Combined Treatment of Heteronemin and Tetrac Induces Antiproliferation in Oral Cancer Cells
Abstract
:1. Introduction
2. Results
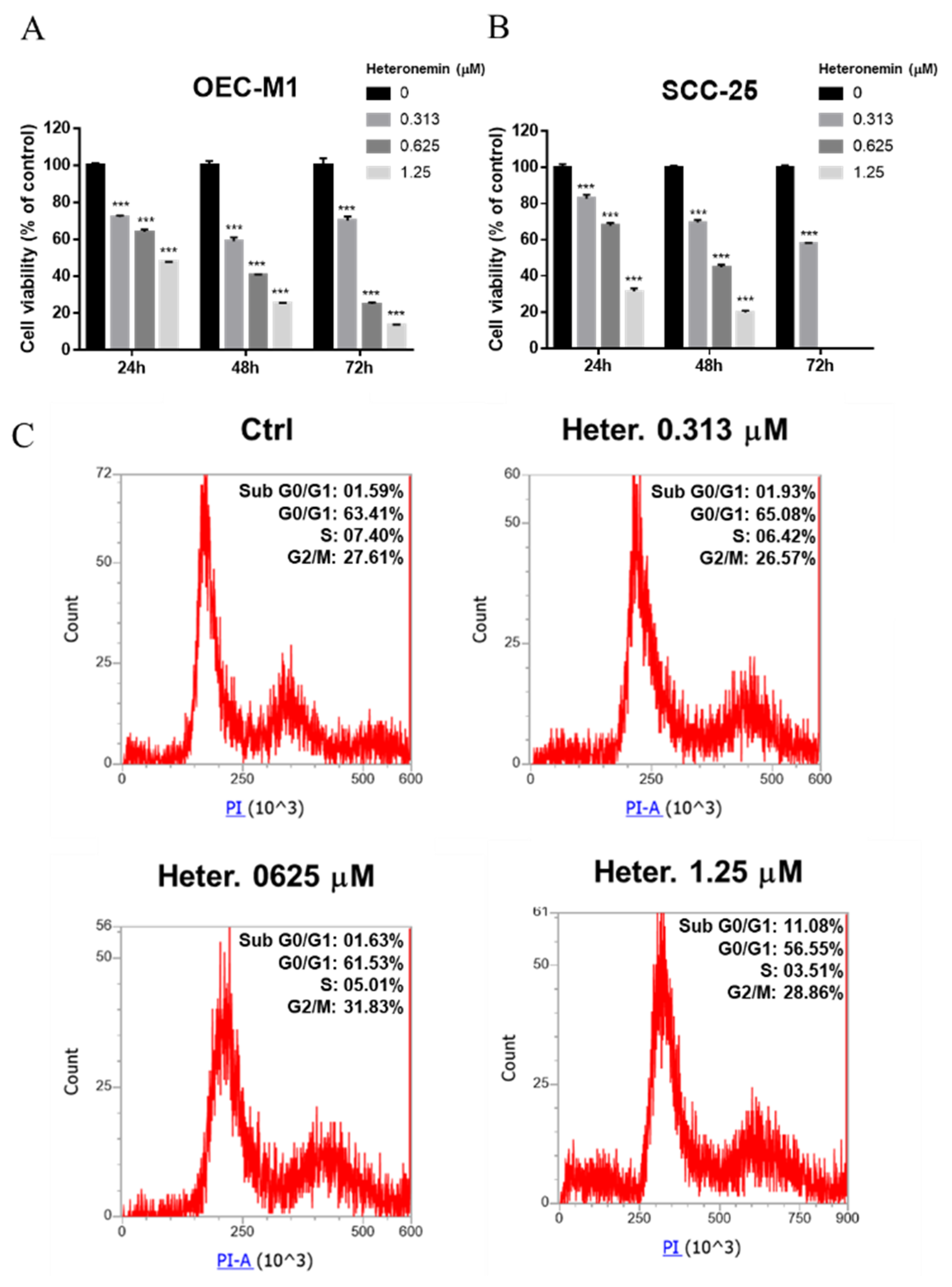
2.1. Heteronemin Induces Antiproliferation and Modulates Gene Expression in Different Types of Oral Cancer Cells
2.2. Tetrac and Heteronemin Inhibit Signal Transduction Pathways in Oral Cancer Cells
2.3. Tetrac and Heteronemin Synergistically Increase p53 Accumulation and Induce Antiproliferation in Oral Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Flow Cytometric Analysis
4.3. Cell Proliferation Assay
4.4. Real-Time Quantitative Polymerase Chain Reaction (qPCR)
4.5. Western Blotting
4.6. Quantification of Results and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tseng, C.H. Oral cancer in Taiwan: Is diabetes a risk factor? Clin. Oral Investig. 2013, 17, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.Y.; Tey, S.L.; Ho, Y.; Chin, Y.T.; Wang, K.; Whang-Peng, J.; Shih, Y.J.; Chen, Y.R.; Yang, Y.N.; Chen, Y.C.; et al. Heteronemin induces anti-proliferation in cholangiocarcinoma cells via inhibiting tgf-beta pathway. Mar. Drugs 2018, 16, 489. [Google Scholar] [CrossRef] [Green Version]
- Nana, A.W.; Chin, Y.T.; Lin, C.Y.; Ho, Y.; Bennett, J.A.; Shih, Y.J.; Chen, Y.R.; Changou, C.A.; Pedersen, J.Z.; Incerpi, S.; et al. Tetrac downregulates beta-catenin and hmga2 to promote the effect of resveratrol in colon cancer. Endocr. Relat. Cancer 2018, 25, 279–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.H.; Kuo, S.M.; Wu, Y.J.; Su, J.H. Improvement and enhancement of antibladder carcinoma cell effects of heteronemin by the nanosized hyaluronan aggregation. Int. J. Nanomed. 2016, 11, 1237–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, M.H.; Huang, H.L.; Lin, Y.Y.; Tsui, K.H.; Chen, P.C.; Cheng, S.Y.; Chong, I.W.; Sung, P.J.; Tai, M.H.; Wen, Z.H.; et al. Ba6 induces apoptosis via stimulation of reactive oxygen species and inhibition of oxidative phosphorylation in human lung cancer cells. Oxid. Med. Cell Longev. 2019, 2019, 6342104. [Google Scholar] [CrossRef]
- Wu, J.C.; Wang, C.T.; Hung, H.C.; Wu, W.J.; Wu, D.C.; Chang, M.C.; Sung, P.J.; Chou, Y.W.; Wen, Z.H.; Tai, M.H. Heteronemin is a novel c-met/stat3 inhibitor against advanced prostate cancer cells. Prostate 2016, 76, 1469–1483. [Google Scholar] [CrossRef]
- Nana, A.W.; Wu, S.Y.; Yang, Y.S.; Chin, Y.T.; Cheng, T.M.; Ho, Y.; Li, W.S.; Liao, Y.M.; Chen, Y.R.; Shih, Y.J.; et al. Nano-diamino-tetrac (ndat) enhances resveratrol-induced antiproliferation by action on the rrm2 pathway in colorectal cancers. Horm. Cancer 2018, 9, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Latteyer, S.; Christoph, S.; Theurer, S.; Hones, G.S.; Schmid, K.W.; Fuehrer, D.; Moeller, L.C. Thyroxine promotes lung cancer growth in an orthotopic mouse model. Endocr. Relat. Cancer 2019. [Google Scholar] [CrossRef]
- Lin, H.Y.; Landersdorfer, C.B.; London, D.; Meng, R.; Lim, C.U.; Lin, C.; Lin, S.; Tang, H.Y.; Brown, D.; Van Scoy, B.; et al. Pharmacodynamic modeling of anti-cancer activity of tetraiodothyroacetic acid in a perfused cell culture system. PLoS Comput. Biol. 2011, 7, e1001073. [Google Scholar] [CrossRef] [Green Version]
- Chin, Y.T.; He, Z.R.; Chen, C.L.; Chu, H.C.; Ho, Y.; Su, P.Y.; Yang, Y.S.H.; Wang, K.; Shih, Y.J.; Chen, Y.R.; et al. Tetrac and ndat induce anti-proliferation via integrin αvβ3 in colorectal cancers with different k-ras status. Front. Endocrinol. 2019, 10, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.S.; Chin, Y.T.; Yang, Y.S.H.; Wei, P.L.; Wu, H.C.; Shih, A.; Lu, Y.T.; Pedersen, J.Z.; Incerpi, S.; Liu, L.F.; et al. The combination of tetraiodothyroacetic acid and cetuximab inhibits cell proliferation in colorectal cancers with different k-ras status. Steroids 2016, 111, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.C.; Chin, Y.T.; Nana, A.W.; Wang, S.H.; Liao, Y.M.; Chen, Y.R.; Shih, Y.J.; Changou, C.A.; Yang, Y.S.; Wang, K.; et al. Enhancement by nano-diamino-tetrac of antiproliferative action of gefitinib on colorectal cancer cells: Mediation by egfr sialylation and pi3k activation. Horm. Cancer 2018, 9, 420–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmohl, K.A.; Müller, A.M.; Wechselberger, A.; Rühland, S.; Salb, N.; Schwenk, N.; Heuer, H.; Carlsen, J.; Göke, B.; Nelson, P.J.; et al. Thyroid hormones and tetrac: New regulators of tumour stroma formation via integrin αvβ3. Endocr. Relat. Cancer 2015, 22, 941–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.R.; Chen, Y.S.; Chin, Y.T.; Li, Z.L.; Shih, Y.J.; Yang, Y.S.H.; ChangOu, C.A.; Su, P.Y.; Wang, S.H.; Wu, Y.H.; et al. Thyroid hormone-induced expression of inflammatory cytokines interfere with resveratrol-induced anti-proliferation of oral cancer cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2019, 132, 110693. [Google Scholar] [CrossRef]
- Ho, Y.; Wu, C.Y.; Chin, Y.T.; Li, Z.L.; Pan, Y.S.; Huang, T.Y.; Su, P.Y.; Lee, S.Y.; Crawford, D.R.; Su, K.W.; et al. Ndat suppresses pro-inflammatory gene expression to enhance resveratrol-induced anti-proliferation in oral cancer cells. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 136, 111092. [Google Scholar] [CrossRef]
- Davis, P.J.; Glinsky, G.V.; Lin, H.Y.; Incerpi, S.; Davis, F.B.; Mousa, S.A.; Tang, H.Y.; Hercbergs, A.; Luidens, M.K. Molecular mechanisms of actions of formulations of the thyroid hormone analogue, tetrac, on the inflammatory response. Endocr. Res. 2013, 38, 112–118. [Google Scholar] [CrossRef]
- Lin, H.Y.; Chin, Y.T.; Yang, Y.C.; Lai, H.Y.; Wang-Peng, J.; Liu, L.F.; Tang, H.Y.; Davis, P.J. Thyroid hormone, cancer, and apoptosis. Compr. Physiol. 2016, 6, 1221–1237. [Google Scholar]
- Lin, H.Y.; Davis, P.J.; Tang, H.Y.; Mousa, S.A.; Luidens, M.K.; Hercbergs, A.H.; Davis, F.B. The pro-apoptotic action of stilbene-induced cox-2 in cancer cells: Convergence with the anti-apoptotic effect of thyroid hormone. Cell Cycle (Georget. Tex.) 2009, 8, 1877–1882. [Google Scholar] [CrossRef]
- Pal, S.K.; Nguyen, C.T.; Morita, K.I.; Miki, Y.; Kayamori, K.; Yamaguchi, A.; Sakamoto, K. Thbs1 is induced by tgfb1 in the cancer stroma and promotes invasion of oral squamous cell carcinoma. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2016, 45, 730–739. [Google Scholar] [CrossRef]
- Lin, H.Y.; Delmas, D.; Vang, O.; Hsieh, T.C.; Lin, S.; Cheng, G.Y.; Chiang, H.L.; Chen, C.E.; Tang, H.Y.; Crawford, D.R.; et al. Mechanisms of ceramide-induced cox-2-dependent apoptosis in human ovarian cancer ovcar-3 cells partially overlapped with resveratrol. J. Cell. Biochem. 2013, 114, 1940–1954. [Google Scholar] [CrossRef]
- Glinskii, A.B.; Glinsky, G.V.; Lin, H.Y.; Tang, H.Y.; Sun, M.; Davis, F.B.; Luidens, M.K.; Mousa, S.A.; Hercbergs, A.H.; Davis, P.J. Modification of survival pathway gene expression in human breast cancer cells by tetraiodothyroacetic acid (tetrac). Cell Cycle (Georget. Tex.) 2009, 8, 3562–3570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yalcin, M.; Dyskin, E.; Lansing, L.; Bharali, D.J.; Mousa, S.S.; Bridoux, A.; Hercbergs, A.H.; Lin, H.Y.; Davis, F.B.; Glinsky, G.V.; et al. Tetraiodothyroacetic acid (tetrac) and nanoparticulate tetrac arrest growth of medullary carcinoma of the thyroid. J. Clin. Endocrinol. Metab. 2010, 95, 1972–1980. [Google Scholar] [CrossRef]
- Yalcin, M.; Lin, H.Y.; Sudha, T.; Bharali, D.J.; Meng, R.; Tang, H.Y.; Davis, F.B.; Stain, S.C.; Davis, P.J.; Mousa, S.A. Response of human pancreatic cancer cell xenografts to tetraiodothyroacetic acid nanoparticles. Horm. Cancer 2013, 4, 176–185. [Google Scholar] [CrossRef]
- Yang, S.H.; Lin, H.Y.; Changou, C.A.; Chen, C.H.; Liu, Y.R.; Wang, J.; Jiang, X.; Luh, F.; Yen, Y. Integrin beta3 and lkb1 are independently involved in the inhibition of proliferation by lovastatin in human intrahepatic cholangiocarcinoma. Oncotarget 2016, 7, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.E. Non-smad pathways in tgf-beta signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Massague, J. Tgfbeta in cancer. Cell 2008, 134, 215–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elston, R.; Inman, G.J. Crosstalk between p53 and tgf-beta signalling. J. Signal Transduct. 2012, 2012, 294097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inman, G.J. Switching tgfbeta from a tumor suppressor to a tumor promoter. Curr. Opin. Genet. Dev. 2011, 21, 93–99. [Google Scholar] [CrossRef]
- Meulmeester, E.; Ten Dijke, P. The dynamic roles of tgf-beta in cancer. J. Pathol. 2011, 223, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; David, L.; Mendoza, V.; Yang, Y.; Villarreal, M.; De, K.; Sun, L.; Fang, X.; Lopez-Casillas, F.; Wrana, J.L.; et al. Tgf-beta signalling is mediated by two autonomously functioning tbetari:Tbetarii pairs. EMBO J. 2011, 30, 1263–1276. [Google Scholar] [CrossRef] [Green Version]
- Massague, J. A very private tgf-beta receptor embrace. Mol. Cell 2008, 29, 149–150. [Google Scholar] [CrossRef] [PubMed]
- Groppe, J.; Hinck, C.S.; Samavarchi-Tehrani, P.; Zubieta, C.; Schuermann, J.P.; Taylor, A.B.; Schwarz, P.M.; Wrana, J.L.; Hinck, A.P. Cooperative assembly of tgf-beta superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol. Cell 2008, 29, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Seoane, J.; Wotton, D. Smad transcription factors. Genes Dev. 2005, 19, 2783–2810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heldin, C.H.; Moustakas, A. Role of smads in tgfbeta signaling. Cell Tissue Res. 2012, 347, 21–36. [Google Scholar] [CrossRef]
- Liu, S.; Chen, S.; Zeng, J. Tgfbeta signaling: A complex role in tumorigenesis (review). Mol. Med. Rep. 2018, 17, 699–704. [Google Scholar]
- Wyllie, F.S.; Dawson, T.; Bond, J.A.; Goretzki, P.; Game, S.; Prime, S.; Wynford-Thomas, D. Correlated abnormalities of transforming growth factor-beta 1 response and p53 expression in thyroid epithelial cell transformation. Mol. Cell. Endocrinol. 1991, 76, 13–21. [Google Scholar] [CrossRef]
- Cordenonsi, M.; Dupont, S.; Maretto, S.; Insinga, A.; Imbriano, C.; Piccolo, S. Links between tumor suppressors: P53 is required for tgf-beta gene responses by cooperating with smads. Cell 2003, 113, 301–314. [Google Scholar] [CrossRef]
- Takebayashi-Suzuki, K.; Funami, J.; Tokumori, D.; Saito, A.; Watabe, T.; Miyazono, K.; Kanda, A.; Suzuki, A. Interplay between the tumor suppressor p53 and tgf beta signaling shapes embryonic body axes in xenopus. Dev. (Camb. Engl.) 2003, 130, 3929–3939. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Weisberg, E.; Fridmacher, V.; Watanabe, M.; Naco, G.; Whitman, M. Smad4 and fast-1 in the assembly of activin-responsive factor. Nature 1997, 389, 85–89. [Google Scholar] [CrossRef]
- Cordenonsi, M.; Montagner, M.; Adorno, M.; Zacchigna, L.; Martello, G.; Mamidi, A.; Soligo, S.; Dupont, S.; Piccolo, S. Integration of tgf-beta and ras/mapk signaling through p53 phosphorylation. Science (N. Y. NY) 2007, 315, 840–843. [Google Scholar] [CrossRef]
- Dupont, S.; Zacchigna, L.; Adorno, M.; Soligo, S.; Volpin, D.; Piccolo, S.; Cordenonsi, M. Convergence of p53 and tgf-beta signaling networks. Cancer Lett. 2004, 213, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Meng, C.L. Regulation of pg synthase by egf and pdgf in human oral, breast, stomach, and fibrosarcoma cancer cell lines. J. Dent. Res. 1994, 73, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.T.; Hsieh, M.T.; Lin, C.Y.; Kuo, P.J.; Yang, Y.C.; Shih, Y.J.; Lai, H.Y.; Cheng, G.Y.; Tang, H.Y.; Lee, C.C.; et al. 2,3,5,4′-tetrahydroxystilbene-2-o-beta-glucoside isolated from polygoni multiflori ameliorates the development of periodontitis. Mediat. Inflamm. 2016, 2016, 6953459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.Y.; Chin, Y.T.; Nana, A.W.; Shih, Y.J.; Lai, H.Y.; Tang, H.Y.; Leinung, M.; Mousa, S.A.; Davis, P.J. Actions of l-thyroxine and nano-diamino-tetrac (nanotetrac) on pd-l1 in cancer cells. Steroids 2016, 114, 59–67. [Google Scholar] [CrossRef] [Green Version]







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-H.; Huang, T.-Y.; Chang, W.-J.; Pan, Y.-s.; Chu, H.-R.; Li, Z.-L.; Unson, S.; Chin, Y.-T.; Lin, C.-Y.; Huang, H.-M.; et al. Combined Treatment of Heteronemin and Tetrac Induces Antiproliferation in Oral Cancer Cells. Mar. Drugs 2020, 18, 348. https://doi.org/10.3390/md18070348
Huang C-H, Huang T-Y, Chang W-J, Pan Y-s, Chu H-R, Li Z-L, Unson S, Chin Y-T, Lin C-Y, Huang H-M, et al. Combined Treatment of Heteronemin and Tetrac Induces Antiproliferation in Oral Cancer Cells. Marine Drugs. 2020; 18(7):348. https://doi.org/10.3390/md18070348
Chicago/Turabian StyleHuang, Chi-Hung, Tung-Yung Huang, Wong-Jin Chang, Yi-shin Pan, Hung-Ru Chu, Zi-Lin Li, Sukanya Unson, Yu-Tang Chin, Chi-Yu Lin, Haw-Ming Huang, and et al. 2020. "Combined Treatment of Heteronemin and Tetrac Induces Antiproliferation in Oral Cancer Cells" Marine Drugs 18, no. 7: 348. https://doi.org/10.3390/md18070348
APA StyleHuang, C.-H., Huang, T.-Y., Chang, W.-J., Pan, Y.-s., Chu, H.-R., Li, Z.-L., Unson, S., Chin, Y.-T., Lin, C.-Y., Huang, H.-M., Hsiung, C.-N., Gionfra, F., De Vito, P., Pedersen, J. Z., Incerpi, S., Chen, Y.-R., Lee, S.-Y., Lin, H.-Y., Davis, P. J., ... Wang, K. (2020). Combined Treatment of Heteronemin and Tetrac Induces Antiproliferation in Oral Cancer Cells. Marine Drugs, 18(7), 348. https://doi.org/10.3390/md18070348








