PHNQ from Evechinus chloroticus Sea Urchin Supplemented with Calcium Promotes Mineralization in Saos-2 Human Bone Cell Line
Abstract
:1. Introduction
2. Results
2.1. Extraction of PHNQs from E. choloticus Spine
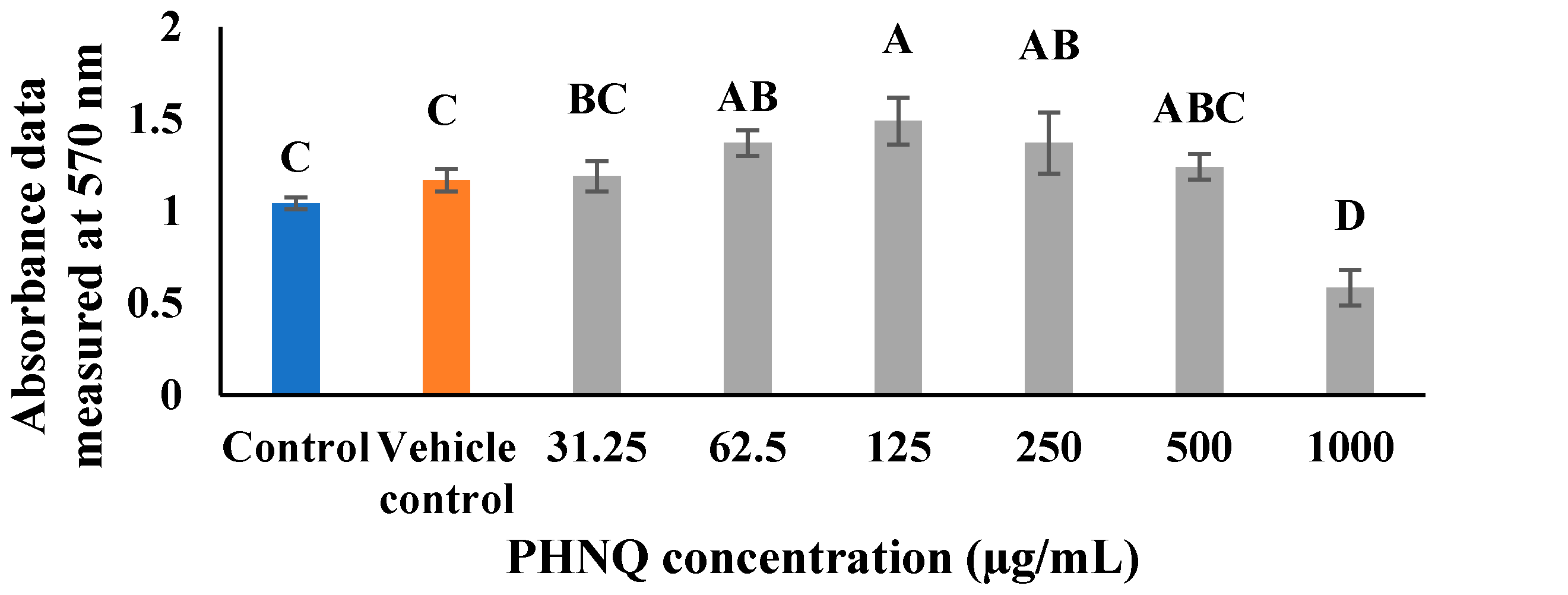
2.2. Cytotoxic Activity of E. chloroticus PHNQ Extract
2.3. The Effect of CaCl2, E. choloroticus PHNQ Extract and Echinochrome A on the Proliferation of Saos-2 Cells
2.4. Effect of Adding CaCl2 with Echinochrome A and E. choloroticus PHNQ Extract on Cell Viability
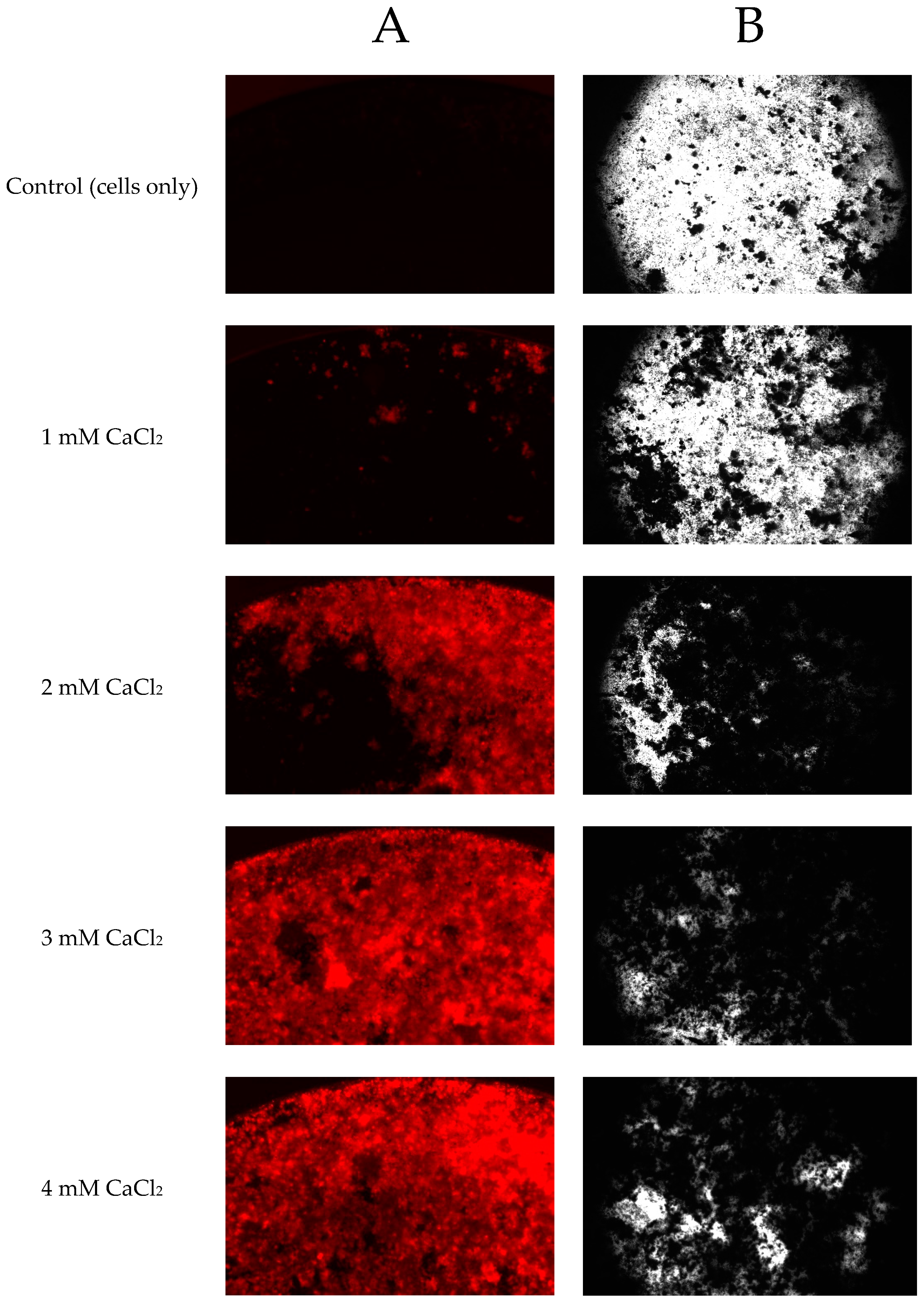
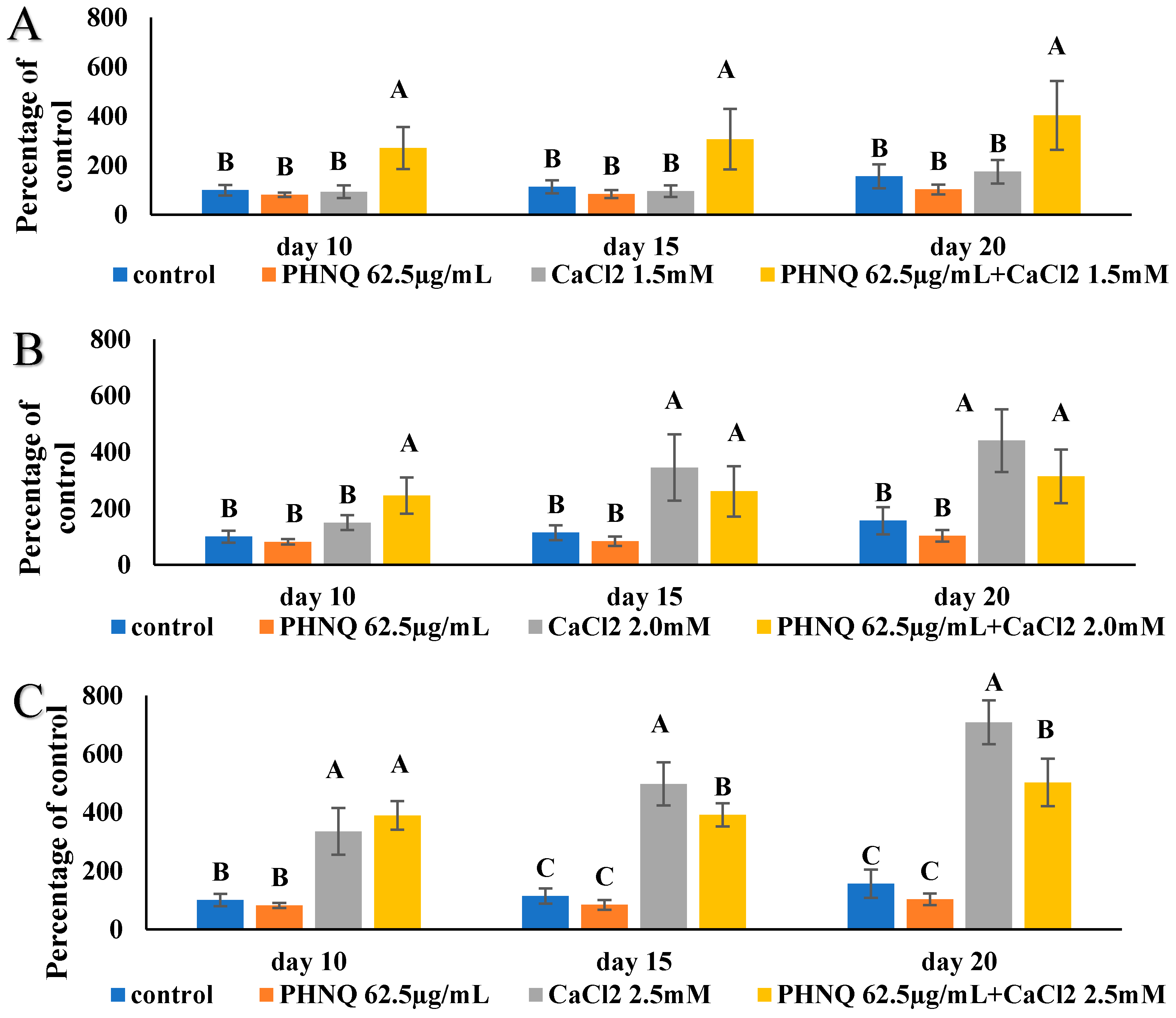
2.5. Effect of CaCl2 on Minerlization of Saos-2 Cells and the Mineralization Assay Evaluation
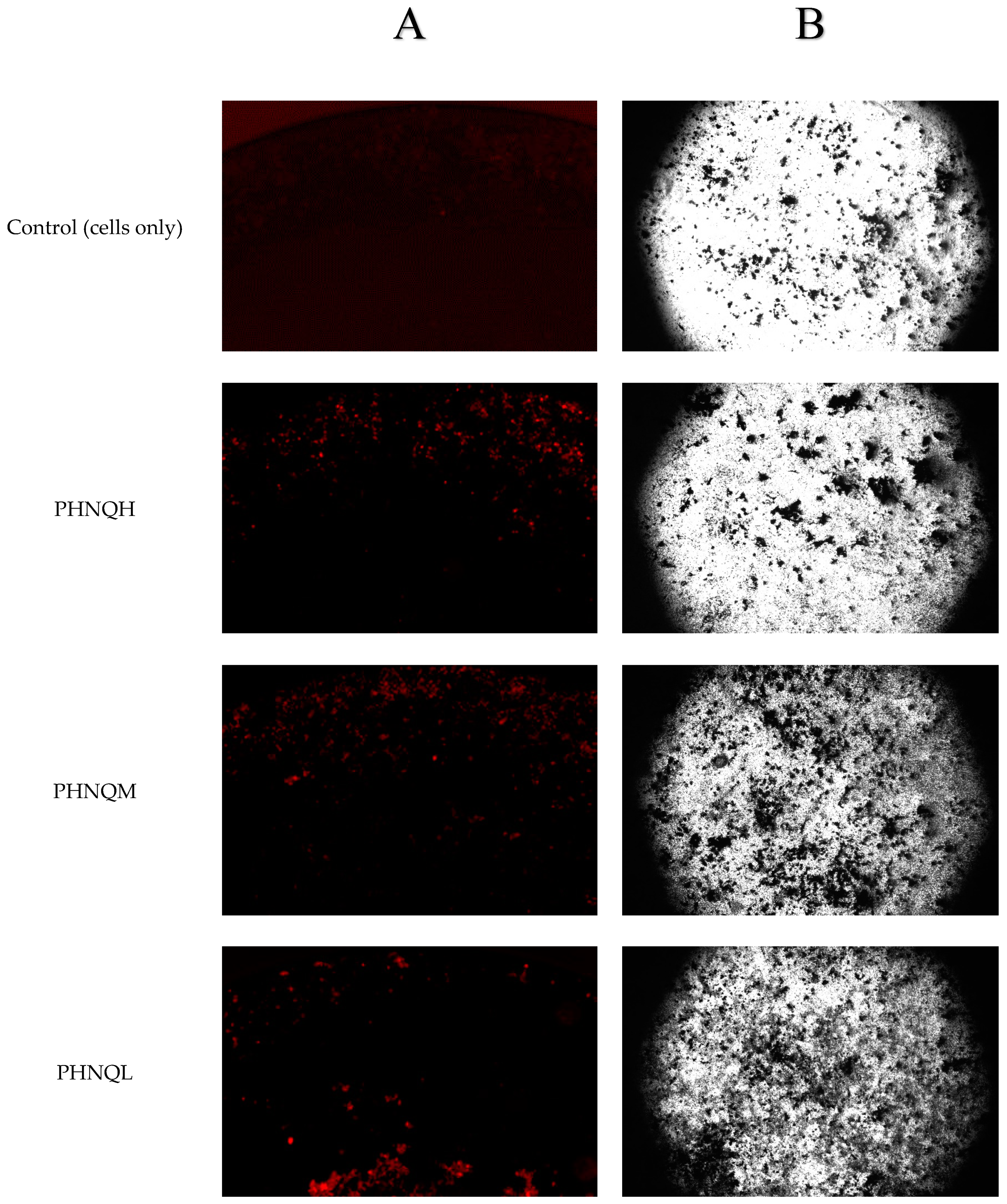
2.6. Effect of PHNQ Extract and Echinochrome A on Mineralization of Saos-2 Cells
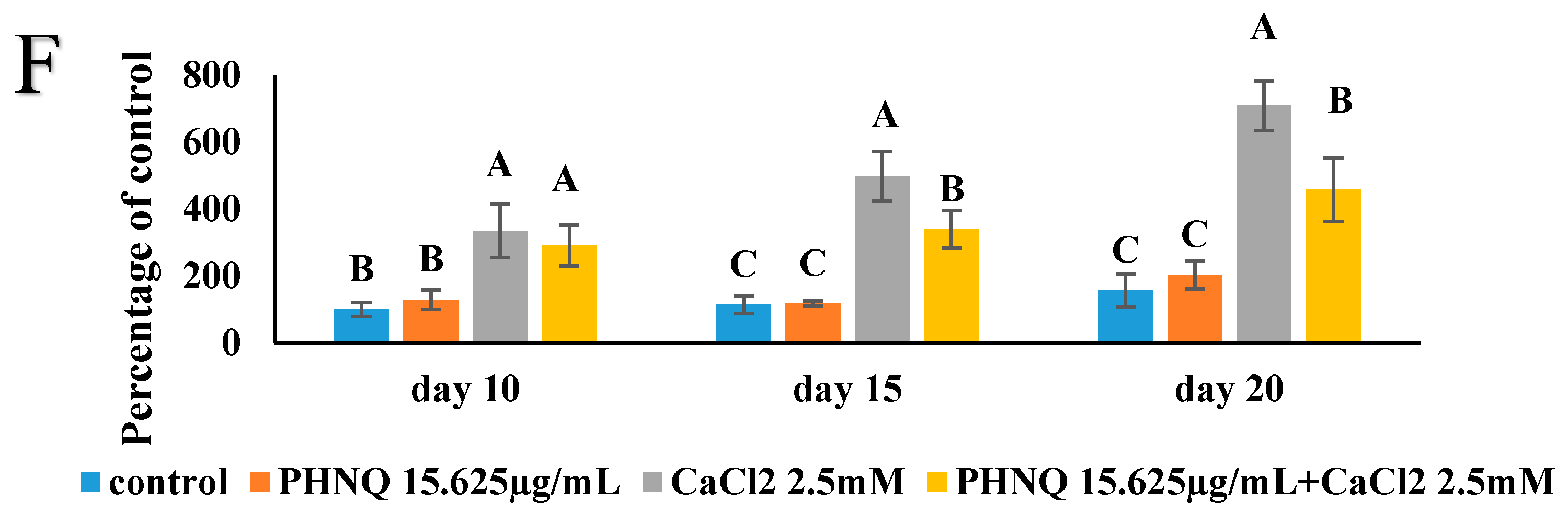
2.7. Effect of PHNQ on Mineralization in the Presence of CaCl2
3. Discussion
4. Materials and Methods
4.1. The Source of Sea Urchin Spine Samples and Echinochrome A Standard
4.2. Preparation of Sea Urchin Spine Crude Extract
4.3. Characterisation of PHNQ Compounds Using HPLC with Diode-Array Detection and Mass Spectrometry (HPLC-DAD/MS)
4.4. Determination of Cytotoxicity of PHNQ by MTT Assay Using Saos-2 Cells (48 h Incubation Time)
4.5. Determination of Cytotoxicity of PHNQ, Echinochrome A, CaCl2, and PHNQ or Echinochrome A Supplemented with CaCl2 by MTT Assay Using Saos-2 Cells (21 days Incubation Time)
4.6. Determination of Mineralized Nodule Formation by Xylenol Orange Staining
4.7. Determination of Mineralized Nodule Formation by von Kossa Staining
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
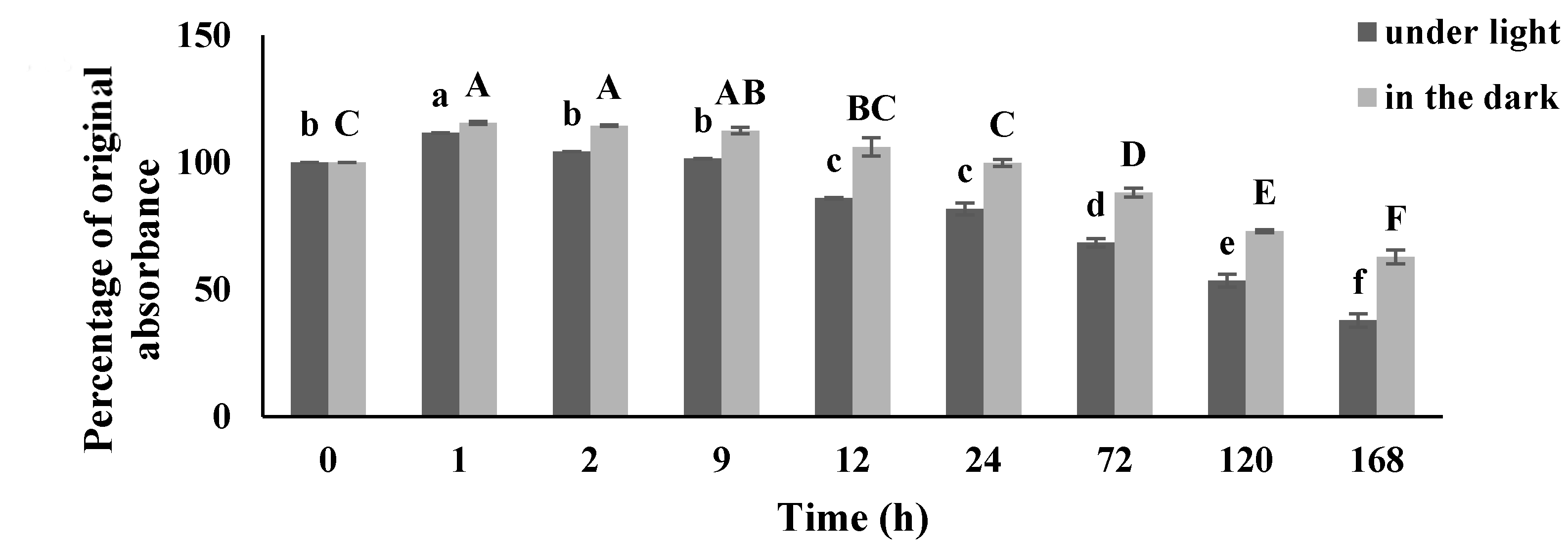
Appendix B.1. PHNQ Stability in Cell Culture Media
Appendix B.2. Results of PHNQ Stability in Cell Culture Media
| CaCl2 (mM)/PHNQ Concentration (µg/mL) | 15.625 (Low) | 31.25 (Medium) | 62.5 (High) |
|---|---|---|---|
| Ca 0 mM | 73.86 ± 14.98 a | 67.21 ± 10.98 b | 84.38 ± 4.93 b |
| Ca 0.5 mM | 77.93 ± 17.65 a | 80.90 ± 10.71 ab | 71.46 ± 3.26 ab |
| Ca 1.0 mM | 69.99 ± 8.80 a | 73.67 ± 3.71 ab | 74.91 ± 3.46 b |
| Ca 1.5 mM | 68.33 ± 19.98 a | 68.66 ± 8.92 b | 57.13 ± 5.27 b |
| Ca 2.0 mM | 66.30 ± 22.70 a | 68.00 ± 7.99 b | 80.85 ± 7.68 b |
| Ca 2.5 mM | 66.19 ± 16.93 a | 70.00 ± 10.38 ab | 63.87 ± 8.10 b |
| Ca 3.0 mM | 65.41 ± 16.98 a | 68.26 ± 13.20 b | 86.25 ± 13.73 b |
| vehicle control ** | 103.2 ± 5.05 a | 106.77 ± 3.19 a | 106.24 ± 8.03 a |
References
- Hou, Y.; Vasileva, E.A.; Carne, A.; McConnell, M.; Bekhit, A.E.D.A.; Mishchenko, N.P. Naphthoquinones of the spinochrome class: Occurrence, isolation, biosynthesis and biomedical applications. RSC Adv. 2018, 8, 32637–32650. [Google Scholar] [CrossRef] [Green Version]
- Shikov, A.; Pozharitskaya, O.; Krishtopina, A.; Makarov, V. Naphthoquinone pigments from sea urchins: Chemistry and pharmacology. Phytochem. Rev. 2018, 17, 1–26. [Google Scholar] [CrossRef]
- Hou, Y.; Vasileva, E.A.; Mishchenko, N.P.; Carne, A.; McConnell, M.; Bekhit, A.E.D.A. Extraction, structural characterization and stability of polyhydroxylated naphthoquinones from shell and spine of New Zealand sea urchin (Evechinus chloroticus). Food Chem. 2019, 272, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, R.; Hatate, H.; Chikami, A.; Murata, H.; Kijidani, Y. Quantitative separation of antioxidant pigments in purple sea urchin shells using a reversed-phase high performance liquid chromatography. LWT Food Sci. Technol. 2010, 43, 1185–1190. [Google Scholar] [CrossRef]
- Li, D.M.; Zhou, D.Y.; Zhu, B.W.; Miao, L.; Qin, L.; Dong, X.P.; Wang, X.D.; Murata, Y. Extraction, structural characterization and antioxidant activity of polyhydroxylated 1,4-naphthoquinone pigments from spines of sea urchin Glyptocidaris crenularis and Strongylocentrotus intermedius. Eur. Food Res. Technol. 2013, 237, 331–339. [Google Scholar] [CrossRef]
- Powell, C.; Hughes, A.; Kelly, M.; Conner, S.; Mcdougall, G. Extraction and identification of antioxidant polyhydroxynaphthoquinone pigments from the sea urchin, Psammechinus miliaris. Eur. Food Res. Technol. 2014, 59, 455–460. [Google Scholar] [CrossRef]
- Brasseur, L.; Hennebert, E.; Fiévez, L.; Caulier, G.; Bureau, F.; Tafforeau, L.; Flammang, P.; Gerbaux, P.; Eeckhaut, I. The roles of spinochromes in four shallow water tropical sea urchins and their potential as bioactive pharmacological agents. Mar. Drugs. 2017, 15, 179. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Carne, A.; Mcconnell, M.; Bekhit, A.; Mros, S.; Amagase, K.; Bekhit, A. In vitro antioxidant and antimicrobial activities, and in vivo anti-inflammatory activity of crude and fractionated PHNQs from sea urchin (Evechinus chloroticus). Food Chem. 2020, 316, 126339. [Google Scholar] [CrossRef] [PubMed]
- Egorov, E.A.; Alekhina, V.A.; Volobueva, T.M.; Fedoreyev, S.; Mishchenko, N.; Kol’tsova, E. Histochrome, a new antioxidant, in the treatment of ocular diseases. Vestnik Oftalmol. 1998, 115, 34–35. [Google Scholar]
- Mishchenko, N.P.; Fedoreev, S.A.; Bagirova, V.L. Histochrome: A new original domestic drug. Pharm. Chem. J. 2003, 37, 48–52. [Google Scholar] [CrossRef]
- Agafonova, I.; Bogdanovich, R.; Kolosova, N. Assessment of nephroprotective potential of histochrome during induced arterial hypertension. Bull. Exp. Biol. Med. 2015, 160, 223–227. [Google Scholar] [CrossRef]
- Agafonova, I.; Kotelnikov, V.; Mishchenko, N.; Kolosova, N. Evaluation of effects of Histochrome and Mexidol on structural and functional characteristics of the brain in senescence-accelerated OXYS Rats by magnetic resonance imaging. Bull. Exp. Biol. Med. 2011, 150, 739–743. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, H.K.; Song, I.S.; Lee, S.; Ko, K.; Rhee, B.; Kim, N.; Mishchenko, N.; Fedoreyev, S.; Stonik, V.; et al. Echinochrome a protects mitochondrial function in cardiomyocytes against cardiotoxic drugs. Mar. Drugs. 2014, 12, 2922–2936. [Google Scholar] [CrossRef] [Green Version]
- Kareva, E.N.; Tikhonov, D.; Mishchenko, N.; Fedoreyev, S.; Shimanovskii, N. Effects of histochrome on P53 expression in mouse red bone marrow cells in a model of chronic Stress. Pharm. Chem. J. 2014, 48, 149–152. [Google Scholar] [CrossRef]
- Mohamed, A.S.; Soliman, A.M.; Marie, M.A.S. Mechanisms of echinochrome potency in modulating diabetic complications in liver. Life Sci. 2016, 151, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Muthusami, S.; Senthilkumar, K.; Vignesh, C.; Ilangovan, R.; Stanley, J.; Selvamurugan, N.; Srinivasan, N. Effects of Cissus quadrangularis on the proliferation, differentiation and matrix mineralization of human osteoblast like SaOS-2 cells. J. Cell. Biochem. 2011, 112, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Marcus, R.; Majumder, S. Chapter 35, the Nature of Osteoporosis. In Osteoporosis, 2nd ed.; Academic Press: San Diego, CA, USA, 2001; pp. 3–17. [Google Scholar]
- Raisz, L.G. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, J.; Yang, H.; Zhang, Q.; Liu, C.; Zhao, J.; Zhang, L.; Chen, B. Natural products for treatment of osteoporosis: The effects and mechanisms on promoting osteoblast-mediated bone formation. Life Sci. 2016, 147, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D. Management of severe osteoporosis. Expert Opin. Pharmacother. 2016, 17, 473–488. [Google Scholar] [CrossRef]
- Suvarna, V.; Sarkar, M.; Chaubey, P.; Khan, T.; Sherje, A.; Patel, K.; Dravyakar, B. Bone health and natural products-An insight. Front Pharmacol. 2018, 9, 981. [Google Scholar] [CrossRef]
- Li, T.M.; Huang, H.C.; Su, C.M.; Ho, T.Y.; Wu, C.M.; Chen, W.C.; Fong, Y.C.; Tang, C.H. Cistanche deserticola extract increases bone formation in osteoblasts. J. Pharm. Pharmacol. 2012, 64, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.H.; Gao, Q.G.; Zhang, Y.; Wong, K.C.; Dai, Y.; Yao, X.S.; Wong, M.S. Vanillic acid exerts oestrogen-like activities in osteoblast-like UMR 106 cells through MAP kinase (MEK/ERK)-mediated ER signaling pathway. J. Steroid Biochem. 2014, 144, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Bankar, R.; Roy, P. Assessment of the role of flavonoids for inducing osteoblast differentiation in isolated mouse bone marrow derived mesenchymal stem cells. Phytomedicine 2013, 20, 683–690. [Google Scholar] [CrossRef]
- Lu, J.; Li, R.; Zhang, W. Sea urchin chemical and pharmacological research. Chin. J. Mar. Drugs. 1994, 2, 38–46. [Google Scholar]
- Shang, X.H.; Liu, X.Y.; Zhang, J.P.; Gao, Y.; Jiao, B.H.; Zheng, H.; Lu, X.L. Traditional Chinese Medicine——Sea urchin. Mini Rev. Med. Chem. 2014, 14, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. In Cancer Cell Culture; Humana Press: Totowa, NJ, USA, 2011; pp. 237–245. [Google Scholar]
- Vasileva, E.A.; Mishchenko, N.P.; Fedoreyev, S.A. Diversity of polyhydroxynaphthoquinone pigments in North Pacific sea urchins. Chem. Biodivers. 2017, 14, e1700182. [Google Scholar] [CrossRef]
- Jeong, S.H.; Kim, H.K.; Song, I.S.; Noh, S.J.; Marquez, J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoreyev, S.A.; et al. Echinochrome a increases mitochondrial mass and function by modulating mitochondrial biogenesis regulatory genes. Mar. Drug. 2014, 12, 4602–4615. [Google Scholar] [CrossRef]
- Chang, Y.L.; Stanford, C.M.; Keller, J.C. Calcium and phosphate supplementation promotes bone cell mineralization: Implications for hydroxyapatite (HA)-enhanced bone formation. J. Biomed. Mater. Res. 2000, 52, 270–278. [Google Scholar] [CrossRef]
- Wang, Y.H.; Liu, Y.; Maye, P.; Rowe, D.W. Examination of mineralized nodule formation in living osteoblastic cultures using fluorescent dyes. Biotechnol. Prog. 2006, 22, 1697–1701. [Google Scholar] [CrossRef]
- Huh, J.E.; Yang, H.R.; Park, D.S.; Choi, D.Y.; Baek, Y.H.; Cho, E.M.; Lee, J.D. Puerariae radix promotes differentiation and mineralization in human osteoblast-like SaOS-2 cells. J. Ethnopharmacol. 2006, 104, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Graef, J.L.; Rendina-Ruedy, E.; Crockett, E.K.; Ouyang, P.; King, J.B.; Cichewicz, R.H.; Lucas, E.A.; Smith, B.J. Select polyphenolic fractions from dried plum enhance osteoblast activity through BMP-2 signaling. J. Nutr. Biochem. 2018, 55, 59–67. [Google Scholar] [CrossRef]
- Zittermann, A. Effects of vitamin K on calcium and bone metabolism. Curr. Opin. Clin. Nutr. 2001, 4, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.P.; Shearer, M.J.; Klenerman, L.; Catterall, A.; Reeve, J.; Sambrook, P.N.; Dodds, R.A.; Bitensky, L.; Chayen, J. Electrochemical detection of pepressed circulating levels of Vitamin K1 in osteoporosis. J. Clin. Endocrinol. Metab. 1985, 60, 1268–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancaster, C.E. Effects of Vitamin D, K1, K2 and Calcium on Bone Formation by Osteoblasts in vitro. Master’ Thesis, University of Toronto, Toronto, ON, Canada, 2015. [Google Scholar]
- Zhu, M.; Ma, J.; Lu, S.; Zhu, Y.; Cui, Y.; Tan, H.; Wu, J.; Xu, Y. Vitamin K2 analog menaquinone-7 shows osteoblastic bone formation activity in vitro. Biomed. Res. 2017, 28, 1364–1369. [Google Scholar]
- Van Thanh, N.; Thao, N.; Huong, P.; Lee, S.H.; Jang, H.; Cuong, N.; Nam, N.; Kiem, P.; Kim, Y.H.; Minh, C. Naphthoquinone and flavonoid constituents from the carnivorous plant Nepenthes mirabilis and their anti-osteoporotic and antioxidant activities. Phytochem. Lett. 2015, 11, 254–259. [Google Scholar] [CrossRef]
- Yamauchi, M.; Yamaguchi, T.; Kaji, H.; Sugimoto, T.; Chihara, K. Involvement of calcium-sensing receptor in osteoblastic differentiation of mouse MC3T3-E1 cells. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E616. [Google Scholar] [CrossRef] [Green Version]
- Sugimoto, T.; Kanatani, M.; Kano, J.; Kaji, H.; Tsukamoto, T.; Yamaguchi, T.; Fukase, M.; Chihara, K. Effects of high calcium concentration on the functions and interactions of osteoblastic cells and monocytes and on the formation of osteoclast-like cells. J. Bone Miner. Res. 1993, 8, 1452. [Google Scholar]
- Lui, E.; Ao, C.; Li, L.; Khong, M.L.; Tanner, J. Inorganic polyphosphate triggers upregulation of interleukin 11 in human osteoblast-like SaOS-2 cells. Biochem. Biophys. Res. Commun. 2016, 479, 771. [Google Scholar] [CrossRef]
- Burrow, K.W. The Effects of New Zealand Sheep Milk on Mineral Absorption and Bone Health. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2019. [Google Scholar]
- Czekanska, E.; Stoddart, M.; Ralphs, J.; Richards, R.; Hayes, J. A phenotypic comparison of osteoblast cell lines versus human primary osteoblasts for biomaterials testing. J. Biomed. Mater. Res. Part A. 2014, 102, 2643. [Google Scholar] [CrossRef]
- Lebedev, A.V.; Ivanova, M.V.; Ruuge, E.K. How do calcium ions induce free radical oxidation of hydroxy-1,4-naphthoquinone? Ca2+ stabilizes the naphthosemiquinone anion-radical of echinochrome A. Arch. Biochem. 2003, 413, 191–198. [Google Scholar] [CrossRef]
- Lebedev, A.V.; Levitskaya, E.L.; Tikhonova, E.V.; Ivanova, M.V. Antioxidant properties, autooxidation, and mutagenic activity of echinochrome a compared with its etherified derivative. Biochemistry (Moscow) 2001, 66, 885–893. [Google Scholar] [CrossRef]







| Sample Name | Final Concentration in Media |
|---|---|
| CaCl2 control | CaCl2 0–4.0 mM |
| High concentration of echinochrome A | 62.5 µg/mL |
| Medium concentration of echinochrome A | 31.25 µg/mL |
| Low concentration of echinochrome A | 15.625 µg/mL |
| High concentration of PHNQ | 62.5 µg/mL |
| Medium concentration of PHNQ | 31.25 µg/mL |
| Low concentration of PHNQ | 15.625 µg/mL |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, Y.; Carne, A.; McConnell, M.; Mros, S.; Vasileva, E.A.; Mishchenko, N.P.; Burrow, K.; Wang, K.; Bekhit, A.A.; Bekhit, A.E.-D.A. PHNQ from Evechinus chloroticus Sea Urchin Supplemented with Calcium Promotes Mineralization in Saos-2 Human Bone Cell Line. Mar. Drugs 2020, 18, 373. https://doi.org/10.3390/md18070373
Hou Y, Carne A, McConnell M, Mros S, Vasileva EA, Mishchenko NP, Burrow K, Wang K, Bekhit AA, Bekhit AE-DA. PHNQ from Evechinus chloroticus Sea Urchin Supplemented with Calcium Promotes Mineralization in Saos-2 Human Bone Cell Line. Marine Drugs. 2020; 18(7):373. https://doi.org/10.3390/md18070373
Chicago/Turabian StyleHou, Yakun, Alan Carne, Michelle McConnell, Sonya Mros, Elena A. Vasileva, Natalia P. Mishchenko, Keegan Burrow, Ke Wang, Adnan A. Bekhit, and Alaa El-Din A. Bekhit. 2020. "PHNQ from Evechinus chloroticus Sea Urchin Supplemented with Calcium Promotes Mineralization in Saos-2 Human Bone Cell Line" Marine Drugs 18, no. 7: 373. https://doi.org/10.3390/md18070373
APA StyleHou, Y., Carne, A., McConnell, M., Mros, S., Vasileva, E. A., Mishchenko, N. P., Burrow, K., Wang, K., Bekhit, A. A., & Bekhit, A. E.-D. A. (2020). PHNQ from Evechinus chloroticus Sea Urchin Supplemented with Calcium Promotes Mineralization in Saos-2 Human Bone Cell Line. Marine Drugs, 18(7), 373. https://doi.org/10.3390/md18070373







