Involvement of Reactive Oxygen Species in the Hepatorenal Toxicity of Actinomycin V In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
2.1. Comparing Cytotoxicity of Act V and Act D in LO-2 and 293T Cells
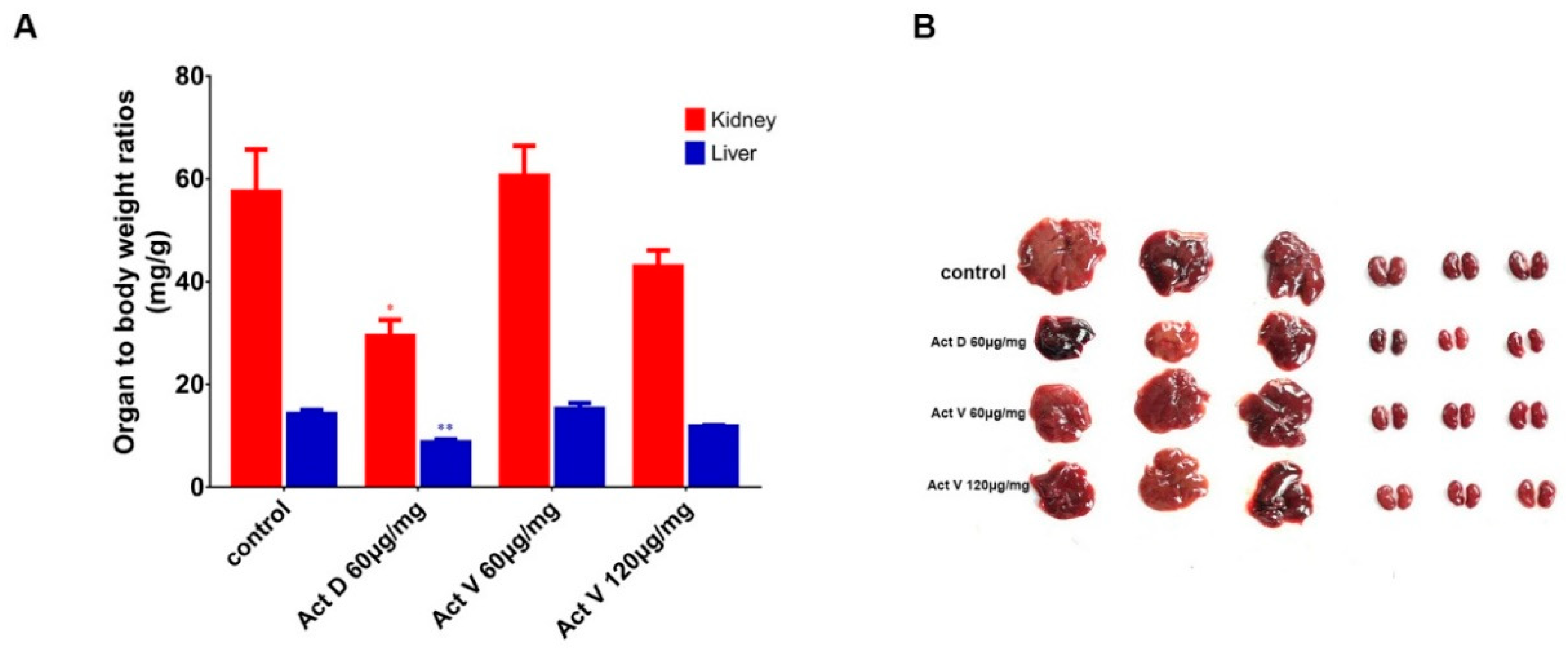
2.2. Effect of Act V and Act D on Organ to Body Weight Ratios in Mice
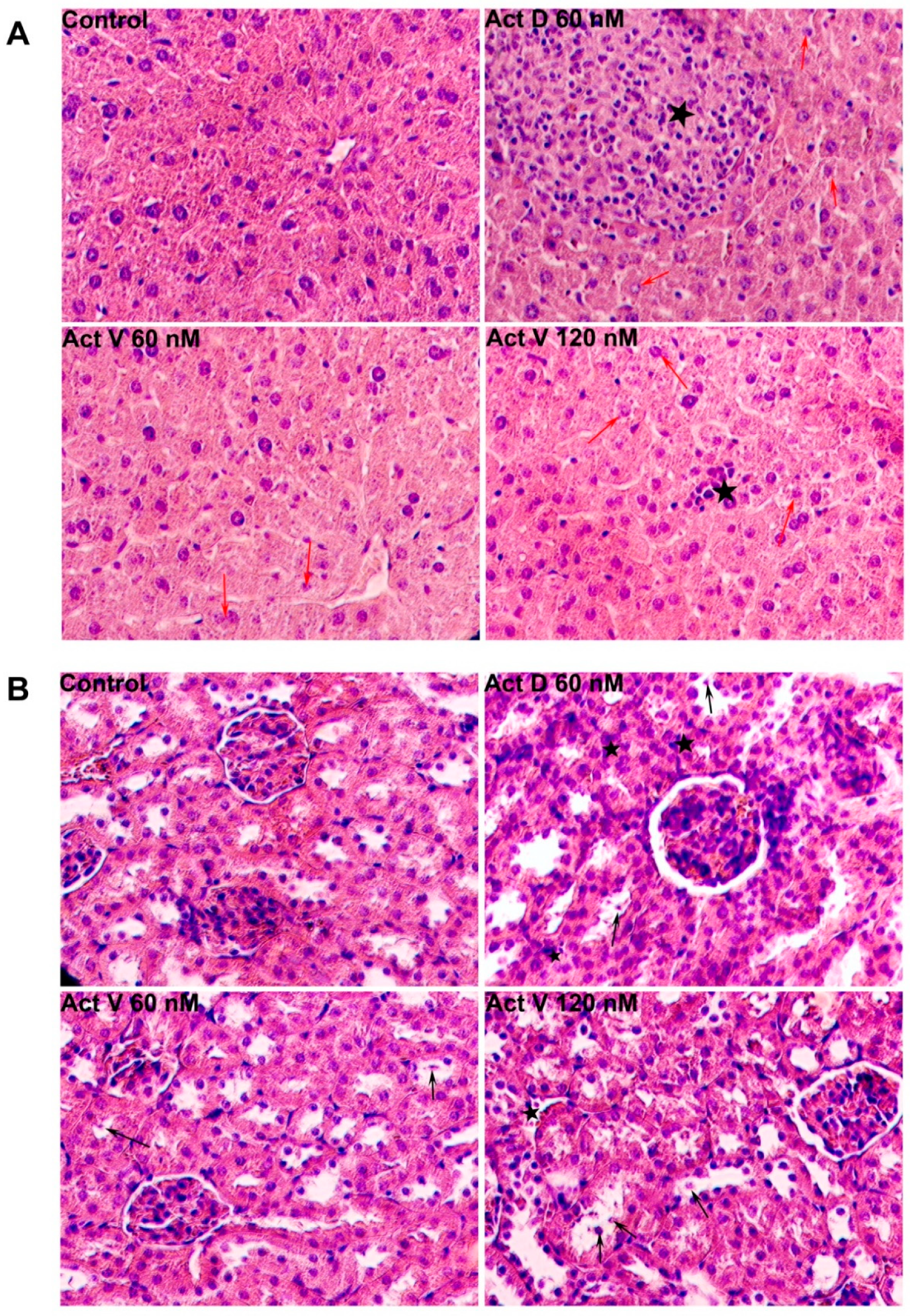
2.3. Effect of Act V and Act D on Liver and Kidney Histopathology
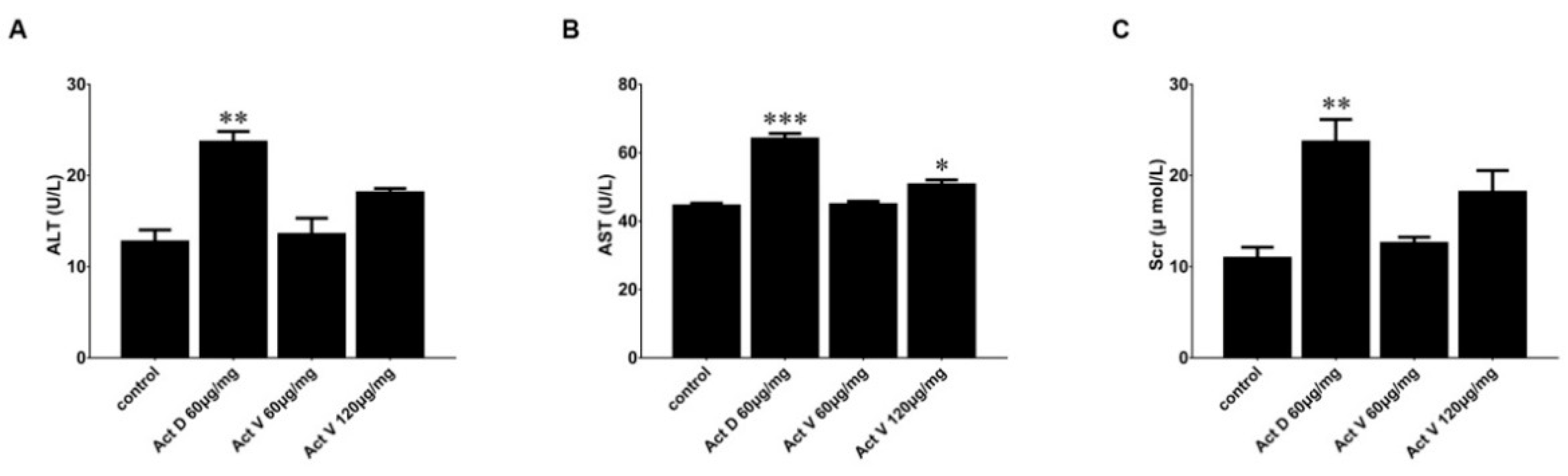
2.4. Effect of Act V and Act D on Liver and Kidney Indicators in Mouse Serum
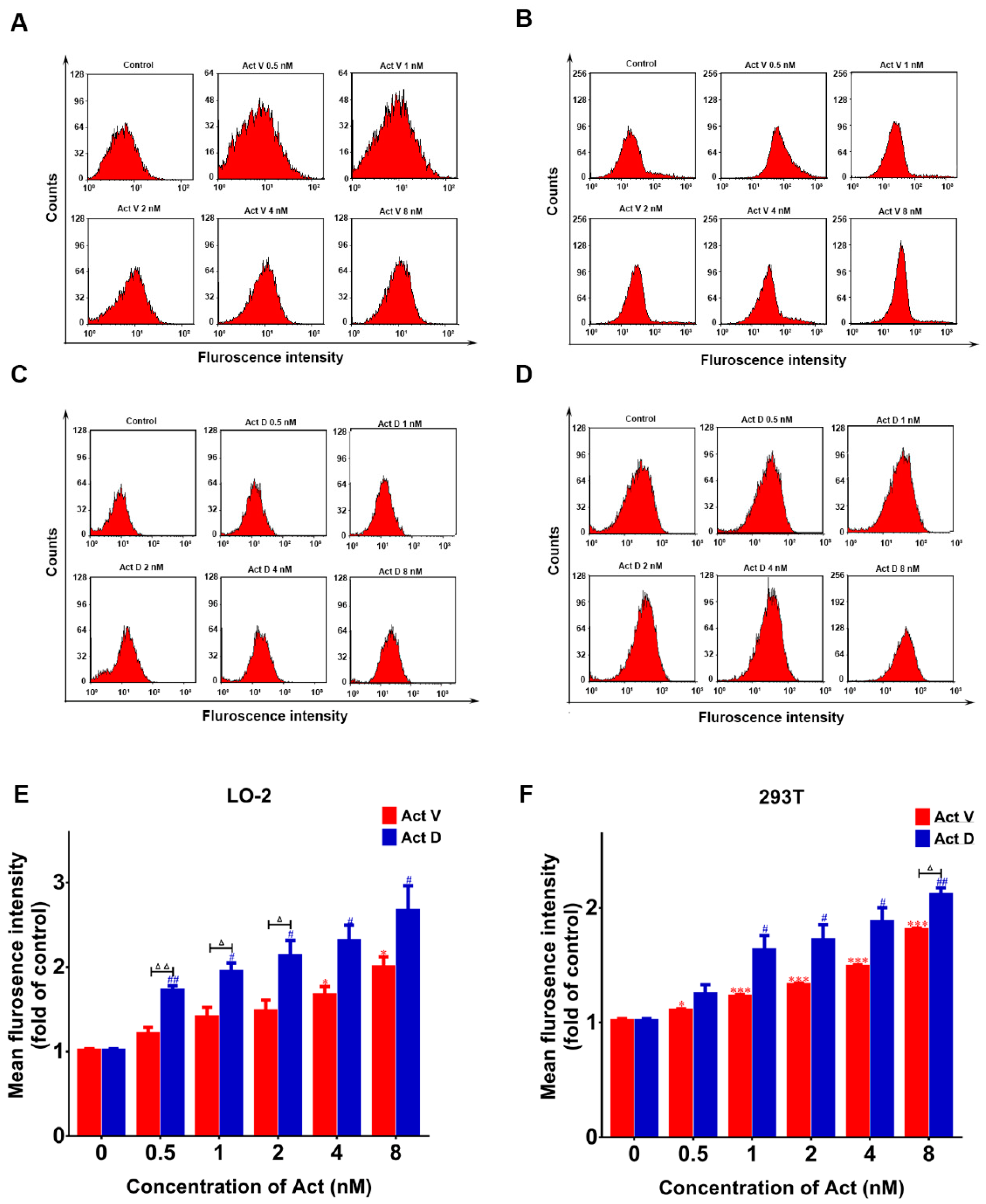
2.5. Act V and Act D Promote the Generation of ROS in LO-2 and 293T Cells
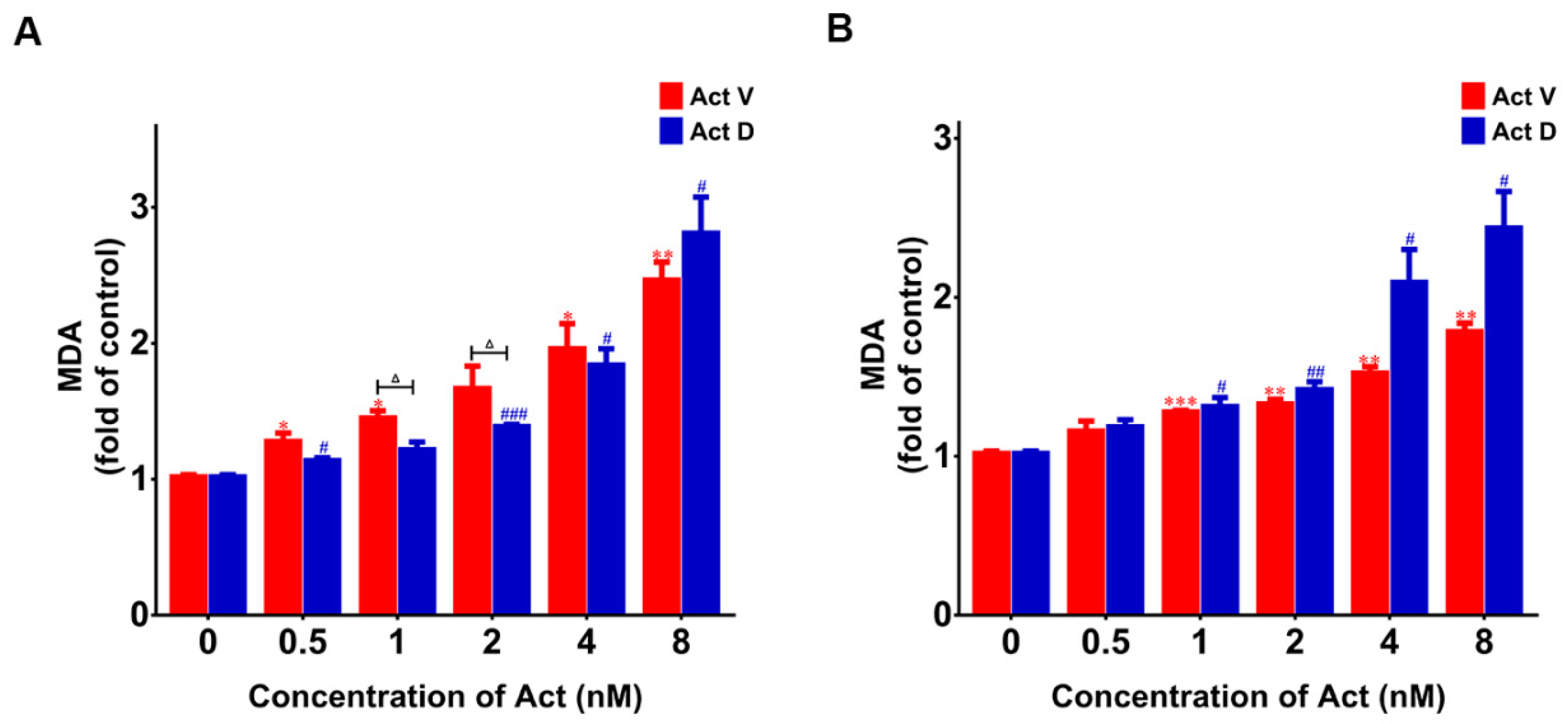
2.6. Act V and Act D Accelerate Lipid Oxidation in LO-2 And 293T Cells
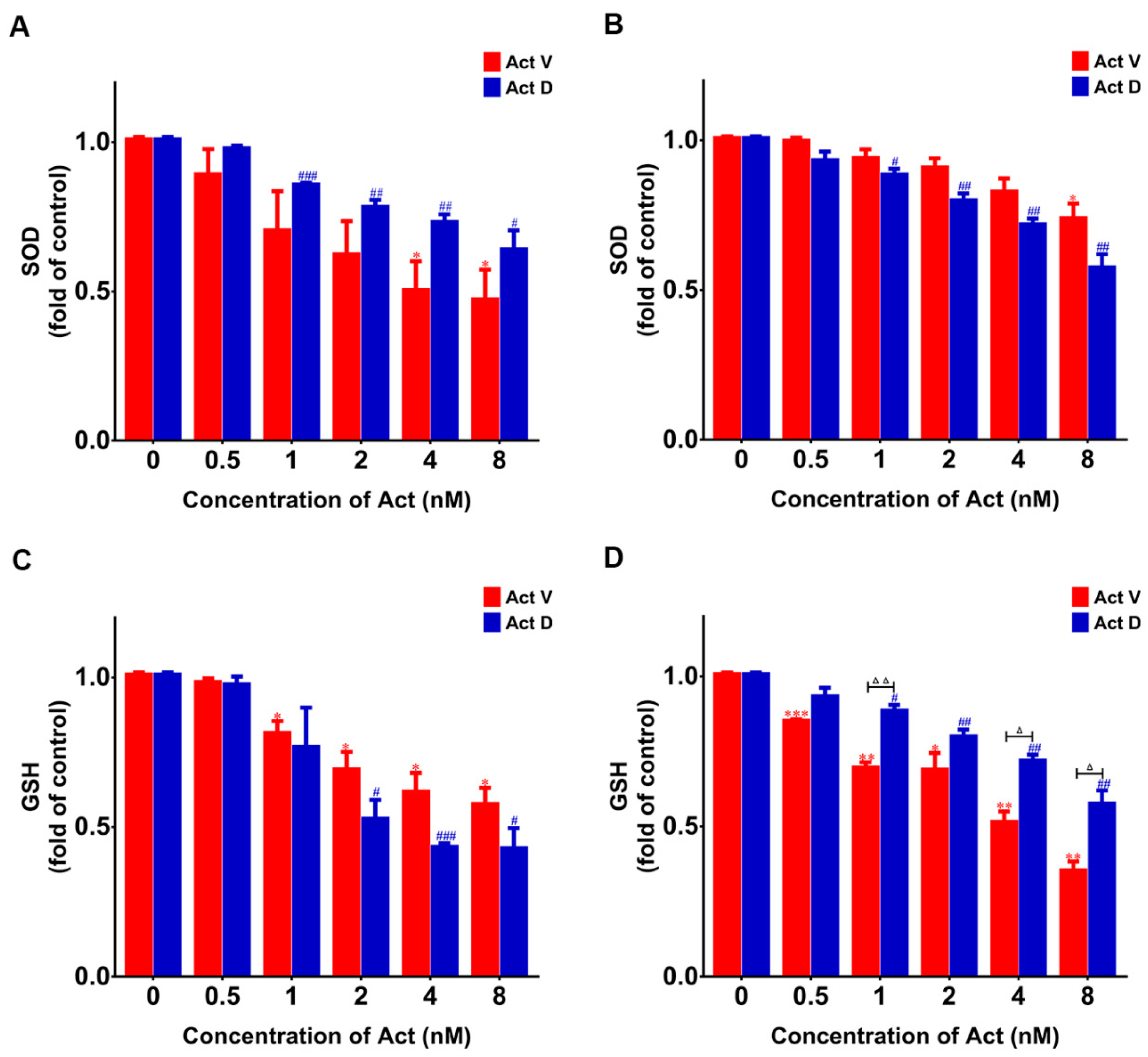
2.7. Act V and Act D Reduce Radical Scavengers Levels in LO-2 and 293T Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals
4.3. Treatments and Sample Collection
4.4. Histopathological Examination
4.5. Estimation of Liver and Kidney Function Markers in Mouse Serum
4.6. Cell Lines
4.7. MTT Assay
4.8. Flow Cytometric Analysis of ROS Levels
4.9. Measurement of MDA, GSH and SOD Content
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, X.; Ye, X.; Chai, W.; Lian, X.-Y.; Zhang, Z. New Metabolites and Bioactive Actinomycins from Marine-Derived Streptomyces sp. ZZ338. Mar. Drugs 2016, 14, 181. [Google Scholar] [CrossRef] [Green Version]
- Perry, R.P.; Kelley, D.E. Inhibition of RNA synthesis by actinomycin-D: Characteristic dose-response of different RNA species. J. Cell. Physiol. 1970, 76, 127–139. [Google Scholar] [CrossRef]
- Malogolowkin, M.; Cotton, C.A.; Green, D.M.; Breslow, N.E.; Perlman, E.; Miser, J.; Ritchey, M.L.; Thomas, P.R.M.; Grundy, P.E.; D’Angio, G.J.; et al. Treatment of Wilms tumor relapsing after initial treatment with vincristine, actinomycin D, and doxorubicin. A report from the National Wilms Tumor Study group. Pediatric Blood Cancer 2008, 50, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Falini, B.; Brunetti, L.; Martelli, M.P. Dactinomycin in NPM1-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2015, 373, 1180–1182. [Google Scholar] [CrossRef]
- Jaffe, N.; Traggis, D.; Salian, S.; Cassady, J.R. Improved outlook for ewings sarcoma with combination chemotherapy (vincristine, actinomycin-d and cyclophosphamide) and radiation-therapy. Cancer 1976, 38, 1925–1930. [Google Scholar] [CrossRef]
- Lertkhachonsuk, A.-A.; Hanvoravongchai, P. Comparison of Cost-Effectiveness between Actinomycin D versus Methotrexate-Folinic Acid in the Treatment of Low-Risk Gestational Trophoblastic Neoplasia. J. Reprod. Med. 2016, 61, 230–234. [Google Scholar] [PubMed]
- Xie, Y.; Liu, D.; Cai, C.; Chen, X.; Zhou, Y.; Wu, L.; Sun, Y.; Dai, H.; Kong, X.; Liu, P. Size-dependent cytotoxicity of Fe3O4 nanoparticles induced by biphasic regulation of oxidative stress in different human hepatoma cells. Int. J. Nanomed. 2016, 11, 3557–3570. [Google Scholar]
- Hill, C.R.; Cole, M.; Errington, J.; Malik, G.; Boddy, A.V.; Veal, G.J. Characterisation of the Clinical Pharmacokinetics of Actinomycin D and the Influence of ABCB1 Pharmacogenetic Variation on Actinomycin D Disposition in Children with Cancer. Clin. Pharmacokinet. 2014, 53, 741–751. [Google Scholar] [CrossRef] [Green Version]
- Goldberg, I.H.; Rabinowicz, M.; Reich, E. Basis of actinomycin action, I. dna binding and inhibition of rna-polymerase synthetic reactions by actinomycin. Proc. Natl. Acad. Sci. USA 1962, 48, 2094–2101. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.; Zhang, C.; Liu, F.; Ma, J.; Jia, F.; Han, Z.; Xie, W.; Li, X. Actinomycin V Inhibits Migration and Invasion via Suppressing Snail/Slug-Mediated Epithelial-Mesenchymal Transition Progression in Human Breast Cancer MDA-MB-231 Cells In Vitro. Mar. Drugs 2019, 17, 305. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.-Q.; Jia, F.-J.; Zhang, C.-Y.; Liu, F.-Y.; Ma, J.-H.; Han, Z.; Xie, W.-D.; Li, X. Actinomycin V Suppresses Human Non-Small-Cell Lung Carcinoma A549 Cells by Inducing G2/M Phase Arrest and Apoptosis via the p53-Dependent Pathway. Mar. Drugs 2019, 17, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartung, T.; Daston, G. Are In Vitro Tests Suitable for Regulatory Use? Toxicol. Sci. 2009, 111, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuykx, M.; Rodrigues, R.M.; Laukens, K.; Vanhaecke, T.; Covaci, A. In vitro assessment of hepatotoxicity by metabolomics: A review. Arch. Toxicol. 2018, 92, 3007–3029. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, A.H.; Ozturk, O.; Ustebay, S.; Adali, Y.; Yagmurdur, H. The beneficial effects of ozone therapy in acetaminophen-induced hepatotoxicity in mice. Pharmacol. Rep. 2018, 70, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fan, H.; Li, Y.; Xiao, X. Research Advances on Hepatotoxicity of Herbal Medicines in China. Biomed Res. Int. 2016. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Meng, X.; Wang, F.; Bao, Y.; Huo, J. Eriodictyol attenuates arsenic trioxide-induced liver injury by activation of Nrf2. Oncotarget 2017, 8, 68668–68674. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chi, X.; Wang, Z.; Bi, S.; Wang, Y.; Shi, F.; Hu, S.; Wang, H. Protective effects of Panax notoginseng saponins on PME-Induced nephrotoxicity in mice. Biomed. Pharmacother. 2019, 116, 108970. [Google Scholar] [CrossRef]
- Gan, L.; Xiang, W.; Xie, B.; Yu, L. Molecular mechanisms of fatty liver in obesity. Front. Med. 2015, 9, 275–287. [Google Scholar] [CrossRef]
- Cortes, C.L.; Veiga, S.R.; Almacellas, E.; Hernandez-Losa, J.; Ferreres, J.C.; Kozma, S.C.; Ambrosio, S.; Thomas, G.; Tauler, A. Effect of low doses of actinomycin D on neuroblastoma cell lines. Mol. Cancer 2016, 15, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.-R.; Bai, Z.-T.; Sun, Y.; Chen, P.; Yang, Z.-G.; Zhi, D.-J.; Li, Y.; Wang, X.; Du, J.-J.; Yang, R.; et al. Protective effects of the bioactive natural product N-trans-Caffeoyldopamine on hepatotoxicity induced by isoniazid and rifampicin. Bioorg. Med. Chem. Lett. 2015, 25, 5424–5426. [Google Scholar] [CrossRef]
- Boroushaki, M.T.; Mollazadeh, H.; Rajabian, A.; Dolati, K.; Hoseini, A.; Paseban, M.; Farzadnia, M. Protective effect of pomegranate seed oil against mercuric chloride-induced nephrotoxicity in rat. Ren. Fail. 2014, 36, 1581–1586. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.M.; Li, J.-M. Evaluation of methods of detecting cell reactive oxygen species production for drug screening and cell cycle studies. J. Pharmacol. Toxicol. Methods 2014, 70, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, L.; Wang, R.; Luo, X.; Li, Y.; Chen, Z. Protective effects of rice dreg protein hydrolysates against hydrogen peroxide-induced oxidative stress in HepG-2 cells. Food Funct. 2016, 7, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.-y.; Nie, S.-p.; Huang, X.-j.; Hu, J.-l.; Cui, S.W.; Xie, M.-y.; Phillips, G.O. Study on Dendrobium officinale O-Acetyl-glucomannan (Dendronan). 7. Improving Effects on Colonic Health of Mice. J. Agric. Food Chem. 2016, 64, 2485–2491. [Google Scholar] [CrossRef] [PubMed]
- Catala, A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids 2009, 157, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.M.; Salem, A.A.M.; Eassawy, M.M.T. Hepatoprotective effect of grape seed oil against carbon tetrachloride induced oxidative stress in liver of gamma-irradiated rat. J. Photochem. Photobiol. B-Biol. 2016, 160, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Adeyemi, D.O.; Ukwenya, V.O.; Obuotor, E.M.; Adewole, S.O. Anti-hepatotoxic activities of Hibiscus sabdariffa L. in animal model of streptozotocin diabetes-induced liver damage. Bmc Complementary Altern. Med. 2014, 14, 277. [Google Scholar] [CrossRef] [Green Version]
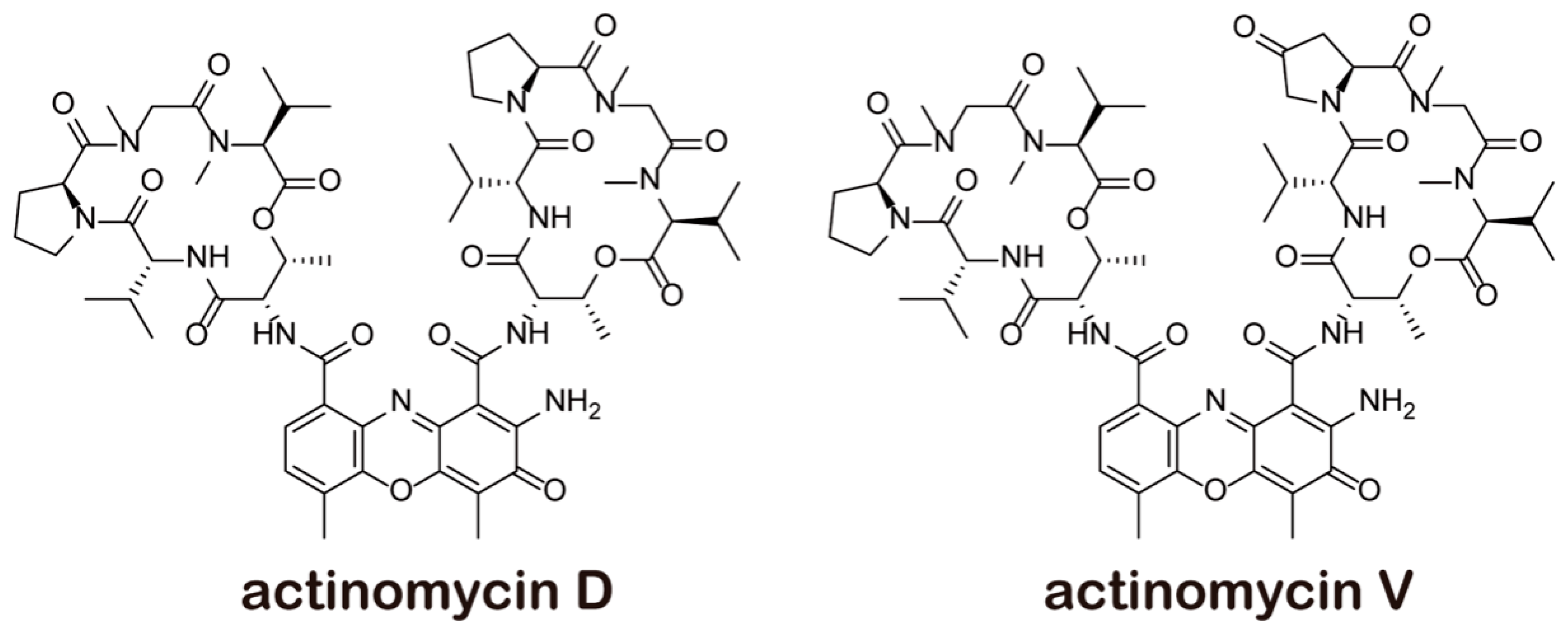
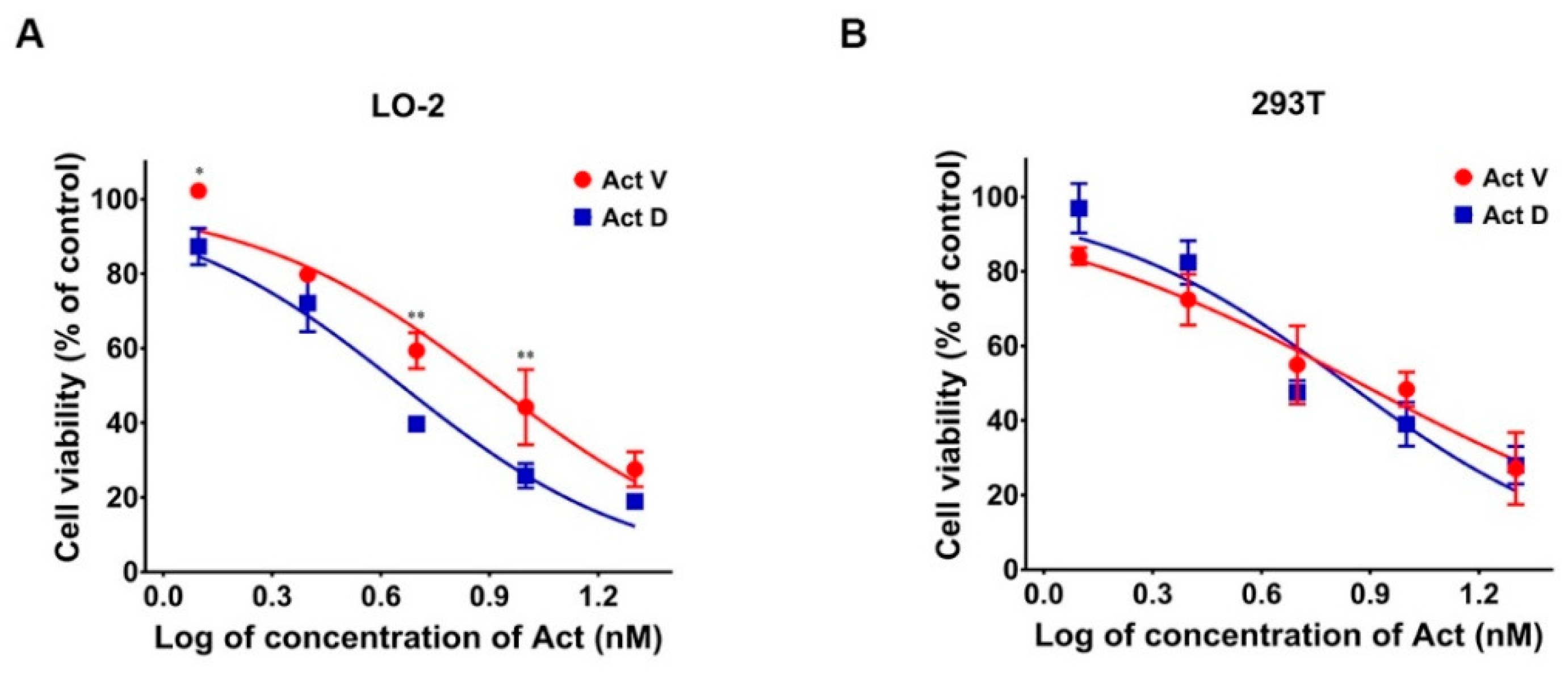
| Cell Lines IC50 (μmol/L) | Act V | Act D |
|---|---|---|
| LO-2 | 0.0828 ± 0.0015 | 0.0382 ± 0.0015 |
| 293T | 0.0750 ± 0.0008 | 0.0675 ± 0.0025 |
| A549 | 0.0068 ± 0.0006 | 0.1284 ± 0.0072 |
| MDA-MB-231 | 0.0083 ± 0.0032 | 0.1515 ± 0.0052 |
| K562 | 0.0046 ± 0.0001 | 0.1272 ± 0.0033 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, F.-j.; Han, Z.; Ma, J.-h.; Jiang, S.-q.; Zhao, X.-m.; Ruan, H.; Xie, W.-d.; Li, X. Involvement of Reactive Oxygen Species in the Hepatorenal Toxicity of Actinomycin V In Vitro and In Vivo. Mar. Drugs 2020, 18, 428. https://doi.org/10.3390/md18080428
Jia F-j, Han Z, Ma J-h, Jiang S-q, Zhao X-m, Ruan H, Xie W-d, Li X. Involvement of Reactive Oxygen Species in the Hepatorenal Toxicity of Actinomycin V In Vitro and In Vivo. Marine Drugs. 2020; 18(8):428. https://doi.org/10.3390/md18080428
Chicago/Turabian StyleJia, Fu-juan, Zhuo Han, Jia-hui Ma, Shi-qing Jiang, Xing-ming Zhao, Hang Ruan, Wei-dong Xie, and Xia Li. 2020. "Involvement of Reactive Oxygen Species in the Hepatorenal Toxicity of Actinomycin V In Vitro and In Vivo" Marine Drugs 18, no. 8: 428. https://doi.org/10.3390/md18080428
APA StyleJia, F.-j., Han, Z., Ma, J.-h., Jiang, S.-q., Zhao, X.-m., Ruan, H., Xie, W.-d., & Li, X. (2020). Involvement of Reactive Oxygen Species in the Hepatorenal Toxicity of Actinomycin V In Vitro and In Vivo. Marine Drugs, 18(8), 428. https://doi.org/10.3390/md18080428





