Comprehensive GCMS and LC-MS/MS Metabolite Profiling of Chlorella vulgaris
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Fatty Acids in Chlorella vulgaris
2.2. Metabolite Profiling of Chlorella vulgaris Ethanolic Extract
2.2.1. Identification of Carotenoids
2.2.2. Identification of Chlorophyll Pigments
2.2.3. Identification of Amino Acids, Fatty Acids, Lipids and Fatty Acyls
2.2.4. Identification of Vitamins
2.2.5. Identification of Other Compounds
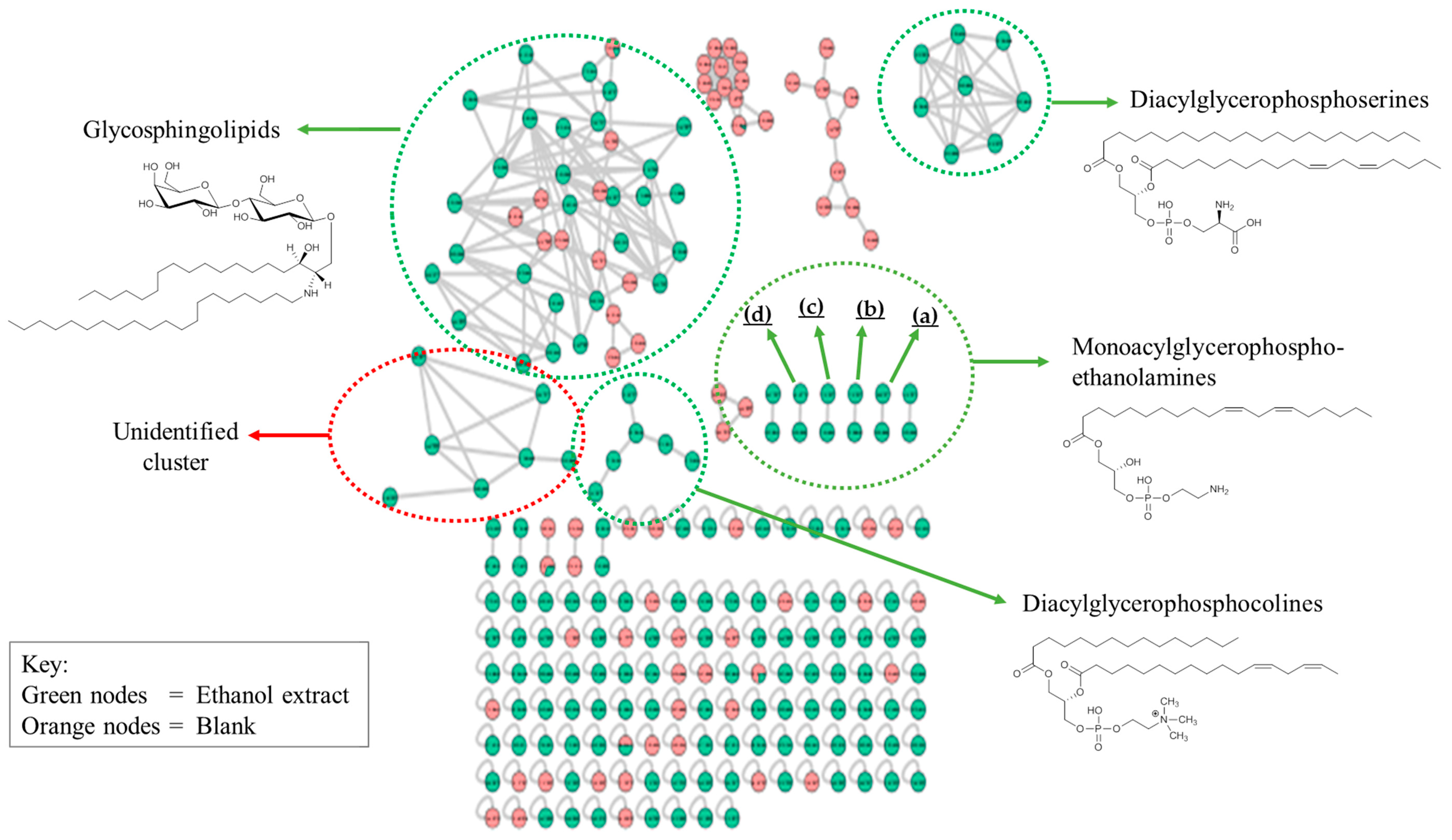
2.2.6. Identification of Lipids via Molecular Networking
3. Materials and Methods
3.1. Fresh Water Microalgae Culture
3.2. Solvents and Chemicals
3.3. Microalgae Solvent Extraction Procedure
3.4. Extraction of Fatty Acids for GC-MS Analysis
3.5. Sample Preparation for UHPLC-MS/MS Analysis
3.6. GC-MS Analysis
3.7. UHPLC-MS/MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tomaselli, L. Biotechnology and phycology. In Handbook of Microalgae Culture, 1st ed.; Richmond, A., Ed.; Blackwell Science: Oxford, UK, 2004; pp. 344–346. [Google Scholar]
- Herrero, M.; Mendiola, J. Screening for bioactive compounds from algae. In Advanced Biofuels and Bioproducts, 1st ed.; Lee, J., Ed.; Springer Scince and Business Media: New York, NY, USA, 2013; pp. 3–12. [Google Scholar]
- Mobin, S.; Alam, F. Some promising microalgal species for commercial applications: A review. Energy Procedia 2017, 110, 510–517. [Google Scholar] [CrossRef]
- Varfolomeev, S.D.; Wasserman, L.A. Microalgae as source of biofuel, food, fodder, and medicines. Appl. Biochem. Microbiol. 2011, 47, 789–807. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Koirala, K.H.; Mishra, A.K.; D’Antoni, J.M.; Mehlhorn, J.E. Energy prices and agricultural commodity prices: Testing correlation using copulas method. Energy 2015, 81, 430–436. [Google Scholar] [CrossRef]
- Satyanarayana, K.G.; Mariano, A.; Vargas, J.V.C. A review on microalgae, a versatile source for sustainable energy and materials. Int. J. Energy Res. 2011, 35, 291–311. [Google Scholar] [CrossRef]
- Sørensen, M.; Berge, G.M.; Reitan, K.I.; Ruyter, B. Microalga Phaeodactylum tricornutum in feed for Atlantic salmon (Salmo salar)-Effect on nutrient digestibility, growth and utilization of feed. Aquaculture 2016, 460, 116–123. [Google Scholar] [CrossRef]
- Teuling, E.; Schrama, J.W.; Gruppen, H.; Wierenga, P.A. Effect of cell wall characteristics on algae nutrient digestibility in nile tilapia (Oreochromis niloticus) and African catfish (Clarus gariepinus). Aquaculture 2017, 479, 490–500. [Google Scholar] [CrossRef]
- Kwak, J.H.; Baek, S.H.; Woo, Y.; Han, J.K.; Kim, B.G.; Kim, O.Y.; Lee, J.H. Beneficial immunostimulatory effect of short-term chlorella supplementation: Enhancement of natural killer cell activity and early inflammatory response (Randomized, double-blinded, placebo-controlled trial). Nutr. J. 2012, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kamat, J.P.; Boloor, K.K.; Devasagayam, T.P.A. Chlorophyllin as an effective antioxidant against membrane damage in vitro and ex vivo. Biochim. Biophys. Acta 2000, 1487, 113–127. [Google Scholar] [CrossRef]
- Zahran, E.; Risha, E. Modulatory role of dietary Chlorella vulgaris powder against arsenic-induced immunotoxicity and oxidative stress in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2014, 41, 654–662. [Google Scholar] [CrossRef]
- FAO. Statistics and Information Branch, Fisheries and Aquaculture Department/FAO. Fishery and Aquaculture Statistics. Global Production by Production Source 1950–2015 (FishStatJ). Rome 2017. Available online: http://www.fao.org/fishery/statistics/software/fishstatj/en (accessed on 29 May 2018).
- Schoepp, N.G.; Ansari, W.S.; Dallwig, J.A.; Gale, D.; Burkart, M.D.; Mayfield, S.P. Rapid estimation of protein, lipid, and dry weight in microalgae using a portable LED fluorometer. Algal. Res. 2015, 11, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Pakravan, S.; Akbarzadeh, A.; Sajjadi, M.; Hajimoradloo, A.; Noori, F. Chlorella vulgaris meal improved growth performance, digestive enzyme activities, fatty acid composition and tolerance of hypoxia and ammonia stress in juvenile pacific white shrimp Litopenaeus vannamei. Aquac Nutr. 2018, 24, 594–604. [Google Scholar] [CrossRef]
- Samuelsen, T.A.; Oterhals, A.; Kousoulaki, K. High lipid microalgae (Schizochytrium sp.) inclusion as a sustainable source of n-3 long-chain PUFA in fish feed—Effects on the extrusion process and physical pellet quality. Anim. Feed Sci. Technol. 2018, 236, 14–28. [Google Scholar] [CrossRef]
- Safafar, H.; Nørregaard, P.U.; Ljubic, A.; Møller, P.; Holdt, S.L.; Jacobsen, C. Enhancement of protein and pigment content in two chlorella species cultivated on industrial process water. J. Mar. Sci. Eng. 2016, 4, 84. [Google Scholar] [CrossRef] [Green Version]
- Hussein, E.E.S.; Dabrowski, K.; El-Saidy, D.M.S.D.; Lee, B.J. Effect of dietary phosphorus supplementation on utilization of algae in the grow-out diet of Nile tilapia Oreochromis niloticus. Aquac. Res. 2012, 45, 1533–1544. [Google Scholar] [CrossRef]
- Sharma, R.; Singh, G.P.; Vijendra, K.S. Comparison of different media formulations on growth, morphology and chlorophyll content of green alga, Chlorella vulgaris. Int. J. Pharm. Biol. Sci. 2011, 2, 509–5016. [Google Scholar]
- Maliwat, G.C.; Velasquez, S.; Robil, J.L.; Chan, M.; Traifalgar, R.F.; Tayamen, M.; Ragaza, J.A. Growth and immune response of giant freshwater prawn Macrobrachium rosenbergii (De Man) postlarvae fed diets containing Chlorella vulgaris (Beijerinck). Aquac. Res. 2016, 48, 1–11. [Google Scholar] [CrossRef]
- Ahmad, M.T.; Shariff, M.; Yusoff, M.F.; Goh, Y.M.; Banerjee, S. Applications of microalga Chlorella vulgaris in aquaculture. Rev. Aquac. 2018, 1–19. [Google Scholar] [CrossRef]
- Arsiya, F.; Sayadi, M.H.; Sobhani, S. Green synthesis of palladium nanoparticles using Chlorella vulgaris. Mater. Lett. 2017, 186, 113–115. [Google Scholar] [CrossRef]
- Lee, W.-L.; Chen, T.-Y.; Chang, J.-S.; Chang, H.-Y.; Chen, C.-Y.; Wu, J.-H. Enhancing microalgal oil/lipid production from Chlorella sorokiniana CY1 using deep-sea water supplemented cultivation medium. Biochem. Eng. J. 2013, 77, 74–81. [Google Scholar] [CrossRef]
- Azizan, A.; Safwan, M.B.A.; Maulidiani, M.; Shaari, K.; Ismail, I.S.; Nagao, N.; Abas, F. Metabolite profiling of the microalgal diatom chaetoceros calcitrans and correlation with antioxidant and nitric oxide inhibitory activities via1H NMR-based metabolomics. Mar. Drugs 2018, 16, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarayloo, E.; Simsek, S.; Unlu, Y.S.; Cevahir, G.; Erkey, C.; Kavakli, I.H. Enhancement of the lipid productivity and fatty acid methyl ester profile of Chlorella vulgaris by two rounds of mutagenesis. Bioresour. Technol. 2018, 250, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef] [Green Version]
- Duk, C.; Young, J.; Hyun, T.; Jun, S. Astaxanthin biosynthesis from simultaneous N and P uptake by the green alga Haematococcus pluvialis in primary-treated wastewater. Biochem. Eng. J. 2006, 31, 234–238. [Google Scholar]
- Ma, N.L.; Aziz, A.; The, K.Y.; Lam, S.S.; Cha, T.S. Metabolites re-programming and physiological changes induced in scenedesmus regularis under nitrate treatment. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Machado, M.; Bromke, M.; Domingues, A.P., Jr.; Vieira Vaz, M.M.G.; Rosa, R.M.; Vinson, C.C.; Sabir, J.S.; Rocha, D.I.; Martins, M.A.; Nunes-Nesi, A. Comprehensive metabolic reprograming in freshwater Nitzschia palea strains undergoing nitrogen starvation is likely associated with its ecological origin. Algal. Res. 2016, 18, 116–126. [Google Scholar] [CrossRef]
- Tang, D.; Han, W.; Li, P.; Miao, X.; Zhong, J. CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour. Technol. 2011, 102, 3071–3076. [Google Scholar] [CrossRef]
- West, N.; West, S. Notes/methodology. J. Lipid Res. 2009, 29, 227–235. [Google Scholar]
- Fernández-Linares, L.C.; Barajas, G.C.; Páramo, D.E.; Corona, B.J.A. Assessment of Chlorella vulgaris and Indigenous microalgae biomass with treated wastewater as growth culture medium. Bioresour. Technol. 2017, 244, 400–406. [Google Scholar] [CrossRef]
- Fu, W.; Guomundsson, Ó.; Paglia, G.; Herjólfsson, G.; Andrésson, Ó.S.; Palsson, B.O.; Brynjólfsson, S. Enhancement of carotenoid biosynthesis in the green microalga Dunaliella salina with light-emitting diodes and adaptive laboratory evolution. Appl. Microbiol. Biotechnol. 2013, 97, 2395–2403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deli, J.; Gonda, S.; Nagy, L.Z.S.; Szabó, I.; Gulyás-Fekete, G.; Agócs, A.; Marton, K.; Vasas, G. Carotenoid composition of three bloom-forming algae species. Food Res. Int. 2014, 65, 215–223. [Google Scholar] [CrossRef]
- Upadhyay, R.K. Plant pigments as dietary anticancer agents. Int. J. Green Pharm. 2018, 12, S93–S107. [Google Scholar]
- Sivathanu, B.; Palaniswamy, S. Purification and characterization of carotenoids from green algae Chlorococcum humicola by HPLC-NMR and LC-MS-APCI. Biomed. Prev. Nutr. 2012, 2, 276–282. [Google Scholar] [CrossRef]
- Patias, L.D.; Fernandes, A.S.; Petry, F.C.; Mercadante, A.Z.; Jacob-Lopes, E.; Zepka, L.Q. Carotenoid profile of three microalgae/cyanobacteria species with peroxyl radical scavenger capacity. Food Res. Int. 2017, 100, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Petry, F.C.; Mercadante, A.Z. Composition by LC-MS/MS of new carotenoid esters in mango and citrus. J. Agric. Food Chem. 2016, 64, 8207–8224. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Naoshige, A.; Takashi, M.; Takahashi, H.; Takeshi, A. Special feature: Tutorial MassBank: A public repository for sharing mass spectral data for life sciences. Int. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Rivera, M.S.; Christou, P.; Canela-Garayoa, R. Identification of carotenoids using mass spectrometry. Mass Spectrom. Rev. 2013, 9999, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Seyfabadi, J.; Ramezanpour, Z.; Khoeyi, Z.A. Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J. Appl. Phycol. 2011, 721–726. [Google Scholar] [CrossRef]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using high performance thin layer chromatography. J. Pharm. Biomed Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, K.; Deuchi, K. Culture of a high-chlorophyll-producing and halotolerant Chlorella vulgaris. J. Biosci. Bioeng. 2014, 117, 617–619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tan, N.G.J.; Li, S.F.Y. NMR-based metabolomics and LC-MS/MS quantification reveal metal-specific tolerance and redox homeostasis in Chlorella vulgaris. Mol. Biosyst. 2014, 10, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Xia, Y.; Zeng, Y.; Li, X.; Zhang, Y. Nitrate concentration-shift cultivation to enhance protein content of heterotrophic microalga Chlorella vulgaris: Over-compensation strategy. Bioresour. Technol. 2017, 233, 247–255. [Google Scholar] [CrossRef]
- Enyidi, U.D. Chlorella vulgaris as protein source in the diets of african catfish Clarias gariepinus. Fishes 2017, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Bouzabata, A.; Bighelli, A.; Abed, L.; Casanova, J.; Tomi, F. Composition and chemical variability of Eucalyptus bosistoana essential oil from Algerian Sahara. Nat. Prod. Commun. 2014, 9, 701–702. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, G.G.; Carnevale, N.F.; Demarque, D.P.; De Sousa, P.-J.J.A.; Filho, S.P.R.C.; De Melo, S.J.; Guedes da Silva Almeida, J.R.; Callegari Lopes, J.L.; Lopes, N.P. Dereplication of Flavonoid Glycoconjugates from Adenocalymma imperatoris-maximilianii by untargeted tandem mass spectrometry-based molecular networking. Planta Med. 2017, 83, 636–646. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Zeng, R.; Zhang, S.X.; Yang, Z.H.; Huang, H. Production of microalgal lipids as biodiesel feedstock with fixation of CO2 by Chlorella vulgaris. Food Technol. Biotechnol. 2014, 52, 285–291. [Google Scholar]
- Converti, A.; Casazza, A.A.; Ortiz, E.Y.; Perego, P.; del Borghi, M. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem. Eng. Process. Process Intensif. 2009, 48, 1146–1151. [Google Scholar] [CrossRef]









| Peak | Systematic Name | Trivial Name | Designation | Composition (%) |
|---|---|---|---|---|
| 1 | Tetradecanoic acid | Myristic acid | C14 | 1.0 |
| 2 | Pentadecanoic acid | - | C15 | 0.4 |
| 3 | Hexadecanoic acid | Palmitic acid | C16 | 16.4 |
| 4 | 9-Hexadecenoic acid | Palmitoleic acid | C16:1 [n-7] | 1.5 |
| 5 | Heptadecanoic acid | Margaric acid | C17 | 1.2 |
| 6 | 7,10-Hexadecadienoic acid | - | C16:2 [n-6] | 20.4 |
| 7 | Octadecanoic acid | Stearic acid | C18 | 2.8 |
| 8 | 5-Octadecenoic acid | - | C18:1 [n-13] | 0.3 |
| 9 | 9-Octadecenoic acid | Oleic acid | C18:1 [n-9] | 4.2 |
| 10 | 11-Octadecenoic acid | - | C18:1 [n-7] | 2.0 |
| 11 | 9,12-Octadecadienoic acid | Linoleic acid | C18:2 [n-6] | 35.1 |
| 12 | Nonadecanoic acid | Nonadecylic acid | C19 | 0.2 |
| 13 | 9,12,15-Octadecatrienoic acid | Linolenic acid | C18:3 [n-6] | 5.2 |
| 14 | Eicosanoic acid | Arachidic acid | C20 | 5.7 |
| 15 | 9-Eicosenoic acid | - | C20:1 [n-9] | 0.4 |
| 16 | Eicosadienoic acid | - | C20:2 | 1.3 |
| 17 | 11,14,17-Eicosatrienoic acid | Homolinolenic acid | C20:3 [n-6] | 0.4 |
| 18 | Heneicosanoic acid | Heneicosylic acid | C21 | 0.2 |
| 19 | 5,8,11,14-Eicosatetraenoic acid | Arachidonic acid | C20:4 [n-6] | 0.6 |
| 20 | Docosanoic acid | Behenic acid | C22 | 0.9 |
| Peak | Carotenoid | tR (min) | λmax (nm) | [M + H]+ (m/z) | MS/MS (m/z) | References |
|---|---|---|---|---|---|---|
| 6 | Vulgaxanthin I | 3.98 | 424, 476 | 340 | 322 (M + H − 18)+ (20%) 209 (100%), 84 (3%) | Not previously reported in C. vulgaris |
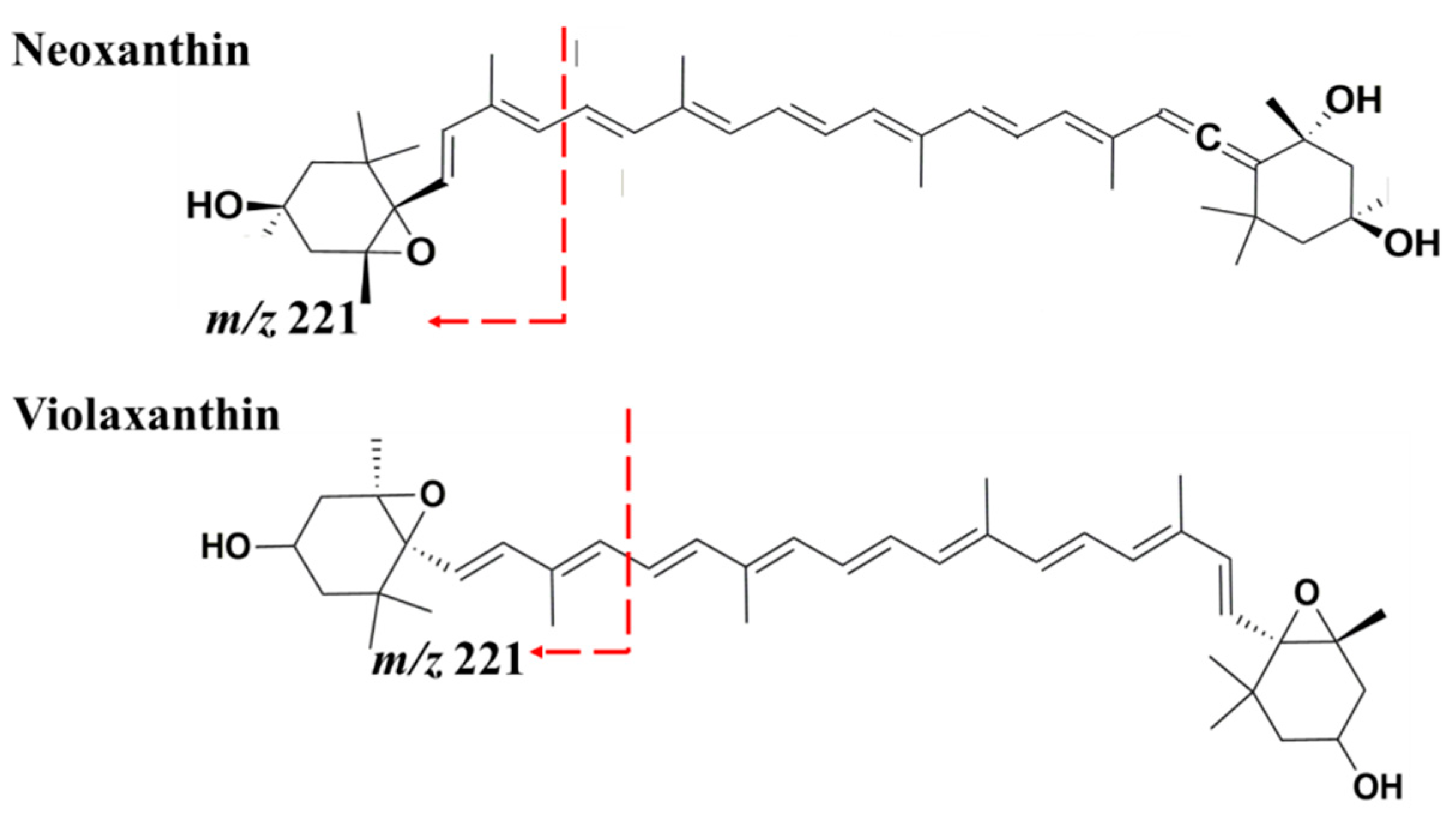
| 16 | Neoxanthin | 14.35 | 414, 436, 462 | 601.4 | 583.4 (M + H − 18)+ (8%) 491.4 (M + H − 92 − 18)+ (10%) 221.2 (100%) | [33,34,36] |
| 17 | Violaxanthin | 14.52 | 416, 442 | 601.4 | 583.4 (M + H − 18)+ (6%) 565.4 (M + H − 18 − 18)+ (4%) 509.4 (M + H − 92)+ (4%) 491.4 (M + H − 92 − 18)+ (7%) 221.2 (100%) | [33,34,36,37,38] |
| 18 | Antheraxanthin | 14.55–14.60 | 400, 424 | 585.4 | 567.4 (M + H − 18)+ (35%) 475.4 (M + H − 92 − 18)+ (10%) 221.2 (100%) | [35,38] |
| 20 | Lutein | 15.35–15.36 | 414, 438, 464 | 569.4 | 551.4 (M + H − 18)+ (25%) 533.4 (M + H − 18 − 18)+ (5%) | [32,33,34,36] |
| 26 | Astaxanthin | 19.21 | 372, 436 | 596.6 | 578.6 (M + H − 18)+ (100%) 560.6 (M + H − 18 − 18)+ (10%) 284.3 (50%) | [39] |
| 29 | β-carotene | 23.15–23.16 | 412, 440, 468 | 536.4 | 444.4 (M + H − 92)+ (20%) 321.3 (18%) | [33,34,39] |
| Peak | Pigment | tR (min) | λmax (nm) | [M + H]+ (m/z) | References |
|---|---|---|---|---|---|
| 19 | Pheophorbide-a | 14.68 | 268, 474, 536 | 593 | [34] |
| 27 | Pheophytin-b | 21.08 | 222, 436, 528 | 885 | [34] |
| 28 | Pheophorbide-b | 21.37 | 202, 222, 372, 436, 528 | 607 | [34] |
| 30 | Pheophytin-a | 24.28 | 204, 408, 536 | 871 | [34] |
| 31 | Chlorophyll-a | 28.66 | 202, 410, 538 | 894 | [34] |
| Peak | Amino Acid | tR (min) | [M + H]+ (m/z) | MS/MS (m/z) |
|---|---|---|---|---|
| 2 | Leucine | 1.44 | 132 | 115, 86, 72, 57 |
| 3 | Phenylalanine | 2.29 | 166 | 165, 120, 103, 93, 91, 79 |
| 5 | Tryptophan | 2.72 | 205 | 159, 146, 144, 143, 142, 132, 118, 91, 74 |
| 7 | Lysophosphatidylethanolamine [Lyso-PE] | 5.44 | 566 | 548, 452, 322, 209, 157, 114, 97, 57 |
| 9 | Disopyramide | 6.30 | 340 | 322, 306, 212, 196, 114, 74 |
| Fatty acids | ||||
| 13 | Fatty acid | 12.22 | 492 | 474, 309, 258, 184, 141, 138, 124, 112, 104, 102, 86, 78, 70, 60 |
| 21 | Nonadecanoyloxyoctadeca- trienoic acid | 15.98 | 575 | 447, 263, 239, 221, 161, 137, 109, 95, 81, 71, 69, 57, 55 |
| 12 | 1-Acetoxy-2-hydroxy-16- heptadecyn-4-one | 11.70 | 325 | 233, 215, 175, 173, 145, 135, 121, 109, 95, 93, 91, 81, 67 |
| 14 | Hexadecatrienal | 12.51 | 235 | 226, 211, 173, 153, 133, 119, 111, 109 105, 95, 91, 81, 79, 67, 55 53 |
| Lipids | ||||
| 15 | 1-[9Z,12Z-octadecadienoyl]-sn- glycero -3-phosphocholine | 13.44 | 520 | 502, 484, 342, 337, 260, 184, 125, 104, 86, 60 |
| 22 | 1-hexadecanoyl-2-[5Z,8Z,11Z, 14Z-eicosatetraenoyl]-sn- glycero-3-phosphocholine | 16.35 | 782 | 782, 184, 124, 184, 86 |
| 23 | 1-tetradecanoyl-2-[11Z,14Z- eicosadienoyl]-glycero-3- phosphocholine | 16.80 | 758 | 553, 357, 199, 184, 124, 86 |
| 24 | 1-hexadecanoyl-2-[11Z,14Z- eicosadienoyl]-glycero-3- phosphocholine | 17.74 | 786 | 688, 552, 501, 474, 215, 184, 125, 104, 87, 86, 60 |
| 25 | 1-octadecanoyl-2-[11Z,14Z- eicosadienoyl]-sn-glycero-3- phosphocholine | 18.74 | 814 | 673, 614, 513, 462, 338, 184, 130, 104, 86 |
| Peak | Vitamin | tR (min) | [M + H]+ (m/z) | MS/MS (m/z) |
|---|---|---|---|---|
| 1 | Vitamin B-3 [nicotinic acid] | 1.33 | 124 | 123, 80, 78, 53, 45 |
| 4 | Vitamin B-5 [Pantothenic acid] | 2.54 | 220 | 142, 116, 103, 90, 87, 86, 73, 72, 70, 57, 55 |
| Peak | Compound | tR (min) | [M + H]+ (m/z) | MS/MS (m/z) |
|---|---|---|---|---|
| 8 | (R)-cryptone | 5.69 | 139 | 138, 111, 83 53 |
| 10 | Glucose | 6.54 | 181 | 163, 145, 121, 119, 117, 115, 105, 83, 59, 57, 55 |
| 11 | Galactose | 7.19 | 181 | 163, 145, 135, 133, 121, 119, 117, 115, 105, 103, 91, 87, 73, 59, 57, 55 |
| No. | Lipids | MF | m/z |
|---|---|---|---|
| Diacylglycerophosphoserines | |||
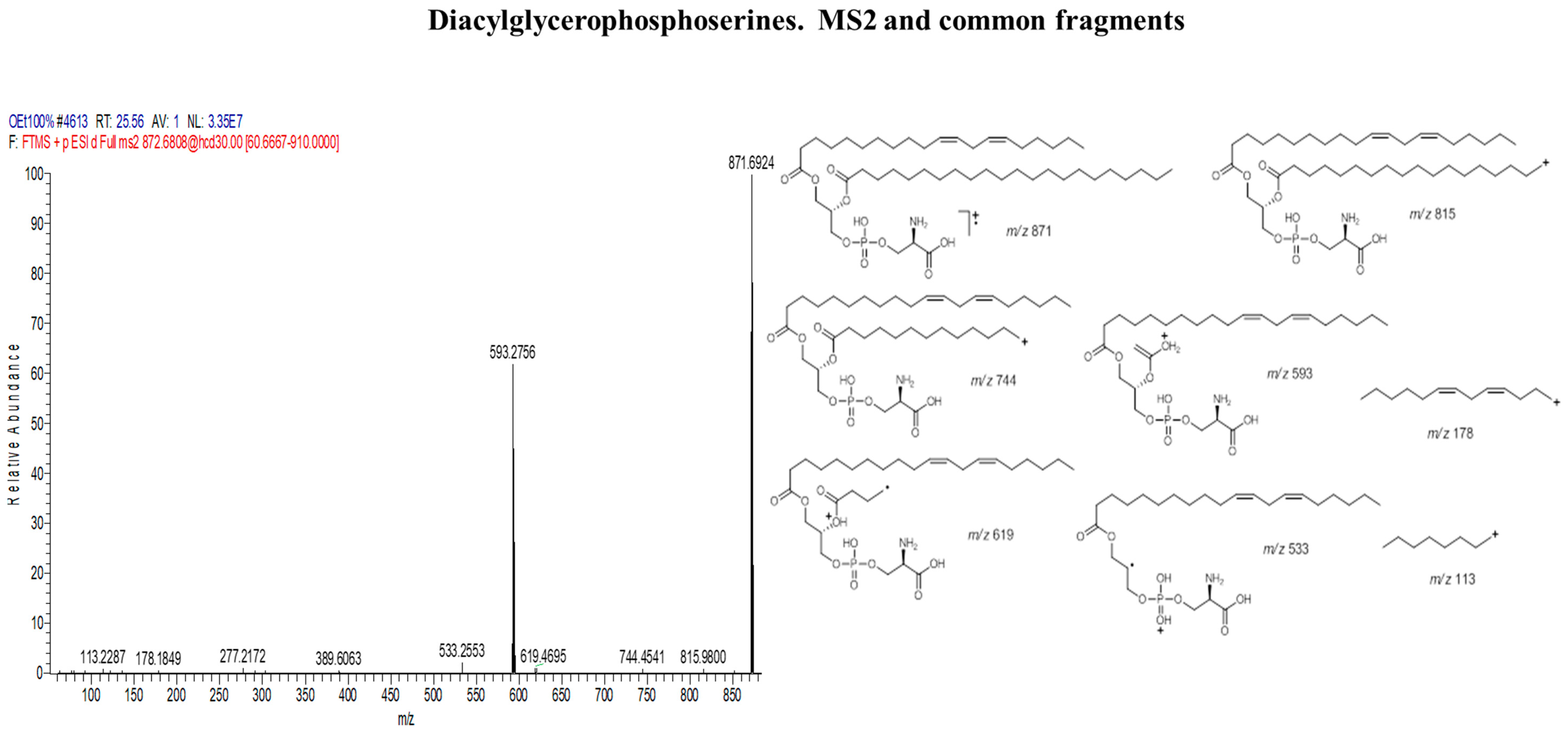
| 1 | 1-[11Z,14Z-eicosadienoyl]-2-docosanoyl-glycero-3-phosphoserine | C48H90NO10P | 872 |
| 2 | 1-[11Z,14Z-eicosadienoyl]-2-heneicosanoyl-glycero-3-phosphoserine | C47H88NO10P | 858 |
| 3 | 1-[11Z,14Z-eicosadienoyl]-2-eicosanoyl-glycero-3-phosphoserine | C46H86NO10P | 844 |
| 4 | 1-[11Z-eicosenoyl]-2-nonadecanoyl-glycero-3-phosphoserine | C45H86NO10P | 832 |
| 5 | 1-[11Z,14Z-eicosadienoyl]-2-nonadecanoyl-glycero-3-phosphoserine | C45H84NO10P | 830 |
| 6 | 1-[11Z,14Z-eicosadienoyl]-2-[9Z-nonadecenoyl]-glycero-3-phosphoserine | C45H82NO10P | 828 |
| 7 | 1-[11Z,14Z-eicosadienoyl]-2-octadecanoyl-glycero-3-phosphoserine | C44H82NO10P | 816 |
| 8 | 1-[11Z,14Z-eicosadienoyl]-2-heptadecanoyl-glycero-3-phosphoserine | C43H80NO10P | 802 |
| Diacylglycerophosphocholines | |||
| 9 | 1-octadecanoyl-2-[11Z,14Z-eicosadienoyl]-sn-glycero-3-phosphocholine | C46H88NO8P | 814 |
| 10 | 1-octadecanoyl-2-(5Z,11Z,14Z-eicosatrienoyl)-sn-glycero-3-phosphocholine | C46H86NO8P | 812 |
| 11 | 1-[9Z-octadecenoyl]-2-[9Z-nonadecenoyl]-glycero-3-phosphocholine | C45H86NO8P | 800 |
| 12 | 1-octadecanoyl-2-[9Z,12Z-heptadecadienoyl]-glycero-3-phosphocholine | C43H82NO8P | 772 |
| 13 | 1-[9Z-octadecenoyl]-2-[9Z-pentadecenoyl]-glycero-3-phosphocholine | C41H78NO8P | 744 |
| 14 | 1-[9Z-octadecenoyl]-2-[9Z-tetradecenoyl]-glycero-3-phosphocholine | C40H76NO8P | 730 |
| Glycosphingolipids | |||
| 15 | N-[eicosanoyl]-1-beta-lactosyl-sphinganine | C50H97NO13 | 920 |
| 16 | N-[docosanoyl]-eicosasphinganine-1-phospho-[1′-myo-inositol] | C48H96NO11P | 894 |
| 17 | N-[octadecanoyl]-1-beta-lactosyl-sphinganine | C48H93NO13 | 892 |
| 18 | 1-[11Z-eicosenoyl]-2-[13Z,16Z-docosadienoyl]-glycero-3-phosphocholine | C50H94NO8P | 868 |
| 19 | N-[docosanoyl]-sphinganine-1-phospho-[1′-myo-inositol]. | C46H92NO11P | 866 |
| 20 | N-[2-hydroxyheptacosanoyl]-1-O-beta-d-glucosyl-15-methylhexadecasphing-4-enine | C50H97NO9 | 856 |
| 21 | N-hexacosanoyl-1-O-beta-D-glucosyl-4-hydroxy-15-methylhexadecasphinganine | C49H97NO9 | 844 |
| 22 | N-[2-hydroxyhexacosanoyl]-1-O-beta-d-glucosyl-15-methylhexadecasphing-4-enine | C49H95NO9 | 842 |
| 23 | N-[2R-hydroxypentacosanoyl]-1-beta-glucosyl-4E,8Z-octadecasphingadienine | C49H93NO9 | 840 |
| 24 | N-[17Z-hexacosenoyl]-1-beta-glucosyl-sphing-4-enine | C50H95NO8 | 838 |
| 25 | N-pentacosanoyl-1-O-beta-d-glucosyl-4-hydroxy-15-methylhexadecasphinganine | C48H95NO9 | 830 |
| 26 | 1-eicosyl-2-eicosyl-sn-glycero-3-phosphocholine | C48H100NO6P | 818 |
| 27 | N-tetracosanoyl-1-O-beta-d-glucosyl-4-hydroxy-15-methylhexadecasphinganine | C47H93NO9 | 816 |
| 28 | N-[2-hydroxytetracosanoyl]-1-O-beta-d-glucosyl-15-methylhexadecasphing-4-enine | C47H91NO9 | 814 |
| 29 | N-[15Z-tetracosenoyl]-1-beta-glucosyl-sphinganine | C48H93NO8 | 812 |
| 30 | 1-[3-hydroxyphytanyl]-2-phytanyl-sn-glycero-3-phosphoethanolamine | C45H94NO7P | 792 |
| 31 | 1-octadecyl-2-docosanoyl-sn-glycero-3-phosphoethanolamine | C45H92NO7P | 790 |
| 32 | 1-[11Z,14Z-eicosadienoyl]-2-[6Z,9Z,12Z-octadecatrienoyl]-glycero-3-phosphoethanolamine | C43H76NO8P | 766 |
| 33 | 1-[9Z,12Z,15Z-octadecatrienoyl]-2-[8Z,11Z,14Z-eicosatrienoyl]-glycero-3-phosphoethanolamine | C43H74NO8P | 764 |
| 34 | N-[2-hydroxyhexacosanoyl]-4R-hydroxysphinganine | C44H89NO5 | 712 |
| 35 | N-[2-hydroxyheptacosanoyl]-15-methylhexadecasphing-4-enine | C44H87NO4 | 694 |
| 36 | N-[2-hydroxypentacosanoyl]-15-methylhexadecasphing-4-enine | C42H83NO4 | 666 |
| 37 | 1,2-Dihexadecyl-sn-glycero-3-phosphoethanolamine | C37H78NO6P | 664 |
| 38 | N-[2-hydroxy-hexacosanoyl]-tetradecasphing-4-enine | C40H79NO4 | 638 |
| 39 | 1,2-ditetradecyl-sn-glycero-3-phospho-N, N-dimethylethanolamine | C35H74NO6P | 636 |
| 40 | N-[2R-hydroxyicosanoyl]-8Z-octadecasphingenine | C38H75NO4 | 610 |
| Separate clusters of monoacylglycerophosphoethanolamines and derivatives | |||
| 41 | (a) N-[2-hydroxyhexacosanoyl]-sphinganine | C44H89NO4 | 696 |
| 42 | N-[2R-hydroxyhexadecanoyl]-4R-hydroxy-20E-hexacosasphingenine | C42H83NO5 | 682 |
| 43 | (b) N-[2R,3-dihydroxyhexacosanoyl]-4R-hydroxy-8E-octadecasphingenine. | C44H87NO6 | 726 |
| 44 | N-[2-hydroxyhexacosanoyl]-4R-hydroxysphinganine | C44H89NO5 | 712 |
| 45 | (c) 1-[11Z,14Z-eicosadienoyl]-2-[9Z-tetradecenoyl]-glycero-3-phospho-[1′-sn-glycerol] | C40H73O10P | 745 |
| 46 | 1-[11Z,14Z-eicosadienoyl]-2-dodecanoyl-glycero-3-phospho-[1′-sn-glycerol] | C38H71O10P | 719 |
| 47 | (d) 1-[11Z,14Z-eicosadienoyl]-glycero-3-phosphoethanolamine | C25H48NO7P | 506 |
| 48 | 2-[11Z,14Z,17Z-eicosatrienoyl]-sn-glycero-3-phosphoethanolamine | C25H46NO7P | 504 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantami, H.A.; Ahamad Bustamam, M.S.; Lee, S.Y.; Ismail, I.S.; Mohd Faudzi, S.M.; Nakakuni, M.; Shaari, K. Comprehensive GCMS and LC-MS/MS Metabolite Profiling of Chlorella vulgaris. Mar. Drugs 2020, 18, 367. https://doi.org/10.3390/md18070367
Pantami HA, Ahamad Bustamam MS, Lee SY, Ismail IS, Mohd Faudzi SM, Nakakuni M, Shaari K. Comprehensive GCMS and LC-MS/MS Metabolite Profiling of Chlorella vulgaris. Marine Drugs. 2020; 18(7):367. https://doi.org/10.3390/md18070367
Chicago/Turabian StylePantami, Hamza Ahmed, Muhammad Safwan Ahamad Bustamam, Soo Yee Lee, Intan Safinar Ismail, Siti Munirah Mohd Faudzi, Masatoshi Nakakuni, and Khozirah Shaari. 2020. "Comprehensive GCMS and LC-MS/MS Metabolite Profiling of Chlorella vulgaris" Marine Drugs 18, no. 7: 367. https://doi.org/10.3390/md18070367
APA StylePantami, H. A., Ahamad Bustamam, M. S., Lee, S. Y., Ismail, I. S., Mohd Faudzi, S. M., Nakakuni, M., & Shaari, K. (2020). Comprehensive GCMS and LC-MS/MS Metabolite Profiling of Chlorella vulgaris. Marine Drugs, 18(7), 367. https://doi.org/10.3390/md18070367






