Preventive Effects of Three Polysaccharides on the Oxidative Stress Induced by Acrylamide in a Saccharomyces cerevisiae Model
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of Acrylamide to S. Cerevisiae
2.2. The Benefit Effect of SIP and WCPP on the Growth of Yeast under Natural Conditions
2.3. Prevention of SIP, FL and WCPP on AA-Mediated Cytotoxicity
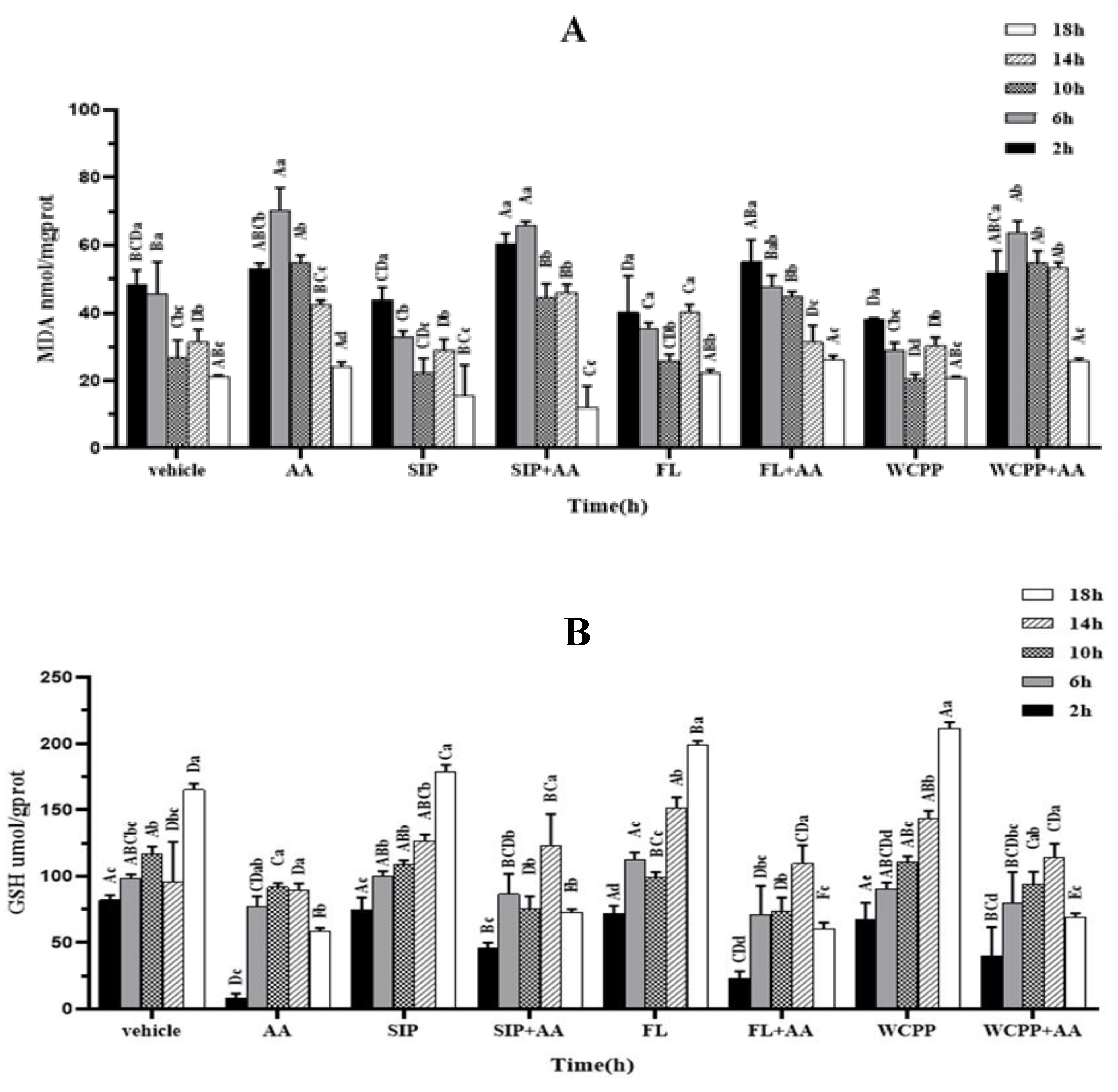
2.4. Attenuation of SIP, FL and WCPP on the Increase in Lipid Peroxidation Caused by AA
2.5. Antagonistic Effects of SIP and WCPP on Intracellular GSH Depletion Induced by AA
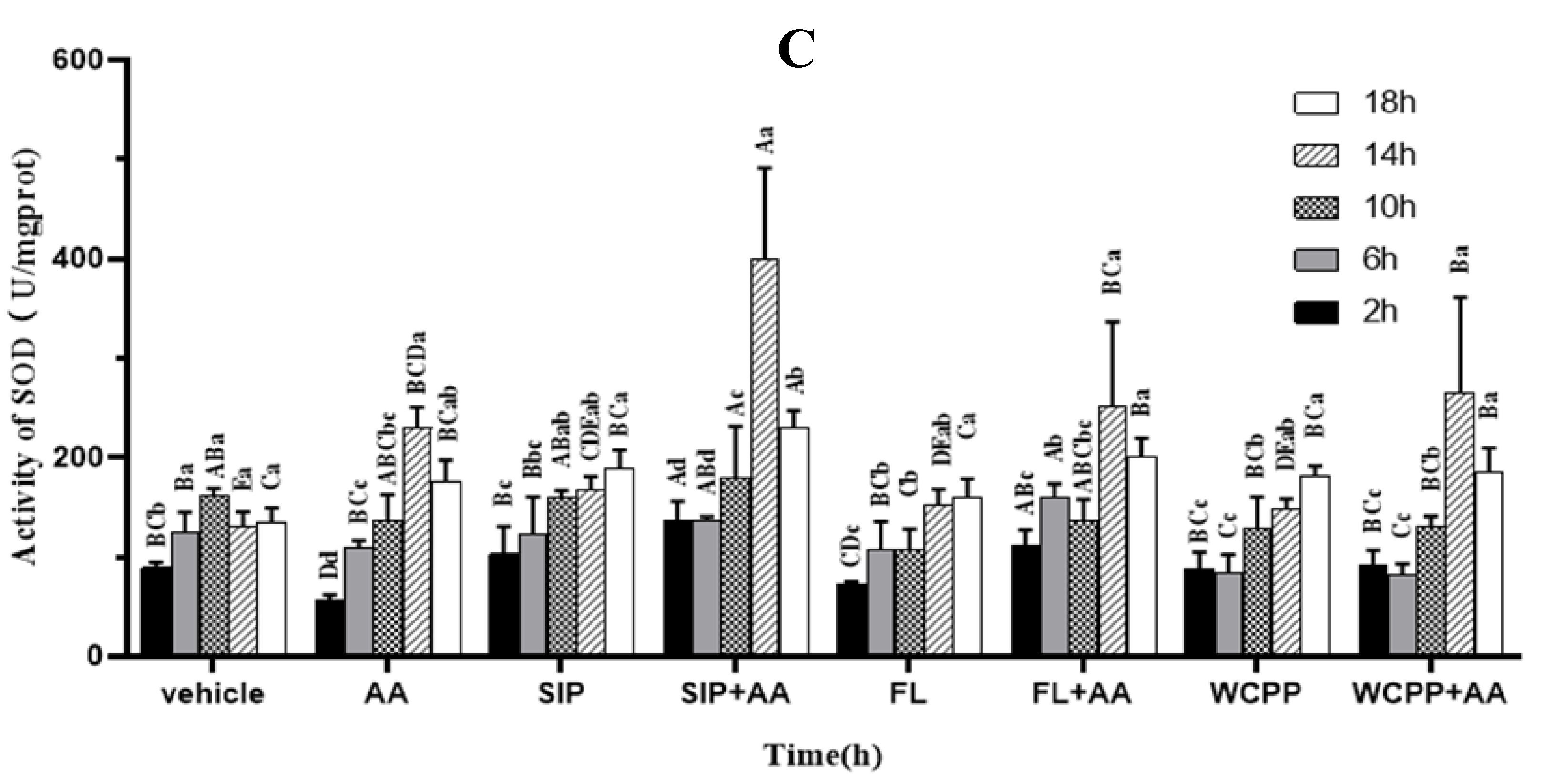
2.6. Prevention of SIP, FL and WCPP on AA Induced SOD Activity Disruption
3. Discussion
4. Materials and Methods
4.1. Preparation of SIP
4.2. Preparation of WCPP
4.3. Preincubation of S. cerevisiae
4.4. Model of AA Induced Cell Damage
4.5. Effect of Polysaccharides on AA Induced Oxidative Damage in Yeast Model
4.6. Determination of Antioxidant Biomarkers in Yeast Model
4.7. Kinetic Parameters Calculation and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Parzefall, W. Minireview on the toxicity of dietary acrylamide. Food Chem. Toxicol. 2008, 46, 1360–1364. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, Y.; Zhang, Y. Cheminform abstract: New research developments on acrylamide: Analytical chemistry, formation mechanism, and mitigation recipes. Cheminform 2010, 41, 4375–4397. [Google Scholar] [CrossRef]
- Kommuguri, U.N.; Satyaprasad Pallem, P.V.; Bodiga, S.; Bodiga, V.L. Effect of dietary antioxidants on the cytostatic effect of acrylamide during copper-deficiency in saccharomyces cerevisiae. Food Funct. 2014, 5, 705. [Google Scholar] [CrossRef] [PubMed]
- Kwolek-Mirek, M.; Zadrag-Tecza, R.; Bednarska, S.; Bartosz, G. Yeast saccharomyces cerevisiae devoid of Cu, Zn-superoxide dismutase as a cellular model to study acrylamide toxicity. Toxicol. Vitro 2011, 25, 573–579. [Google Scholar] [CrossRef]
- Jiang, L.; Cao, J.; An, Y.; Geng, C.; Qu, S.; Jiang, L.; Zhong, L. Genotoxicity of acrylamide in human hepatoma G2 (HepG2) cells. Toxicol. Vitro 2007, 21, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Mei, N.; Hu, J.; Churchwell, M.I.; Guo, L.; Moore, M.M.; Doerge, D.R.; Chen, T. Genotoxic effects of acrylamide and glycidamide in mouse lymphoma cells. Food Chem. Toxicol. 2008, 46, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Dinda, B.; Dinda, S.; Dassharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457–480. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, K.; Tancharoen, S.; Takeshige, N.; Yoshitomi, M.; Morioka, M.; Murai, Y.; Tanaka, E. The efficacy of edaravone (radicut), a free radical scavenger, for cardiovascular disease. Int. J. Mol. Sci. 2013, 14, 13909–13930. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on mechanisms of in vitro antioxidant activity of polysaccharides. Oxid. Med. Cell. Longev. 2016, 5692852. [Google Scholar] [CrossRef] [Green Version]
- Gu, Y.P.; Yang, X.M.; Luo, P.; Li, Y.Q.; Tao, Y.X.; Duan, Z.H.; Xiao, W.; Zhang, D.Y.; Liu, H.Z. Inhibition of acrolein-induced autophagy and apoptosis by a glycosaminoglycan from sepia esculenta ink in mouse leydig cells. Carbohyd. Polym. 2017, 163, 270–279. [Google Scholar] [CrossRef]
- Liu, H.Z.; Tao, Y.X.; Luo, P.; Deng, C.M.; Gu, Y.P.; Yang, L.; Zhong, J.P. Preventive effects of a novelty polysaccharide from sepia esculenta ink on ovarian failure and its action mechanisms in cyclophosphamide-treated mice. J. Agric. Food Chem. 2016, 64, 5759–5766. [Google Scholar] [CrossRef] [PubMed]
- Le, X.Y.; Luo, P.; Gu, Y.P.; Tao, Y.X.; Liu, H.Z. Interventional effects of squid ink polysaccharides on cyclophosphamide-associated testicular damage in mice. Bratisl. Med. J. 2015, 116, 334–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, X.Y.; Luo, P.; Gu, Y.P.; Tao, Y.X.; Liu, H.Z. Squid ink polysaccharide reduces cyclophosphamide-induced testicular damage via Nrf2/ARE activation pathway in mice. Iran. J. Basic Med. Sci. 2015, 18, 827–831. [Google Scholar] [PubMed]
- Wang, J.; Geng, L.; Yue, Y.; Zhang, Q. Use of fucoidan to treat renal diseases: A review of 15 years of clinic studies. Prog. Mol. Biol. Transl. 2019, 163, 95–111. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H.; Luo, P.; Li, Y. Production inhibition and excretion promotion of urate by fucoidan from Laminaria japonica in adenine-induced hyperuricemic mice. Mar. Drugs 2018, 16, 472. [Google Scholar] [CrossRef] [Green Version]
- Zhan, G.; Pan, L.; Tu, K.; Jiao, S. Antitumor, antioxidant, and nitrite scavenging effects of chinese water chestnut (eleocharis dulcis) peel flavonoids. J. Food Sci. 2016, 81, H2578–H2586. [Google Scholar] [CrossRef]
- Zhan, G.; Pan, L.Q.; Mao, S.B.; Zhang, W.; Wei, Y.Y.; Tu, K. Study on antibacterial properties and major bioactive constituents of chinese water chestnut (eleocharis dulcis) peels extracts/fractions. Eur. Food Res. Technol. 2014, 238, 789–796. [Google Scholar] [CrossRef]
- Semla, M.; Goc, Z.; Martiniaková, M.; Omelka, R.; Formicki, G. Acrylamide a common food toxin related to physiological functions and health. Physiol. Res. 2017, 66, 205–217. [Google Scholar] [CrossRef]
- Erdemli, M.E.; Arif Aladag, M.; Altinoz, E.; Demirtas, S.; Turkoz, Y.; Yigitcan, B.; Bag, H.G. Acrylamide applied during pregnancy causes the neurotoxic effect by lowering BDNF levels in the fetal brain. Neurotoxicol. Teratol. 2018, 67, 37–43. [Google Scholar] [CrossRef]
- Marta, K.; Muchacka, R.; Czech, J.; Batoryna, M.; Formicki, G. Acrylamide toxicity and cholinergic nervous system. J. Physiol. Pharmacol. 2019, 69, 847–858. [Google Scholar] [CrossRef]
- Pennisi, M.; Malaguarnera, G.; Puglisi, V.; Vinciguerra, L.; Vacante, M.; Malaguarnera, M. Neurotoxicity of acrylamide in exposed workers. Int. J. Environ. Res. Public Health 2013, 10, 3843–3854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LoPachin, R.M. Acrylamide neurotoxicity: Neurological, morhological and molecular endpoints in animal models. Adv. Exp. Med. Biol. 2005, 561, 21–37. [Google Scholar] [CrossRef]
- Batoryna, M.; Lis, M.; Formicki, G. Acrylamide-induced disturbance of the redox balance in the chick embryonic brain. J. Environ. Sci. Health B 2017, 52, 600–606. [Google Scholar] [CrossRef]
- Pan, X.; Wu, X.; Yan, D.; Peng, C.; Rao, C.; Yan, H. Acrylamide-induced oxidative stress and inflammatory response are alleviated by N-acetylcysteine in PC12 cells: Involvement of the crosstalk between Nrf2 and NF-κB pathways regulated by MAPKs. Toxicol. Lett. 2018, 288, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lewis Wang, F.S.; Hu, X.; Chen, F.; Chan, H.M. Acrylamide-induced neurotoxicity in primary astrocytes and microglia: Roles of the Nrf2-ARE and NF-κB pathways. Food Chem. Toxicol. 2017, 106, 25–35. [Google Scholar] [CrossRef]
- Pan, X.; Yan, D.; Wang, D.; Wu, X.; Zhao, W.; Lu, Q.; Yan, H. Mitochondrion-mediated apoptosis induced by acrylamide is regulated by a balance between Nrf2 antioxidant and MAPK signaling pathways in PC12 Cells. Mol. Neurobiol. 2017, 54, 4781–4794. [Google Scholar] [CrossRef] [PubMed]
- Azari, A.; Shokrzadeh, M.; Zamani, E.; Amani, N.; Shaki, F. Cerium oxide nanoparticles protects against acrylamide induced toxicity in HepG2 cells through modulation of oxidative stress. Drug Chem. Toxicol. 2019, 42, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Bo, L.; Liu, Y.; Jia, S.; Liu, Y.; Zhang, M.; Li, S.; Zhao, X.; Sun, C. Metabonomics analysis of quercetin against the nephrotoxicity of acrylamide in rats. Food Funct. 2018, 9, 5965–5974. [Google Scholar] [CrossRef]
- Elhelaly, A.E.; AlBasher, G.; Alfarraj, S.; Almeer, R.; Bahbah, E.I.; Fouda, M.; Bungău, S.G.; Aleya, L.; Abdel-Daim, M.M. Protective effects of hesperidin and diosmin against acrylamide-induced liver, kidney, and brain oxidative damage in rats. Environ. Sci. Pollut. Res. 2019, 26, 35151–35162. [Google Scholar] [CrossRef]
- Karimi, M.Y.; Fatemi, I.; Kalantari, H.; Mombeini, M.A.; Mehrzadi, S.; Goudarzi, M. Ellagic acid prevents oxidative stress, inflammation, and histopathological alterations in acrylamide-induced hepatotoxicity in wistar rats. J. Diet. Suppl. 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zamani, E.; Shokrzadeh, M.; Ziar, A.; Abedian-Kenari, S.; Shaki, F. Acrylamide attenuated immune tissues’ function via induction of apoptosis and oxidative stress: Protection by l-carnitine. Hum. Exp. Toxicol. 2018, 37, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Kunnel, S.G.; Subramanya, S.; Satapathy, P.; Sahoo, I.; Zameer, F. Acrylamide induced toxicity and the propensity of phytochemicals in amelioration: A review. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Mirzadeh, M.; Arianejad, M.R.; Khedmat, L. Antioxidant, antiradical, and antimicrobial activities of polysaccharides obtained by microwave-assisted extraction method: A review. Carbohyd. Polym. 2020, 229, 115421. [Google Scholar] [CrossRef]
- Kommuguri, U.N.; Bodiga, S.; Sankuru, S.; Bodiga, V.L. Copper deprivation modulates CTR1 and CUP1 expression and enhances cisplatin cytotoxicity in Saccharomyces cerevisiae. J. Trace Elem. Med. Biol. 2012, 26, 13–19. [Google Scholar] [CrossRef]
- Hassan, H.A.; El-Kholy, W.M.; El-Sawi, M.; Galal, N.A.; Ramadan, M.F. Myrtle (Myrtus communis) leaf extract suppresses hepatotoxicity induced by monosodium glutamate and acrylamide through obstructing apoptosis, DNA fragmentation, and cell cycle arrest. Environ. Sci. Pollut. Res. 2020, 27, 23188–23198. [Google Scholar] [CrossRef]
- Zhu, H.; Jia, Z.; Misra, B.R.; Zhang, L.; Cao, Z.; Yamamoto, M.; Trush, M.A.; Misra, H.P.; Li, Y. Nuclear factor E2-related factor 2-dependent myocardiac cytoprotection against oxidative and electrophilic stress. Cardiovasc. Toxicol. 2008, 8, 71–85. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Q.; Wei, J.G.; Tu, M.J.; Gu, J.G.; Zhang, W. Fucoidan alleviates acetaminophen-induced hepatotoxicity via oxidative stress inhibition and Nrf2 translocation. Int. J. Mol. Sci. 2018, 19, 4050. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhang, L.; Jiang, G.; Lei, A.; Yu, Q.; Xie, J.; Chen, Y. Evaluation of the protective effects of Ganoderma atrum polysaccharide on acrylamide-induced injury in small intestine tissue of rats. Food Funct. 2019, 10, 5863–5872. [Google Scholar] [CrossRef]
- Albalawi, A.; Alhasani, R.; Biswas, L.; Reilly, J.; Akhtar, S.; Shu, X. Carnosic acid attenuates acrylamide-induced retinal toxicity in zebrafish embryos. Exp. Eye Res. 2018, 175, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghorbel, I.; Elwej, A.; Fendri, N.; Mnif, H.; Jamoussi, K.; Boudawara, T.; Grati Kamoun, N.; Zeghal, N. Olive oil abrogates acrylamide induced nephrotoxicity by modulating biochemical and histological changes in rats. Ren. Fail. 2017, 39, 236–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdy, S.M.; Shabaan, A.M.; Abdel Latif, A.; Abdel-Aziz, A.M.; Amin, A.M. Protective effect of hesperidin and tiger nut against acrylamide toxicity in female rats. Exp. Toxicol. Pathol. 2017, 69, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.J.; Zeng, T.; Zhu, Y.B.; Yu, S.F.; Wang, Q.S.; Zhang, L.P.; Guo, X.; Xie, K.Q. Effects of acrylamide on the nervous tissue antioxidant system and sciatic nerve electrophysiology in the rat. Neurochem. Res. 2008, 33, 2310–2317. [Google Scholar] [CrossRef]
- Braconi, D.; Bernardini, G.; Santucci, A. Saccharomyces cerevisiae as a model in ecotoxicological studies: A post-genomics perspective. J. Proteomics 2016, 137, 19–34. [Google Scholar] [CrossRef]
- Granato, D.; Shahidi, F.; Wrolstad, R.; Kilmartin, P.; Melton, L.D.; Hidalgo, F.J.; Miyashita, K.; Camp, J.V.; Alasalvar, C.; Ismail, A.B.; et al. Antioxidant activity, total phenolics and flavonoids contents: Should we ban in vitro screening methods? Food Chem. 2018, 264, 471–475. [Google Scholar] [CrossRef]
- Wang, Z.J.; Xie, J.H.; Nie, S.P.; Xie, M.Y. Review on cell models to evaluate the potential antioxidant activity of polysaccharides. Food Funct. 2017, 8, 915–926. [Google Scholar] [CrossRef] [PubMed]
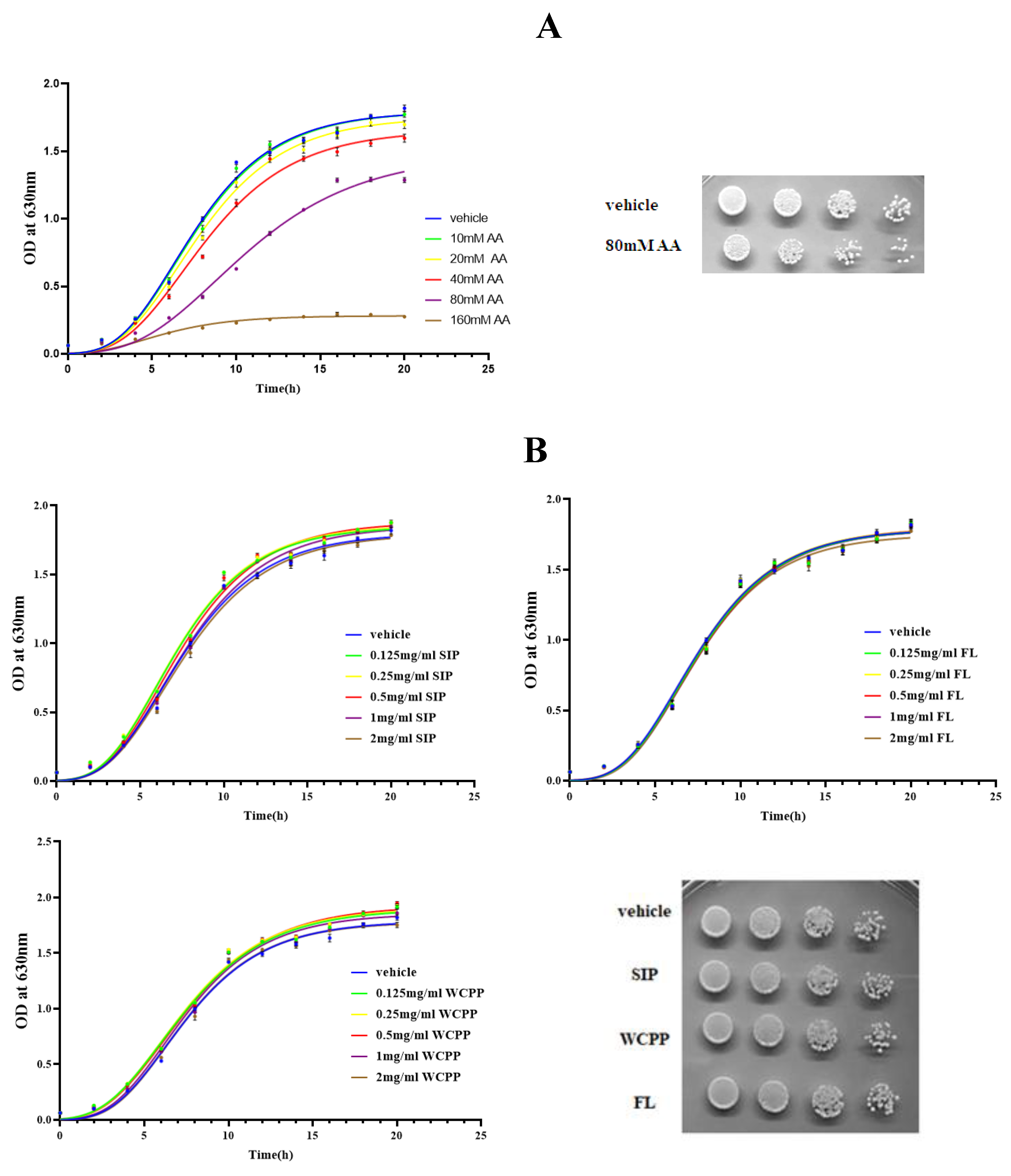

| Groups | Vehicle | AA | IC50 (mM) | ||||
|---|---|---|---|---|---|---|---|
| Inhibition rate (%) | 0 | Concentration (mM) | 92.64 ± 3.30 | ||||
| 10 | 20 | 40 | 80 | 160 | |||
| 0.41 ± 2.6 | 4.73 ± 3.07 | 12.65 ± 2.58 * | 36.83 ± 1.52 * | 80.91 ± 0.17 * | |||
| Groups | Vehicle | AA | Polysaccharide | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration (mM) | Concentration (mg/mL) | ||||||||||||
| 10 | 20 | 40 | 80 | 160 | 0.125 | 0.25 | 0.5 | 1 | 2 | ||||
| SIP | 4.66 ± 0.02 * | 4.65 ± 0.01 * | 4.78 ± 0.05 * | 4.88 ± 0.02 | 4.99 ± 0.04 | ||||||||
| Dt (h) | 4.91 ± 0.02 | 4.95 ± 0.05 | 5.12 ± 0.08 * | 5.43 ± 0.06 * | 7.02 ± 0.08 * | 12.26 ± 0.20 * | FL | 4.96 ± 0.08 | 4.93 ± 0.09 | 4.96 ± 0.05 | 4.98 ± 0.05 | 5.00 ± 0.05 | |
| WCPP | 4.68 ± 0.01 * | 4.66 ± 0.02 * | 4.71 ± 0.05 * | 4.81± 0.02 * | 4.90 ± 0.03 | ||||||||
| Groups | Vehicle | AA | Polysaccharide | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ym | 1.80 ± 0.02 | Concentration (mM) | Concentration (mg/mL) | |||||||||
| 10 | 20 | 40 | 80 | 160 | 0.125 | 0.25 | 0.5 | 1 | 2 | |||
| 1.80 ± 0.02 | 1.76 ± 0.03 | 1.66 ± 0.03 * | 1.49 ± 0.02 * | 0.31 ± 0.01 * | SIP | 1.85 ± 0.00 * | 1.86 ± 0.01 * | 1.88 ± 0.01 * | 1.85 ± 0.00 * | 1.79 ± 0.01 | ||
| FL | 1.80 ± 0.01 | 1.81 ± 0.02 | 1.81 ± 0.02 | 1.80 ± 0.01 | 1.77 ± 0.01 | |||||||
| WCPP | 1.87 ± 0.00 * | 1.88 ± 0.01 * | 1.90 ± 0.01 * | 1.86 ± 0.02 * | 1.79 ± 0.01 | |||||||
| 1/K (h) | 3.32 ± 0.02 | 3.36 ± 0.04 | 3.48 ± 0.03 * | 3.65 ± 0.02 * | 4.77 ± 0.02 * | 5.60 ± 0.15 * | SIP | 3.15 ± 0.01 * | 3.14 ± 0.01 * | 3.27 ± 0.03 | 3.36 ± 0.02 | 3.39 ± 0.02 * |
| FL | 3.36 ± 0.05 | 3.34 ± 0.05 | 3.39 ± 0.03 | 3.38 ± 0.03 | 3.36 ± 0.03 | |||||||
| WCPP | 3.20 ± 0.00 * | 3.19 ± 0.01 * | 3.26 ± 0.04 | 3.28 ± 0.04 | 3.31 ± 0.03 | |||||||
| R2 | 0.9919 | 0.9936 | 0.993 | 0.9888 | 0.9884 | 0.9796 | SIP | 0.9921 | 0.9928 | 0.9938 | 0.9945 | 0.9921 |
| FL | 0.9907 | 0.9907 | 0.9912 | 0.9926 | 0.9911 | |||||||
| WCPP | 0.9915 | 0.9916 | 0.9916 | 0.9907 | 0.9916 | |||||||
| Groups | Vehicle | 80 mM AA | Combination | |||||
|---|---|---|---|---|---|---|---|---|
| Concentration of Polysaccharide (mg/mL) | ||||||||
| 0.125 | 0.25 | 0.5 | 1 | 2 | ||||
| SIP + 80 mM AA | 6.60 ± 0.05 * | 6.75 ± 0.01 * | 6.84 ± 0.13 * | 7.30 ± 0.05 * | 7.82 ± 0.02 | |||
| Dt (h) | 5.23 ± 0.02 * | 7.73 ± 0.04 | FL + 80 mM AA | 7.63 ± 0.20 | 7.23 ± 0.18 * | 7.16 ± 0.15 * | 7.41 ± 0.08 * | 7.54 ± 0.07 * |
| WCPP + 80 mM AA | 7.07 ± 0.19 * | 7.09 ± 0.09 * | 7.23 ± 0.07 * | 7.63 ± 0.03 * | 7.87 ± 0.06 * | |||
| SIP + 80 mM AA | 29.69 ± 0.32 * | 31.31 ± 0.46 * | 31.91 ± 0.01 * | 35.84 ± 0.98 * | 40.35 ± 0.17 | |||
| Inhibition rate (%) | 0 * | 39.76 ± 0.50 | FL + 80 mM AA | 38.04 ± 1.15 | 34.69 ± 1.41 * | 34.10 ± 1.12 * | 36.13 ± 0.67 * | 37.04 ± 0.35 * |
| WCPP + 80 mM AA | 34.96 ± 1.07 * | 34.16 ± 0.86 * | 34.41 ± 0.01 * | 37.31 ± 0.34 * | 39.96 ± 0.25 | |||
| Groups | Vehicle | 80 mM AA | Combination | |||||
|---|---|---|---|---|---|---|---|---|
| Concentration of Polysaccharide (mg/mL) | ||||||||
| 0.125 | 0.25 | 0.5 | 1 | 2 | ||||
| SIP + 80 mM AA | 1.57 ± 0.17 * | 1.58 ± 0.00 * | 1.60 ± 0.01 * | 1.59 ± 0.01 * | 1.58 ± 0.01 * | |||
| Ym | 1.83 ± 0.02 * | 1.53 ± 0.00 | FL + 80 mM AA | 1.60 ± 0.02 * | 1.57 ± 0.02 | 1.56 ± 0.02 | 1.57 ± 0.02 | 1.53 ± 0.02 |
| WCPP + 80 mM AA | 1.55 ± 0.03 | 1.60 ± 0.00 * | 1.62 ± 0.02 * | 1.61 ± 0.02 * | 1.60 ± 0.01 * | |||
| SIP + 80 mM AA | 5.17 ± 0.03 * | 5.32 ± 0.01 * | 5.42 ± 0.09 * | 5.70 ± 0.01 * | 6.03 ± 0.05 * | |||
| 1/K (h) | 3.91 ± 0.01 * | 5.82 ± 0.01 | FL + 80 mM AA | 5.89 ± 0.08 | 5.53 ± 0.10 * | 5.44 ± 0.05 * | 5.61 ± 0.03 * | 5.58 ± 0.03 * |
| WCPP + 80 mM AA | 5.52 ± 0.13 * | 5.60 ± 0.03 * | 5.66 ± 0.02 * | 5.85 ± 0.05 | 6.04 ± 0.07 * | |||
| SIP + 80 mM AA | 0.988 | 0.9897 | 0.9907 | 0.991 | 0.991 | |||
| R2 | 0.9896 | 0.9898 | FL + 80 mM AA | 0.9892 | 0.9886 | 0.9893 | 0.9892 | 0.9885 |
| WCPP + 80 mM AA | 0.9891 | 0.9876 | 0.9864 | 0.9848 | 0.9861 | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Zhang, Y.; Li, F.; Tan, X.; Luo, P.; Liu, H. Preventive Effects of Three Polysaccharides on the Oxidative Stress Induced by Acrylamide in a Saccharomyces cerevisiae Model. Mar. Drugs 2020, 18, 395. https://doi.org/10.3390/md18080395
Lin Z, Zhang Y, Li F, Tan X, Luo P, Liu H. Preventive Effects of Three Polysaccharides on the Oxidative Stress Induced by Acrylamide in a Saccharomyces cerevisiae Model. Marine Drugs. 2020; 18(8):395. https://doi.org/10.3390/md18080395
Chicago/Turabian StyleLin, Zhen, Yu Zhang, Fangping Li, Xiaohui Tan, Ping Luo, and Huazhong Liu. 2020. "Preventive Effects of Three Polysaccharides on the Oxidative Stress Induced by Acrylamide in a Saccharomyces cerevisiae Model" Marine Drugs 18, no. 8: 395. https://doi.org/10.3390/md18080395
APA StyleLin, Z., Zhang, Y., Li, F., Tan, X., Luo, P., & Liu, H. (2020). Preventive Effects of Three Polysaccharides on the Oxidative Stress Induced by Acrylamide in a Saccharomyces cerevisiae Model. Marine Drugs, 18(8), 395. https://doi.org/10.3390/md18080395





