Seaweed Phenolics: From Extraction to Applications
Abstract
:1. Introduction
2. Phenols Found in Seaweeds
2.1. Phenolic Acids
2.2. Phlorotannins
2.3. Bromophenols
2.4. Flavonoids
2.5. Phenolic Terpenoids
2.6. Mycosporine-Like Aminoacids (MAA)
2.7. Non-Typical Phenolic Compounds
3. Phenolic Compounds Extractions and Purification Methodologies
3.1. Pre-Treatment
3.2. Extraction
3.3. Purification, Quantification, and Characterization
4. Seaweed Phenolic Compounds and their Bioactivities
4.1. Green Seaweeds
4.1.1. Bromophenols
4.1.2. Flavonoids
4.2. Red Seaweeds
4.2.1. Bromophenols
4.2.2. Flavonoids
4.2.3. Phenolic Terpenoids
4.2.4. Mycosporine-Like Amino Acid
4.3. Brown Seaweeds
4.3.1. Phlorotannins
4.3.2. Bromophenols
4.3.3. Flavonoids
4.3.4. Phenolic Terpenoids
5. Seaweed Phenolics: Commercial and Potential New Applications
5.1. Food Applications
5.2. Cosmetic Applications
- -
- Ulva compressa (formerly known as Enteromorpha compressa) Extract (Green Confertii Extract-NS by Natural Solution in Flemington, NJ, USA) contains flavonoids, tannins, polysaccharides, and acrylic acid as active compounds. It displays an antioxidant effect and anti-allergic effect and acts as an anti-microbial, antioxidant, and anti-allergic agent [294].
- -
- Fucus vesiculosus Extract (Bladderwrack Extract-NS by Natural Solution) contains fucoidan and phlorotannins as active compounds and acts as an anti-aging, antioxidant, anti-fungal, and anti-bacterial agent [295].
5.3. Pharmaceutical and Biomedical Applications
5.3.1. Cardiovascular Disease
5.3.2. Neurodegenerative and Mental Disorders
5.3.3. Anticancer Properties
5.3.4. Diabetes
5.3.5. Anti-Microbial Function
5.3.6. Tissue and Bone Regeneration
5.3.7. Anti-Inflammatory
5.3.8. Other Medical Applications
5.4. Feed and Animal Health
5.5. Agriculture
5.6. Other Applications
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Fallarero, A.; Peltoketo, A.; Loikkanen, J.; Tammela, P.; Vidal, A.; Vuorela, P. Effects of the aqueous extract of Bryothamnion triquetrum on chemical hypoxia and aglycemia-induced damage in GT1-7 mouse hypothalamic immortalized cells. Phytomedicine 2006, 13, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Pinto, E.; Andrade, P.B.; Valentão, P. Antifungal activity of phlorotannins against dermatophytes and yeasts: Approaches to the mechanism of action and influence on Candida albicans virulence factor. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jónsdóttir, R.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- Audibert, L.; Fauchon, M.; Blanc, N.; Hauchard, D.; Ar Gall, E. Phenolic compounds in the brown seaweed Ascophyllum nodosum: Distribution and radical-scavenging activities. Phytochem. Anal. 2010, 21, 399–405. [Google Scholar] [CrossRef]
- Ferreres, F.; Lopes, G.; Gil-Izquierdo, A.; Andrade, P.B.; Sousa, C.; Mouga, T.; Valentão, P. Phlorotannin extracts from fucales characterized by HPLC-DAD-ESI-MS n: Approaches to hyaluronidase inhibitory capacity and antioxidant properties. Mar. Drugs 2012, 10, 2766–2781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuesta, R.G.; González García, K.L.; Del, O.; Iglesias, R.V.; Rivera, Y.H.; Suárez, Y.A. Seaweeds as sources of bioactive compounds in the benefit of human health: A review. Rev. Cienc. Biológicas Y La Salud 2016, 18, 20–27. [Google Scholar] [CrossRef]
- Pacheco, D.; García-Poza, S.; Cotas, J.; Gonçalves, A.M.; Pereira, L. Fucoidan—A valuable source from the ocean to pharmaceutical. Front. Drug Chem. Clin. Res. 2020, 3, 1–4. [Google Scholar] [CrossRef]
- Wijesekara, I.; Kim, S.K.; Li, Y.X.; Li, Y.X. Phlorotannins as bioactive agents from brown algae. Process Biochem. 2011, 46, 2219–2224. [Google Scholar] [CrossRef]
- Yuan, Y.V.; Bone, D.E.; Carrington, M.F. Antioxidant activity of dulse (Palmaria palmata) extract evaluated in vitro. Food Chem. 2005, 91, 485–494. [Google Scholar] [CrossRef]
- Cox, S.; Gupta, S.; Abu-Ghannam, N. Effect of different rehydration temperatures on the moisture, content of phenolic compounds, antioxidant capacity and textural properties of edible Irish brown seaweed. LWT Food Sci. Technol. 2012, 47, 300–307. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joseph, D.; Praveen, N.K. Antioxidant activities and phenolic contents of three red seaweeds (Division: Rhodophyta) harvested from the Gulf of Mannar of Peninsular India. J. Food Sci. Technol. 2015, 52, 1924–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, D.C.; Reddy, C.R.K.; Balar, N.; Suthar, P.; Gajaria, T.; Gadhavi, D.K. Assessment of the nutritive, biochemical, antioxidant and antibacterial potential of eight tropical macro algae along kachchh coast, india as human food supplements. J. Aquat. Food Prod. Technol. 2018, 27, 61–79. [Google Scholar] [CrossRef]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in traditional chinese medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef] [PubMed]
- Mekinić, I.G.; Skroza, D.; Šimat, V.; Hamed, I.; Čagalj, M.; Perković, Z.P. Phenolic content of brown algae (Pheophyceae) species: Extraction, identification, and quantification. Biomolecules 2019, 9, 244. [Google Scholar] [CrossRef] [Green Version]
- Cotas, J.; Leandro, A.; Pacheco, D.; Gonçalves, A.M.M.; Pereira, L. A comprehensive review of the nutraceutical and therapeutic applications of red seaweeds (Rhodophyta). Life 2020, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Domínguez, H. Algae as a source of biologically active ingredients for the formulation of functional foods and nutraceuticals. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 1–19. ISBN 9780857095121. [Google Scholar]
- Singh, I.P.; Sidana, J. Phlorotannins. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 181–204. ISBN 9780857095121. [Google Scholar]
- Dimitrios, B. Sources of natural phenolic antioxidants. Trends Food Sci. Technol. 2006, 17, 505–512. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.; Ólafsdóttir, G. Total phenolic compounds, radical scavenging and metal chelation of extracts from icelandic seaweeds. Food Chem. 2009, 116, 240–248. [Google Scholar] [CrossRef]
- Swanson, B.G. Tannins and polyphenols. In Encyclopedia of Food Sciences and Nutrition; Caballero, B., Ed.; Academic Press: Cambridge, MA, USA, 2003; pp. 5729–5733. [Google Scholar]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 2009, 56, 317–333. [Google Scholar] [CrossRef]
- Shahidi, F.; Naczk, M. Phenolics in Food and Nutraceuticals; CRC Press: Boca Raton, FL, USA, 2003; ISBN 9780367395094. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Wells, M.L.; Potin, P.; Craigie, J.S.; Raven, J.A.; Merchant, S.S.; Helliwell, K.E.; Smith, A.G.; Camire, M.E.; Brawley, S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017, 29, 949–982. [Google Scholar] [CrossRef]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Coman, M.M.; Guo, Y.; Hotchkiss, S.; Gill, C.; Yaqoob, P.; Spencer, J.P.E.; Rowland, I. Effect of simulated gastrointestinal digestion and fermentation on polyphenolic content and bioactivity of brown seaweed phlorotannin-rich extracts. Mol. Nutr. Food Res. 2017, 61, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guzmán, M.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J. Potential role of seaweed polyphenols in cardiovascular-associated disorders. Mar. Drugs 2018, 16, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairhead, V.A.; Amsler, C.D.; McClintock, J.B.; Baker, B.J. Variation in phlorotannin content within two species of brown macroalgae (Desmarestia anceps and D. menziesii) from the Western Antarctic Peninsula. Polar Biol. 2005, 28, 680–686. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Li, Z.; Mou, Q. The anti-allergic activity of polyphenol extracted from five marine algae. J. Ocean Univ. China 2015, 14, 681–684. [Google Scholar] [CrossRef]
- Murray, M.; Dordevic, A.; Ryan, L.; Bonham, M. The Impact of a single dose of a polyphenol-rich seaweed extract on postprandial glycaemic control in healthy adults: A randomised cross-over trial. Nutrients 2018, 10, 270. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Kim, S.-K. Neuroprotective effects of marine algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [Green Version]
- Thomas, N.V.; Kim, S.K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Env. Toxicol. Pharm. 2011, 32, 325–335. [Google Scholar] [CrossRef]
- Tanna, B.; Brahmbhatt, H.R.; Mishra, A. Phenolic, flavonoid, and amino acid compositions reveal that selected tropical seaweeds have the potential to be functional food ingredients. J. Food Process. Preserv. 2019, 43, 1–10. [Google Scholar] [CrossRef]
- Nwosu, F.; Morris, J.; Lund, V.A.; Stewart, D.; Ross, H.A.; McDougall, G.J. Anti-proliferative and potential anti-diabetic effects of phenolic-rich extracts from edible marine algae. Food Chem. 2011, 126, 1006–1012. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Silva, L.R.; Pinto, E.; Andrade, P.B.; Bernardo, J.; Mouga, T.; Valentão, P. Can phlorotannins purified extracts constitute a novel pharmacological alternative for microbial infections with associated inflammatory conditions? PLoS ONE 2012, 7, e31145. [Google Scholar] [CrossRef] [PubMed]
- Urquiaga, I.; Leighton, F. Plant polyphenol antioxidants and oxidative stress. Biol. Res. 2000, 33, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L. Therapeutic and Nutritional Uses of Algae; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781498755382. [Google Scholar]
- Ravikumar, S.; Jacob Inbaneson, S.; Suganthi, P. Seaweeds as a source of lead compounds for the development of new antiplasmodial drugs from South East coast of India. Parasitol. Res. 2011, 109, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.C.; Hunter, M.D.; Appel, H.M. Antimicrobial activity of polyphenols mediates plant-herbivore interactions. In Plant Polyphenols; Hemingway, R.W., Laks, P.E., Eds.; Springer US: Boston, MA, USA, 1992; pp. 621–637. [Google Scholar]
- Maqsood, S.; Benjakul, S.; Shahidi, F. Emerging Role of phenolic compounds as natural food additives in fish and fish products. Crit. Rev. Food Sci. Nutr. 2013, 53, 162–179. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural phenol polymers: Recent advances in food and health applications. Antioxidants 2017, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Leandro, A.; Pereira, L.; Gonçalves, A.M.M. Diverse applications of marine macroalgae. Mar. Drugs 2019, 18, 17. [Google Scholar] [CrossRef] [Green Version]
- Freile-Pelegrín, Y.; Robledo, D. Bioactive phenolic compounds from algae. Bioact. Compd. Mar. Foods Plant Anim. Sources 2013, 113–129. [Google Scholar] [CrossRef]
- Bilal Hussain, M.; Hassan, S.; Waheed, M.; Javed, A.; Adil Farooq, M.; Tahir, A. Bioavailability and metabolic pathway of phenolic compounds. Plant Physiol. Asp. Phenolic Compd. 2019, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Maqsood, S.; Benjakul, S.; Abushelaibi, A.; Alam, A. Phenolic compounds and plant phenolic extracts as natural antioxidants in prevention of lipid oxidation in seafood: A detailed review. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1125–1140. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Félix, R.; Pais, A.C.S.; Rocha, S.M.; Silvestre, A.J.D. The quest for phenolic compounds from macroalgae: A review of extraction and identification methodologies. Biomolecules 2019, 9, 847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, T.M.; Targett, N.M. Marine tannins: The importance of a mechanistic framework for predicting ecological roles. J. Chem. Ecol. 2002, 28, 1919–1934. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Santos, T.; Duarte, A.C. Introduction to the analysis of bioactive compounds in marine samples. In Comprehensive Analytical Chemistry; Rocha-Santos, T., Duarte, A.C., Eds.; Elsevier B.V.: Amsterdam, Netherlands, 2014; Volume 65, pp. 1–13. ISBN 9780444633590. [Google Scholar]
- Mukherjee, P.K. Bioactive phytocomponents and their analysis. In Quality Control and Evaluation of Herbal Drugs; Mukherjee, P.K., Ed.; Elsevier Inc.: Amsterdam, Netherlands, 2019; pp. 237–328. ISBN 9780128133743. [Google Scholar]
- Pietta, P.; Minoggio, M.; Bramati, L. Plant polyphenols: Structure, occurrence and bioactivity. Stud. Nat. Prod. Chem. 2003, 28, 257–312. [Google Scholar] [CrossRef]
- Liwa, A.C.; Barton, E.N.; Cole, W.C.; Nwokocha, C.R. Bioactive Plant Molecules, Sources and Mechanism of Action in the Treatment of Cardiovascular Disease; Elsevier Inc.: Amsterdam, Netherlands, 2017; ISBN 9780128020999. [Google Scholar]
- Luna-Guevara, M.L.; Luna-Guevara, J.J.; Hernández-Carranza, P.; Ruíz-Espinosa, H.; Ochoa-Velasco, C.E. Phenolic compounds: A good choice against chronic degenerative diseases. Stud. Nat. Prod. Chem. 2018, 59, 79–108. [Google Scholar] [CrossRef]
- Mancini-Filho, J.; Novoa, A.V.; González, A.E.B.; de Andrade-Wartha, E.R.S.; Mancini, D.A.P. Free phenolic acids from the seaweed Halimeda monile with antioxidant effect protecting against liver injury. Z. Für Nat. C 2009, 64, 657–663. [Google Scholar] [CrossRef]
- Feng, Y.; Carroll, A.R.; Addepalli, R.; Fechner, G.A.; Avery, V.M.; Quinn, R.J. Vanillic acid derivatives from the green algae Cladophora socialis as potent protein tyrosine phosphatase 1B inhibitors. J. Nat. Prod. 2007, 70, 1790–1792. [Google Scholar] [CrossRef]
- Agregán, R.; Munekata, P.E.S.; Franco, D.; Dominguez, R.; Carballo, J.; Lorenzo, J.M. Phenolic compounds from three brown seaweed species using LC-DAD–ESI-MS/MS. Food Res. Int. 2017, 99, 979–985. [Google Scholar] [CrossRef]
- Farvin, K.H.S.; Jacobsen, C.; Sabeena Farvin, K.H.; Jacobsen, C. Phenolic compounds and antioxidant activities of selected species of seaweeds from Danish coast. Food Chem. 2013, 138, 1670–1681. [Google Scholar] [CrossRef]
- Xu, T.; Sutour, S.; Casabianca, H.; Tomi, F.; Paoli, M.; Garrido, M.; Pasqualini, V.; Aiello, A.; Castola, V.; Bighelli, A. Rapid screening of chemical compositions of Gracilaria dura and Hypnea mucisformis (Rhodophyta) from Corsican Lagoon. Int. J. Phytocosmetics Nat. Ingred. 2015, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Souza, B.W.S.; Cerqueira, M.A.; Martins, J.T.; Quintas, M.A.C.; Ferreira, A.C.S.; Teixeira, J.A.; Vicente, A.A. Antioxidant potential of two red seaweeds from the brazilian coasts. J. Agric. Food Chem. 2011, 59, 5589–5594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef] [PubMed]
- Imbs, T.I.; Zvyagintseva, T.N. Phlorotannins are polyphenolic metabolites of brown algae. Russ. J. Mar. Biol. 2018, 44, 263–273. [Google Scholar] [CrossRef]
- Achkar, J.; Xian, M.; Zhao, H.; Frost, J.W. Biosynthesis of phloroglucinol. J. Am. Chem. Soc. 2005, 127, 5332–5333. [Google Scholar] [CrossRef] [PubMed]
- Katsui, N.; Suzuki, Y.; Kitamura, S.; Irie, T. 5,6-dibromoprotocatechualdehyde and 2,3-dibromo-4,5-dihydroxybenzyl methyl ether: New dibromophenols from Rhodomela larix. Tetrahedron 1967, 23, 1185–1188. [Google Scholar] [CrossRef]
- Fan, X.; Xu, N.J.; Shi, J.G. Bromophenols from the red alga Rhodomela confervoides. J. Nat. Prod. 2003, 66, 455–458. [Google Scholar] [CrossRef]
- Ko, S.C.; Ding, Y.; Kim, J.; Ye, B.R.; Kim, E.A.; Jung, W.K.; Heo, S.J.; Lee, S.H. Bromophenol (5-bromo-3,4-dihydroxybenzaldehyde) isolated from red alga Polysiphonia morrowii inhibits adipogenesis by regulating expression of adipogenic transcription factors and AMP-activated protein kinase activation in 3T3-L1 adipocytes. Phyther. Res. 2019, 33, 737–744. [Google Scholar] [CrossRef]
- Flodin, C.; Helidoniotis, F.; Whitfield, F.B. Seasonal variation in bromophenol content and bromoperoxidase activity in Ulva lactuca. Phytochemistry 1999, 51, 135–138. [Google Scholar] [CrossRef]
- Colon, M.; Guevara, P.; Gerwick, W.H.; Ballantine, D. 5’-hydroxyisoavrainvilleol, a new diphenylmethane derivative from the tropical green alga Avrainvillea Nigricans. J. Nat. Prod. 1987, 50, 368–374. [Google Scholar] [CrossRef]
- Flodin, C.; Whitfield, F.B. 4-hydroxybenzoic acid: A likely precursor of 2,4,6-tribromophenol in Ulva lactuca. Phytochemistry 1999, 51, 249–255. [Google Scholar] [CrossRef]
- Shi, D.; Li, X.; Li, J.; Guo, S.; Su, H.; Fan, X. Antithrombotic effects of bromophenol, an alga-derived thrombin inhibitor. Chin. J. Oceanol. Limnol. 2010, 28, 96–98. [Google Scholar] [CrossRef]
- Xu, X.-L.; Fan, X.; Song, F.-H.; Zhao, J.-L.; Han, L.-J.; Yang, Y.-C.; Shi, J.-G. Bromophenols from the brown alga Leathesia nana. J. Asian Nat. Prod. Res. 2004, 6, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.Y.; Ma, W.C.J.; Ang, P.O.; Kim, J.S.; Chen, F. Seasonal variations of bromophenols in brown algae (Padina arboroscens, Sargassum siliquastrum, and Lobophora variegata) collected in Hong Kong. J. Agric. Food Chem. 2003, 51, 2619–2624. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.E.; Wani, M.C.; Manikumar, G.; Taylor, H.; Hughes, T.J.; Gaetano, K.; Gerwick, W.H.; McPhail, A.T.; McPhail, D.R. Plant antimutagenic agents, 7. Structure and antimutagenic properties of cymobarbatol and 4-isocymobarbatol, new cymopols from green alga (Cymopolia barbata). J. Nat. Prod. 1989, 52, 1092–1099. [Google Scholar] [CrossRef]
- Flodin, C.; Whitfield, F.B. Biosynthesis of bromophenols in marine algae. Water Sci. Technol. 1999, 40, 53–58. [Google Scholar] [CrossRef]
- Peng, J.; Li, J.; Hamann, M.T. The marine bromotyrosine derivatives. Alkaloids Chem. Biol. 2005, 61, 59–262. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zhang, W.; Wei, J.; Qiu, L.; Lin, X. Marine bromophenol bis(2,3-dibromo-4,5-dihydroxybenzyl) ether, induces mitochondrial apoptosis in K562 cells and inhibits topoisomerase I in vitro. Toxicol. Lett. 2012, 211, 126–134. [Google Scholar] [CrossRef]
- Jesus, A.; Correia-da-Silva, M.; Afonso, C.; Pinto, M.; Cidade, H. Isolation and potential biological applications of haloaryl secondary metabolites from macroalgae. Mar. Drugs 2019, 17, 73. [Google Scholar] [CrossRef] [Green Version]
- Yoshie-Stark, Y.; Hsieh, Y. Distribution of flavonoids and related compounds from seaweeds in Japan. Tokyo Univ. Fish. 2003, 89, 1–6. [Google Scholar]
- Markham, K.R.; Porter, L.J. Flavonoids in the green algae (chlorophyta). Phytochemistry 1969, 8, 1777–1781. [Google Scholar] [CrossRef]
- Yonekura-Sakakibara, K.; Higashi, Y.; Nakabayashi, R. The origin and evolution of plant flavonoid metabolism. Front. Plant Sci. 2019, 10, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Bowman, J.L.; Kohchi, T.; Yamato, K.T.; Jenkins, J.; Shu, S.; Ishizaki, K.; Yamaoka, S.; Nishihama, R.; Nakamura, Y.; Berger, F.; et al. Insights into land plant evolution garnered from the Marchantia polymorpha genome. Cell 2017, 171, 287–304.e15. [Google Scholar] [CrossRef] [PubMed]
- Goiris, K.; Muylaert, K.; Voorspoels, S.; Noten, B.; De Paepe, D.; E Baart, G.J.; De Cooman, L. Detection of flavonoids in microalgae from different evolutionary lineages. J. Phycol. 2014, 50, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Alseekh, S.; Fernie, A.R. Conservation and diversification of flavonoid metabolism in the plant kingdom. Curr. Opin. Plant Biol. 2020, 55, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Osuna-Ruíz, I.; Salazar-Leyva, J.A.; López-Saiz, C.M.; Burgos-Hernández, A.; Hernández-Garibay, E.; Lizardi-Mendoza, J.; Hurtado-Oliva, M.A. Enhancing antioxidant and antimutagenic activity of the green seaweed Rhizoclonium riparium by bioassay-guided solvent partitioning. J. Appl. Phycol. 2019, 31, 3871–3881. [Google Scholar] [CrossRef]
- Kumar, J.G.S.; Umamaheswari, S.; Kavimani, S.; Ilavarasan, R. Pharmacological potential of green algae Caulerpa: A review. Int. J. Pharm. Sci. Res. 2019, 10, 1014. [Google Scholar] [CrossRef]
- Haq, S.H.; Al-Ruwaished, G.; Al-Mutlaq, M.A.; Naji, S.A.; Al-Mogren, M.; Al-Rashed, S.; Ain, Q.T.; Al-Amro, A.A.; Al-Mussallam, A. Antioxidant, anticancer activity and phytochemical analysis of green algae, Chaetomorpha collected from the Arabian Gulf. Sci. Rep. 2019, 9, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pangestuti, R.; Getachew, A.T.; Siahaan, E.A.; Chun, B.S. Characterization of functional materials derived from tropical red seaweed Hypnea musciformis produced by subcritical water extraction systems. J. Appl. Phycol. 2019, 31, 2517–2528. [Google Scholar] [CrossRef]
- Suganya, S.; Ishwarya, R.; Jayakumar, R.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Al-anbr, M.N.; Vaseeharan, B. New insecticides and antimicrobials derived from Sargassum wightii and Halimeda gracillis seaweeds: Toxicity against mosquito vectors and antibiofilm activity against microbial pathogens. S. Afr. J. Bot. 2019, 125, 466–480. [Google Scholar] [CrossRef]
- Ismail, M.M.; Gheda, S.F.; Pereira, L. Variation in bioactive compounds in some seaweeds from Abo Qir bay, Alexandria, Egypt. Rend. Lincei 2016, 27, 269–279. [Google Scholar] [CrossRef]
- Abirami, R.G.; Kowsalya, S. Nutrient and nutraceutical potentials of seaweed biomass Ulva lactuca and Kappaphycus alvarezii. Agric. Sci. Technol. 2011, 5, 109. [Google Scholar]
- Yoshie, Y.; Wang, W.; Petillo, D.; Suzuki, T. Distribution of catechins in Japanese seaweeds. Fish. Sci. 2000, 66, 998–1000. [Google Scholar] [CrossRef]
- Reddy, P.; Urban, S. Meroditerpenoids from the southern Australian marine brown alga Sargassum fallax. Phytochemistry 2009, 70, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Lane, A.L.; Stout, E.P.; Lin, A.S.; Prudhomme, J.; Le Roch, K.; Fairchild, C.R.; Franzblau, S.G.; Hay, M.E.; Aalbersberg, W.; Kubanek, J. Antimalarial bromophyeolides J-Q from the Fijian red alga Callophycus serratus. J. Org. Chem. 2009, 74, 2736–2742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perveen, S. Introductory chapter: Terpenes and terpenoids. In Terpenes and Terpenoids; Perveen, S., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C Toxicol. Pharm. 2007, 146, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, C.A.; Airs, R.L. Distribution and abundance of MAAs in 33 species of microalgae across 13 classes. Mar. Drugs 2010, 8, 1273–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreto, J.I.; Carignan, M.O. Mycosporine-like amino acids: Relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs 2011, 9, 387–446. [Google Scholar] [CrossRef]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef]
- Green, D.; Kashman, Y.; Miroz, A. Colpol, a new cytotoxic C6-C4-C6 metabolite from the alga Colpomenia sinuosa. J. Nat. Prod. 1993, 56, 1201–1202. [Google Scholar] [CrossRef]
- Ishii, T.; Okino, T.; Suzuki, M.; Machiguchi, Y. Tichocarpols A and B, two novel phenylpropanoids with feeding-deterrent activity from the reel alga Tichocarpus crinitus. J. Nat. Prod. 2004, 67, 1764–1766. [Google Scholar] [CrossRef]
- Martone, P.T.; Estevez, J.M.; Lu, F.; Ruel, K.; Denny, M.W.; Somerville, C.; Ralph, J. Discovery of lignin in seaweed reveals convergent evolution of cell-wall architecture. Curr. Biol. 2009, 19, 169–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.J.; Li, X.M.; Wang, B.G. Highly brominated mono- and bis-phenols from the marine red alga Symphyocladia latiuscula with radical-scavenging activity. J. Nat. Prod. 2007, 70, 1210–1213. [Google Scholar] [CrossRef] [PubMed]
- Machu, L.; Misurcova, L.; Ambrozova, J.V.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, N.; Fu, X.; Duan, D.; Xu, J.; Gao, X.; Zhao, L. Evaluation of bioactivity of phenolic compounds from the brown seaweed of Sargassum fusiforme and development of their stable emulsion. J. Appl. Phycol. 2018, 30, 1955–1970. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the polyphenols: Status and controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S. Natural products from marine algae: Methods and protocols. Nat. Prod. Mar. Algae Methods Protoc. 2015, 1308, 1–439. [Google Scholar] [CrossRef]
- Pádua, D.; Rocha, E.; Gargiulo, D.; Ramos, A.A. Bioactive compounds from brown seaweeds: Phloroglucinol, fucoxanthin and fucoidan as promising therapeutic agents against breast cancer. Phytochem. Lett. 2015, 14, 91–98. [Google Scholar] [CrossRef]
- Michalak, I. Experimental processing of seaweeds for biofuels. Wiley Interdiscip. Rev. Energy Env. 2018, 7, 1–25. [Google Scholar] [CrossRef]
- Koivikko, R.; Loponen, J.; Pihlaja, K.; Jormalainen, V. High-performance liquid chromatographic analysis of phlorotannins from the brown alga Fucus vesiculosus. Phytochem. Anal. 2007, 18, 326–332. [Google Scholar] [CrossRef]
- Onofrejová, L.; Vašíčková, J.; Klejdus, B.; Stratil, P.; Mišurcová, L.; Kráčmar, S.; Kopecký, J.; Vacek, J. Bioactive phenols in algae: The application of pressurized-liquid and solid-phase extraction techniques. J. Pharm. Biomed. Anal. 2010, 51, 464–470. [Google Scholar] [CrossRef]
- Sugiura, Y.; Matsuda, K.; Yamada, Y.; Nishikawa, M.; Shioya, K.; Katsuzaki, H.; Imai, K.; Amano, H. Isolation of a new anti-allergic phlorotannin, phlorofucofuroeckol-B, from an edible brown alga, Eisenia arborea. Biosci. Biotechnol. Biochem. 2006, 70, 2807–2811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, A.; Ganzera, M.; Karsten, U.; Skhirtladze, A.; Stuppner, H. Phytochemical and analytical characterization of novel sulfated coumarins in the marine green macroalga Dasycladus vermicularis (Scopoli) krasser. Molecules 2018, 23, 2753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koivikko, R.; Loponen, J.; Honkanen, T.; Jormalainen, V. Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. J. Chem. Ecol. 2005, 31, 195–212. [Google Scholar] [CrossRef] [Green Version]
- Michalak, I.; Chojnacka, K. Algal extracts: Technology and advances. Eng. Life Sci. 2014, 14, 581–591. [Google Scholar] [CrossRef]
- Kim, S.M.; Kang, S.W.; Jeon, J.S.; Jung, Y.J.; Kim, W.R.; Kim, C.Y.; Um, B.H. Determination of major phlorotannins in Eisenia bicyclis using hydrophilic interaction chromatography: Seasonal variation and extraction characteristics. Food Chem. 2013, 138, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Ospina, M.; Castro-Vargas, H.I.; Parada-Alfonso, F. Antioxidant capacity of Colombian seaweeds: 1. extracts obtained from Gracilaria mammillaris by means of supercritical fluid extraction. J. Supercrit. Fluids 2017. [Google Scholar] [CrossRef]
- Ibañez, E.; Herrero, M.; Mendiola, J.A.; Castro-Puyana, M. Extraction and characterization of bioactive compounds with health benefits from marine resources: Macro and micro algae, cyanobacteria, and invertebrates. In Marine Bioactive Compounds; Hayes, M., Ed.; Springer: Boston, MA, USA, 2012; Volume 9781461412, pp. 55–98. [Google Scholar]
- Vinatoru, M. An overview of the ultrasonically assisted extraction of bioactive principles from herbs. Ultrason. Sonochem. 2001, 8, 303–313. [Google Scholar] [CrossRef]
- Rajbhar, K.; Dawda, H.; Mukundan, U. Polyphenols: Methods of extraction. Sci. Revs. Chem. Commun. 2015, 5, 1–6. [Google Scholar]
- Yuan, Y.; Zhang, J.; Fan, J.; Clark, J.; Shen, P.; Li, Y.; Zhang, C. Microwave assisted extraction of phenolic compounds from four economic brown macroalgae species and evaluation of their antioxidant activities and inhibitory effects on α-amylase, α-glucosidase, pancreatic lipase and tyrosinase. Food Res. Int. 2018, 113, 288–297. [Google Scholar] [CrossRef]
- Kadam, S.U.; Tiwari, B.K.; O’Donnell, C.P. Application of novel extraction technologies for bioactives from marine algae. J. Agric. Food Chem. 2013, 61, 4667–4675. [Google Scholar] [CrossRef]
- Plaza, M.; Amigo-Benavent, M.; del Castillo, M.D.; Ibáñez, E.; Herrero, M. Facts about the formation of new antioxidants in natural samples after subcritical water extraction. Food Res. Int. 2010, 43, 2341–2348. [Google Scholar] [CrossRef] [Green Version]
- Huie, C.W. A review of modern sample-preparation techniques for the extraction and analysis of medicinal plants. Anal. Bioanal. Chem. 2002, 373, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Supercritical CO2 extraction and purification of compounds with antioxidant activity. J. Agric. Food Chem. 2006, 54, 2441–2469. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, M.H.; Han, J.S.; Jeong, Y.; Kim, M.; Jeon, Y.J. Bioactive compounds extracted from gamtae (Ecklonia cava) by using enzymatic hydrolysis, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Food Sci. Biotechnol. 2012, 21, 1149–1155. [Google Scholar] [CrossRef]
- Žuvela, P.; Skoczylas, M.; Jay Liu, J.; Ba̧czek, T.; Kaliszan, R.; Wong, M.W.; Buszewski, B. Column characterization and selection systems in reversed-phase high-performance liquid chromatography. Chem. Rev. 2019, 119, 3674–3729. [Google Scholar] [CrossRef]
- Moll, F. Principles of Adsorption Chromatography. The Separation of Nonionic Organic Compounds. Vol. 3, Chromatographic Science Series. VonL. R. Snyder. 424 S. Marcel Dekker, Inc., New York 1968. Preis: $ 17.50. Arch. Pharm. (Weinh.) 1969, 302, 475. [Google Scholar] [CrossRef]
- Snyder, L.R. Role of the solvent in liquid-solid chromatography—A review. Anal. Chem. 1974, 46, 1384–1393. [Google Scholar] [CrossRef]
- Rafferty, J.L.; Zhang, L.; Siepmann, J.I.; Schure, M.R. Retention mechanism in reversed-phase liquid chromatography: A molecular perspective. Anal. Chem. 2007, 79, 6551–6558. [Google Scholar] [CrossRef]
- Lijun, H.; Nianjun, X.; Jiangong, S.; Xiaojun, Y.; Chengkui, Z. Isolation and pharmacological activities of bromophenols from Rhodomela confervoides. Chin. J. Oceanol. Limnol. 2005, 23, 226–229. [Google Scholar] [CrossRef]
- Sherma, J.; Fried, B. Thin layer chromatographic analysis of biological samples. A review. J. Liq. Chromatogr. Relat. Technol. 2005, 28, 2297–2314. [Google Scholar] [CrossRef]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.Y.; Park, K.E.; Yoon, N.Y.; Kim, J.S.; Choi, J.S.; Jang, B.C.; Byun, D.S.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Li, S.P.; Lisak, G. Advanced sensing technologies of phenolic compounds for pharmaceutical and biomedical analysis. J. Pharm. Biomed. Anal. 2020, 179, 112913. [Google Scholar] [CrossRef] [PubMed]
- Olate-Gallegos, C.; Barriga, A.; Vergara, C.; Fredes, C.; García, P.; Giménez, B.; Robert, P. Identification of polyphenols from chilean brown seaweeds extracts by LC-DAD-ESI-MS/MS. J. Aquat. Food Prod. Technol. 2019, 28, 375–391. [Google Scholar] [CrossRef]
- Maheswari, M.U.; Reena, A.; Sivaraj, C. Analysis, antioxidant and antibacterial activity of the brown algae, Padina tetrastromatica. Int. J. Pharm. Sci. Res 2018, 9, 298–304. [Google Scholar]
- Principles of MALDI-TOF Mass Spectrometry: SHIMADZU (Shimadzu Corporation). Available online: https://www.shimadzu.com/an/lifescience/maldi/princpl1.html (accessed on 14 April 2020).
- Karthik, R.; Manigandan, V.; Sheeba, R.; Saravanan, R.; Rajesh, P.R. Structural characterization and comparative biomedical properties of phloroglucinol from Indian brown seaweeds. J. Appl. Phycol. 2016, 28, 3561–3573. [Google Scholar] [CrossRef]
- Lopes, G.; Barbosa, M.; Vallejo, F.; Gil-Izquierdo, Á.; Andrade, P.B.; Valentão, P.; Pereira, D.M.; Ferreres, F. Profiling phlorotannins from Fucus spp. of the Northern Portuguese coastline: Chemical approach by HPLC-DAD-ESI/MS and UPLC-ESI-QTOF/MS. Algal Res. 2018, 29, 113–120. [Google Scholar] [CrossRef]
- Nerantzaki, A.A.; Tsiafoulis, C.G.; Charisiadis, P.; Kontogianni, V.G.; Gerothanassis, I.P. Novel determination of the total phenolic content in crude plant extracts by the use of 1H NMR of the -OH spectral region. Anal. Chim. Acta 2011, 688, 54–60. [Google Scholar] [CrossRef]
- Pauli, G.F.; Jaki, B.U.; Lankin, D.C. Quantitative 1H NMR: Development and potential of a method for natural products analysis. J. Nat. Prod. 2005, 68, 133–149. [Google Scholar] [CrossRef]
- Blümich, B.; Singh, K. Desktop NMR and its applications from materials science to organic chemistry. Angew. Chem. Int. Ed. 2018, 57, 6996–7010. [Google Scholar] [CrossRef]
- Wekre, M.E.; Kåsin, K.; Underhaug, J.; Jordheim, M.; Holmelid, B. Quantification of polyphenols in seaweeds: A case study of Ulva intestinalis. Antioxidants 2019, 8, 612. [Google Scholar] [CrossRef] [Green Version]
- Farasat, M.; Khavari-Nejad, R.A.; Nabavi, S.M.B.; Namjooyan, F. Antioxidant activity, total phenolics and flavonoid contents of some edible green seaweeds from northern coasts of the Persian Gulf. Iran. J. Pharm. Res. 2014, 13, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Kang, I.-J.; Won, M.-H.; Lee, H.-S.; You, S. The antioxidant properties of ethanol extracts and their solvent-partitioned fractions from various green seaweeds. J. Med. Food 2010, 13, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Inoue, M.; Uzawa, Y.; Kawamura, Y. Anti-tumorigenic components of a sea weed, Enteromorpha clathrata. BioFactors 2004, 22, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Khanavi, M.; Gheidarloo, R.; Sadati, N.; Shams Ardekani, M.R.; Bagher Nabavi, S.M.; Tavajohi, S.; Ostad, S.N. Cytotoxicity of fucosterol containing fraction of marine algae against breast and colon carcinoma cell line. Pharm. Mag. 2012, 8, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Lavoie, S.; Sweeney-Jones, A.M.; Mojib, N.; Dale, B.; Gagaring, K.; McNamara, C.W.; Quave, C.L.; Soapi, K.; Kubanek, J. Antibacterial oligomeric polyphenols from the green alga Cladophora socialis. J. Org. Chem. 2019, 84, 5035–5045. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L. Portuguese Seaweeds Website (MACOI). Available online: http://www.flordeutopia.pt/macoi/default.php (accessed on 7 June 2020).
- Carte, B.K.; Troupe, N.; Chan, J.A.; Westley, J.W.; Faulkner, D.J. Rawsonol, an inhibitor of HMG-CoA reductase from the tropical green alga Avrainvillea rawsoni. Phytochemistry 1989, 28, 2917–2919. [Google Scholar] [CrossRef]
- Estrada, D.M.; Martin, J.D.; Perez, C. A new brominated monoterpenoid quinol from Cymopolm barbata. J. Nat. Prod. 1987, 50, 735–737. [Google Scholar] [CrossRef]
- Yan, X.; Yang, C.; Lin, G.; Chen, Y.; Miao, S.; Liu, B.; Zhao, C. Antidiabetic potential of green seaweed Enteromorpha prolifera flavonoids regulating insulin signaling pathway and gut microbiota in type 2 diabetic mice. J. Food Sci. 2019, 84, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Lin, G.; Liu, X.; Yan, X.; Liu, D.; Yang, C.; Liu, B.; Huang, Y.; Zhao, C. Role of green macroalgae Enteromorpha prolifera polyphenols in the modulation of gene expression and intestinal microflora profiles in type 2 diabetic mice. Int. J. Mol. Sci. 2018, 20, 25. [Google Scholar] [CrossRef] [Green Version]
- Tanna, B.; Choudhary, B.; Mishra, A. Metabolite profiling, antioxidant, scavenging and anti-proliferative activities of selected tropical green seaweeds reveal the nutraceutical potential of Caulerpa spp. Algal Res. 2018, 36, 96–105. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; dos Santos, D.Y.A.C. A comprehensive review of traditional uses, bioactivity potential, and chemical diversity of the genus Gracilaria (Gracilariales, Rhodophyta). Algal Res. 2019, 37, 288–306. [Google Scholar] [CrossRef]
- Namvar, F.; Mohamed, S.; Fard, S.G.; Behravan, J.; Mustapha, N.M.; Alitheen, N.B.M.; Othman, F. Polyphenol-rich seaweed (Eucheuma cottonii) extract suppresses breast tumour via hormone modulation and apoptosis induction. Food Chem. 2012, 130, 376–382. [Google Scholar] [CrossRef]
- Choi, J.S.; Park, H.J.; Jung, H.A.; Chung, H.Y.; Jung, J.H.; Choi, W.C. A cyclohexanonyl bromophenol from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2000, 63, 1705–1706. [Google Scholar] [CrossRef]
- Popplewell, W.L.; Northcote, P.T. Colensolide A: A new nitrogenous bromophenol from the New Zealand marine red alga Osmundaria colensoi. Tetrahedron Lett. 2009, 50, 6814–6817. [Google Scholar] [CrossRef]
- Qi, X.; Liu, G.; Qiu, L.; Lin, X.; Liu, M. Marine bromophenol bis (2,3-dibromo-4,5-dihydroxybenzyl) ether, represses angiogenesis in HUVEC cells and in zebrafish embryos via inhibiting the VEGF signal systems. Biomed. Pharm. 2015, 75, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Luo, J.; Jiang, B.; Wang, L.; Wang, S.; Wang, C.; Fu, C.; Li, J.; Shi, D. Marine bromophenol bis (2,3-dibromo-4,5-dihydroxy-phenyl)-methane inhibits the proliferation, migration, and invasion of hepatocellular carcinoma cells via modulating β1-integrin/FAK signaling. Mar. Drugs 2015, 13, 1010–1025. [Google Scholar] [CrossRef]
- Ma, M.; Zhao, J.; Wang, S.; Li, S.; Yang, Y.; Shi, J.; Fan, X.; He, L. Bromophenols coupled with methyl γ-ureidobutyrate and bromophenol sulfates from the red alga Rhodomela confervoides. J. Nat. Prod. 2006, 69, 206–210. [Google Scholar] [CrossRef]
- Paudel, P.; Seong, S.; Park, H.; Jung, H.; Choi, J. Anti-Diabetic activity of 2,3,6-tribromo-4,5-dihydroxybenzyl derivatives from Symphyocladia latiuscula through PTP1B downregulation and α-glucosidase inhibition. Mar. Drugs 2019, 17, 166. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Okada, Y.; Shi, H.; Wang, Y.; Okuyama, T. Structures and aldose reductase inhibitory effects of bromophenols from the red alga Symphyocladia latiuscula. J. Nat. Prod. 2005, 68, 620–622. [Google Scholar] [CrossRef]
- Shi, D.; Xu, F.; He, J.; Li, J.; Fan, X.; Han, L. Inhibition of bromophenols against PTP1B and anti-hyperglycemic effect of Rhodomela confervoides extract in diabetic rats. Chin. Sci. Bull. 2008, 53, 2476–2479. [Google Scholar] [CrossRef] [Green Version]
- Mikami, D.; Kurihara, H.; Ono, M.; Kim, S.M.; Takahashi, K. Inhibition of algal bromophenols and their related phenols against glucose 6-phosphate dehydrogenase. Fitoterapia 2016, 108, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, N.; Fan, X.; Yan, X.; Li, X.; Niu, R.; Tseng, C.K. Antibacterial bromophenols from the marine red alga Rhodomela confervoides. Phytochemistry 2003, 62, 1221–1224. [Google Scholar] [CrossRef]
- Barreto, M.; Meyer, J.J.M. Isolation and antimicrobial activity of a lanosol derivative from Osmundaria serrata (Rhodophyta) and a visual exploration of its biofilm covering. S. Afr. J. Bot. 2006, 72, 521–528. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Wang, G.; Xiao, L.; Xu, X.; Liu, X.; Xu, P.; Lin, X. Bis (2,3-dibromo-4,5-dihydroxybenzyl) ether, a marine algae derived bromophenol, inhibits the growth of botrytis cinerea and interacts with DNA molecules. Mar. Drugs 2014, 12, 3838–3851. [Google Scholar] [CrossRef]
- Park, H.-J.; Kurokawa, M.; Shiraki, K.; Nakamura, N.; Choi, J.-S.; Hattori, M. Antiviral activity of the marine alga Symphyocladia latiuscula against Herpes simplex virus (HSV-1) in Vitro and its therapeutic efficacy against HSV-1 infection in mice. Biol. Pharm. Bull. 2005, 28, 2258–2262. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Kim, S.R.; Oh, M.J.; Jung, S.J.; Kang, S.Y. In Vitro antiviral activity of red alga, Polysiphonia morrowii extract and its bromophenols against fish pathogenic infectious hematopoietic necrosis virus and infectious pancreatic necrosis virus. J. Microbiol. 2011, 49, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, T.H.; Ji, H.L.; Chae, C.S.; Chung, S.C.; Shin, D.S.; Shin, J.; Oh, K.B. Inhibition of the pathogenicity of Magnaporthe grisea by bromophenols, isocitrate lyase inhibitors, from the red alga Odonthalia corymbifera. J. Agric. Food Chem. 2007, 55, 6923–6928. [Google Scholar] [CrossRef]
- Jeyaprakash, R.R.K. HPLC Analysis of flavonoids in acanthophora specifera (red seaweed) collected from Gulf of Mannar, Tamilnadu, India. Int. J. Sci. Res. 2017, 6, 69–72. [Google Scholar]
- Bilanglod, A.; Petthongkhao, S.; Churngchow, N. Phenolic and flavonoid contents isolated from the red seaweed, Acanthophora spicifera. In Proceedings of the 31st Annual Meeting of the Thai Society for Biotechnology and International Conference (TSB2019), Phuket, Thailand, 10–12 November 2019; pp. 189–196. [Google Scholar]
- Ben Saad, H.; Gargouri, M.; Kallel, F.; Chaabene, R.; Boudawara, T.; Jamoussi, K.; Magné, C.; Mounir Zeghal, K.; Hakim, A.; Ben Amara, I. Flavonoid compounds from the red marine alga Alsidium corallinum protect against potassium bromate-induced nephrotoxicity in adult mice. Env. Toxicol. 2017, 32, 1475–1486. [Google Scholar] [CrossRef]
- Davyt, D.; Fernandez, R.; Suescun, L.; Mombrú, A.W.; Saldaña, J.; Domínguez, L.; Coll, J.; Fujii, M.T.; Manta, E. New sesquiterpene derivatives from the red alga Laurencia scoparia. Isolation, structure determination, and anthelmintic activity. J. Nat. Prod. 2001, 64, 1552–1555. [Google Scholar] [CrossRef]
- Stout, E.P.; Prudhomme, J.; Le Roch, K.; Fairchild, C.R.; Franzblau, S.G.; Aalbersberg, W.; Hay, M.E.; Kubanek, J. Unusual antimalarial meroditerpenes from tropical red macroalgae. Bioorg. Med. Chem. Lett. 2010, 20, 5662–5665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makkar, F.; Chakraborty, K. Highly oxygenated antioxidative 2H-chromen derivative from the red seaweed Gracilaria opuntia with pro-inflammatory cyclooxygenase and lipoxygenase inhibitory properties. Nat. Prod. Res. 2018, 32, 2756–2765. [Google Scholar] [CrossRef] [PubMed]
- Guihéneuf, F.; Gietl, A.; Stengel, D.B. Temporal and spatial variability of mycosporine-like amino acids and pigments in three edible red seaweeds from western Ireland. J. Appl. Phycol. 2018, 30, 2573–2586. [Google Scholar] [CrossRef]
- Torres, P.; Santos, J.P.; Chow, F.; Pena Ferreira, M.J.; dos Santos, D.Y.A.C. Comparative analysis of in vitro antioxidant capacities of mycosporine-like amino acids (MAAs). Algal Res. 2018, 34, 57–67. [Google Scholar] [CrossRef]
- Athukorala, Y.; Trang, S.; Kwok, C.; Yuan, Y. Antiproliferative and antioxidant activities and mycosporine-like amino acid profiles of wild-harvested and cultivated edible canadian marine red macroalgae. Molecules 2016, 21, 119. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.V.; Westcott, N.D.; Hu, C.; Kitts, D.D. Mycosporine-like amino acid composition of the edible red alga, Palmaria palmata (dulse) harvested from the west and east coasts of Grand Manan Island, New Brunswick. Food Chem. 2009, 112, 321–328. [Google Scholar] [CrossRef]
- Reef, R.; Kaniewska, P.; Hoegh-Guldberg, O. Coral skeletons defend against ultraviolet radiation. PLoS ONE 2009, 4, e7995. [Google Scholar] [CrossRef] [Green Version]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Bioactive components from seaweeds. In Advances in Botanical Research; Bourgougnon, N., Ed.; Academic Press Inc.: Cambridge, MA, USA, 2014; Volume 71, pp. 345–378. [Google Scholar]
- Ying, R.; Zhang, Z.; Zhu, H.; Li, B.; Hou, H. The protective effect of mycosporine-like amino acids (MAAs) from Porphyra yezoensis in a mouse model of UV irradiation-induced photoaging. Mar. Drugs 2019, 17, 470. [Google Scholar] [CrossRef] [Green Version]
- Suh, S.S.; Oh, S.K.; Lee, S.G.; Kim, I.C.; Kim, S. Porphyra-334, a mycosporine-like amino acid, attenuates UV-induced apoptosis in HaCaT cells. Acta Pharm. 2017, 67, 257–264. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, K.P.; Long, P.F.; Young, A.R. Mycosporine-Like Amino Acids for Skin Photoprotection. Curr. Med. Chem. 2017, 25, 5512–5527. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Alilou, M.; Gelbrich, T.; Planchenault, P.; Derbré, S.; Schinkovitz, A.; Richomme, P.; Hensel, A.; Ganzera, M. Absolute configuration of mycosporine-like amino acids, their wound healing properties and in vitro anti-aging effects. Mar. Drugs 2020, 18, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, K.; Hartmann, A.; Ganzera, M.; Fuchs, D.; Gostner, J.M. Immunomodulatory effects of the mycosporine-like amino acids shinorine and porphyra-334. Mar. Drugs 2016, 14, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.A.; Lee, K.H.; Chae, S.; Koh, Y.S.; Yoo, B.S.; Kim, J.H.; Ham, Y.M.; Baik, J.S.; Lee, N.H.; Hyun, J.W. Triphlorethol-A from Ecklonia cava protects V79-4 lung fibroblast against hydrogen peroxide induced cell damage. Free Radic. Res. 2005, 39, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Béress, A.; Wassermann, O.; Bruhn, T.; Béress, L.; Kraiselburd, E.N.; Gonzalez, L.V.; de Motta, G.E.; Chavez, P.I. A new procedure for the isolation of anti-HIV compounds (polysaccharides and polyphenols) from the marine alga Fucus vesiculosus. J. Nat. Prod. 1993, 56, 478–488. [Google Scholar] [CrossRef]
- Eom, S.-H.; Moon, S.-Y.; Lee, D.-S.; Kim, H.-J.; Park, K.; Lee, E.-W.; Kim, T.H.; Chung, Y.-H.; Lee, M.-S.; Kim, Y.-M. In vitro antiviral activity of dieckol and phlorofucofuroeckol-A isolated from edible brown alga Eisenia bicyclis against murine norovirus. ALGAE 2015, 30, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.; Kim, E.; Moon, S.; Choi, J.-D.; Lee, M.-S.; Kim, Y.-M. Phaeophyta Extracts Exhibit Antiviral Activity against Feline Calicivirus. Fish. Aquat. Sci. 2014, 17, 155–158. [Google Scholar] [CrossRef] [Green Version]
- Shibata, T.; Ishimaru, K.; Kawaguchi, S.; Yoshikawa, H.; Hama, Y. Antioxidant activities of phlorotannins isolated from Japanese Laminariaceae. J. Appl. Phycol. 2008, 20, 705–711. [Google Scholar] [CrossRef]
- Besednova, N.N.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Makarenkova, I.D.; Smolina, T.P.; Fedyanina, L.N.; Kryzhanovsky, S.P.; Zaporozhets, T.S. Marine Algae Metabolites as Promising Therapeutics for the Prevention and Treatment of HIV/AIDS. Metabolites 2019, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Kim, A.R.; Lee, M.S.; Shin, T.S.; Hua, H.; Jang, B.C.; Choi, J.S.; Byun, D.S.; Utsuki, T.; Ingram, D.; Kim, H.R. Phlorofucofuroeckol A inhibits the LPS-stimulated iNOS and COX-2 expressions in macrophages via inhibition of NF-κB, Akt, and p38 MAPK. Toxicol. Vitr. 2011, 25, 1789–1795. [Google Scholar] [CrossRef]
- Chang, M.Y.; Byon, S.H.; Shin, H.C.; Han, S.E.; Kim, J.Y.; Byun, J.Y.; Lee, J.D.; Park, M.K. Protective effects of the seaweed phlorotannin polyphenolic compound dieckol on gentamicin-induced damage in auditory hair cells. Int. J. Pediatr. Otorhinolaryngol. 2016, 83, 31–36. [Google Scholar] [CrossRef]
- Kong, C.S.; Kim, J.A.; Yoon, N.Y.; Kim, S.K. Induction of apoptosis by phloroglucinol derivative from Ecklonia Cava in MCF-7 human breast cancer cells. Food Chem. Toxicol. 2009, 47, 1653–1658. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-K.; Tang, Y.; Kim, Y.-S.; Hwang, J.-W.; Choi, E.-J.; Lee, J.-H.; Lee, S.-H.; Jeon, Y.-J.; Park, P.-J. First Evidence that Ecklonia cava-Derived Dieckol Attenuates MCF-7 Human Breast Carcinoma Cell Migration. Mar. Drugs 2015, 13, 1785–1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, J.H.; Yang, Y.I.; Lee, K.T.; Choi, J.H. Dieckol, isolated from the edible brown algae Ecklonia cava, induces apoptosis of ovarian cancer cells and inhibits tumor xenograft growth. J. Cancer Res. Clin. Oncol. 2014, 141, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Park, J.H.; Park, S.A.; Joo, N.R.; Lee, B.H.; Lee, K.B.; Oh, S.M. Dieckol or phlorofucofuroeckol extracted from Ecklonia cava suppresses lipopolysaccharide-mediated human breast cancer cell migration and invasion. J. Appl. Phycol. 2020, 32, 631–640. [Google Scholar] [CrossRef]
- LI, Y.; Qian, Z.-J.; Kim, M.-M.; Kim, S.-K. Cytotoxic activities of phlorethol and fucophlorethol derivates isolated from Laminariaceae Ecklonia cava. J. Food Biochem. 2011, 35, 357–369. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Jin, L.; Zhao, Y.; Zhu, G.; Shen, W. Dieckol inhibits non-small–cell lung cancer cell proliferation and migration by regulating the PI3K/AKT signaling pathway. J. Biochem. Mol. Toxicol. 2019, 33. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Zhou, W.; Zhao, S.; Li, S.; Yan, D.; Wang, J. Eckol inhibits Reg3A-induced proliferation of human SW1990 pancreatic cancer cells. Exp. Med. 2019, 18, 2825–2832. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.S.; Kasin Yadunandam, A.; Kim, S.J.; Woo, H.C.; Kim, H.R.; Kim, G. Do Dieckol, isolated from Ecklonia stolonifera, induces apoptosis in human hepatocellular carcinoma Hep3B cells. J. Nat. Med. 2013, 67, 519–527. [Google Scholar] [CrossRef]
- Moon, C.; Kim, S.-H.; Kim, J.-C.; Hyun, J.W.; Lee, N.H.; Park, J.W.; Shin, T. Protective effect of phlorotannin components phloroglucinol and eckol on radiation-induced intestinal injury in mice. Phyther. Res. 2008, 22, 238–242. [Google Scholar] [CrossRef]
- Park, E.; Lee, N.H.; Joo, H.G.; Jee, Y. Modulation of apoptosis of eckol against ionizing radiation in mice. Biochem. Biophys. Res. Commun. 2008, 372, 792–797. [Google Scholar] [CrossRef]
- Zhang, R.; Kang, K.A.; Piao, M.J.; Ko, D.O.; Wang, Z.H.; Lee, I.K.; Kim, B.J.; Jeong, I.Y.; Shin, T.; Park, J.W.; et al. Eckol protects V79-4 lung fibroblast cells against γ-ray radiation-induced apoptosis via the scavenging of reactive oxygen species and inhibiting of the c-Jun NH2-terminal kinase pathway. Eur. J. Pharm. 2008, 591, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Ahn, G.; Yun, J.S.; Kim, M.J.; Bing, S.J.; Kim, D.S.; Lee, J.; Lee, N.H.; Park, J.W.; Jee, Y. Dieckol rescues mice from lethal irradiation by accelerating hemopoiesis and curtailing immunosuppression. Int. J. Radiat. Biol. 2010, 86, 848–859. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.A.; Zhang, R.; Lee, K.H.; Chae, S.; Kim, B.J.; Kwak, Y.S.; Park, J.W.; Lee, N.H.; Hyun, J.W. Protective Effect of Triphlorethol-A from Ecklonia cava against Ionizing Radiation in vitro. J. Radiat. Res. 2006, 47, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Zheng, Y.; Zhou, J.; Geng, Y.; Zou, P.; Li, Y.; Zhang, C. Polyphenol-Rich Extracts from Brown Macroalgae Lessonia trabeculata Attenuate Hyperglycemia and Modulate Gut Microbiota in High-Fat Diet and Streptozotocin-Induced Diabetic Rats. J. Agric. Food Chem. 2019, 67, 12472–12480. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.; Andrade, P.; Valentão, P. Phlorotannins: Towards New Pharmacological Interventions for Diabetes Mellitus Type 2. Molecules 2016, 22, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kellogg, J.; Grace, M.; Lila, M. Phlorotannins from Alaskan Seaweed Inhibit Carbolytic Enzyme Activity. Mar. Drugs 2014, 12, 5277–5294. [Google Scholar] [CrossRef]
- Husni, A.; Wijayanti, R. Ustadi Inhibitory activity of α-Amylase and α-Glucosidase by Padina pavonica extracts. J. Biol. Sci. 2014, 14, 515–520. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Park, M.H.; Heo, S.J.; Kang, S.M.; Ko, S.C.; Han, J.S.; Jeon, Y.J. Dieckol isolated from Ecklonia cava inhibits α-glucosidase and α-amylase in vitro and alleviates postprandial hyperglycemia in streptozotocin-induced diabetic mice. Food Chem. Toxicol. 2010, 48, 2633–2637. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, S.-K. Biological Phlorotannins of Eisenia bicyclis. In Marine Algae Extracts; Kim, S., Chojnacka, K., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; Volume 2, pp. 453–464. [Google Scholar]
- Moon, H.E.; Islam, M.N.; Ahn, B.R.; Chowdhury, S.S.; Sohn, H.S.; Jung, H.A.; Choi, J.S. Protein tyrosine phosphatase 1B and α-glucosidase inhibitory phlorotannins from edible brown algae, Ecklonia stolonifera and Eisenia bicyclis. Biosci. Biotechnol. Biochem. 2011, 75, 1472–1480. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Ko, S.C.; Kang, M.C.; Lee, D.H.; Jeon, Y.J. Octaphlorethol A, a marine algae product, exhibits antidiabetic effects in type 2 diabetic mice by activating AMP-activated protein kinase and upregulating the expression of glucose transporter 4. Food Chem. Toxicol. 2016, 91, 58–64. [Google Scholar] [CrossRef]
- Kawamura-Konishi, Y.; Watanabe, N.; Saito, M.; Nakajima, N.; Sakaki, T.; Katayama, T.; Enomoto, T. Isolation of a new phlorotannin, a potent inhibitor of carbohydrate-hydrolyzing enzymes, from the brown alga Sargassum patens. J. Agric. Food Chem. 2012, 60, 5565–5570. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Son, M.; Choi, J.; Choi, C.H.; Park, K.Y.; Son, K.H.; Byun, K. Phlorotannins from Ecklonia cava Attenuates Palmitate-Induced Endoplasmic Reticulum Stress and Leptin Resistance in Hypothalamic Neurons. Mar. Drugs 2019, 17, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.H.; Heo, S.J.; Park, P.J.; Moon, S.H.; Sung, S.H.; Jeon, B.T.; Lee, S.H. 6,6′-Bieckol isolated from Ecklonia cava protects oxidative stress through inhibiting expression of ROS and proinflammatory enzymes in high-glucose-induced human umbilical vein endothelial cells. Appl. Biochem. Biotechnol. 2014, 174, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Heo, S.J.; Kim, K.N.; Ahn, G.; Park, P.J.; Moon, S.H.; Jeon, B.T.; Lee, S.H. 6,6′-Bieckol protects insulinoma cells against high glucose-induced glucotoxicity by reducing oxidative stress and apoptosis. Fitoterapia 2015, 106, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.J.; Yoon, K.D.; Min, S.Y.; Lee, J.S.; Kim, J.H.; Kim, T.G.; Kim, S.H.; Kim, N.G.; Huh, H.; Kim, J. Inhibition of HIV-1 reverse transcriptase and protease by phlorotannins from the brown alga Ecklonia cava. Biol. Pharm. Bull. 2004. [Google Scholar] [CrossRef] [Green Version]
- Artan, M.; Li, Y.; Karadeniz, F.; Lee, S.H.; Kim, M.M.; Kim, S.K. Anti-HIV-1 activity of phloroglucinol derivative, 6,6′-bieckol, from Ecklonia cava. Bioorg. Med. Chem. 2008, 16, 7921–7926. [Google Scholar] [CrossRef] [PubMed]
- Karadeniz, F.; Kang, K.-H.; Park, J.W.; Park, S.-J.; Kim, S.-K. Anti-HIV-1 activity of phlorotannin derivative 8,4‴-dieckol from Korean brown alga Ecklonia cava. Biosci. Biotechnol. Biochem. 2014, 78, 1151–1158. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.H.; Kwon, J.M.; Kwon, H.J.; Jeong, H.J.; Kim, Y.M.; Kim, D.; Lee, W.S.; Ryu, Y.B. Dieckol, a SARS-CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorg. Med. Chem. 2013, 21, 3730–3737. [Google Scholar] [CrossRef]
- Ryu, Y.B.; Jeong, H.J.; Yoon, S.Y.; Park, J.Y.; Kim, Y.M.; Park, S.J.; Rho, M.C.; Kim, S.J.; Lee, W.S. Influenza virus neuraminidase inhibitory activity of phlorotannins from the edible brown alga Ecklonia cava. J. Agric. Food Chem. 2011, 59, 6467–6473. [Google Scholar] [CrossRef]
- Gentile, D.; Patamia, V.; Scala, A.; Sciortino, M.T.; Piperno, A.; Rescifina, A. Putative Inhibitors of SARS-CoV-2 Main Protease from A Library of Marine Natural Products: A Virtual Screening and Molecular Modeling Study. Mar. Drugs 2020, 18, 225. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.; Kwak, J. Antiviral phlorotannin from Eisenia bicyclis against human papilloma virus in vitro. Planta Med. 2015, 81, PW_22. [Google Scholar] [CrossRef]
- Lee, M.H.; Lee, K.B.; Oh, S.M.; Lee, B.H.; Chee, H.Y. Antifungal activities of dieckol isolated from the marine brown alga Ecklonia cava against Trichophyton rubrum. J. Appl. Biol. Chem. 2010, 53, 504–507. [Google Scholar] [CrossRef]
- Choi, J.-G.; Kang, O.-H.; Brice, O.-O.; Lee, Y.-S.; Chae, H.-S.; Oh, Y.-C.; Sohn, D.-H.; Park, H.; Choi, H.-G.; Kim, S.-G.; et al. Antibacterial Activity of Ecklonia cava Against Methicillin-Resistant Staphylococcus aureus and Salmonella spp. Foodborne Pathog. Dis. 2010, 7, 435–441. [Google Scholar] [CrossRef]
- Lee, D.S.; Kang, M.S.; Hwang, H.J.; Eom, S.H.; Yang, J.Y.; Lee, M.S.; Lee, W.J.; Jeon, Y.J.; Choi, J.S.; Kim, Y.M. Synergistic effect between dieckol from Ecklonia stolonifera and β-lactams against methicillin-resistant Staphylococcus aureus. Biotechnol. Bioprocess Eng. 2008, 13, 758–764. [Google Scholar] [CrossRef]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, B.M.; Li, Y.; Qian, Z.J.; Kim, M.M.; Kim, S.K. Differentiation of human osteosarcoma cells by isolated phlorotannins is subtly linked to COX-2, iNOS, MMPs, and MAPK signaling: Implication for chronic articular disease. Chem. Biol. Interact. 2009, 179, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Ahn, B.-N.; Karadeniz, F.; Kim, J.-A.; Lee, J.I.; Seo, Y.; Kong, C.-S. Phlorofucofuroeckol A from Edible Brown Alga Ecklonia Cava Enhances Osteoblastogenesis in Bone Marrow-Derived Human Mesenchymal Stem Cells. Mar. Drugs 2019, 17, 543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Youn, K.; Kim, D.; Ahn, M.-R.; Yoon, E.; Kim, O.-Y.; Jun, M. Anti-Neuroinflammatory Property of Phlorotannins from Ecklonia cava on Aβ25-35-Induced Damage in PC12 Cells. Mar. Drugs 2018, 17, 7. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.A.; Kim, J.-I.; Choung, S.Y.; Choi, J.S. Protective effect of the edible brown alga Ecklonia stolonifera on doxorubicin-induced hepatotoxicity in primary rat hepatocytes. J. Pharm. Pharm. 2014, 66, 1180–1188. [Google Scholar] [CrossRef]
- Lee, J.; Jun, M. Dual BACE1 and Cholinesterase Inhibitory Effects of Phlorotannins from Ecklonia cava—An In Vitro and In Silico Study. Mar. Drugs 2019, 17, 91. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zheng, J.; Huang, C.; Zhao, J.; Lin, J.; Zhou, X.; Naman, C.B.; Wang, N.; Gerwick, W.H.; Wang, Q.; et al. Eckmaxol, a Phlorotannin Extracted from Ecklonia maxima, Produces Anti-β-amyloid Oligomer Neuroprotective Effects Possibly via Directly Acting on Glycogen Synthase Kinase 3β. Acs Chem. Neurosci. 2018, 9, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.H.; Paudel, P.; Choi, J.-W.; Ahn, D.H.; Nam, T.-J.; Jung, H.A.; Choi, J.S. Probing Multi-Target Action of Phlorotannins as New Monoamine Oxidase Inhibitors and Dopaminergic Receptor Modulators with the Potential for Treatment of Neuronal Disorders. Mar. Drugs 2019, 17, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, S.Y.; Choi, J.S.; Byun, D.S. Inhibitory effects of phloroglucinol derivatives isolated from Ecklonia stolonifera on FcεRI expression. Bioorg. Med. Chem. 2009, 17, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Ahn, G.; Amagai, Y.; Matsuda, A.; Kang, S.-M.; Lee, W.; Jung, K.; Oida, K.; Jang, H.; Ishizaka, S.; Matsuda, K.; et al. Dieckol, a phlorotannin of Ecklonia cava, suppresses IgE-mediated mast cell activation and passive cutaneous anaphylactic reaction. Exp. Derm. 2015, 24, 968–970. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Lee, J.; Lee, H.; Kim, B.; Lew, J.; Baek, N.; Kim, S.H. MicroRNA134 Mediated Upregulation of JNK and Downregulation of NFkB Signalings Are Critically Involved in Dieckol Induced Antihepatic Fibrosis. J. Agric. Food Chem. 2016, 64, 5508–5514. [Google Scholar] [CrossRef]
- Jung, H.A.; Hyun, S.K.; Kim, H.R.; Choi, J.S. Angiotensin-converting enzyme I inhibitory activity of phlorotannins from Ecklonia stolonifera. Fish. Sci. 2006, 72, 1292–1299. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Ko, S.C.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on angiotensin I-converting enzyme (ACE) inhibitory activity. Nutr. Res. Pract. 2011, 5, 93–100. [Google Scholar] [CrossRef] [Green Version]
- Kurihara, H.; Konno, R.; Takahashi, K. Fucophlorethol C, a phlorotannin as a lipoxygenase inhibitor. Biosci. Biotechnol. Biochem. 2015, 79, 1954–1956. [Google Scholar] [CrossRef] [Green Version]
- Ryu, B.M.; Ahn, B.N.; Kang, K.H.; Kim, Y.S.; Li, Y.X.; Kong, C.S.; Kim, S.K.; Kim, D.G. Dioxinodehydroeckol protects human keratinocyte cells from UVB-induced apoptosis modulated by related genes Bax/Bcl-2 and caspase pathway. J. Photochem. Photobiol. B Biol. 2015, 153, 352–357. [Google Scholar] [CrossRef]
- Shibata, T.; Fujimoto, K.; Nagayama, K.; Yamaguchi, K.; Nakamura, T. Inhibitory activity of brown algal phlorotannins against hyaluronidase. Int. J. Food Sci. Technol. 2002, 37, 703–709. [Google Scholar] [CrossRef]
- Kang, H.S.; Kim, H.R.; Byun, D.S.; Son, B.W.; Nam, T.J.; Choi, J.S. Tyrosinase inhibitors isolated from the edible brown alga Ecklonia stolonifera. Arch. Pharm. Res. 2004, 27, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Yoon, N.Y.; Eom, T.K.; Kim, M.M.; Kim, S.K. Inhibitory effect of phlorotannins isolated from Ecklonia cava on mushroom tyrosinase activity and melanin formation in mouse B16F10 melanoma cells. J. Agric. Food Chem. 2009, 57, 4124–4129. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kang, S.M.; Sok, C.H.; Hong, J.T.; Oh, J.Y.; Jeon, Y.J. Cellular activities and docking studies of eckol isolated from Ecklonia cava (Laminariales, Phaeophyceae) as potential tyrosinase inhibitor. Algae 2015, 30, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Heo, S.J.; Ko, S.C.; Cha, S.H.; Kang, D.H.; Park, H.S.; Choi, Y.U.; Kim, D.; Jung, W.K.; Jeon, Y.J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Manandhar, B.; Wagle, A.; Seong, S.H.; Paudel, P.; Kim, H.R.; Jung, H.A.; Choi, J.S. Phlorotannins with Potential Anti-tyrosinase and Antioxidant Activity Isolated from the Marine Seaweed Ecklonia stolonifera. Antioxidants 2019, 8, 240. [Google Scholar] [CrossRef] [Green Version]
- Bak, S.S.; Sung, Y.K.; Kim, S.K. 7-Phloroeckol promotes hair growth on human follicles in vitro. Naunyn. Schmiedebergs. Arch. Pharm. 2014, 387, 789–793. [Google Scholar] [CrossRef]
- Xi, M.; Dragsted, L.O. Biomarkers of seaweed intake. Genes Nutr. 2019, 14, 24. [Google Scholar] [CrossRef] [Green Version]
- Nagayama, K.; Shibata, T.; Fujimoto, K.; Honjo, T.; Nakamura, T. Algicidal effect of phlorotannins from the brown alga Ecklonia kurome on red tide microalgae. Aquaculture 2003, 218, 601–611. [Google Scholar] [CrossRef]
- Kwon, H.J.; Ryu, Y.B.; Kim, Y.M.; Song, N.; Kim, C.Y.; Rho, M.C.; Jeong, J.H.; Cho, K.O.; Lee, W.S.; Park, S.J. In vitro antiviral activity of phlorotannins isolated from Ecklonia cava against porcine epidemic diarrhea coronavirus infection and hemagglutination. Bioorg. Med. Chem. 2013, 21, 4706–4713. [Google Scholar] [CrossRef]
- Hussain, E.; Wang, L.J.; Jiang, B.; Riaz, S.; Butt, G.Y.; Shi, D.Y. A review of the components of brown seaweeds as potential candidates in cancer therapy. RSC Adv. 2016, 6, 12592–12610. [Google Scholar] [CrossRef]
- Xu, X.; Song, F.; Wang, S.; Li, S.; Xiao, F.; Zhao, J.; Yang, Y.; Shang, S.; Yang, L.; Shi, J. Dibenzyl bromophenols with diverse dimerization patterns from the brown alga Leathesia nana. J. Nat. Prod. 2004, 67, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Li, J.; Guo, S.; Su, H.; Fan, X. The antitumor effect of bromophenol derivatives in vitro and Leathesia nana extract in vivo. Chin. J. Oceanol. Limnol. 2009, 27, 277–282. [Google Scholar] [CrossRef]
- Shi, D.; Li, J.; Guo, S.; Han, L. Antithrombotic effect of bromophenol, the alga-derived thrombin inhibitor. J. Biotechnol. 2008, 136, S579. [Google Scholar] [CrossRef]
- Karuppaiah, J.; Dhanraj, V.; Karuppaiah, J.; Balakrishnan, R.; Elangovan, N. Myricetin attenuates neurodegeneration and cognitive impairment in Parkinsonism. Front. Biosci. 2018, 10, 835. [Google Scholar] [CrossRef]
- Mokrini, R.; Ben Mesaoud, M.; Daoudi, M.; Hellio, C.; Maréchal, J.P.; El Hattab, M.; Ortalo-Magné, A.; Piovetti, L.; Culioli, G. Meroditerpenoids and derivatives from the brown alga Cystoseira baccata and their antifouling properties. J. Nat. Prod. 2008, 71, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Gerald Culioli, A.O.M.; Valls, R.; Hellio, C.; Clare, A.S.; Piovetti, L. Antifouling activity of meroditerpenoids from the marine brown alga Halidrys siliquosa. J. Nat. Prod. 2008, 71, 1121–1126. [Google Scholar] [CrossRef]
- Sabry, O.M.M.; Andrews, S.; McPhail, K.L.; Goeger, D.E.; Yokochi, A.; LePage, K.T.; Murray, T.F.; Gerwick, W.H. Neurotoxic meroditerpenoids from the tropical marine brown alga Stypopodium flabelliforme. J. Nat. Prod. 2005, 68, 1022–1030. [Google Scholar] [CrossRef]
- Pereira, D.M.; Cheel, J.; Areche, C.; San-Martin, A.; Rovirosa, J.; Silva, L.R.; Valentao, P.; Andrade, P.B. Anti-proliferative activity of meroditerpenoids isolated from the brown alga Stypopodium flabelliforme against several cancer cell lines. Mar. Drugs 2011, 9, 852–862. [Google Scholar] [CrossRef]
- Mendes, G.; Soares, A.R.; Sigiliano, L.; Machado, F.; Kaiser, C.; Romeiro, N.; Gestinari, L.; Santos, N.; Romanos, M.T.V. In vitro anti-HMPV activity of meroditerpenoids from marine alga Stypopodium zonale (Dictyotales). Molecules 2011, 16, 8437–8450. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, H.; Koyama, T.; Yamada, S.; Lipton, S.A.; Satoh, T. Zonarol, a sesquiterpene from the brown algae Dictyopteris undulata, provides neuroprotection by activating the Nrf2/ARE pathway. Biochem. Biophys. Res. Commun. 2015, 457, 718–722. [Google Scholar] [CrossRef] [Green Version]
- Ali, Y.; Kim, D.H.; Seong, S.H.; Kim, H.R.; Jung, H.A.; Choi, J.S. α-Glucosidase and protein tyrosine phosphatase 1b inhibitory activity of plastoquinones from marine brown alga Sargassum serratifolium. Mar. Drugs 2017, 15, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The potential of seaweeds as a source of functional ingredients of prebiotic and antioxidant value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, K.H.; Cheung, P.C.K. Nutritional evaluation of some subtropical red and green seaweeds Part II. In vitro protein digestibility and amino acid profiles of protein concentrates. Food Chem. 2001, 72, 11–17. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Nutritional quality of some wild and cultivated seaweeds: Nutrient composition, total phenolic content and in vitro digestibility. J. Appl. Phycol. 2016, 28, 3575–3585. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Apostolidis, E.; Kwon, Y.I.; Shetty, K. Inhibition of Listeria monocytogenes by oregano, cranberry and sodium lactate combination in broth and cooked ground beef systems and likely mode of action through proline metabolism. Int. J. Food Microbiol. 2008, 128, 317–324. [Google Scholar] [CrossRef]
- Lin, Y.T.; Labbe, R.G.; Shetty, K. Inhibition of Listeria monocytogenes in fish and meat systems by use of oregano and cranberry phytochemical synergies. Appl. Env. Microbiol. 2004, 70, 5672–5678. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Jónsdóttir, R.; Kristinsson, H.G.; Thorkelsson, G.; Jacobsen, C.; Hamaguchi, P.Y.; Ólafsdóttir, G. Inhibition of haemoglobin-mediated lipid oxidation in washed cod muscle and cod protein isolates by Fucus vesiculosus extract and fractions. Food Chem. 2010, 123, 321–330. [Google Scholar] [CrossRef]
- Eom, S.H.; Kim, Y.M.; Kim, S.K. Antimicrobial effect of phlorotannins from marine brown algae. Food Chem. Toxicol. 2012, 50, 3251–3255. [Google Scholar] [CrossRef]
- Whitfield, F.B.; Helidoniotis, F.; Shaw, K.J.; Svoronos, D. Distribution of Bromophenols in Species of Marine Algae from Eastern Australia. J. Agric. Food Chem. 1999, 47, 2367–2373. [Google Scholar] [CrossRef]
- Origin of SEANOL. Available online: http://seanolinstitute.org/ssc/origin.html (accessed on 30 April 2020).
- Yeo, A.R.; Lee, J.; Tae, I.H.; Park, S.R.; Cho, Y.H.; Lee, B.H.; Cheol Shin, H.; Kim, S.H.; Yoo, Y.C. Anti-hyperlipidemic effect of polyphenol extract (SeapolynolTM) and dieckol isolated from Ecklonia cava in in vivo and in vitro models. Prev. Nutr. Food Sci. 2012, 17, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.-S.; Lee, D.-H.; Kim, T.-J.; Shin, H.-C.; Jeon, H.-K. Cardioprotective Effects of a Phlorotannin Extract Against Doxorubicin-Induced Cardiotoxicity in a Rat Model. J. Med. Food 2017, 20, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.-J.; Yoon, K.-Y.; Koh, E.-J.; Choi, J.; Kim, K.-J.; Choi, H.-S.; Lee, B.-Y. Seapolynol and Dieckol Improve Insulin Sensitivity through the Regulation of the PI3K Pathway in C57BL/KsJ-db/db Mice. J. Food Nutr. Res. 2015, 3, 648–652. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.-J.; Choi, H.-S.; Lee, Y.-J.; Hwang, J.-H.; Lee, O.-H.; Seo, M.-J.; Kim, K.-J.; Lee, B.-Y. Seapolynol Extracted from Ecklonia cava Inhibits Adipocyte Differentiation in Vitro and Decreases Fat Accumulation in Vivo. Molecules 2015, 20, 21715–21731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turck, D.; Bresson, J.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of Ecklonia cava phlorotannins as a novel food pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15. [Google Scholar] [CrossRef]
- Hwang, H.J. Skin Elasticity and Sea Polyphenols. Seanol Sci. Cent. Rev. 2010, 1, 1–10. [Google Scholar]
- Pimentel, F.; Alves, R.; Rodrigues, F.; Oliveira, M.P.P. Macroalgae-Derived Ingredients for Cosmetic Industry—An Update. Cosmetics 2017, 5, 2. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L. Seaweeds as Source of Bioactive Substances and Skin Care Therapy—Cosmeceuticals, Algotheraphy, and Thalassotherapy. Cosmetics 2018, 5, 68. [Google Scholar] [CrossRef] [Green Version]
- Cosmetics Ingredients Database | Online Raw Materials Search. Available online: https://cosmetics.specialchem.com/ (accessed on 9 April 2020).
- Default Web Site Page. Available online: https://sealgae.pt/ (accessed on 9 April 2020).
- Exclusive Worldwide Licence—Aethic. Available online: https://aethic.com/aethic-granted-exclusive-worldwide-license-use-seaweed-compound/ (accessed on 13 April 2020).
- Aethic Wins Exclusive License to Use Novel Seaweed Compound. Available online: https://www.cosmeticsbusiness.com/news/article_page/Aethic_wins_exclusive_license_to_use_novel_seaweed_compound/132704 (accessed on 13 April 2020).
- 5 Best Eco-friendly Suncreens | The Independent. Available online: https://www.independent.co.uk/extras/indybest/fashion-beauty/sun-care-tanning/best-eco-friendly-sunscreen-natural-organic-mineral-for-face-baby-sensitive-skin-a8340881.html (accessed on 13 April 2020).
- ECKLEXT® for Natural Cosmetic Ingredients, NOF EUROPE GmbH. Available online: https://nofeurope.com/index.php?dispatch=categories.view&category_id=270 (accessed on 10 April 2020).
- Ingredients & Formulas | SEPPIC. Available online: https://www.seppic.com/ingredients-formulas (accessed on 13 April 2020).
- The Garden of Naturalsolution. Available online: http://www.naturalsolution.co.kr/eng/home.php (accessed on 10 April 2020).
- Green Confertii Extract-NS—The Garden of Naturalsolution—Datasheet. Available online: https://cosmetics.specialchem.com/product/i-natural-solution-green-confertii-extract-ns (accessed on 10 April 2020).
- Bladderwrack Extract-NS—The Garden of Naturalsolution—Datasheet. Available online: https://cosmetics.specialchem.com/product/i-natural-solution-bladderwrack-extract-ns (accessed on 10 April 2020).
- DE10259966A1—Cosmetic or Pharmaceutical Composition Containing Mycosporin-Like Amino Acids, Useful for Treatment and Prevention of Hypoxia and Associated Conditions, Improves Oxygen Uptake—Google Patents. Available online: https://patents.google.com/patent/DE10259966A1/en?q=mycosporine-like+amino+acid&oq=mycosporine-like+amino+acid&page=2 (accessed on 13 April 2020).
- GB2472021A—Cosmetic Sunscreen Composition—Google Patents. Available online: https://patents.google.com/patent/GB2472021A/en?q=mycosporine-like+amino+acid&oq=mycosporine-like+amino+acid&page=2 (accessed on 13 April 2020).
- EP1473028A1—Cosmetic Skin Care Products and Cosmetic Agents for Protecting Skin Against Premature Aging—Google Patents. Available online: https://patents.google.com/patent/EP1473028A1/en?q=mycosporine-like+amino+acid&oq=mycosporine-like+amino+acid&page=2 (accessed on 13 April 2020).
- Jarald, E.; Joshi, S.B.; Jain, D.C. Diabetes and Herbal Medicines. Iran. J. Pharm. 2008, 7, 97–106. [Google Scholar]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Freile-Pelegrín, Y.; Tasdemir, D. Seaweeds to the rescue of forgotten diseases: A review. Bot. Mar. 2019, 62, 211–226. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Croft, K.D. Dietary flavonoids: Effects on endothelial function and blood pressure. J. Sci. Food Agric. 2006, 86, 2492–2498. [Google Scholar] [CrossRef]
- Park, B.G.; Shin, W.S.; Oh, S.; Park, G.M.; Kim, N.I.; Lee, S. A novel antihypertension agent, sargachromenol D from marine brown algae, Sargassum siliquastrum, exerts dual action as an L-type Ca2+ channel blocker and endothelin A/B2 receptor antagonist. Bioorg. Med. Chem. 2017, 25, 4649–4655. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, E.; Lee, C.M. Brown Seaweed-Derived Phenolic Phytochemicals and Their Biological Activities for Functional Food Ingredients with Focus on Ascophyllum nodosum. In Handbook of Marine Macroalgae; Kim, S., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2011; pp. 356–370. [Google Scholar]
- Jung, H.A.; Oh, S.H.; Choi, J.S. Molecular docking studies of phlorotannins from Eisenia bicyclis with BACE1 inhibitory activity. Bioorg. Med. Chem. Lett. 2010, 20, 3211–3215. [Google Scholar] [CrossRef]
- Um, M.Y.; Lim, D.W.; Son, H.J.; Cho, S.; Lee, C. Phlorotannin-rich fraction from Ishige foliacea brown seaweed prevents the scopolamine-induced memory impairment via regulation of ERK-CREB-BDNF pathway. J. Funct. Foods 2018, 40, 110–116. [Google Scholar] [CrossRef]
- Catarino, M.D.; Silva, A.M.S.; Mateus, N.; Cardoso, S.M. Optimization of phlorotannins extraction from Fucus vesiculosus and evaluation of their potential to prevent metabolic disorders. Mar. Drugs 2019, 17, 162. [Google Scholar] [CrossRef] [Green Version]
- Hussain, S.P.; Hofseth, L.J.; Harris, C.C. Radical causes of cancer. Nat. Rev. Cancer 2003, 3, 276–285. [Google Scholar] [CrossRef]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.-K.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. BioFactors 2010, 36, 408–414. [Google Scholar] [CrossRef]
- Liu, E.-H.; Qi, L.-W.; Wu, Q.; Peng, Y.-B.; Li, P. Anticancer Agents Derived from Natural Products. Mini-Rev. Med. Chem. 2009, 9, 1547–1555. [Google Scholar] [CrossRef]
- Xu, N.; Fan, X.; Yan, X.; Tseng, C.K. Screening marine algae from China for their antitumor activities. J. Appl. Phycol. 2004, 16, 451–456. [Google Scholar] [CrossRef]
- Ganesan, P.; Kumar, C.S.; Bhaskar, N. Antioxidant properties of methanol extract and its solvent fractions obtained from selected Indian red seaweeds. Bioresour. Technol. 2008, 99, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K.; Ming, C.H. Antioxidant activities and phenolics content of eight species of seaweeds from north Borneo. J. Appl. Phycol. 2008, 20, 367–373. [Google Scholar] [CrossRef]
- Qin, J.; Su, H.; Zhang, Y.; Gao, J.; Zhu, L.; Wu, X.; Pan, H.; Li, X. Highly brominated metabolites from marine red alga Laurencia similis inhibit protein tyrosine phosphatase 1B. Bioorg. Med. Chem. Lett. 2010, 20, 7152–7154. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.Y.; Nam, K.A.; Kurihara, H.; Kim, S.M. Potent α-glucosidase inhibitors purified from the red alga Grateloupia elliptica. Phytochemistry 2008, 69, 2820–2825. [Google Scholar] [CrossRef]
- Roy, M.C.; Anguenot, R.; Fillion, C.; Beaulieu, M.; Bérubé, J.; Richard, D. Effect of a commercially-available algal phlorotannins extract on digestive enzymes and carbohydrate absorption in vivo. Food Res. Int. 2011, 44, 3026–3029. [Google Scholar] [CrossRef]
- Gwynn, M.N.; Portnoy, A.; Rittenhouse, S.F.; Payne, D.J. Challenges of antibacterial discovery revisited. Ann. N. Y. Acad. Sci. 2010, 1213, 5–19. [Google Scholar] [CrossRef]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef] [Green Version]
- JP2003277203A—Antibacterial Agent Based on Phlorotannins—Google Patents. Available online: https://patents.google.com/patent/JP2003277203A/en?q=phlorotannins&oq=phlorotannins (accessed on 10 April 2020).
- US20130338218A1—HIV-1 Inhibiting Pharmaceutical Composition Containing an Ecklonia Cava-Derived Phloroglucinol Polymer Compound—Google Patents. Available online: https://patents.google.com/patent/US20130338218A1/en (accessed on 29 April 2020).
- Im, J.H.; Choi, C.H.; Mun, F.; Lee, J.H.; Kim, H.; Jung, W.K.; Jang, C.H.; Kim, G.H. A polycaprolactone/fish collagen/alginate biocomposite supplemented with phlorotannin for hard tissue regeneration. RSC Adv. 2017, 7, 2009–2018. [Google Scholar] [CrossRef] [Green Version]
- Khademhosseini, A.; Du, Y.; Rajalingam, B.; Vacanti, J.P.; Langer, R.S. Microscale technologies for tissue engineering. Adv. Tissue Eng. 2008, 103, 349–369. [Google Scholar] [CrossRef]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001, 7, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.J.; Meredith, C.; Johnson, C.; Galis, Z.S. The effect of scaffold degradation rate on three-dimensional cell growth and angiogenesis. Biomaterials 2004, 25, 5735–5742. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W.; Schantz, T.; Zein, I.; Ng, K.W.; Teoh, S.H.; Tan, K.C. Mechanical properties and cell cultural response of polycaprolactone scaffolds designed and fabricated via fused deposition modeling. J. Biomed. Mater. Res. 2001, 55, 203–216. [Google Scholar] [CrossRef]
- Douglas, T.E.L.; Dokupil, A.; Reczyńska, K.; Brackman, G.; Krok-Borkowicz, M.; Keppler, J.K.; Božič, M.; Van Der Voort, P.; Pietryga, K.; Samal, S.K.; et al. Enrichment of enzymatically mineralized gellan gum hydrogels with phlorotannin-rich Ecklonia cava extract Seanol® to endow antibacterial properties and promote mineralization. Biomed. Mater. 2016, 11, 045015. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.K.; Lichtman, A.H.H.; Pillai, S. Cellular and Molecular Immunology, 8th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2014; ISBN 9780323316149. [Google Scholar]
- Kazłowska, K.; Hsu, T.; Hou, C.C.; Yang, W.C.; Tsai, G.J. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J. Ethnopharmacol. 2010, 128, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.-C.; Hwang, H.J.; Kang, K.J.; Lee, B.H. An antioxidative and antiinflammatory agent for potential treatment of osteoarthritis from Ecklonia cava. Arch. Pharm. Res. 2006, 29, 165–171. [Google Scholar] [CrossRef]
- Kwon, S.; Yoon, M.; Lee, J.; Moon, K.D.; Kim, D.; Kim, S.B.; Cho, S. A standardized phlorotannin supplement attenuates caffeine-induced sleep disruption in mice. Nutrients 2019, 11, 556. [Google Scholar] [CrossRef] [Green Version]
- Park, H.-H.; Ko, S.-C.; Oh, G.-W.; Heo, S.-J.; Kang, D.-H.; Bae, S.-Y.; Jung, W.-K. Fabrication and characterization of phlorotannins/poly (vinyl alcohol) hydrogel for wound healing application. J. Biomater. Sci. Polym. Ed. 2018, 29, 972–983. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [Green Version]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [Green Version]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Keyrouz, R.; Abasq, M.L.; Le Bourvellec, C.; Blanc, N.; Audibert, L.; Argall, E.; Hauchard, D. Total phenolic contents, radical scavenging and cyclic voltammetry of seaweeds from Brittany. Food Chem. 2011, 126, 831–836. [Google Scholar] [CrossRef] [Green Version]
- Chojnacka, K. Biologically Active Compounds in Seaweed Extracts—The Prospects for the Application. Open Conf. Proc. J. 2012, 3, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Galleano, M.; Pechanova, O.; G. Fraga, C. Hypertension, Nitric Oxide, Oxidants, and Dietary Plant Polyphenols. Curr. Pharm. Biotechnol. 2010, 11, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2016, 212, 1–17. [Google Scholar] [CrossRef]
- Saker, K.E.; Allen, V.G.; Fontenot, J.P.; Bagley, C.P.; Ivy, R.L.; Evans, R.R.; Wester, D.B. Tasco-Forage: II. Monocyte immune cell response and performance of beef steers grazing tall fescue treated with a seaweed extract. J. Anim. Sci. 2001, 79, 1022. [Google Scholar] [CrossRef] [Green Version]
- Spiers, D.E.; Eichen, P.A.; Leonard, M.J.; Wax, L.E.; Rottinghaus, G.E.; Williams, J.E.; Colling, D.P. Benefit of dietary seaweed (Ascophyllum nodosum) extract in reducing heat strain and fescue toxicosis: A comparative evaluation. J. Biol. 2004, 29, 753–757. [Google Scholar] [CrossRef]
- Bach, S.J.; Wang, Y.; McAllister, T.A. Effect of feeding sun-dried seaweed (Ascophyllum nodosum) on fecal shedding of Escherichia coli O157:H7 by feedlot cattle and on growth performance of lambs. Anim. Feed Sci. Technol. 2008, 142, 17–32. [Google Scholar] [CrossRef]
- Braden, K.W.; Blanton, J.R.; Allen, V.G.; Pond, K.R.; Miller, M.F. Ascophyllum nodosum supplementation: A preharvest intervention for reducing Escherichia coli O157:H7 and Salmonella spp. in feedlot steers. J. Food Prot. 2004, 67, 1824–1828. [Google Scholar] [CrossRef]
- Braden, K.W.; Blanton, J.R.; Montgomery, J.L.; van Santen, E.; Allen, V.G.; Miller, M.F. Tasco supplementation: Effects on carcass characteristics, sensory attributes, and retail display shelf-life. J. Anim. Sci. 2007, 85, 754–768. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Z.; Bach, S.J.; McAllister, T.A. Effects of phlorotannins from Ascophyllum nodosum (brown seaweed) on in vitro ruminal digestion of mixed forage or barley grain. Anim. Feed Sci. Technol. 2008, 145, 375–395. [Google Scholar] [CrossRef]
- Wiseman, M. Evaluation of Tasco ® as a Candidate Prebiotic in Broiler Chickens. Master’s Thesis, Dalhousie University Halifax, Nova Scotia, NS, Canada, 2012. [Google Scholar]
- McHugh, D.J. A Guide to the Seaweed Industry; Food and Agriculture Organization of the United Nations, Ed.; FAO Fisher.: Rome, Italy, 2003; ISBN 9251049580. [Google Scholar]
- Leupp, J.L.; Caton, J.S.; Soto-Navarro, S.A.; Lardy, G.P. Effects of cooked molasses blocks and fermentation extract or brown seaweed meal inclusion on intake, digestion, and microbial efficiency in steers fed low-quality hay. J. Anim. Sci. 2005, 83, 2938–2945. [Google Scholar] [CrossRef] [PubMed]
- Producers, Manufacturers, Seaweed Processors, Exporters, Specialists in Seaweed Harvesting and Cultivation, Renewable Resource Technologies and Marine Food Safety—Acadian Seaplants. Available online: https://www.acadianseaplants.com/marine-plant-seaweed-manufacturers/#environmental_sustainability (accessed on 11 April 2020).
- Evans, F.D.; Critchley, A.T. Seaweeds for animal production use. J. Appl. Phycol. 2014, 26, 891–899. [Google Scholar] [CrossRef]
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Exploiting biological activities of brown seaweed Ecklonia cava for potential industrial applications: A review. Int. J. Food Sci. Nutr. 2012, 63, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Q.; Zhang, Z.; Zhang, J.; Li, P. Synthesized phosphorylated and aminated derivatives of fucoidan and their potential antioxidant activity in vitro. Int. J. Biol. Macromol. 2009, 44, 170–174. [Google Scholar] [CrossRef]
- WO2017032954A1—Use of Phlorotannins as a Stimulant for Mychorrhizal and Rhizobial Symbioses—Google Patents. Available online: https://patents.google.com/patent/WO2017032954A1/en (accessed on 10 April 2020).
- JP2004189532A—Stable Liquid Organic Fertilizer Having Plant Disease Protection Effect—Google Patents. Available online: https://patents.google.com/patent/JP2004189532A/en?q=phlorotannins&oq=phlorotannins&page=2 (accessed on 10 April 2020).
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Glombitza, K.-W. Highly Hydroxylated Phenols of the Phaeophyceae. In Marine Natural Products Chemistry; Faulkner, D.J., Fenical, W.H., Eds.; Springer: Boston, MA, USA, 1977; pp. 191–204. [Google Scholar]
- Jaillet, F.; Nouailhas, H.; Boutevin, B.; Caillol, S. Synthesis of novel vinylester from biobased phloroglucinol. Green Mater. 2016, 4. [Google Scholar] [CrossRef]

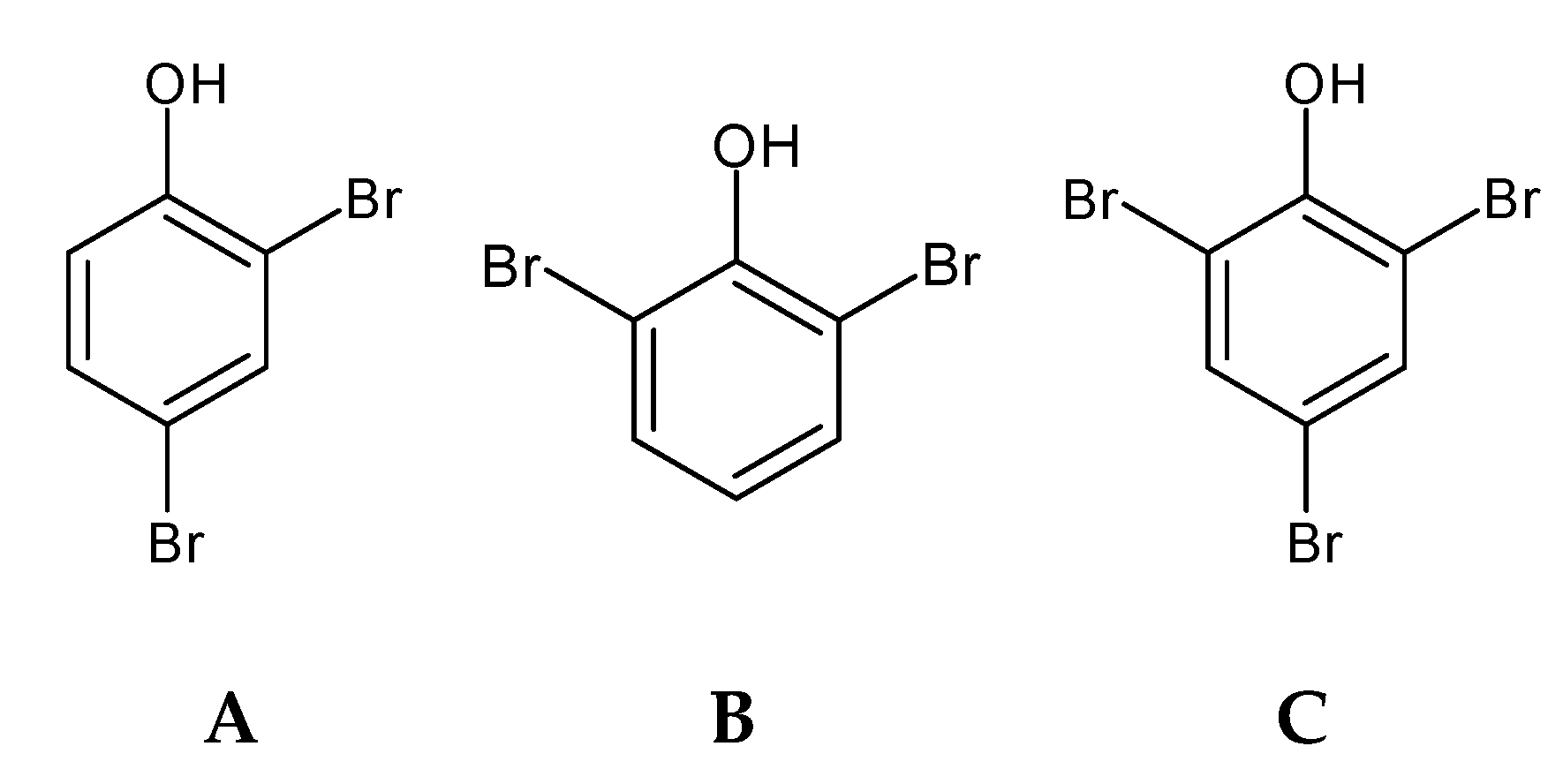




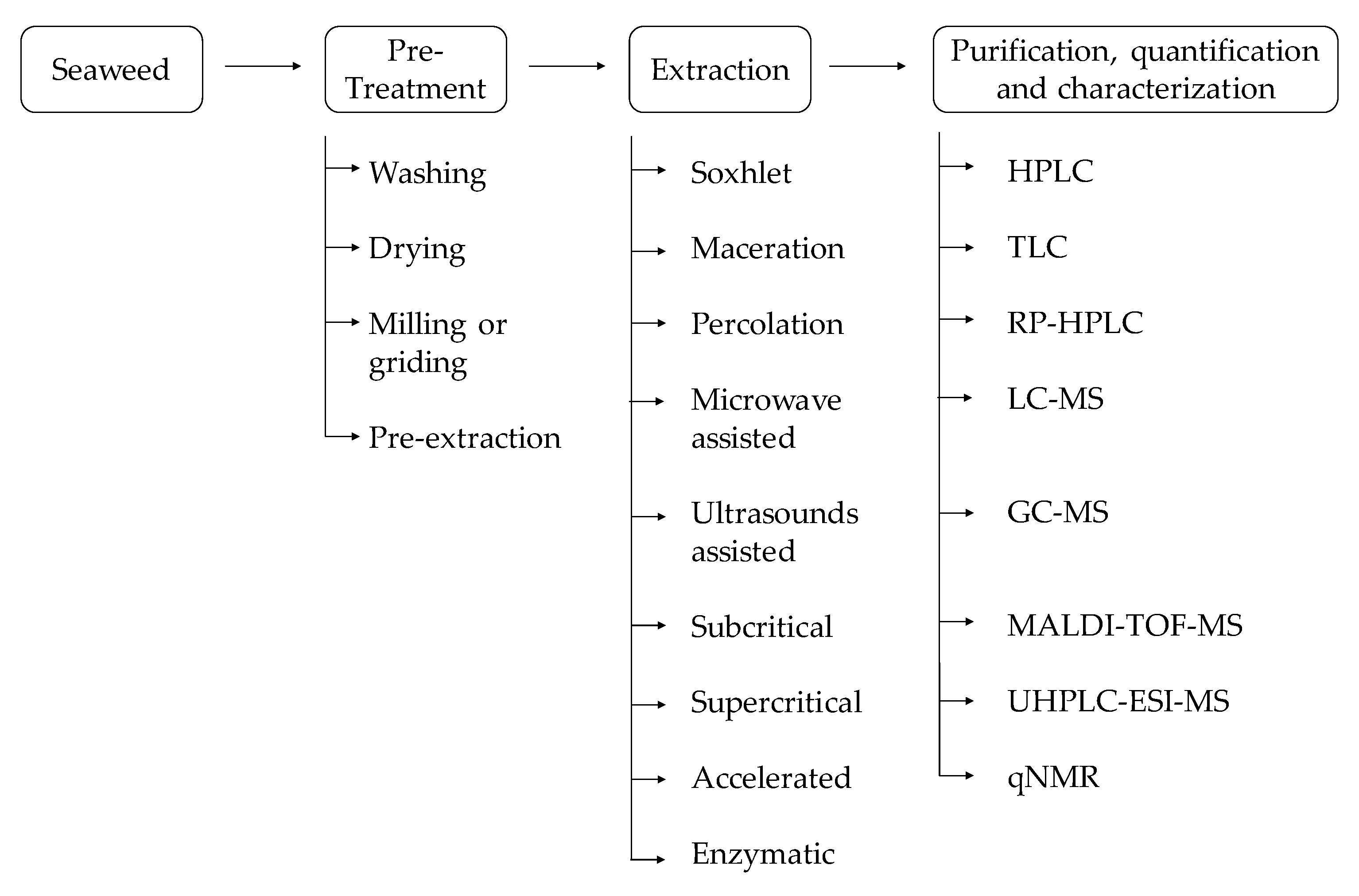
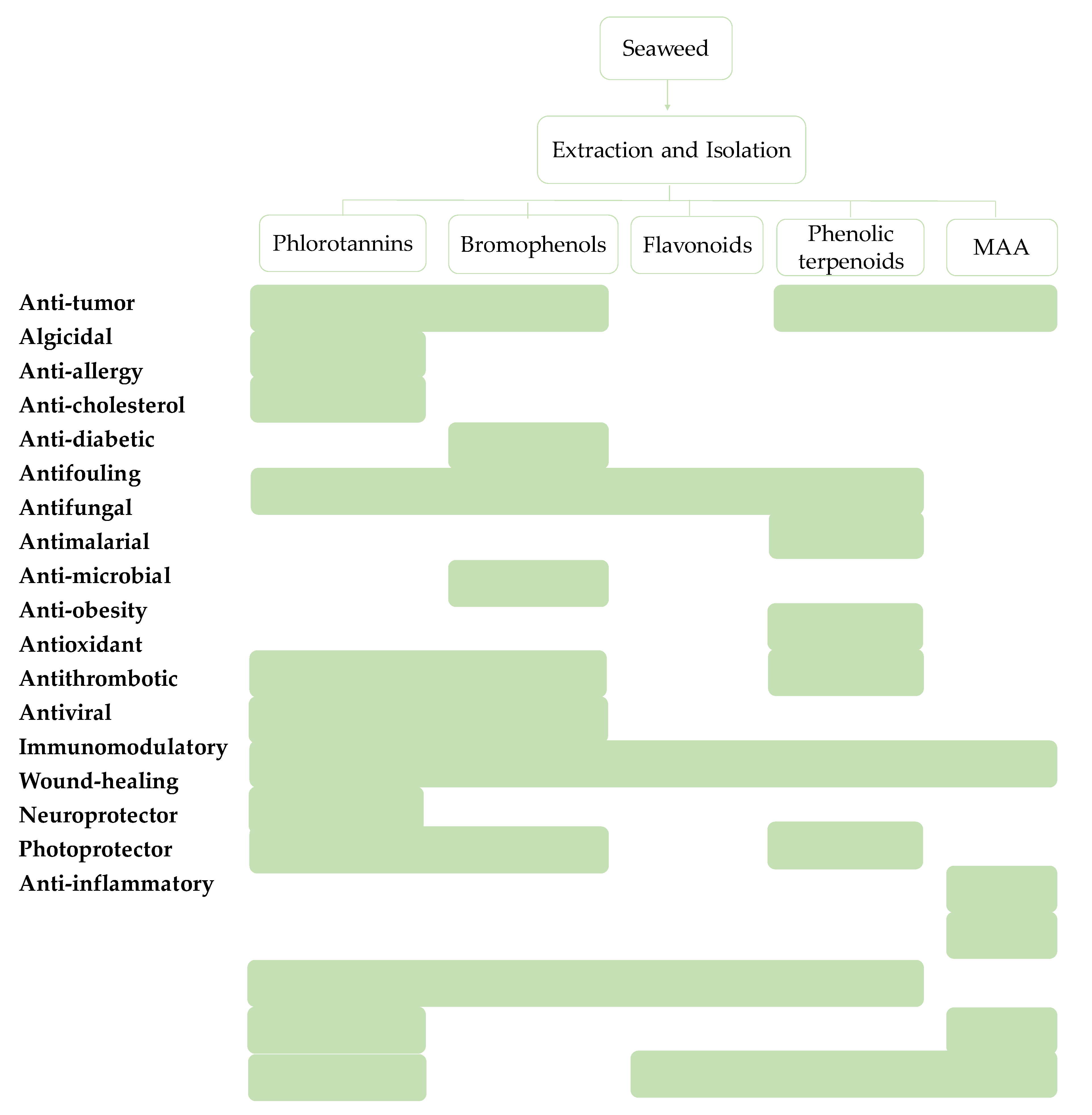




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed Phenolics: From Extraction to Applications. Mar. Drugs 2020, 18, 384. https://doi.org/10.3390/md18080384
Cotas J, Leandro A, Monteiro P, Pacheco D, Figueirinha A, Gonçalves AMM, da Silva GJ, Pereira L. Seaweed Phenolics: From Extraction to Applications. Marine Drugs. 2020; 18(8):384. https://doi.org/10.3390/md18080384
Chicago/Turabian StyleCotas, João, Adriana Leandro, Pedro Monteiro, Diana Pacheco, Artur Figueirinha, Ana M. M. Gonçalves, Gabriela Jorge da Silva, and Leonel Pereira. 2020. "Seaweed Phenolics: From Extraction to Applications" Marine Drugs 18, no. 8: 384. https://doi.org/10.3390/md18080384
APA StyleCotas, J., Leandro, A., Monteiro, P., Pacheco, D., Figueirinha, A., Gonçalves, A. M. M., da Silva, G. J., & Pereira, L. (2020). Seaweed Phenolics: From Extraction to Applications. Marine Drugs, 18(8), 384. https://doi.org/10.3390/md18080384








