Lee, K.W.; Cho, M.G.; Jeon, Y.J. Cytoprotective effect of fucoxanthin isolated from brown algae Sargassum siliquastrum against H2O2-induced cell damage. Eur. Food Res. Technol. 2008, 228, 145–151. [Google Scholar] [CrossRef]
- Zhao, D.; Kwon, S.H.; Chun, Y.S.; Gu, M.Y.; Yang, H.O. Anti-Neuroinflammatory Effects of Fucoxanthin via Inhibition of Akt/NF-κB and MAPKs/AP-1 Pathways and Activation of PKA/CREB Pathway in Lipopolysaccharide-Activated BV-2 Microglial Cells. Neurochem. Res. 2017, 42, 667–677. [Google Scholar] [CrossRef]
- Lee, S.; Lee, Y.S.; Jung, S.H.; Kang, S.S.; Shin, K.H. Anti-oxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch. Pharm. Res. 2003, 26, 719–722. [Google Scholar] [CrossRef]
- Jung, H.A.; Jin, S.E.; Ahn, B.R.; Lee, C.M.; Choi, J.S. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem. Toxicol. 2013, 59, 199–206. [Google Scholar] [CrossRef]
- Choi, J.S.; Han, Y.R.; Byeon, J.S.; Choung, S.Y.; Sohn, H.S.; Jung, H.A. Protective effect of fucosterol isolated from the edible brown algae, Ecklonia stolonifera and Eisenia bicyclis, on tert-butyl hydroperoxide- and tacrine-induced HepG2 cell injury. J. Pharm. Pharmacol. 2015, 67, 1170–1178. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Jayawardena, T.U.; Kim, H.S.; Lee, W.W.; Vaas, A.P.J.P.; De Silva, H.I.C.; Abayaweera, G.S.; Nanayakkara, C.M.; Abeytunga, D.T.U.; Lee, D.S.; et al. Beijing urban particulate matter-induced injury and inflammation in human lung epithelial cells and the protective effects of fucosterol from Sargassum binderi (Sonder ex J. Agardh). Environ. Res. 2019, 172, 150–158. [Google Scholar] [CrossRef]
- Rafiquzzaman, S.M.; Kim, E.Y.; Lee, J.M.; Mohibbullah, M.; Alam, M.B.; Soo Moon, I.; Kim, J.M.; Kong, I.S. Anti-Alzheimers and anti-inflammatory activities of a glycoprotein purified from the edible brown alga Undaria pinnatifida. Food Res. Int. 2015, 77, 118–124. [Google Scholar] [CrossRef]
- Zou, Y.; Qian, Z.J.; Li, Y.; Kim, M.M.; Lee, S.H.; Kim, S.K. Antioxidant Effects of Phlorotannins Isolated from Ishige okamurae in Free Radical Mediated Oxidative Systems. J. Agric. Food Chem. 2008, 56, 7001–7009. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, P.; Li, Z.; Zhang, H.; Xu, Z.; Li, P. Antioxidant activities of sulfated polysaccharide fractions from Porphyra haitanesis. J. Appl. Phycol. 2003, 15, 305–310. [Google Scholar] [CrossRef]
- Isaka, S.; Cho, K.; Nakazono, S.; Abu, R.; Ueno, M.; Kim, D.; Oda, T. Antioxidant and anti-inflammatory activities of porphyran isolated from discolored nori (Porphyra yezoensis). Int. J. Biol. Macromol. 2015, 74, 68–75. [Google Scholar] [CrossRef]
- Anggadiredja, J.; Andyani, R.; Hayati, M. Antioxidant activity of Sargassum polycystum (Phaeophyta) and Laurencia obtusa (Rhodophyta) from Seribu Islands. J. Appl. Phycol. 1997, 9, 477. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Amoo, S.O.; Aremu, A.O.; Stirk, W.A.; Gruz, J.; Šubrtová, M.; Doležal, K.; Van Staden, J. Phenolic profiles, antioxidant capacity, and acetylcholinesterase inhibitory activity of eight South African seaweeds. J. Appl. Phycol. 2015, 27, 1599–1605. [Google Scholar] [CrossRef]
- Hannan, M.A.; Sohag, A.A.M.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Oktaviani, D.F.; Hossain, M.T.; Choi, H.J.; Moon, I.S. Phytosterols of marine algae: Insights into the potential health benefits and molecular pharmacology. Phytomedicine 2020, 69, 153201. [Google Scholar] [CrossRef]
- Sun, Z.; Mohamed, M.A.A.; Park, S.Y.; Yi, T.H. Fucosterol protects cobalt chloride induced inflammation by the inhibition of hypoxia-inducible factor through PI3K/Akt pathway. Int. Immunopharmacol. 2015, 29, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Yoo, M.S.; Shin, J.S.; Choi, H.E.; Cho, Y.W.; Bang, M.H.; Baek, N.I.; Lee, K.T. Fucosterol isolated from Undaria pinnatifida inhibits lipopolysaccharide-induced production of nitric oxide and pro-inflammatory cytokines via the inactivation of nuclear factor-kappaB and p38 mitogen-activated protein kinase in RAW264.7 macrophages. Food Chem. 2012, 135, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.H.; Gan, S.Y.; Tan, S.C.; Gany, S.A.; Ying, T.; Gray, A.I.; Igoli, J.; Chan, E.W.L.; Phang, S.M. Fucosterol inhibits the cholinesterase activities and reduces the release of pro-inflammatory mediators in lipopolysaccharide and amyloid-induced microglial cells. J. Appl. Phycol. 2018, 30, 3261–3270. [Google Scholar] [CrossRef]
- Jung, W.K.; Heo, S.J.; Jeon, Y.J.; Lee, C.M.; Park, Y.M.; Byun, H.G.; Choi, Y.H.; Park, S.G.; Choi, I.W. Inhibitory effects and molecular mechanism of dieckol isolated from marine brown alga on COX-2 and iNOS in microglial cells. J. Agric. Food Chem. 2009, 57, 4439–4446. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, M.S.; Choi, J.W.; Utsuki, T.; Kim, J.I.; Jang, B.C.; Kim, H.R. Phlorofucofuroeckol A suppresses expression of inducible nitric oxide synthase, cyclooxygenase-2, and pro-inflammatory cytokines via inhibition of nuclear factor-κB, c-Jun NH2-terminal kinases, and Akt in microglial cells. Inflammation 2013, 36, 259–271. [Google Scholar] [CrossRef]
- Yu, D.K.; Lee, B.; Kwon, M.; Yoon, N.; Shin, T.; Kim, N.G.; Choi, J.S.; Kim, H.R. Phlorofucofuroeckol B suppresses inflammatory responses by down-regulating nuclear factor κB activation via Akt, ERK, and JNK in LPS-stimulated microglial cells. Int. Immunopharmacol. 2015, 28, 1068–1075. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, B.; Joung, E.J.; Gwon, W.G.; Utsuki, T.; Kim, N.G.; Kim, H.R. 6,6′-Bieckol suppresses inflammatory responses by down-regulating nuclear factor-κB activation via Akt, JNK, and p38 MAPK in LPS-stimulated microglial cells. Immunopharmacol. Immunotoxicol. 2016, 38, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.I.; Jung, S.H.; Lee, K.T.; Choi, J.H. 8,8′-Bieckol, isolated from edible brown algae, exerts its anti-inflammatory effects through inhibition of NF-κB signaling and ROS production in LPS-stimulated macrophages. Int. Immunopharmacol. 2014, 23, 460–468. [Google Scholar] [CrossRef]
- Florez, N.; Gonzalez-Munoz, M.J.; Ribeiro, D.; Fernandes, E.; Dominguez, H.; Freitas, M. Algae Polysaccharides’ Chemical Characterization and their Role in the Inflammatory Process. Curr. Med. Chem. 2017, 24, 149–175. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Han, M.H.; Park, C.; Jin, C.Y.; Kim, G.Y.; Choi, I.W.; Kim, N.D.; Nam, T.J.; Kwon, T.K.; Choi, Y.H. Anti-inflammatory effects of fucoidan through inhibition of NF-κB, MAPK and Akt activation in lipopolysaccharide-induced BV2 microglia cells. Food Chem. Toxicol. 2011, 49, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.Q.; Jia, Y.J.; Zhang, T.; Zhang, Q.B.; Wang, X.M. Fucoidan Protects against Lipopolysaccharide-Induced Rat Neuronal Damage and Inhibits the Production of Proinflammatory Mediators in Primary Microglia. CNS Neurosci. Ther. 2012, 18, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.A.; Xu, L.; Wu, H.G. Immunomodulatory function of κ-carrageenan oligosaccharides acting on LPS-activated microglial cells. Neurochem. Res. 2014, 39, 333–343. [Google Scholar] [CrossRef]
- Jiang, Z.; Hama, Y.; Yamaguchi, K.; Oda, T. Inhibitory effect of sulphated polysaccharide porphyran on nitric oxide production in lipopolysaccharide-stimulated RAW264.7 macrophages. J. Biochem. 2012, 151, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.Y.; Liu, D.; Lin, G.P.; Wu, Y.J.; Gao, L.Y.; Ai, C.; Huang, Y.F.; Wang, M.F.; El-Seedi, H.R.; Chen, X.H.; et al. Anti-ageing and antioxidant effects of sulfate oligosaccharides from green algae Ulva lactuca and Enteromorpha prolifera in SAMP8 mice. Int. J. Biol. Macromol. 2019, 139, 342–351. [Google Scholar] [CrossRef]
- Zhou, R.; Shi, X.Y.; Bi, D.C.; Fang, W.S.; Wei, G.B.; Xu, X. Alginate-Derived Oligosaccharide Inhibits Neuroinflammation and Promotes Microglial Phagocytosis of β-Amyloid. Mar. Drugs 2015, 13, 5828–5846. [Google Scholar] [CrossRef] [Green Version]
- Bi, D.; Lai, Q.; Han, Q.; Cai, N.; He, H.; Fang, W.; Yi, J.; Li, X.; Xu, H.; Li, X.; et al. Seleno-polymannuronate attenuates neuroinflammation by suppressing microglial and astrocytic activation. J. Funct. Foods 2018, 51, 113–120. [Google Scholar] [CrossRef]
- Yang, E.J.; Ham, Y.M.; Yang, K.W.; Lee, N.H.; Hyun, C.G. Sargachromenol from Sargassum micracanthum inhibits the lipopolysaccharide-induced production of inflammatory mediators in RAW 264.7 macrophages. Sci. World J. 2013, 2013, 712303. [Google Scholar] [CrossRef] [Green Version]
- Kang, G.J.; Han, S.C.; Yoon, W.J.; Koh, Y.S.; Hyun, J.W.; Kang, H.K.; Youl Cho, J.; Yoo, E.S. Sargaquinoic acid isolated from Sargassum siliquastrum inhibits lipopolysaccharide-induced nitric oxide production in macrophages via modulation of nuclear factor-κB and c-Jun N-terminal kinase pathways. Immunopharm. Immunot. 2013, 35, 80–87. [Google Scholar] [CrossRef]
- Kim, M.; Li, Y.X.; Dewapriya, P.; Ryu, B.; Kim, S.K. Floridoside suppresses pro-inflammatory responses by blocking MAPK signaling in activated microglia. BMB Rep. 2013, 46, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Souza, C.R.M.; Bezerra, W.P.; Souto, J.T. Marine Alkaloids with Anti-Inflammatory Activity: Current Knowledge and Future Perspectives. Mar. Drugs 2020, 18, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, W.K.; Ahn, Y.W.; Lee, S.H.; Choi, Y.H.; Kim, S.K.; Yea, S.S.; Choi, I.; Park, S.G.; Seo, S.K.; Lee, S.W.; et al. Ecklonia cava ethanolic extracts inhibit lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression in BV2 microglia via the MAP kinase and NF-kappaB pathways. Food Chem. Toxicol. 2009, 47, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.I.; Choi, J.W.; Kim, M.; Yoon, N.Y.; Choi, C.G.; Choi, J.S.; Kim, H.R. Anti-inflammatory effect of hexane fraction from Myagropsis myagroides ethanolic extract in lipopolysaccharide-stimulated BV-2 microglial cells. J. Pharm. Pharmacol. 2013, 65, 895–906. [Google Scholar] [CrossRef]
- Kim, S.; Lee, M.S.; Lee, B.; Gwon, W.G.; Joung, E.J.; Yoon, N.Y.; Kim, H.R. Anti-inflammatory effects of sargachromenol-rich ethanolic extract of Myagropsis myagroides on lipopolysaccharide-stimulated BV-2 cells. BMC Complement. Altern. Med. 2014, 14, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, S.J.; Joung, E.J.; Kwon, M.S.; Lee, B.; Utsuki, T.; Oh, C.W.; Kim, H.R. Anti-Inflammatory Effect of Ethanolic Extract of Sargassum serratifolium in Lipopolysaccharide-Stimulated BV2 Microglial Cells. J. Med. Food 2016, 19, 1023–1031. [Google Scholar] [CrossRef]
- Gany, S.A.; Tan, S.C.; Gan, S.Y. Antioxidative, anticholinesterase and anti-neuroinflammatory properties of Malaysian brown and green seaweeds. World Acad. Sci. Eng. Technol. 2015, 8, 1269–1275. [Google Scholar]
- Jin, D.Q.; Lim, C.S.; Sung, J.Y.; Choi, H.G.; Ha, I.; Han, J.S. Ulva conglobata, a marine algae, has neuroprotective and anti-inflammatory effects in murine hippocampal and microglial cells. Neurosci. Lett. 2006, 402, 154–158. [Google Scholar] [CrossRef]
- Gwon, W.G.; Lee, M.S.; Kim, J.S.; Kim, J.I.; Lim, C.W.; Kim, N.G.; Kim, H.R. Hexane fraction from Sargassum fulvellum inhibits lipopolysaccharide-induced inducible nitric oxide synthase expression in RAW 264.7 cells via NF-κB pathways. Am. J. Chin. Med. 2013, 41, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.E.; Jung, Y.C.; Jung, I.; Lee, H.W.; Youn, H.Y.; Lee, J.S. Anti-inflammatory effects of ethanolic extract from Sargassum horneri (Turner) C. Agardh on lipopolysaccharide-stimulated macrophage activation via NF-κB pathway regulation. Immunol. Investig. 2015, 44, 137–146. [Google Scholar] [CrossRef]
- Joung, E.J.; Lee, M.S.; Choi, J.W.; Kim, J.S.; Shin, T.; Jung, B.M.; Yoon, N.Y.; Lim, C.W.; Kim, J.I.; Kim, H.R. Anti-inflammatory effect of ethanolic extract from Myagropsis myagroides on murine macrophages and mouse ear edema. BMC Complement. Altern. Med. 2012, 12, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joung, E.J.; Lee, M.S.; Choi, J.W.; Kim, J.S.; Shin, T.; Jung, B.M.; Kim, J.I.; Kim, H.R. Anti-inflammatory effects of phlorofucofuroeckol B-rich ethyl acetate fraction obtained from Myagropsis myagroides on lipopolysaccharide-stimulated RAW 264.7 cells and mouse edema. Int. Immunopharmacol. 2012, 14, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Joung, E.J.; Gwon, W.G.; Shin, T.; Jung, B.M.; Choi, J.; Kim, H.R. Anti-inflammatory action of the ethanolic extract from Sargassum serratifolium on lipopolysaccharide-stimulated mouse peritoneal macrophages and identification of active components. J. Appl. Phycol. 2017, 29, 563–573. [Google Scholar] [CrossRef]
- Yoon, N.Y.; Chung, H.Y.; Kim, H.R.; Choi, J.S. Acetyl- and butyrylcholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fish. Res. 2008, 74, 200–207. [Google Scholar] [CrossRef]
- Castro-Silva, E.S.; Bello, M.; Hernandez-Rodriguez, M.; Correa-Basurto, J.; Murillo-Alvarez, J.I.; Rosales-Hernandez, M.C.; Munoz-Ochoa, M. In vitro and in silico evaluation of fucosterol from Sargassum horridum as potential human acetylcholinesterase inhibitor. J. Biomol. Struct. Dyn. 2019, 37, 3259–3268. [Google Scholar] [CrossRef]
- Kawee-ai, A.; Kuntiya, A.; Kim, S.M. Anticholinesterase and antioxidant activities of fucoxanthin purified from the microalga Phaeodactylum tricornutum. Nat. Prod. Commun. 2013, 8, 1381–1386. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Huang, L.; Yu, J.; Xiang, S.; Wang, J.; Zhang, J.; Yan, X.; Cui, W.; He, S.; Wang, Q. Fucoxanthin, a Marine Carotenoid, Reverses Scopolamine-Induced Cognitive Impairments in Mice and Inhibits Acetylcholinesterase in vitro. Mar. Drugs 2016, 14, 67. [Google Scholar] [CrossRef] [Green Version]
- Shanmuganathan, B.; Sheeja Malar, D.; Sathya, S.; Pandima Devi, K. Antiaggregation Potential of Padina gymnospora against the Toxic Alzheimer’s Beta-Amyloid Peptide 25-35 and Cholinesterase Inhibitory Property of Its Bioactive Compounds. PLoS ONE 2015, 10, e0141708. [Google Scholar] [CrossRef] [Green Version]
- Kannan, R.R.R.; Aderogba, M.A.; Ndhlala, A.R.; Stirk, W.A.; Van Staden, J. Acetylcholinesterase inhibitory activity of phlorotannins isolated from the brown alga, Ecklonia maxima (Osbeck) Papenfuss. Food Res. Int. 2013, 54, 1250–1254. [Google Scholar] [CrossRef]
- Myung, C.S.; Shin, H.C.; Bao, H.Y.; Yeo, S.J.; Lee, B.H.; Kang, J.S. Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: Possible involvement of the inhibition of acetylcholinesterase. Arch. Pharm. Res. 2005, 28, 691–698. [Google Scholar] [CrossRef]
- Choi, B.W.; Ryu, G.; Park, S.H.; Kim, E.S.; Shin, J.; Roh, S.S.; Shin, H.C.; Lee, B.H. Anticholinesterase activity of plastoquinones from Sargassum sagamianum: Lead compounds for Alzheimer’s disease therapy. Phytother. Res. 2007, 21, 423–426. [Google Scholar] [CrossRef]
- Rengasamy, K.R.R.; Aderogba, M.A.; Amoo, S.O.; Stirk, W.A.; Van Staden, J. Macrocystis angustifolia is a potential source of enzyme inhibitors linked to type 2 diabetes and dementia. J. Appl. Phycol. 2014, 26, 1557–1563. [Google Scholar] [CrossRef]
- Seong, S.H.; Ali, M.Y.; Kim, H.R.; Jung, H.A.; Choi, J.S. BACE1 inhibitory activity and molecular docking analysis of meroterpenoids from Sargassum serratifolium. Bioorg. Med. Chem. 2017, 25, 3964–3970. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jun, M. Dual BACE1 and Cholinesterase Inhibitory Effects of Phlorotannins from Ecklonia cava-An In Vitro and in Silico Study. Mar. Drugs 2019, 17, 91. [Google Scholar] [CrossRef] [Green Version]
- Murugan, A.C.; Vallal, D.; Karim, M.R.; Govindan, N.; Yusoff, M.; Rahman, M.J. In vitro antiradical and neuroprotective activity of polyphenolic extract from marine algae Padina australis H. J. Chem. Pharm. Res. 2015, 7, 355–362. [Google Scholar]
- Custódio, L.; Soares, F.; Pereira, H.; Rodrigues, M.J.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Botryococcus braunii and Nannochloropsis oculata extracts inhibit cholinesterases and protect human dopaminergic SH-SY5Y cells from H2O2-induced cytotoxicity. J. Appl. Phycol. 2015, 27, 839–848. [Google Scholar] [CrossRef]
- Custódio, L.; Silvestre, L.; Rocha, M.I.; Rodrigues, M.J.; Vizetto-Duarte, C.; Pereira, H.; Barreira, L.; Varela, J. Methanol extracts from Cystoseira tamariscifolia and Cystoseira nodicaulis are able to inhibit cholinesterases and protect a human dopaminergic cell line from hydrogen peroxide-induced cytotoxicity. Pharm. Biol. 2016, 54, 1687–1696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.E.; Son, H.J.; Lim, D.W.; Yoon, M.; Lee, J.; Kim, Y.T.; Han, D.; Lee, C.; Um, M.Y. Memory-enhancing effects of Ishige foliacea extract: In vitro and in vivo study. J. Food Biochem. 2020, 44, e13162. [Google Scholar] [CrossRef]
- Nunes, N.; Rosa, G.P.; Ferraz, S.; Barreto, M.C.; de Carvalho, M.A.A.P. Fatty acid composition, TLC screening, ATR-FTIR analysis, anti-cholinesterase activity, and in vitro cytotoxicity to A549 tumor cell line of extracts of 3 macroalgae collected in Madeira. J. Appl. Phycol. 2019. [Google Scholar] [CrossRef]
- De Souza, E.T.; de Lira, D.P.; de Queiroz, A.C.; da Silva, D.J.C.; de Aquino, A.B.; Mella, E.A.C.; Lorenzo, V.P.; de Miranda, G.E.C.; de Araújo-Júnior, J.X.; de Oliveira Chaves, M.C.; et al. The antinociceptive and anti-inflammatory activities of caulerpin, a bisindole alkaloid isolated from seaweeds of the genus Caulerpa. Mar. Drugs 2009, 7, 689–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cengiz, S.; Cavas, L.; Yurdakoc, K.; Pohnert, G. The Sesquiterpene Caulerpenyne from Caulerpa spp. is a Lipoxygenase Inhibitor. Mar. Biotechnol. 2011, 13, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.; O’Gorman, D.M.; Nolan, Y.M. Evidence that the marine-derived multi-mineral Aquamin has anti-inflammatory effects on cortical glial-enriched cultures. Phytother. Res. 2011, 25, 765–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, G.; Park, S.H.; Kim, E.S.; Choi, B.W.; Ryu, S.Y.; Lee, B.H. Cholinesterase inhibitory activity of two farnesylacetone derivatives from the brown alga Sargassum sagamianum. Arch. Pharm. Res. 2003, 26, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Ali, M.Y.; Choi, R.J.; Jeong, H.O.; Chung, H.Y.; Choi, J.S. Kinetics and molecular docking studies of fucosterol and fucoxanthin, BACE1 inhibitors from brown algae Undaria pinnatifida and Ecklonia stolonifera. Food Chem. Toxicol. 2016, 89, 104–111. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, F.; Lin, J.; Chen, H.; Huang, C.; Chen, L.; Zhou, Y.; Ye, L.; Zhang, K.; Jin, J.; et al. Fucoxanthin Inhibits β-Amyloid Assembly and Attenuates β-Amyloid Oligomer-Induced Cognitive Impairments. J. Agric. Food Chem. 2017, 65, 4092–4102. [Google Scholar] [CrossRef]
- Alghazwi, M.; Smid, S.; Musgrave, I.; Zhang, W. In vitro studies of the neuroprotective activities of astaxanthin and fucoxanthin against amyloid beta (Aβ(1-42)) toxicity and aggregation. Neurochem. Int. 2019, 124, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Shanmuganathan, B.; Suryanarayanan, V.; Sathya, S.; Narenkumar, M.; Singh, S.K.; Ruckmani, K.; Pandima Devi, K. Anti-amyloidogenic and anti-apoptotic effect of α-bisabolol against Aβ induced neurotoxicity in PC12 cells. Eur. J. Med. Chem. 2018, 143, 1196–1207. [Google Scholar] [CrossRef]
- Hoang, M.H.; Jia, Y.; Jun, H.J.; Lee, J.H.; Lee, B.Y.; Lee, S.J. Fucosterol Is a Selective Liver X Receptor Modulator That Regulates the Expression of Key Genes in Cholesterol Homeostasis in Macrophages, Hepatocytes, and Intestinal Cells. J. Agric. Food Chem. 2012, 60, 11567–11575. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, J.; Fu, Z.; Ye, C.; Zhang, R.; Song, Y.; Zhang, Y.; Li, H.; Ying, H.; Liu, H. 24(S)-Saringosterol from edible marine seaweed Sargassum fusiforme is a novel selective LXRbeta agonist. J. Agric. Food Chem. 2014, 62, 6130–6137. [Google Scholar] [CrossRef]
- Seong, S.H.; Paudel, P.; Choi, J.W.; Ahn, D.H.; Nam, T.J.; Jung, H.A.; Choi, J.S. Probing multi-target action of phlorotannins as new monoamine oxidase inhibitors and dopaminergic receptor modulators with the potential for treatment of neuronal disorders. Mar. Drugs 2019, 17, 377. [Google Scholar] [CrossRef] [Green Version]
- Oktaviani, D.F.; Bae, Y.S.; Meinita, M.D.N.; Moon, I.S.; Hong, Y.K. An ethanol extract of the brown seaweed Hizikia fusiformis and its active constituent, fucosterol, extend the lifespan of the nematode Caenorhabditis elegans. J. Life Sci. 2019, 29, 1120–1125. [Google Scholar]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Moon, I.S. Deciphering molecular mechanism of the neuropharmacological action of fucosterol through integrated system pharmacology and in silico analysis. Mar. Drug 2019, 17, 639. [Google Scholar] [CrossRef] [Green Version]
- Dezsi, L.; Vecsei, L. Monoamine Oxidase B Inhibitors in Parkinson’s Disease. CNS Neurol. Disord. DrugTargets 2017, 16, 425–439. [Google Scholar] [CrossRef] [PubMed]
- Abdul, Q.A.; Choi, R.J.; Jung, H.A.; Choi, J.S. Health benefit of fucosterol from marine algae: A review. J. Sci. Food Agric. 2016, 96, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Tsang, C.K.; Ina, A.; Goto, T.; Kamei, Y. Sargachromenol, a novel nerve growth factor-potentiating substance isolated from Sargassum macrocarpum, promotes neurite outgrowth and survival via distinct signaling pathways in PC12D cells. Neuroscience 2005, 132, 633–643. [Google Scholar] [CrossRef]
- Kamei, Y.; Tsang, C.K. Sargaquinoic acid promotes neurite outgrowth via protein kinase A and MAP kinases-mediated signaling pathways in PC12D cells. Int. J. Dev. Neurosci. 2003, 21, 255–262. [Google Scholar] [CrossRef]
- Ina, A.; Hayashi, K.I.; Nozaki, H.; Kamei, Y. Pheophytin a, a low molecular weight compound found in the marine brown alga Sargassum fulvellum, promotes the differentiation of PC12 cells. Int. J. Dev. Neurosci. 2007, 25, 63–68. [Google Scholar] [CrossRef]
- Ina, A.; Kamei, Y. Vitamin B(12), a chlorophyll-related analog to pheophytin a from marine brown algae, promotes neurite outgrowth and stimulates differentiation in PC12 cells. Cytotechnology 2006, 52, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Wichmann, H.; Brinkhoff, T.; Simon, M.; Richter-Landsberg, C. Dimethylsulfoniopropionate promotes process outgrowth in neural cells and exerts protective effects against tropodithietic acid. Mar. Drugs 2016, 14, 89. [Google Scholar] [CrossRef] [Green Version]
- Mohibbullah, M.; Choi, J.S.; Bhuiyan, M.M.H.; Haque, M.N.; Rahman, M.K.; Moon, I.S.; Hong, Y.K. The red alga Gracilariopsis chorda and its active constituent arachidonic acid promote spine dynamics via dendritic filopodia and potentiate functional synaptic plasticity in hippocampal neurons. J. Med. Food 2018, 21, 481–488. [Google Scholar] [CrossRef]
- Kamei, Y.; Sagara, A. Neurite outgrowth promoting activity of marine algae from Japan against rat adrenal medulla pheochromocytoma cell line, PC12D. Cytotechnology 2002, 40, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mohibbullah, M.; Bhuiyan, M.M.; Hannan, M.A.; Getachew, P.; Hong, Y.K.; Choi, J.S.; Choi, I.S.; Moon, I.S. The Edible Red alga Porphyra yezoensis promotes neuronal survival and cytoarchitecture in primary hippocampal neurons. Cell. Mol. Neurobiol. 2016, 36, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Haque, M.N.; Mohibbullah, M.; Dash, R.; Hong, Y.K.; Moon, I.S. Gelidium amansii Attenuates hypoxia/reoxygenation-induced oxidative injury in primary hippocampal neurons through suppressing glun2b expression. Antioxidants 2020, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannan, M.A.; Mohibbullah, M.; Hong, Y.K.; Moon, I.S. Proteomic analysis of the neurotrophic effect of Gelidium amansii in primary cultured neurons. J. Med. Food 2017, 20, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Kang, J.Y.; Hong, Y.K.; Lee, H.; Choi, J.S.; Choi, I.S.; Moon, I.S. The marine alga Gelidium amansii promotes the development and complexity of neuronal cytoarchitecture. Phytother. Res. 2013, 27, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Mohibbullah, M.; Hong, Y.K.; Nam, J.H.; Moon, I.S. Gelidium amansii promotes dendritic spine morphology and synaptogenesis, and modulates NMDA receptor-mediated postsynaptic current. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 445–452. [Google Scholar] [CrossRef]
- Hannan, M.A.; Kang, J.Y.; Hong, Y.K.; Lee, H.; Chowdhury, M.T.; Choi, J.S.; Choi, I.S.; Moon, I.S. A brown alga Sargassum fulvellum facilitates neuronal maturation and synaptogenesis. In Vitro Cell. Dev. Biol. Anim. 2012, 48, 535–544. [Google Scholar] [CrossRef]
- Hannan, M.A.; Mohibbullah, M.; Hwang, S.Y.; Lee, K.; Kim, Y.C.; Hong, Y.K.; Moon, I.S. Differential neuritogenic activities of two edible brown macroalgae, Undaria pinnatifida and Saccharina japonica. Am. J. Chin. Med. 2014, 42, 1371–1384. [Google Scholar] [CrossRef]
- Mohibbullah, M.; Hannan, M.A.; Park, I.S.; Moon, I.S.; Hong, Y.K. The edible red seaweed Gracilariopsis chorda promotes axodendritic architectural complexity in hippocampal neurons. J. Med. Food 2016, 19, 638–644. [Google Scholar] [CrossRef]
- Tirtawijaya, G.; Mohibbullah, M.; Meinita, M.D.N.; Moon, I.S.; Hong, Y.K. The tropical carrageenophyte Kappaphycus alvarezii extract promotes axodendritic maturation of hippocampal neurons in primary culture. J. Appl. Phycol. 2018, 30, 3233–3241. [Google Scholar] [CrossRef]
- Tirtawijaya, G.; Mohibbullah, M.; Meinita, M.D.N.; Moon, I.S.; Hong, Y.K. The ethanol extract of the rhodophyte Kappaphycus alvarezii promotes neurite outgrowth in hippocampal neurons. J. Appl. Phycol. 2016, 28, 2515–2522. [Google Scholar] [CrossRef]
- Tirtawijaya, G.; Haque, M.N.; Choi, J.S.; Moon, I.S.; Meinita, M.D.N.; Choi, J.S.; Hong, Y.K.; Science, F. Spinogenesis and synaptogenesis effects of the red seaweed Kappaphycus alvarezii and its isolated cholesterol on hippocampal neuron cultures. Prev. Nutr. Food Sci. 2019, 24, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Tirtawijaya, G.; Meinita, M.D.N.; Marhaeni, B.; Haque, M.N.; Moon, I.S.; Hong, Y.K. Neurotrophic activity of the Carrageenophyte Kappaphycus alvarezii cultivated at different depths and for different growth periods in various areas of indonesia. Evid. Based Complement. Alternat. Med. 2018, 2018, 1098076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Yu, J.; Zhao, J.; Zhang, K.; Zheng, J.; Wang, J.; Huang, C.; Zhang, J.; Yan, X.; Gerwick, W.H.; et al. Fucoxanthin, a marine carotenoid, attenuates β-amyloid oligomer-induced neurotoxicity possibly via regulating the PI3K/Akt and the ERK pathways in SH-SY5Y cells. Oxid. Med. Cell. Longev. 2017, 2017, 6792543. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Lin, J.J.; Yu, R.; He, S.; Wang, Q.W.; Cui, W.; Zhang, J.R. Fucoxanthin prevents H(2)O(2)-induced neuronal apoptosis via concurrently activating the PI3-K/Akt cascade and inhibiting the ERK pathway. Food Nutr. Res. 2017, 61, 1304678. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, K.; Kitamura, A.; Machida, H.; Watanabe, M.; Negishi, H.; Hiraoka, J.; Nakano, T. Effect of Undaria pinnatifida (Wakame) on the development of cerebrovascular diseases in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 2003, 30, 44–48. [Google Scholar] [CrossRef]
- Mohibbullah, M.; Haque, M.N.; Khan, M.N.A.; Park, I.S.; Moon, I.S.; Hong, Y.K. Neuroprotective effects of fucoxanthin and its derivative fucoxanthinol from the phaeophyte Undaria pinnatifida attenuate oxidative stress in hippocampal neurons. J. Appl. Phycol. 2018, 30, 3243–3252. [Google Scholar] [CrossRef]
- Hu, L.; Chen, W.; Tian, F.; Yuan, C.; Wang, H.; Yue, H. Neuroprotective role of fucoxanthin against cerebral ischemic/reperfusion injury through activation of Nrf2/HO-1 signaling. Biomed. Pharmacother. 2018, 106, 1484–1489. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Fan, Y.; Gao, Y.; Li, X.; Hu, Z.; Ding, K.; Wang, Y.; Wang, X. Fucoxanthin provides neuroprotection in models of traumatic brain injury via the Nrf2-ARE and Nrf2-autophagy pathways. Sci. Rep. 2017, 7, 46763. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.A.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2015, 457, 718–722. [Google Scholar] [CrossRef] [Green Version]
- Shanmuganathan, B.; Sathya, S.; Balasubramaniam, B.; Balamurugan, K.; Devi, K.P. Amyloid-β induced neuropathological actions are suppressed by Padina gymnospora (Phaeophyceae) and its active constituent α-bisabolol in Neuro2a cells and transgenic Caenorhabditis elegans Alzheimer’s model. Nitric Oxide 2019, 91, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Choi, J.S.; Nam, T.J. Fucosterol from an edible brown alga Ecklonia stolonifera prevents soluble amyloid beta-induced cognitive dysfunction in aging rats. Mar. Drugs 2018, 16, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, S.Y.; Wong, L.Z.; Wong, J.W.; Tan, E.L. Fucosterol exerts protection against amyloid β-induced neurotoxicity, reduces intracellular levels of amyloid β and enhances the mRNA expression of neuroglobin in amyloid β-induced SH-SY5Y cells. Int. J. Biol. Macromol. 2019, 121, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Liu, D.Q.; Liang, T.J.; Li, J.; Zhang, H.Y.; Liu, A.H.; Guo, Y.W.; Mao, S.C. Bioactive constituents from the green alga Caulerpa racemosa. Bioorg. Med. Chem. 2015, 23, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.R.; Moon, H.E.; Kim, H.R.; Jung, H.A.; Choi, J.S. Neuroprotective effect of edible brown alga Eisenia bicyclis on amyloid beta peptide-induced toxicity in PC12 cells. Arch. Pharm. Res. 2012, 35, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Amarsanaa, K.; Lee, J.H.; Rhim, J.K.; Kwon, J.M.; Kim, S.H.; Park, J.M.; Jung, S.C.; Eun, S.Y. Neuroprotective mechanisms of dieckol against glutamate toxicity through reactive oxygen species scavenging and nuclear factor-like 2/heme oxygenase-1 pathway. Korean J. Physiol. Pharmacol. 2019, 23, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.J.; Ahn, S.; Ryu, J.; Choi, M.S.; Choi, S.; Chong, Y.H.; Hyun, J.W.; Chang, M.J.; Kim, H.S. Phloroglucinol Attenuates the Cognitive Deficits of the 5XFAD Mouse Model of Alzheimer’s Disease. PLoS ONE 2015, 10, e0135686. [Google Scholar] [CrossRef]
- Kang, S.M.; Cha, S.H.; Ko, J.Y.; Kang, M.C.; Kim, D.; Heo, S.J.; Kim, J.S.; Heu, M.S.; Kim, Y.T.; Jung, W.K.; et al. Neuroprotective effects of phlorotannins isolated from a brown alga, Ecklonia cava, against H2O2-induced oxidative stress in murine hippocampal HT22 cells. Environ. Toxicol. Pharmacol. 2012, 34, 96–105. [Google Scholar] [CrossRef]
- Heo, S.J.; Cha, S.H.; Kim, K.N.; Lee, S.H.; Ahn, G.; Kang, D.H.; Oh, C.; Choi, Y.U.; Affan, A.; Kim, D.; et al. Neuroprotective effect of phlorotannin isolated from Ishige okamurae against H2O2 -induced oxidative stress in murine hippocampal neuronal cells, HT22. Appl. Biochem. Biotechnol. 2012, 166, 1520–1532. [Google Scholar] [CrossRef]
- Kim, J.J.; Kang, Y.J.; Shin, S.A.; Bak, D.H.; Lee, J.W.; Lee, K.B.; Yoo, Y.C.; Kim, D.K.; Lee, B.H.; Kim, D.W.; et al. Phlorofucofuroeckol improves glutamate-induced neurotoxicity through modulation of oxidative stress-mediated mitochondrial dysfunction in PC12 cells. PLoS ONE 2016, 11, e0163433. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Huang, C.; Zhao, J.; Lin, J.; Zhou, X.; Naman, C.B.; Wang, N.; Gerwick, W.H.; Wang, Q.; et al. Eckmaxol, a phlorotannin extracted from Ecklonia maxima, Produces anti-beta-amyloid oligomer neuroprotective effects possibly via directly acting on glycogen synthase kinase 3 beta. ACS. Chem. Neurosci. 2018, 9, 1349–1356. [Google Scholar] [CrossRef]
- Jhamandas, J.H.; Wie, M.B.; Harris, K.; MacTavish, D.; Kar, S. Fucoidan inhibits cellular and neurotoxic effects of beta-amyloid (A beta) in rat cholinergic basal forebrain neurons. Eur. J. Neurosci. 2005, 21, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Zhang, Q.; Wang, H.; Cui, Y.; Sun, Z.; Yang, J.; Zheng, Y.; Jia, J.; Yu, F.; Wang, X.; et al. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 2009, 617, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Dong, C.; Yin, J.; Shen, J.; Tian, J.; Li, C. Neuroprotective effect of fucoidan on H2O2-induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cell. Mol. Neurobiol. 2012, 32, 523–529. [Google Scholar] [CrossRef]
- Wei, H.; Gao, Z.; Zheng, L.; Zhang, C.; Liu, Z.; Yang, Y.; Teng, H.; Hou, L.; Yin, Y.; Zou, X. Protective effects of fucoidan on Aβ25-35 and d-Gal-induced neurotoxicity in PC12 cells and d-Gal-induced cognitive dysfunction in mice. Mar. Drugs 2017, 15, 77. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Liu, Z.; Sun, X.; Tao, M.; Xiao, X.; Yu, G.; Wang, X. The Effect of Fucoidan on cellular oxidative stress and the CatD-Bax signaling axis in MN9D cells damaged by 1-Methyl-4-Phenypyridinium. Front. Aging Neurosci. 2019, 10, 429. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Gao, S.; Terakawa, S. Inhibitory effects of fucoidan on NMDA receptors and l-type Ca(2+) channels regulating the Ca(2+) responses in rat neurons. Pharm. Biol. 2019, 57, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Deng, Z.; Geng, L.; Wang, J.; Zhang, Q. In vitro evaluation of the neuroprotective effect of oligo-porphyran from Porphyra yezoensis in PC12 cells. J. Appl. Phycol. 2019, 31, 2559–2571. [Google Scholar] [CrossRef]
- Wang, S.; Li, J.; Xia, W.; Geng, M. A marine-derived acidic oligosaccharide sugar chain specifically inhibits neuronal cell injury mediated by beta-amyloid-induced astrocyte activation in vitro. Neurol. Res. 2007, 29, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Q.; Mao, S.C.; Zhang, H.Y.; Yu, X.Q.; Feng, M.T.; Wang, B.; Feng, L.H.; Guo, Y.W. Racemosins A and B, two novel bisindole alkaloids from the green alga Caulerpa racemosa. Fitoterapia 2013, 91, 15–20. [Google Scholar] [CrossRef]
- Wu, S.; Yue, Y.; Tian, H.; Tao, L.; Wang, Y.; Xiang, J.; Wang, S.; Ding, H. Tramiprosate protects neurons against ischemic stroke by disrupting the interaction between PSD95 and nNOS. Neuropharmacology 2014, 83, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, L.; Li, X. κ-carrageenan-derived pentasaccharide attenuates Aβ25-35-induced apoptosis in SH-SY5Y cells via suppression of the JNK signaling pathway. Mol. Med. Rep. 2017, 15, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, C.; Yin, J.; Shen, J.; Wang, H.; Wu, Y.; Jin, H. Fucoidan, a sulfated polysaccharide from brown algae, improves cognitive impairment induced by infusion of Aβ peptide in rats. Environ. Toxicol. Pharmacol. 2012, 33, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yi, K.; Zhao, Y. Fucoidan inhibits amyloid-β-induced toxicity in transgenic Caenorhabditis elegans by reducing the accumulation of amyloid-β and decreasing the production of reactive oxygen species. Food Funct. 2018, 9, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Chung, D.H.; Kim, D.O.; Kim, G.H.; Heo, H.J. Fucoidan-rich substances from ecklonia cava improve trimethyltin-induced cognitive dysfunction via down-regulation of amyloid β production/Tau hyperphosphorylation. Mar. Drugs 2019, 17, 591. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Ahn, J.H.; Song, M.; Kim, D.W.; Lee, T.K.; Lee, J.C.; Kim, Y.M.; Kim, J.D.; Cho, J.H.; Hwang, I.K.; et al. Pretreated fucoidan confers neuroprotection against transient global cerebral ischemic injury in the gerbil hippocampal CA1 area via reducing of glial cell activation and oxidative stress. Biomed. Pharmacother. 2019, 109, 1718–1727. [Google Scholar] [CrossRef]
- Lee, T.K.; Ahn, J.H.; Park, C.W.; Kim, B.; Park, Y.E.; Lee, J.C.; Park, J.H.; Yang, G.E.; Shin, M.C.; Cho, J.H.; et al. Pre-treatment with laminarin protects hippocampal ca1 pyramidal neurons and attenuates reactive gliosis following transient forebrain ischemia in gerbils. Mar. Drugs 2020, 18, 52. [Google Scholar] [CrossRef] [Green Version]
- Park, J.H.; Ahn, J.H.; Lee, T.K.; Park, C.W.; Kim, B.; Lee, J.C.; Kim, D.W.; Shin, M.C.; Cho, J.H.; Lee, C.H.; et al. Laminarin pretreatment provides neuroprotection against forebrain ischemia/reperfusion injury by reducing oxidative stress and neuroinflammation in aged gerbils. Mar. Drugs 2020, 18, 213. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Geng, L.; Zhang, J.; Wang, J.; Zhang, Q.; Duan, D.; Zhang, Q. Oligo-Porphyran ameliorates neurobehavioral deficits in parkinsonian mice by regulating the PI3K/Akt/Bcl-2 pathway. Mar. Drugs 2018, 16, 82. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, X.; Pan, Y.; Wang, G.; Mao, G. The degraded polysaccharide from Pyropia haitanensis represses amyloid beta peptide-induced neurotoxicity and memory in vivo. Int. J. Biol. Macromol. 2020, 146, 725–729. [Google Scholar] [CrossRef]
- Pentón-Rol, G.; Marín-Prida, J.; Pardo-Andreu, G.; Martínez-Sánchez, G.; Acosta-Medina, E.F.; Valdivia-Acosta, A.; Lagumersindez-Denis, N.; Rodríguez-Jiménez, E.; Llópiz-Arzuaga, A.; López-Saura, P.A.; et al. C-Phycocyanin is neuroprotective against global cerebral ischemia/reperfusion injury in gerbils. Brain. Res. Bull. 2011, 86, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.B.; Frota, A.F.; Sousa, R.S.; Cezario, N.A.; Santos, T.B.; Souza, L.M.; Coura, C.O.; Monteiro, V.S.; Cristino Filho, G.; Vasconcelos, S.M.; et al. Neuroprotective effects of sulphated agaran from marine alga Gracilaria cornea in Rat 6-Hydroxydopamine parkinson’s disease model: Behavioural, neurochemical and transcriptional alterations. Basic Clin. Pharmacol. Toxicol. 2017, 120, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yi, M.; Ding, L.; He, S.; Yan, X. Isolation and purification of a neuroprotective phlorotannin from the marine algae Ecklonia maxima by size exclusion and high-speed counter-current chromatography. Mar. Drugs 2019, 17, 212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Potential beneficial actions of fucoidan in brain and liver injury, disease, and intoxication—Potential implication of sirtuins. Mar. Drugs 2020, 18, 242. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.N.; Chen, P.W.; Huang, C.Y. Compositional characteristics and in vitro evaluations of antioxidant and neuroprotective properties of crude extracts of fucoidan prepared from compressional puffing-pretreated Sargassum crassifolium. Mar. Drugs 2017, 15, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.Y.; Kuo, C.H.; Chen, P.W. Compressional-puffing pretreatment enhances neuroprotective effects of fucoidans from the brown seaweed Sargassum hemiphyllum on 6-Hydroxydopamine-induced apoptosis in SH-SY5Y cells. Molecules 2017, 23, 78. [Google Scholar] [CrossRef] [Green Version]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; de Almeida, R.R.; Pedrosa, R.; et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef]
- Tsang, C.K.; Kamei, Y. Sargaquinoic acid supports the survival of neuronal PC12D cells in a nerve growth factor-independent manner. Eur. J. Pharmacol. 2004, 488, 11–18. [Google Scholar] [CrossRef]
- Tsolaki, M. Future strategies of management of Alzheimer’s Disease. The role of homotaurine. Hell. J. Nucl. Med. 2019, 22, 82–94. [Google Scholar]
- Ricciardi, L.; De Nigris, F.; Specchia, A.; Fasano, A. Homotaurine in Parkinson’s disease. Neurol. Sci. 2015, 36, 1581–1587. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, E.Y.; Nam, T.J. Phycoerythrin peptide from Pyropia yezoensis alleviates endoplasmic reticulum stress caused by perfluorooctane sulfonate-induced calcium dysregulation. Mar. Drugs 2018, 16, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.H.; Kim, E.Y.; Nam, T.J. Phycoerythrin-derived tryptic peptide of a red alga Pyropia yezoensis attenuates glutamate-induced er stress and neuronal senescence in primary rat hippocampal neurons. Mol. Nutr. Food Res. 2018, 62, e1700469. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L.; Zhang, H.; Feng, M.; Yang, H.; Yang, P.; Lin, K.; Guo, Y.; Mao, S. The fatty acids of green alga Caulerpa racemosa and their bioactivities. Zhongguo Haiyang Yaowu 2013, 32, 13–20. [Google Scholar]
- Silva, J.; Alves, C.; Pinteus, S.; Mendes, S.; Pedrosa, R. Neuroprotective effects of seaweeds against 6-hydroxidopamine-induced cell death on an in vitro human neuroblastoma model. BMC Complement. Altern. Med. 2018, 18, 58. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.; Alves, C.; Freitas, R.; Martins, A.; Pinteus, S.; Ribeiro, J.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Antioxidant and neuroprotective potential of the brown seaweed Bifurcaria bifurcata in an in vitro Parkinson’s Disease model. Mar. Drugs 2019, 17, 85. [Google Scholar] [CrossRef] [Green Version]
- Chacko, A.; Ittiyavirah, S.P. Pharmacology, neuroprotective effect of against aluminium-induced Gracilaria corticata neurotoxicity in the hippocampus and cerebral cortex of rat brain: Biochemical and histological approach. J. Pharm. Pharmacol. 2019, 5, 604–613. [Google Scholar]
- Alghazwi, M.; Smid, S.; Zhang, W. In vitro protective activity of South Australian marine sponge and macroalgae extracts against amyloid beta (Aβ(1-42)) induced neurotoxicity in PC-12 cells. Neurotoxicol. Teratol. 2018, 68, 72–83. [Google Scholar] [CrossRef]
- Kim, J.; Moon, I.S.; Goo, T.W.; Moon, S.S.; Seo, M. Algae Undaria pinnatifida protects hypothalamic neurons against endoplasmic reticulum stress through Akt/mTOR signaling. Molecules 2015, 20, 20998–21009. [Google Scholar] [CrossRef] [Green Version]
- Mohibbullah, M.; Hannan, M.A.; Choi, J.Y.; Bhuiyan, M.M.H.; Hong, Y.K.; Choi, J.S.; Choi, I.S.; Moon, I.S. The edible marine alga Gracilariopsis chorda alleviates hypoxia/reoxygenation-induced oxidative stress in cultured hippocampal neurons. J. Med. Food 2015, 18, 960–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Yang, I.; Cao, M.; Su, Z.Y.; Wu, R.; Guo, Y.; Fang, M.; Kong, A.N. Fucoxanthin elicits epigenetic modifications, nrf2 activation and blocking transformation in mouse skin JB6 P+ Cells. AAPS J. 2018, 20, 32. [Google Scholar] [CrossRef]
- Oh, J.H.; Nam, T.J. Hydrophilic glycoproteins of an edible green alga Capsosiphon fulvescens prevent aging-induced spatial memory impairment by suppressing gsk-3beta-mediated er stress in dorsal hippocampus. Mar. Drugs 2019, 17, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, J.H.; Nam, T.J.; Choi, Y.H. Capsosiphon fulvescens Glycoproteins Enhance Probiotics-Induced Cognitive Improvement in Aged Rats. Nutrients 2020, 12, 837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisha, S.A.; Devi, K.P. Gelidiella acerosa protects against Aβ 25-35-induced toxicity and memory impairment in Swiss Albino mice: An in vivo report. Pharm. Biol. 2017, 55, 1423–1435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siddiqui, P.J.A.; Khan, A.; Uddin, N.; Khaliq, S.; Rasheed, M.; Nawaz, S.; Dar, A.; Hanif, M. Sargassum swartzii extracts ameliorate memory functions by neurochemical modulation in a rat model. Food Sci. Biotechnol. 2017, 26, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Mohibbullah, M.; Park, I.S.; Moon, I.S.; Hong, Y.K. An ethanol extract from the phaeophyte Undaria pinnatifida improves learning and memory impairment and dendritic spine morphology in hippocampal neurons. J. Appl. Phycol. 2018, 30, 129–136. [Google Scholar] [CrossRef]
- El-Baz, F.K.; Aly, H.F.; Ali, G.H. Haematococcus pluvialis modulating effect on neurotransmitters, hormones and oxidative damage-associated with alzheimers disease in experimental rats model. Int. J. Pharm. Pharm. Sci. 2017, 9, 198–206. [Google Scholar] [CrossRef]
- Choi, W.Y.; Kang, D.H.; Heo, S.J.; Lee, H.Y. Enhancement of the neuroprotective effect of fermented Spirulina maxima associated with antioxidant activities by ultrasonic extraction. Appl. Sci. 2018, 8, 2469. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Lee, N.S.; Jeong, Y.G.; Lee, J.H.; Kim, E.J.; Han, S.Y. Protective efficacy of an Ecklonia cava extract used to treat transient focal ischemia of the rat brain. Anat. Cell. Biol. 2012, 45, 103–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, R.W.; Du, X.G.; Zhang, X.; Wang, X.; Hu, D.Y.; Meng, T.; Chen, Y.L.; Geng, M.Y.; Shen, J.K. Synthesis and bioassay of β-(1,4)-D-mannans as potential agents against Alzheimer’s disease. Acta Pharmacol. Sin. 2013, 34, 1585–1591. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Sun, G.; Feng, T.; Zhang, J.; Huang, X.; Wang, T.; Xie, Z.; Chu, X.; Yang, J.; Wang, H.; et al. Sodium oligomannate therapeutically remodels gut microbiota and suppresses gut bacterial amino acids-shaped neuroinflammation to inhibit Alzheimer’s disease progression. Cell Res. 2019, 29, 787–803. [Google Scholar] [CrossRef]
- Xiao, S. A phase II clinical trial on GV-971 in patients with Alzheimer’s [abstract no. OC 3]. J. Prev. Alz. Dis. 2014, 1, 214–296. [Google Scholar]
- Xiao, S.; Zhang, Z.; Geng, M. Phase 3 Clinical trial of a novel and multi-targeted oligosaccharide in patients with mildmoderate ad in china. China J. Prev. Alzheimers Dis. 2018, 5, S10. [Google Scholar]
- Davis, G.D.; Vasanthi, A.H. Seaweed metabolite database (SWMD): A database of natural compounds from marine algae. Bioinformation 2011, 5, 361–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, J.; Zhou, J. A marine natural product database. J. Chem. Inf. Comput. Sci. 2002, 42, 742–748. [Google Scholar] [CrossRef]
- Babu, P.A.; Puppala, S.S.; Aswini, S.L.; Vani, M.R.; Kumar, C.N.; Prasanna, T. A database of natural products and chemical entities from marine habitat. Bioinformation 2008, 3, 142–143. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, A.J.M.; Roque, A.C.A. Free marine natural products databases for biotechnology and bioengineering. Biotechnol. J. 2019, 14, 1800607. [Google Scholar] [CrossRef]
- Dictionary of Marine Natural Products. Available online: http://dmnp.chemnetbase.com/faces/chemical/ChemicalSearch.xhtml (accessed on 20 May 2020).
- MarinLit, A Database of the Marine Natural Products Literature. Available online: http://pubs.rsc.org/marinlit (accessed on 20 May 2020).
- Keller, T.H.; Pichota, A.; Yin, Z. A practical view of ‘druggability’. Curr. Opin. Chem. Biol. 2006, 10, 357–361. [Google Scholar] [CrossRef]
- Wale, N.; Karypis, G. Target fishing for chemical compounds using target-ligand activity data and ranking based methods. J. Chem. Inf. Model. 2009, 49, 2190–2201. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, G.; Zhou, Y.; Lin, C.; Chen, S.; Lin, Y.; Mai, S.; Huang, Z. Reverse screening methods to search for the protein targets of chemopreventive compounds. Front. Chem. 2018, 6, 138. [Google Scholar] [CrossRef]
- Dunkel, M.; Fullbeck, M.; Neumann, S.; Preissner, R. SuperNatural: A searchable database of available natural compounds. Nucleic Acids Res. 2006, 34, D678–D683. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Xie, D.; Yu, Y.; Liu, H.; Shi, Y.; Shi, T.; Wen, C. TCMID 2.0: A comprehensive resource for TCM. Nucleic Acids Res. 2017, 46, D1117–D1120. [Google Scholar] [CrossRef] [PubMed]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, F.; Aires-de-Sousa, J. Computational methodologies in the exploration of marine natural product leads. Mar. Drugs 2018, 16, 236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorokina, M.; Steinbeck, C. Review on natural products databases: Where to find data in 2020. J. Cheminform. 2020, 12, 20. [Google Scholar] [CrossRef] [Green Version]
- Vitale, R.M.; D’Aniello, E.; Gorbi, S.; Martella, A.; Silvestri, C.; Giuliani, M.E.; Fellous, T.; Gentile, A.; Carbone, M.; Cutignano, A.; et al. Fishing for targets of alien metabolites: A novel peroxisome proliferator-activated receptor (PPAR) agonist from a marine pest. Mar. Drugs 2018, 16, 431. [Google Scholar] [CrossRef] [Green Version]
- Chamberlin, S.R.; Blucher, A.; Wu, G.; Shinto, L.; Choonoo, G.; Kulesz-Martin, M.; McWeeney, S. Natural product target network reveals potential for cancer combination therapies. Front. Pharmacol. 2019, 10, 557. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.A.; Roy, A.; Choi, J.S. In vitro monoamine oxidase A and B inhibitory activity and molecular docking simulations of fucoxanthin. Fish. Sci. 2017, 83, 123–132. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.H.; Jung, H.A.; Choi, J.S. Characterizing fucoxanthin as a selective dopamine D(3)/D(4) receptor agonist: Relevance to Parkinson’s disease. Chem. Biol. Interact. 2019, 310, 108757. [Google Scholar] [CrossRef]
- Paudel, P.; Park, S.E.; Seong, S.H.; Jung, H.A.; Choi, J.S. Bromophenols from symphyocladia latiuscula target human monoamine oxidase and dopaminergic receptors for the management of neurodegenerative diseases. J. Agric. Food Chem. 2020, 68, 2426–2436. [Google Scholar] [CrossRef]
- Floresta, G.; Amata, E.; Barbaraci, C.; Gentile, D.; Turnaturi, R.; Marrazzo, A.; Rescifina, A. A Structure- and Ligand-Based Virtual Screening of a Database of “Small” Marine Natural Products for the Identification of “Blue” Sigma-2 Receptor Ligands. Mar. Drugs 2018, 16, 10. [Google Scholar] [CrossRef] [Green Version]
- Sá Monteiro, M.; Sloth, J.J.; Holdt, S.L.; Hansen, M. Analysis and risk assessment of seaweed. EFSA J. 2019, 17, e170915. [Google Scholar]
- Hwang, P.A.; Yan, M.D.; Lin, H.T.; Li, K.L.; Lin, Y.C. Toxicological evaluation of low molecular weight fucoidan in vitro and in vivo. Mar. Drugs 2016, 14, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Zhang, Q.; Song, J. Toxicological evaluation of fucoidan extracted from Laminaria japonica in Wistar rats. Food Chem. Toxicol. 2005, 43, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Jeun, J.; Houng, S.J.; Jun, H.J.; Kweon, D.K.; Lee, S.J. Toxicological evaluation of fucoidan from Undaria pinnatifida in vitro and in vivo. Phytother. Res. 2010, 24, 1078–1083. [Google Scholar] [PubMed]
- Kim, K.J.; Lee, O.H.; Lee, B.Y. Genotoxicity studies on fucoidan from Sporophyll of Undaria pinnatifida. Food Chem. Toxicol. 2010, 48, 1101–1104. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, O.H.; Lee, H.H.; Lee, B.Y. A 4-week repeated oral dose toxicity study of fucoidan from the Sporophyll of Undaria pinnatifida in Sprague-Dawley rats. Toxicology 2010, 267, 154–158. [Google Scholar] [CrossRef]
- Myers, S.P.; O’Connor, J.; Fitton, J.H.; Brooks, L.; Rolfe, M.; Connellan, P.; Wohlmuth, H.; Cheras, P.A.; Morris, C. A combined phase I and II open label study on the effects of a seaweed extract nutrient complex on osteoarthritis. Biologics 2010, 4, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Myers, S.P.; Mulder, A.M.; Baker, D.G.; Robinson, S.R.; Rolfe, M.I.; Brooks, L.; Fitton, J.H. Effects of fucoidan from fucus vesiculosus in reducing symptoms of osteoarthritis: A randomized placebo-controlled trial. Biologics 2016, 10, 81–88. [Google Scholar]
- Weiner, M.L. Food additive carrageenan: Part II: A critical review of carrageenan in vivo safety studies. Crit. Rev. Toxicol. 2014, 44, 244–269. [Google Scholar] [CrossRef]
- Hebar, A.; Koller, C.; Seifert, J.M.; Chabicovsky, M.; Bodenteich, A.; Bernkop-Schnurch, A.; Grassauer, A.; Prieschl-Grassauer, E. Non-clinical safety evaluation of intranasal iota-carrageenan. PLoS ONE 2015, 10, e0122911. [Google Scholar] [CrossRef]
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadekaru, T.; Toyama, H.; Yasumoto, T. Safety Evaluation of Fucoxanthin purified from Undaria pinnatifida. Nippon Shokuhin Kagaku Kogaku Kaishi 2008, 55, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Beppu, F.; Niwano, Y.; Sato, E.; Kohno, M.; Tsukui, T.; Hosokawa, M.; Miyashita, K. In vitro and in vivo evaluation of mutagenicity of fucoxanthin (FX) and its metabolite fucoxanthinol (FXOH). J. Toxicol. Sci. 2009, 34, 693–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Pharmacological Effects | Compound (Class) | Algal Source If Any) | Effective Concentration | Experimental Model (In Vivo/In Vitro) | Cellular Effects/Significant Findings | Signaling Pathways Involved | Pharmacological Markers | Reference |
|---|---|---|---|---|---|---|---|---|
| Antioxidant activity | Fucoxanthin (carotenoids) | Sargassum siliquastrum | 50 and 100 μM | H2O2-induced cell damage in kidney fibroblast cells | Attenuates oxidative stress | n.d. | ↓ROS level | [69] |
| Fucoxanthin | 5, 10, and 50 μM | H2O2 induced BV2 microglial cells | Antioxidation | Antioxidant pathway | ↓ROS ↑SOD and GSH | [36] | ||
| Fucosterol, 3,6,17-trihydroxy-stigmasta-4,7,24(28)-triene and 14,15,18,20-diepoxyturbinarin (sterols) | Pelvetia siliquosa | A seven day-dose regimen at 30 mg/kg/day before carbon tetrachloride (CCl4) administration | Rat model | Antioxidation | n.d. | ↑SOD, CAT, and GPx | [71] | |
| Fucosterol | Eisenia bicyclis, brown alga | 25, 50, 100, 200, and 400 μM | RAW 264.7 murine macrophages (t-BHP stimulated) | Protects against oxidative stress | n.d. | ↓ROS generation | [72] | |
| Fucosterol | Ecklonia stolonifera and Eisenia bicyclis; Brown algae | 25, 50, and 100 μM | tert-Butyl hydroperoxide- and tacrine-induced HepG2cell injury model | Antioxidation | n.d. | ↓ROS generation ↑GSH level | [73] | |
| Fucosterol | Sargassum Binderi; brown alga | 3.125, 6.25, 12.5, 25, 50, and 100 μg /mL | Particulate matter-induced injury and inflammation in A549 human lung epithelial cells | Attenuates oxidative stress | ↓ROS level ↑SOD, CAT, and HO-1 in the cytosol, and NRF2 in the nucleus | [74]. | ||
| Glycoprotein | U. pinnatifida | SOD activity and Xox activity at a concentration of 5 mg/mL and 1 mg/mL, respectively | In vitro enzyme assay | ↑SOD and↓Xox | [75] | |||
| Sulfated oligosaccharides | Ulva lactuca and Enteromorpha prolifera; green algae | 150 mg/kg·day | Aging model (male senescence-accelerated prone (SAMP8) and male senescence resistant (SAMR1) mice) | Antioxidantion | n.d. | ↑GSH, SOD, CAT, telomerase levels, ↑Total antioxidant capacity, ↓MDA and AGEPs | [96] | |
| Anti-inflammatory activity | Fucoxanthin | 5, 10, and 50 μM | Aβ42-induced BV2 microglia cells | Anti-inflammation | MAPK pathway | ↓iNOS, COX-2 ↓TNF-α, IL-6, IL-1β, PGE2 ↓JNK, ERK, and p38 MAPK phosphorylation | [36] | |
| Fucoxanthin | - | LPS-activated BV-2 microglia | Anti-inflammation and antioxidation | Akt/NF-κB and MAPKs/AP-1 pathways; PKA/CREB pathway | ↓iNOS, COX-2, ↓TNF-α, IL-6, PGE2, NO, ROS ↓IL-6, TNF-α, iNOS, and COX-2 mRNA expression ↓Akt, NF-κB, ERK, p38 MAPK and AP-1 phosphorylation ↑Nrf2, HO-1 ↑PKA, CREB ↑BDNF | [70] | ||
| Fucosterol | E. bicyclis; brown alga | 5–20 μM for NO | RAW 264.7 murine macrophages (t-BHP 200 μM, LPS-1μM stimulated) | ↓Inflammatory response | ↓NF-κB pathway | ↓NO production ↓iNOS and COX-2 | [72] | |
| Fucosterol | U. pinnatifida | 10, 25, or 50 μM | LPS-induced RAW 264.7 macrophages and THP-1 human monocyte cell line | ↓Inflammatory response | ↓NF-κB pathway | ↓iNOS, TNF-α, and IL-6 ↓DNA binding ↓phosphorylation of NF-κB, MKK3/6 and MK2 | [83] | |
| Fucosterol | Hizikia fusiformis | 1–10 μM | CoCl2 induced hypoxia in keratinocytes | ↓Inflammatory response | n.d. | ↓IL-6, IL-1β and TNF-α ↓pPI3K and pAkt and HIF1-α accumulation | [82] | |
| Fucosterol | Panida. australis | 0.004,0.2, and 10 μM | LPS or Aβ-induced BV2 (microglial) cells | Protects against LPS or Aβ-mediated neuroinflammation | n.d. | ↓IL-6, IL-1β, TNF-α, NO, and PGE2 | [85] | |
| Fucosterol | S. Binderi; brown alga | 3.125, 6.25, 12.5, 25, 50, 100 μg/mL | Particulate matter-induced injury and inflammation in A549 human lung epithelial cells | ↓Inflammatory response | n.d. | ↓COX-2, PGE2, TNF-α and IL-6 | [74] | |
| Dieckol (phlorotannin) | E. cava | 50–300 µg/mL | LPS-stimulated murine BV2 microglia | Anti-inflammation and antioxidation | p-38 MAPK/ NF-κB pathway | ↓NO and PGE2; ↓iNOS and COX-2; ↓IL-1β and TNF-α; ↓ROS | [86] | |
| Phloroglucinol, eckol, dieckol, 7-phloroeckol, phlorofucofuroeckol A and dioxinodehydroeckol (phlorotannin) | E. bicyclis; brown alga | 5–20 μM for NO | LPS-stimulated RAW 264.7 murine macrophages | ↓Inflammatory response | ↓NF-κB pathway | ↓NO production | [72] | |
| Phlorofucofuroeckol A | E. stolonifera | 20 μM | LPS-activated BV2 and primary microglial cells | Anti-inflammation | NF-κB, JNKs, p38 MAPK, and Akt pathways | ↓NO and PGE2; ↓iNOS and COX-2; ↓IL-1β, IL-6 and TNF-α; ↓NF-κB activation and IκB-α degradation ↓JNK, p38, and Akt | [87] | |
| Phlorofucofuroeckol B (phlorotannin) | E. stolonifera | 10–40 µM | LPS-stimulated murine BV2 microglia | Anti-inflammation | IκB-α/NF-κB and Akt/ERK/JNK pathways | ↓TNF-α, IL-1β and IL-6; ↓COX-2 and iNOS ↓NF-κB activation and IκB-α degradation ↓Akt, ERK, and JNK phosphorylation | [88] | |
| 8,8’-bieckol (phlorotannin) | E. cava | LPS-stimulated primary macrophages and RAW 264.7 macrophages & LPS-induced septic mice | Anti-inflammation; Protects mice from endotoxin shock | NF-κB pathway | ↓NO and PGE2; ↓iNOS mRNA and protein expression; ↓IL-6; ↓Transactivation of NF-κB and nuclear translocation of the NF-κB p65 subunit ↓ROS | [90] | ||
| 6,6′-bieckol (phlorotannin) | E.stolonifera | LPS-stimulated BV2 and murine primary microglial cells | Anti-inflammation | IκB-α/NF-κB and JNK/p38 MAPK/Akt pathways | ↓COX-2 and iNOS; ↓NO and PGE2, ↓IL-6 ↓Transactivation of NF-κB and nuclear translocation of the NF-κB p65 subunit ↓Akt, JNK and p38 MAPK phosphorylation | [89] | ||
| Fucoidan (sulfated polysaccharide) | Brown seaweed | 25, 50, and 100 µg/mL | LPS-stimulated murine BV2 microglia | Anti-inflammation | NF-κB and JNK/p38 MAPK/Akt pathways | ↓NO and PGE2; ↓COX-2, iNOS and MCP-1; ↓TNF-α and IL-1β; ↓NF-κB activation; ↓Akt, ERK, p38 MAPK and JNK phosphorylation | [92] | |
| Fucoidan | - | 125 µg/mL | LPS-activated primary microglia | Anti-inflammation | n.d. | ↓TNF-α and ROS | [93] | |
| κ-carrageenan oligosaccharides and desulfated derivatives | Red algae | LPS-activated microglia | Anti-inflammation | n.d. | ↓TNF-α | [94] | ||
| Sulfated oligosaccharides | U. lactuca and E. prolifera; green algae | 150 mg/kg·day | Aging model (male senescence-accelerated prone (SAMP8) and male senescence resistant (SAMR1) mice) | ↓Inflammatory response | n.d. | ↓IFN-γ, TNF-α, and IL-6 | [96] | |
| Alginate-derived oligosaccharide | Brown algae | 50–500 µg/mL | LPS/Aβ-stimulated BV2 microglia | Anti-inflammation | TLR4/NF-κB signaling pathway | ↓NO and PGE2; ↓COX-2 and iNOS; ↓TNF-α, IL-6 and IL-12; ↓TLR4; ↑NF-κB/p65 subunit translocation | [97] | |
| Seleno-polymannuronate | Brown algae | 0.8 mg/mL | LPS-activated primary microglia and astrocytes; mouse model of acute inflammation | Anti-inflammation | NF-κB and MAPK signaling | ↓NO and PGE2; ↓COX-2 and iNOS; ↓TNF-α, IL-1β and IL-6; ↑IκB-α, p65, p38, ERK and JNK phosphorylation | [98] | |
| Sargachromenol (plastoquinone) | Sargassum micracanthum | 30.2 μM (IC50) | LPS-stimulated RAW 264.7 macrophages | Anti-inflammation | NF-κB signaling | ↓NO and PGE2; ↓COX-2 and iNOS; ↑IκB-α | [99] | |
| Sargaquinoic acid (plastoquinone) | Sargassum siliquastrum | LPS-stimulated RAW 264.7 macrophages | Anti-inflammation | NF-κB signaling | ↓NO; ↓iNOS; ↑IκB-α; ↓nuclear translocation of NF-κB; ↓JNK1/2 MAPK | [100] | ||
| Floridoside (glycerol glycosides) | Laurencia undulate; red alga | 50 μM | LPS-stimulated murine BV2 microglia | Anti-inflammation | MAPK Signaling | ↓NO, ROS; ↓iNOS and COX-2; ↓p38 MAPK and ERK phosphorylation | [101] | |
| Glycoprotein | U. pinnatifida | COX-1 and COX-2 inhibition with IC50 values of 53.03 ± 1.03 μg/mL and 193.35 ± 3.08 μg/mL, respectively | LPS-stimulated RAW 264.7 macrophages | Anti-inflammation | n.d. | ↓COX-1 and COX-2 ↓NO | [75] | |
| Caulerpin (bisindole alkaloid) | Caulerpa racemosa | 100 µM/kg body wt | Capsaicin-induced ear edema and carrageenan-induced peritonitis | Inhibition of nociception | n.d. | n.d. | [130] | |
| Caulerpenyne (sesquiterpene) | C. prolifera and C. racemosa | 5.1 μM | Lipoxygenase (LOX) enzyme activity assay | Inhibitory activity against LOX | - | Un-competitive type of inhibition | [131] | |
| Aquamin (multi-mineral complex) | Lithothamnion corallioides; red alga | LPS-stimulated, glial-enriched primary cultures of rat cortex | Anti-inflammation | n.d. | ↓TNF-α and IL-1β | [132] | ||
| Anticholinesterase activity | Fucosterol and 24-hydroperoxy 24-vinylcholesterol | E. stolonifera | IC50 values of 421.72 ± 1.43, 176.46 ± 2.51 µM, respectively | In vitro enzymatic assay | ↓BChE activity | - | Selective inhibition of BChE | [114] |
| Fucosterol | Panida australis | inhibition against AChE (10.99–20.71%) and BChE (4.53–17.53%) with concentrations ≤ 56 μM, | In vitro enzymatic assay | ↓AChE and BChE activities | - | Nonselective cholinesterase inhibition | [85] | |
| Fucosterol | Sargassum horridum | - | In vitro enzymatic assay | ↓AChE activity | - | Non-competitive inhibition | [115] | |
| Fucoxanthin | - | IC50 value 1.97 mM | In vitro BChE activity assay | ↓BChE activity | Mixed inhibition type | [116]. | ||
| Fucoxanthin | Brown seaweed | IC50 value of 81.2 μM | In vitro AChE activity assay; Molecular docking analysis | ↓AChE activity | Fucoxanthin likely interacts with the peripheral anionic site within AChE | Non-competitive manner | [117] | |
| α-Bisabolol | Padina gymnospora | IC50 value < 10 μg/mL | In vitro enzymatic assay | ↓AChE and BChE activity | - | - | [118] | |
| Glycoprotein | U. pinnatifida | AChE and BChE inhibitory activities with IC50 values of 63.56 ± 1.86 and 99.03 ± 4.64, respectively | In vitro enzymatic assay | ↓AChE and BChE activity | - | - | [75] | |
| Phloroglucinol, dibenzo [1,4] dioxine-2,4,7,9-tetraol and eckol | Ecklonia maxima; Brown alga | IC50 value: 76.70 to 579.32 μM | In vitro AChE activity assay | ↓AChE activity | - | - | [119] | |
| Dieckol and phlorofucofuroeckol | E. cava | Ethanol-intoxicated memory impairment in mice | ↓AChE activity | n.d. | ↑Acetylcholine | [120] | ||
| Sargaquinoic acid and sargachromenol (plastoquinones) | Sargassum sagamianum | IC50 value for anti-AChE: 23.2 and 32.7 μM, respectively; IC50 value for anti-BChE of sargaquinoic acid 26 nm | In vitro ChE activity assay | Sargaquinoic acid shows potent inhibitory activity against BuChE and moderate inhibitory activity against AChE | -. | - | [121] | |
| (5E,10Z)-6,10,14-trimethylpentadeca-5,10-dien-2,12-dione and (5E,9E,13E)-6,10,14-trimethylpentadeca-5,9,13-trien-2,12-dione (Sesquiterpenes) | S. sagamianum | IC50 values of 65.0 and 48.0, and 34.0 and 23.0 μM, respectively | In vitro ChE activity assay | Moderate inhibitory activity against AChE and BuChE | - | - | [133] | |
| Anti-amyloidogenic and aggregation inhibition activity | Fucoxanthin | E. stolonifera and U. pinnatifida | ↓β-secretase activity; Binding energy (-7.0 kcal/mol) | - | mixed-type inhibition | [134] | ||
| Fucoxanthin | - | 0.1–30 μM | Suppresses the formation of Aβ1-42 fibrils and Aβ1–42 oligomers, and inhibits Aβ aggregation | - | - | [135] | ||
| Fucoxanthin | - | 2 μM | ThT assay | Inhibits Aβ1-42 fibril and aggregate formation | - | - | [136] | |
| Fucosterol | E. stolonifera and U. pinnatifida | 10–100 μM (IC50 value of 64.12 ± 1.0 μM) | In vitro enzyme assay; In silico analysis | ↓β-secretase activity; Binding energy (−10.1 kcal/mol) | - | Noncompetitive inhibition | [134] | |
| α-Bisabolol | Padina gymnospora | 5 μg/mL | Thioflavin T (ThT), Confocal laser scanning microscopy (CLSM) analysis, Transmission electron microscopy (TEM), Fourier transform infrared (FTIR) spectroscopic analysis and molecular dynamics simulation | Prevents oligomers formation as well as disaggregates the matured fibrils | - | - | [137] | |
| Glycoprotein | U. pinnatifida | IC50 values of 73.35 ± 2.54 μg/mL | In vitro enzymatic assay | ↓BACE1 activity | - | - | [75] | |
| Cholesterol homeostasis and Aβ clearance activity | Fucosterol | - | 100 and 200 μM (HEK293 cell cultures); 100 or 200 μM (macrophages and HepG2, H4IIE, and Caco2 cells) | HEK293 cell cultures (Reporter system); THP-1-derived macrophages; Caco-2 cells HepG2 cells | Reverses cholesterol transport. No accumulation of triglyceride in HepG2 | n.d. | Dual-LXR agonist (LXR-α and LXR-β) ↑ABCA1, ABCG1, and ApoE; ↑Intestinal NPC1L1 and ABCA1; ↑Insig-2a, that delays nuclear translocation of SREBP-1c | [138] |
| Saringosterol | Sargassum fusiforme | 30 μM | Luciferase reporter assay system; HEK293T, THP-1 monocytes, HepG2, RAW264.7, THP-1 macrophages and Caco-2 cells | n.d. | n.d. | Selective LXRβ agonist; ↑ABCA1, ABCG1, and SREBP-1c | [139] | |
| Alginate-derived oligosaccharide | Marine brown algae | BV2 microglial cells | Microglial phagocytosis of Aβ | Toll-like receptor signaling | ↑TLR4 | [97]. | ||
| Monoamine oxidase inhibition and affinity to dopaminergic receptors | Phlorofucofuroeckol-A and dieckol (phlorotannin) | - | In vitro enzyme assay and functional assay for GPCR screening; Docking analysis | ↓hMAO activity; D3R and D4R stimulation | - | - | [140]. | |
| Antiaging | Sulfated oligosaccharides | U. lactuca and E. prolifera; green algae | 150 mg/kg/day | Aging model (male senescence-accelerated prone (SAMP8) and male senescence resistant (SAMR1) mice) | Antioxidant and anti-inflammation | n.d. | ↑GSH, SOD, CAT, telomerase levels, ↑Total antioxidant capacity, ↓MDA and AGEPs ↓IFN-γ, TNF-α, and IL-6 ↑BDNF and ChAT; ↑Sirt1, ↑p53 and FOXO1 | [96] |
| Fucosterol | Hizikia fusiformis | 50 µg/mL | Culture model of C. elegans | Extends lifespan | ↑Antioxidant mechanism | n.d. | [141] |
| Compound | Algal Origin (If Any) | Dosage | Experimental Model (In Vivo/In Vitro) | Cellular Effects/Significant Findings | Pharmacological Markers | References |
|---|---|---|---|---|---|---|
| Sargachromenol | Sargassum macrocarpum (Brown alga, Japan) | ED50 9 μM (with 10 ng/mL NGF) | PC12D cells | NGF-dependent neurite outgrowth and survival | ↑PKA and MAPK1/2 ↑PI3K | [145] |
| Sargaquinoic acid | S. macrocarpum (Brown alga, Japan) | 3 µg/mL (with 10 ng/mL NGF) | Cell differentiation | Protein Kinase A and MAP Kinases-Mediated Signaling Pathways | [146] | |
| Vitamin B12 (chlorophyll-related analog to pheophytin) | Sargassum fulvellum (Brown alga, Japan) | PC12 cells | Cell differentiation | MAPK signal transduction pathway | [148] | |
| Pheophytin A | S. fulvellum (Brown alga, Japan) | 3.9 µg/mL | PC12 cells | NGF-independent neurite outgrowth | ↑PKA and MAPK1/2 ↑PI3K | [147] |
| Dimethylsulfoniopropionate | - | 7.4 mM | Neuronal N2a and glial OLN-93 cells | Process outgrowth; microtubule reorganization and bundling | ↑α-tubulin acetylation | [149] |
| Fucoxanthin | - | 0.1–2 μM | PC-12 cells | NGF-independent neurite outgrowth | n.d. | [136] |
| Compound (Class) | Algal Origin (If Any) | Effective Concentration | Experimental Model (In Vivo/In Vitro) | Cellular Effects/Significant Findings | Signaling Pathways Involved | Pharmacological Markers | References |
|---|---|---|---|---|---|---|---|
| In Vitro Experimental Models | |||||||
| Zonarol (p-hydroquinone sesquiterpene) | Dictyopteris undulate (Brown alga, Japan) | ED50 0.22 µM (therapeutic index, defined as the ratio of ED50 to LD50, is 14.2-fold) | HT22 hippocampal neuronal cells (glutamate-induced oxidative stress) & Cerebrocortical neurons (glutamate or rotenone-induced oxidative stress) | Neuronal survival against oxidative stress | Nrf2/ARE pathway | ↑NQO-1, HO-1, and PRDX4 | [170] |
| Fucoxanthin | Undaria pinnatifida | 0.15–1.5 µmol/L | Hypoxia/reoxygenation-induced neuronal injury | Neuronal survival against oxidative stress | n.d. | n.d. | [166] |
| Fucoxanthin | - | 20 μM | In Vitro model of TBI (primary culture of mouse cortical neurons scratched manually) | Neuronal survival against secondary injury (oxidative stress) | Nrf2-ARE and Nrf2-autophagy pathways | ↓ROS ↑Beclin-1 (Atg6), LC3 (Atg8) and↓p62 ↓Cleaved caspase-3 ↑Nrf2 nuclear translocation ↑HO-1 and NQO-1 | [169] |
| Fucoxanthin | - | 3 μM | β-Amyloid oligomer-induced neurotoxicity in SH-SY5Y Cells | Neuronal survival against oxidative stress | PI3K/Akt and ERK Pathways | ↓ROS ↑pSer473-Akt and pSer9-GSK3β ↓pERK | [164] |
| Fucoxanthin | - | 1-3 μM | H2O2-induced toxicity in SH-SY5Y Cells and primary cerebellar granule neurons | Neuronal survival against oxidative stress | PI3K/Akt and ERK Pathways | ↓ROS ↑pSer473-Akt and pSer9-GSK3β ↓pERK | [165] |
| Fucoxanthin | - | 0.3 μM | Fucoxanthin-modified Aβ1–42 oligomers-induced neurotoxicity in SH-SY5Y Cells | Neuronal survival | n.d. | n.d. | [135] |
| Fucoxanthin | - | 5 μM, 10 μM, and 20 μM | Oxygen-glucose deprivation and reoxygenation (OGD/R) model of cultured neurons | Neuronal survival against oxidative stress | Nrf2/HO-1 signaling | ↑Nrf2 nuclear translocation ↑HO-1 | [168] |
| Fucoxanthin | Undaria pinnatifida | 0.075 μg/mL | H/R-induced excitotoxicity in primary hippocampal neurons | Neuronal survival against oxidative stress | n.d. | n.d. | [167] |
| Fucoxanthin | - | <2 μM (against Aβ1-42-mediated toxicity) 0.5–2 μM(H2O2-induced cytotoxicity) | Aβ1-42-mediated toxicity in PC12 cells H2O2-induced cytotoxicity | Cell survival | n.d. | n.d. | [136] |
| α-Bisabolol | Padina gymnospora | 5 μg/mL | Aβ25-35-induced neurotoxicity in PC-12 cells | Antiapoptosis | n.d. | n.d. | [137] |
| α-Bisabolol | Padina gymnospora | 5 and 10 μg/mL | Aβ25-35-induced neurotoxicity in Neuro2a cells and transgenic C. elegans | Antioxidation Antiapoptosis; Protection against Aβ induced proteotoxicity | Aβ mediated pathway | ↓ROS, NOS ↓Bax and caspase-3 ↓ace-1, hsp-4 and Aβ | [171] |
| Fucosterol | Ecklonia stolonifera | 1–10 µM at 24 h before sAβ1-42 exposure (effective fucosterol conc. 5–10 µM) | sAβ1-42 (10 µM)-induced ER stress model of primary neurons | Attenuates Aβ1-42-induced neurotoxicity | n.d. | ↑TrkB-mediated ERK1/2 signaling ↓GRP78 expression ↑BDNF expression | [172] |
| Fucosterol | - | 0.0032 to 20 μM | Aβ-induced cytotoxicity in SH-SY5Y cells | Reduces apoptosis in Aβ-induced SH-SY5Y cells | n.d. | ↑Ngb mRNA ↓APP mRNA and intracellular Aβ levels | [173] |
| Eckol, dieckol and 8,8′-bieckol | Ecklonia cava | 1–50 µM | Aβ25-35-stimulated PC12 cells | Antioxidation, anti-inflammation, anti-apoptotic properties | NF-κB pathway | ↓COX-2, iNOS; ↓TNF-α, IL-1β and PGE2 production; ↓p38, ERK and JNK | [96] |
| Phloroglucinol, eckol, triphloroethol A, eckstolonol, and dieckol | Ecklonia cava | 50 μM | H2O2-induced oxidative stress in murine hippocampal HT22 cells | ↓Lipid peroxidation; ↓apoptosis | n.d. | ↓ROS ↓Ca2+ release | [178] |
| Diphlorethohydroxycarmalol | Ishige okamurae | 50 μM | H2O2-induced oxidative stress in murine hippocampal HT22 cells | Antioxidation; ↓Lipid peroxidation; ↓Apoptosis | n.d. | ↓Bax ↑Bcl-xL ↓Poly (ADP-ribose) polymerase-1 (PARP) cleavage ↓ROS ↓Ca2+ release | [179] |
| Phloroglucinol, dioxinodehydroeckol, eckol, phlorofucofuroeckol A, dieckol, and 7-phloroeckol | Eisenia bicyclis | 2.5, 5, 10 and 20 µg/mL | Aβ peptide-induced toxicity in PC12 cells | Antioxidation | n.d. | ↓ROS ↓Ca2+ release | [175] |
| Phlorofucofuroeckol | Brown algae | 10 µm | Glutamate-induced cytotoxicity in PC12 | Antioxidation | n.d. | ↓Caspase-3, -8, and PARP | [180] |
| Eckmaxol (phlorotannin) | Ecklonia maxima | 20 µm | β-amyloid oligomer -induced neuronal apoptosis in SH-SY5Y cells | ↓Apoptosis | GSK-3β and ERK pathways | ↑pGSK-3β ↓pERK ↑HO-1 | [181] |
| Fucoidan | - | 0.1–1.0 µm | Aβ1−42-induced neurotoxicity in rat cholinergic basal forebrain neurons | Restores Aβ-induced reduction in whole-cell currents | n.d. | ↑pPKC ↓ROS ↓caspases 9 and 3 | [182] |
| Fucoidan (sulfated polysaccharide) | - | 0.1 and 1.0 mg/mL | MPP(+)-induced injury in MN9D cells | Antioxidation; Protects cellular injury | n.d. | n.d. | [183] |
| Fucoidan (sulfated polysaccharide) | - | 60 and 30 μg/mL | H2O2-induced apoptosis in PC12 cells | ↑Cell viability; antioxidation | PI3K/Akt signaling | ↓ROS; ↑SOD and GPx activities; ↓MDA; ↑Bcl-2/Bax ratio; ↓caspase-3; ↑p-Akt | [184] |
| Fucoidan (sulfated polysaccharide) | - | 100, 200, 400 μg/mL | Aβ25–35 and d-Gal-induced neurotoxicity in PC12 cells | ↓Apoptosis | Caspase-dependent apoptosis pathway | ↓Cytochrome c release; ↓Caspase activation; ↑Livin and XIAP; ↑SOD ↑GSH | [185] |
| Fucoidan (sulfated polysaccharide) | - | 100 μM | MPP(+)-induced injury in dopaminergic precursor cell line(MN9D) cells | ↓Apoptosis; Antioxidation; | CatD-Bax signaling axis | ↓LC3-II and CatD; ↓Bax; ↑SOD ↑GSH | [186] |
| Fucoidan (sulfated polysaccharide) | Fucus vesiculosus Linn., brown alga | 0.5 mg/mL or 1.5 mg/mL | NMDA-induced Ca2+ responses in culture rat neurons | Suppresses the intracellular Ca2+ responses by selectively inhibiting NMDA receptors in cortical neurons and l-type Ca2+ channels in hippocampal neurons. | n.d. | ↓GluNR1 mRNA and l-type Ca2+ channels, PR1/PR2 | [187] |
| Oligo-porphyran | Synthesized from porphyran (isolated from Pyropia yezoensis) through acidolysis reaction | 200 μg/mL | 6-OHDA-induced cytotoxicity in PC12 cells | ↓Apoptosis; Antioxidation; Anti-inflammation | PI3K/ Akt/PKC pathway | ↓ROS; ↑MMP ↑SOD and GSH; ↑Bcl-2/Bax ratio; ↓caspase-3 and -9 ↑p-Akt, p-PI3K, PKC ↑DAT and TH ↓TNF-α, IL-1β, and IL-6 | [188] |
| Acidic oligosaccharide sugar chain | Echlonia kurome Okam | 50, 75, 100 μg/mL | Inflammatory responses and cytotoxicity in SH-SY5Y cell line induced by Aβ-stimulated astrocytes conditioned medium | Oxidative stress | n.d. | ↓TNF-α and IL-6; ↓Ca2+ influx | [189] |
| Racemosins A (bisindole alkaloid) | Caulerpa racemosa, green alga | 10μM | Aβ25–35-induced SH-SY5Y cell damage | ↑Cell viability; ↓apoptosis | n.d. | [190] | |
| Tramiprosate (small aminosulphonate compound) | Red marine algae | 50 mg/kg | OGD- or NMDA-induced injury in NGF-differentiated PC12 cells and primary cortical neurons | Protects against neuronal injury | n.d. | [191] | |
| Dimethylsulfoniopropionate | - | 1 mg/mL | Tropodithietic acid -induced cytotoxicity in OLN-93 and N2a cells | Protects against neurotoxicity; Attenuates stress responses and mitochondrial damage | n.d. | ↓ERK1/2 activation and HSP32 induction | [149] |
| κ-Carrageenan-derived pentasaccharide | marine red algae | 25, 50, or 100 µM | Aβ25-35-induced neurotoxicity in SH-SY5Y cells | ↑Cell viability; ↓Apoptosis | JNK signaling pathway | ↓Cleaved caspase 3 ↓p-JNK | [192] |
| In vivo experimental models | |||||||
| Fucoidan (sulfated polysaccharide) | - | 25 mg/kg | MPTP-induced animal model of Parkinsonism in C57/BL mice in vivo | ↓Behavioral deficits; ↓TH-positive neuronal loss | n.d. | ↑Dopamine, DOPAC and HVA; ↑Tyrosine hydroxylase; ↑GSH; ↑SOD, GPx, and catalase activity and total antioxidant capacity; | [183] |
| Fucoidan (sulfated polysaccharide) | - | 7.5 and 15 mg/kg body wt (intranigral injection) | LPS-induced neurotoxicity in rat | Ameliorates behavioral deficits, prevents the loss of dopaminergic neurons and inhibits the deleterious activation of microglia in the substantia nigra pars compacta | n.d. | ↓CD11b | [93] |
| Fucoidan (sulfated polysaccharide) | - | 50, 100, 200 mg kg−1 | Aβ1-40-induced learning and memory impairment in rats | Ameliorates learning and memory impairment; ↓oxidative stress; ↓apoptosis | Antioxidation | ↑ChAT, SOD and GPx activity; ↑Ach; ↓AchE activity; ↓MDA; ↑Bcl-2/Bax ratio; ↓caspase-3 activity | [193] |
| Fucoidan (sulfated polysaccharide) | - | 100 and 200 mg/kg on day 2–6, 50 mg/kg on day 4–6 | d-Gal-induced cognitive dysfunction in mice | ↓Apoptosis; ameliorate the learning and memory impairment | Caspase-dependent apoptosis pathway | ↑Ach level and ChAT activity; ↓AChE activity; ↑SOD; ↑GSH | [185] |
| Fucoidan (sulfated polysaccharide) | - | 100–500 ng/mL | Transgenic C. elegans AD model | Alleviates the paralyzed phenotype; ↓Aβ deposits | n.d. | ↑Proteasomal activity (proteolysis); ↓ROS | [194] |
| Fucoidan-rich substances | E. cava | Polyphenol/fucoidan extract and mixture (4:6) | Trimethyltin-induced cognitive dysfunction model | Ameliorates learning and memory impairment | n.d. | ↓ROS; ↑MMP; ↓BAX and cytochrome C release; ↓Amyloid β production; ↓Tau hyperphosphorylation | [195] |
| Fucoidan | - | 50 mg/kg | Transient global cerebral ischemia (tGCI) model of gerbils | ↓Oxidative stress and glial activation | n.d. | ↑SOD1 and SOD2 | [196] |
| Laminarin (polysaccharide) | - | 50 or 100 mg/kg (i.p) for seven days before IR (5-min transient ischemia) surgery | Forebrain I/R injury in young gerbils (6 months) | ↓Reactive gliosis (M1 microglia) and neuroinflammation | n.d. | ↓IL-2 | [197] |
| Laminarin (polysaccharide) | Brown algae | 50 mg/kg/day (i.p) for seven days before IR (5-min transient ischemia) surgery | Forebrain I/R injury in aged gerbils (22–24 months) | ↓Oxidative stress and neuroinflammation | n.d. | ↓Superoxide anions and 4-hydroxy-2-nonenal (HNE) ↓IL-1β and TNF-α ↑SOD1 and SOD2 ↑IL-4 and IL-13 | [198] |
| Oligo-porphyran | Synthesized From porphyran (isolated from Pyropia yezoensis) through acidolysis reaction | 25 and 50 mg/kg | 6-OHDA-induced Parkinsonian mice model | ↓Apoptosis; Ameliorates behavioral deficits | PI3K/Akt/Bcl-2 pathway | ↑DAT and TH; ↓caspase-3 and -9 ↑DA, NE, 5-HT, DOPAC ↑p-ERK1/2, DRD2 ↑p-Akt, p-PI3K, GSK-3β ↑Bcl-2/Bax ratio; ↓PARP and cytC ↑p-TrkA and NGF | [199] |
| Porphyran (polysaccharide) | Degraded polysaccharide from Pyropia haitanensis | 75, 150, 300 mg/kg | Aβ1-40-induced mice AD model | Improved learning and memory deficits | n.d. | ↑ChAT activity; ↓AChE activity; ↑Ach | [200] |
| Fucoxanthin | Brown seaweed | 50, 100, 200 mg/kg | Scopolamine-induced cognitive impairments in mice | Memory enhancement; anticholinesterase | n.d. | ↓AChE and choline acetyltransferase ↑BDNF | [117] |
| Fucoxanthin | - | 0.1−30 μM | Aβ oligomer-induced cognitive impairments in mice | Memory enhancement, attenuation of oxidative stress | n.d. | ↑BDNF | [135] |
| Fucoxanthin | - | 5 μM, 10 μM, and 20 μM | Middle cerebral artery occlusion (MCAO) rat model (cerebral ischemic/reperfusion (I/R) injury) | Improves the neurologic deficit score and reduces the infarct volume | n.d. | ↑SOD activity ↓ROS, MDA ↓cleaved caspase-3 ↑Bcl-2/Bax ratio | [168] |
| Fucoxanthin | 100 mg/kg and 0.05 mmol/L | Traumatic brain injury (TBI) model | Anti-apoptosis, attenuation of oxidative stress, induction of autophagy | Nrf2-ARE and Nrf2-autophagy pathways | ↑GPx ↓MDA ↓Cleaved caspase-3, PARP, cytosolic cytochrome c ↑Mitochondrial cytochrome c ↑Beclin-1 (Atg6), LC3 (Atg8) and↓p62 ↑Nrf2 nuclear translocation ↑HO-1 and NQO-1 | [169] | |
| Fucosterol | Ecklonia stolonifera | 1–10 µM | sAβ1-42-induced memory dysfunction in aging rats | Ameliorates Aβ1-42-induced memory impairment | n.d. | ↑TrkB-mediated ERK1/2 signaling ↓GRP78 expression ↑BDNF expression | [172] |
| Dieckol and phlorofucofuroeckol | Ecklonia cava | PFF (0.2 and 2 mg/kg) and dieckol (1 and 10 mg/kg) | Ethanol-intoxicated memory-impaired mice | ↓AChE activity; reduces the inhibition of latency | n.d. | ↑ACh | [120] |
| C-Phycocyanin | 200 mg/kg | Global cerebral ischemia/reperfusion (I/R) injury in gerbils | Reduces the infarct volume and improves the neurologic deficit score; protects neurons, improves the functional outcome (locomotor behavior) and promotes survival | n.d. | ↓MDA | [201] | |
| Tramiprosate (small aminosulphonate compound) | Red marine algae | 50 mg/kg | Intraluminal filament model of MCAO | Reduces infarct volume | PSD95/nNOS signaling | Disruption of the interaction between PSD95 and nNOS; ↓nNOS translocation | [191] |
| Sulfated agaran | Gracilaria cornea, red alga | 60 μg, single intrastriatal injection | Rat 6-hydroxydopamine Parkinson’s disease model | ↓Oxidative/ nitrosative stress; restores behavioral deficits and locomotor performance; improves weight | n.d. | ↑DA, DOPAC and HVA; ↓5-HT; ↓NO2/NO3 and TBARS; ↑GSH; ↓p65, IL-1β and iNOS; ↑BDNF | [202] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hannan, M.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Sohag, A.A.M.; Rahman, M.A.; Uddin, M.J.; Alam, M.; Moon, I.S. Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Mar. Drugs 2020, 18, 347. https://doi.org/10.3390/md18070347
Hannan MA, Dash R, Haque MN, Mohibbullah M, Sohag AAM, Rahman MA, Uddin MJ, Alam M, Moon IS. Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Marine Drugs. 2020; 18(7):347. https://doi.org/10.3390/md18070347
Chicago/Turabian StyleHannan, Md. Abdul, Raju Dash, Md. Nazmul Haque, Md. Mohibbullah, Abdullah Al Mamun Sohag, Md. Ataur Rahman, Md Jamal Uddin, Mahboob Alam, and Il Soo Moon. 2020. "Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances" Marine Drugs 18, no. 7: 347. https://doi.org/10.3390/md18070347
APA StyleHannan, M. A., Dash, R., Haque, M. N., Mohibbullah, M., Sohag, A. A. M., Rahman, M. A., Uddin, M. J., Alam, M., & Moon, I. S. (2020). Neuroprotective Potentials of Marine Algae and Their Bioactive Metabolites: Pharmacological Insights and Therapeutic Advances. Marine Drugs, 18(7), 347. https://doi.org/10.3390/md18070347