Current Research Landscape of Marine-Derived Anti-Atherosclerotic Substances
Abstract
1. Introduction
2. Polysaccharides
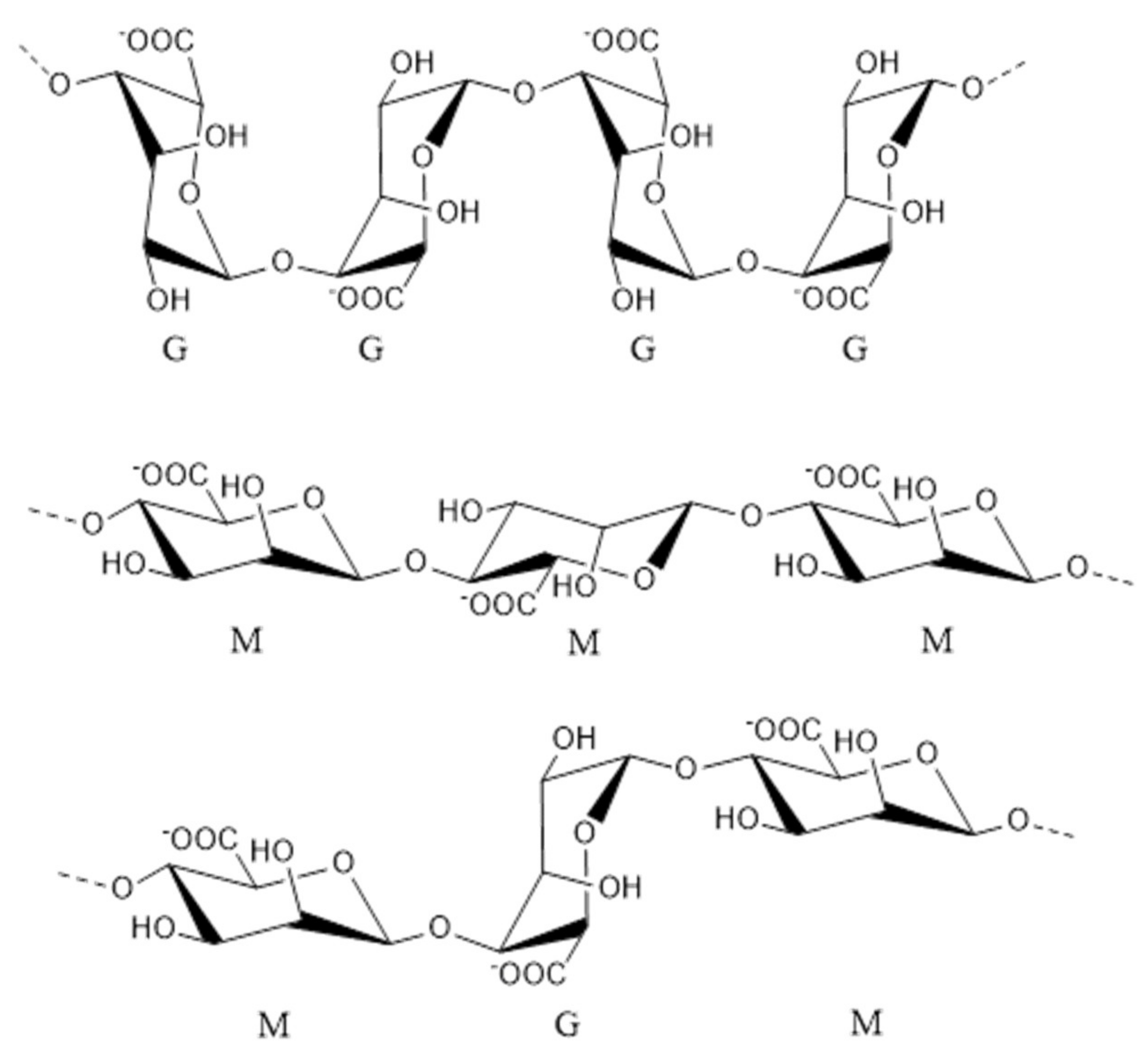
2.1. Fucoidan
2.2. Alginate
2.3. Ulvan
2.4. Enteromorpha Prolifera Polysaccharides
2.5. Porphyra Polysaccharides
2.6. Chondroitin Sulfate
2.7. Chitosan
2.8. Summary
3. Proteins and Peptides
4. Polyunsaturated Fatty Acids
5. Small Molecule Compounds
5.1. Astaxanthin
5.2. Sponge Extract
5.3. Sea Cucumber Saponins
5.4. Asperlin
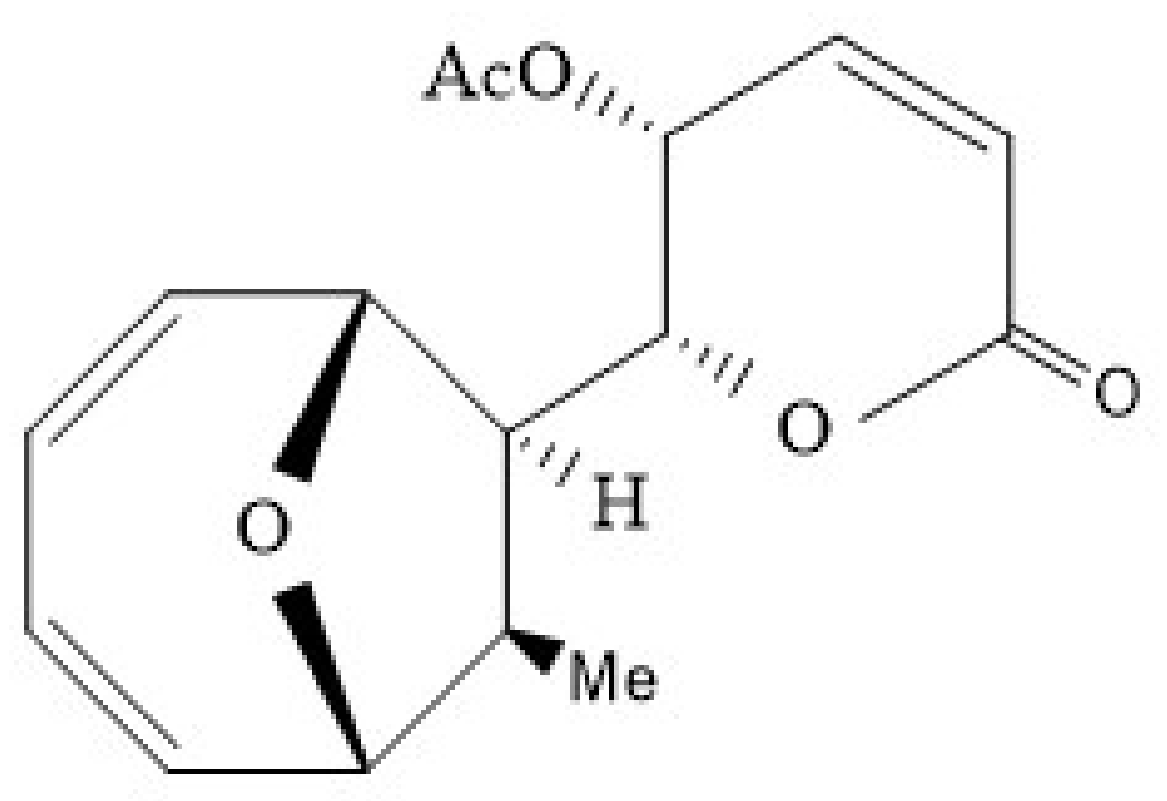
5.5. Mycoepoxydiene
5.6. Xyloketal B
5.7. Summary
6. Concluding Remarks and Future Outlooks
Author Contributions
Funding
Conflicts of Interest
References
- Mcmahan, C.A.; Gidding, S.S.; McGill, H.C., Jr. Coronary heart disease risk factors and atherosclerosis in young people. J. Clin. Lipidol. 2008, 2, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Staffa, J.A.; Shatin, D.; Andrade, S.E.; Schech, S.D.; La Grenade, L.; Gurwitz, J.H.; Chan, K.A.; Goodman, M.J.; Platt, R. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004, 292, 2585–2590. [Google Scholar] [CrossRef] [PubMed]
- Magni, P.; Macchi, C.; Morlotti, B.; Sirtori, C.R.; Ruscica, M. Risk identification and possible countermeasures for muscle adverse effects during statin therapy. Eur. J. Intern. Med. 2015, 26, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Patil, N.P.; Le, V.; Sligar, A.D.; Mei, L.; Chavarria, D.; Yang, E.Y.; Baker, A.B. Algal polysaccharides as therapeutic agents for atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 153. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C. Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Haslam, S.M.; McDowell, R.A.; Shashkov, A.S.; Nifant’Ev, N.E.; Khatuntseva, E.A.; Usov, A.I. A study of fucoidan from the brown seaweed Chorda filum. Carbohydr. Res. 1999, 320, 108–119. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Li, Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008, 42, 127–132. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar] [CrossRef]
- Deniaud-Bouët, E.; Hardouin, K.; Potin, P.; Kloareg, B.; Hervé, C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017, 175, 395–408. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [PubMed]
- Van Weelden, G.; Bobiński, M.; Okła, K.; Van Weelden, W.J.; Romano, A.; Pijnenborg, J.M. Fucoidan structure and activity in relation to anti-cancer mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhu, W.; Wang, T.; Jin, L.; Liu, T.; Li, X.; Guan, Z.; Jiang, Z.; Meng, X.; Wang, J.; et al. Low molecule weight fucoidan mitigates atherosclerosis in ApoE (-/-) mouse model through activating multiple signal pathway. Carbohydr. Polym. 2019, 206, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, J.; Ge, K.; Tian, Q.; Zhao, P.; Guo, Y. Anti-inflammatory effect of low molecular weight fucoidan from Saccharina japonica on atherosclerosis in apoE-knockout mice. Int. J. Biol. Macromol. 2018, 118, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Yokota, T.; Nomura, K.; Nagashima, M.; Kamimura, N. Fucoidan alleviates high-fat diet-induced dyslipidemia and atherosclerosis in ApoE(shl) mice deficient in apolipoprotein E expression. J. Nutr. Biochem. 2016, 32, 46–54. [Google Scholar] [CrossRef]
- Fulcher, J.; O’Connell, R.; Voysey, M.; Emberson, J.R.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; Colhoun, H.M.; et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174 000 participants in 27 randomised trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [CrossRef]
- Park, J.; Yeom, M.; Hahm, D.H. Fucoidan improves serum lipid levels and atherosclerosis through hepatic SREBP-2-mediated regulation. J. Pharmacol. Sci. 2016, 131, 84–92. [Google Scholar] [CrossRef]
- Krizshanovsky, S.P.; Kuznetsova, T.A.; Geltser, B.I.; Zaporozhets, T.S.; Ermakova, S.P.; Besednova, N.N. Fucoidan from brown algae fucus evanescens: New perspectives in the treatment of atherosclerosis. Russ. J. Biotherapy 2017, 16, 82–87. [Google Scholar] [CrossRef]
- Huang, L.; Wen, K.; Gao, X.; Liu, Y. Hypolipidemic effect of fucoidan from Laminaria japonica in hyperlipidemic rats. Pharm. Biol. 2010, 48, 422–426. [Google Scholar] [CrossRef]
- Xu, H.; Wang, J.; Chang, Y.; Xu, J.; Wang, Y.; Long, T.; Xue, C. Fucoidan from the sea cucumber Acaudina molpadioides exhibits anti-adipogenic activity by modulating the Wnt/beta-catenin pathway and down-regulating the SREBP-1c expression. Food Funct. 2014, 5, 1547–1555. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Mao, G.; Wu, T.; Hu, Y.; Ye, X.; Tian, D.; Linhardt, R.J.; Chen, S.; Ding, T. A fucoidan from sea cucumber Pearsonothuria graeffei with well-repeated structure alleviates gut microbiota dysbiosis and metabolic syndromes in HFD-fed mice. Food Funct. 2018, 9, 5371–5380. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, J.; Li, F.; Yang, Z.; Yang, X.; Sun, W.; Xia, B.; Li, T.; Song, W.; Guo, S. The fucoidan from the brown seaweed Ascophyllum nodosum ameliorates atherosclerosis in apolipoprotein E-deficient mice. Food Funct. 2019, 10, 5124–5139. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, G.; Wang, Y.; Yin, J.; Wang, J.; Xia, B.; Li, T.; Yang, X.; Hou, P.; Hu, S.; et al. Fucoidan A2 from the brown seaweed ascophyllum nodosum lowers lipid by improving reverse cholesterol transport in C57BL/6J mice fed a high-fat diet. J. Agric. Food Chem. 2019, 67, 5782–5791. [Google Scholar] [CrossRef]
- Yang, Z.; Yin, J.; Wang, Y.; Wang, J.; Xia, B.; Li, T.; Yang, X.; Hu, S.; Ji, C.; Yin, J. The fucoidan A3 from the seaweed Ascophyllum nodosum enhances RCT-related genes expression in hyperlipidemic C57BL/6J mice. Int. J. Biol. Macromol. 2019, 134, 759–769. [Google Scholar] [CrossRef]
- Wang, X.; Pei, L.L.; Liu, H.B.; Qv, K.; Xian, W.W.; Liu, J.; Zhang, G.M. Fucoidan attenuates atherosclerosis in LDLR-/- mice through inhibition of inflammation and oxidative stress. Int. J. Clin. Exp. Patho. 2016, 9, 6896–6904. [Google Scholar]
- Kuznetsova, T.A.; Ivanushko, L.A.; Persiyanova, E.V.; Ermakova, S.P.; Besednova, N.N. Markers of systemic inflammation in experimental dyslipidemia induced by P-407: Modulation with fucoidan from brown alga fucus evanescens. Bull. Exp. Biol. Med. 2019, 166, 766–769. [Google Scholar] [CrossRef]
- Yin, J.; Yang, X.; Xia, B.; Yang, Z.; Wang, Z.; Wang, J.; Li, T.; Lin, P.; Song, X.; Yin, J. The fucoidan from sea cucumber Apostichopus japonicus attenuates lipopolysaccharide-challenged liver injury in C57BL/6J mice. J. Funct. Foods 2019, 61, 103493. [Google Scholar] [CrossRef]
- Lee, S.H.; Ko, C.I.; Ahn, G.; You, S.; Kim, J.S.; Heu, M.S.; Kim, J.; Jee, Y.; Jeon, Y.J. Molecular characteristics and anti-inflammatory activity of the fucoidan extracted from Ecklonia cava. Carbohydr. Polym. 2012, 89, 599–606. [Google Scholar] [CrossRef]
- Sanjeewa, K.; Jayawardena, T.U.; Kim, S.Y.; Kim, H.S.; Ahn, G.; Kim, J.I.; Jeon, Y.J. Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-κB and MAPK pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Fernando, I.S.; Sanjeewa, K.; Kim, S.Y.; Lee, J.S.; Jeon, Y.J.; Sanjeewa, K.K.A. Reduction of heavy metal (Pb2+) biosorption in zebrafish model using alginic acid purified from Ecklonia cava and two of its synthetic derivatives. Int. J. Biol. Macromol. 2018, 106, 330–337. [Google Scholar] [CrossRef]
- Park, J.; Kwak, C.H.; Ha, S.H.; Kwon, K.M.; Abekura, F.; Cho, S.H.; Chang, Y.C.; Lee, Y.C.; Ha, K.T.; Chung, T.W.; et al. Ganglioside GM3 suppresses lipopolysaccharide-induced inflammatory responses in rAW 264.7 macrophage cells through NF-κB, AP-1 and MAPKs signaling. J. Cell. Biochem. 2017, 119, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Massberg, S.; Brand, K.; Grüner, S.; Page, S.; Müller, E.; Müller, I.; Bergmeier, W.; Richter, T.; Lorenz, M.; Konrad, I.; et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J. Exp. Med. 2002, 196, 887–896. [Google Scholar] [CrossRef] [PubMed]
- Jacobin-Valat, M.; Deramchia, K.; Mornet, S.; Hagemeyer, C.E.; Bonetto, S.; Robert, R.; Biran, M.; Massot, P.; Miraux, S.; Sanchez, S.; et al. MRI of inducible P-selectin expression in human activated platelets involved in the early stages of atherosclerosis. NMR Biomed. 2010, 24, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Von Andrian, U.H.; Hansell, P.; Chambers, J.D.; Berger, E.M.; Filho, I.T.; Butcher, E.C.; Arfors, K.E. L-selectin function is required for beta 2-integrin-mediated neutrophil adhesion at physiological shear rates in vivo. Am. J. Physiol. Circ. Physiol. 1992, 263, H1034–H1044. [Google Scholar] [CrossRef] [PubMed]
- Kubo, H.; Doyle, N.A.; Graham, L.; Bhagwan, S.D.; Quinlan, W.M.; Doerschuk, C.M. L- and P-selectin and CD11/CD18 in intracapillary neutrophil sequestration in rabbit lungs. Am. J. Respir. Crit. Care Med. 1999, 159, 267–274. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Q.; Luo, D.; Wang, J.; Duan, D. Low molecular weight fucoidan modulates P-selectin and alleviates diabetic nephropathy. Int. J. Biol. Macromol. 2016, 91, 233–240. [Google Scholar] [CrossRef]
- Preobrazhenskaya, M.E.; Berman, A.E.; Mikhailov, V.I.; Ushakova, N.A.; Mazurov, A.V.; Semenov, A.V.; Usov, A.I.; Nifant’Ev, N.E.; Bovin, N.V. Fucoidan inhibits leukocyte recruitment in a model peritonial inflammation in rat and blocks interaction of P-selectin with its carbohydrate ligand. Biochem. Mol. Biol. Int. 1997, 43, 443–451. [Google Scholar] [CrossRef]
- Thorlacius, H.; Vollmar, B.; Seyfert, U.T.; Vestweber, D.; Menger, M.D. The polysaccharide fucoidan inhibits microvascular thrombus formation independently from P- and l-selectin function in vivo. Eur. J. Clin. Investig. 2000, 30, 804–810. [Google Scholar] [CrossRef]
- Woollard, K.J.; Chin-Dusting, J. Therapeutic targeting of p-selectin in atherosclerosis. Inflamm. Allergy Drug Targets 2007, 6, 69–74. [Google Scholar] [CrossRef]
- Yamamoto, K.; Nishimura, N.; Doi, T.; Imanishi, T.; Kodama, T.; Suzuki, K.; Tanaka, T. The lysine cluster in the collagen-like domain of the scavenger receptor provides for its ligand binding and ligand specificity. FEBS Lett. 1997, 414, 182–186. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Hajjar, D.P.; Khan, K.M.F.; Falcone, D.J. Ligand binding to macrophage scavenger receptor-A induces urokinase-type plasminogen activator expression by a protein kinase-dependent signaling pathway. J. Biol. Chem. 1998, 273, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Chiu, S.L.; Wen, M.H.; Chen, K.Y.; Hua, K.F. Ligands of macrophage scavenger receptor induce cytokine expression via differential modulation of protein kinase signaling pathways. J. Biol. Chem. 2001, 276, 28719–28730. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Suzuki, H.; Wada, Y.; Kodama, T.; Doi, T. Fucoidan induces nitric oxide production via p38 mitogen-activated protein kinase and NF-kappaB-dependent signaling pathways through macrophage scavenger receptors. Biochem. Biophys. Res. Commun. 2006, 343, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxidative Med. Cell. Longev. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Panth, N.; Paudel, K.R.; Parajuli, K. Reactive oxygen species: A key hallmark of cardiovascular disease. Adv. Med. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Religa, P.; Kazi, M.; Thyberg, J.; Gaciong, Z.; Swedenborg, J.; Hedin, U. Fucoidan inhibits smooth muscle cell proliferation and reduces mitogen-activated protein kinase activity. Eur. J. Vasc. Endovasc. Surg. 2000, 20, 419–426. [Google Scholar] [CrossRef]
- Vreeland, V.; Laetsch, W.M. Identification of associating carbohydrate sequences with labeled oligosaccharides–Localization of alginate-gelling subunits in cells walls of a brown alga. Planta 1989, 177, 423–434. [Google Scholar] [CrossRef]
- Li, L.; Jiang, X.; Guan, H.; Wang, P. Preparation, purification and characterization of alginate oligosaccharides degraded by alginate lyase from Pseudomonas sp. HZJ 216. Carbohydr. Res. 2011, 346, 794–800. [Google Scholar] [CrossRef]
- Augst, A.D.; Kong, H.J.; Mooney, D.J. Alginate hydrogels as biomaterials. Macromol. Biosci. 2006, 6, 623–633. [Google Scholar] [CrossRef]
- Haug, A.; Larsen, B.; Smidsrød, O.; Eriksson, G.; Blinc, R.; Pausak, S.; Ehrenberg, L.; Dumanović, J. Studies on the sequence of uronic acid residues in alginic acid. Acta Chem. Scand. 1967, 21, 691–704. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Jiang, X.; Hwang, H.; Liu, S.; Guan, H. Antitumour activities of alginate-derived oligosaccharides and their sulfated substitution derivatives. Eur. J. Phycol. 2004, 39, 67–71. [Google Scholar] [CrossRef]
- Hou, W.; Han, L.; Li, M.; Chen, J.; Chen, Y. Effectiveness evaluation of Alginate Oligosaccharides antibacterial gel for bacterial vaginosis. Life Sci. J. 2014, 11, 528–531. [Google Scholar]
- Zhou, R.; Shi, X.; Gao, Y.; Cai, N.; Jiang, Z.; Xu, X. Anti-inflammatory activity of guluronate oligosaccharides obtained by oxidative degradation from alginate in lipopolysaccharide-activated murine macrophage RAW 264.7 cells. J. Agric. Food Chem. 2015, 63, 160–168. [Google Scholar] [CrossRef]
- Tusi, S.K.; Khalaj, L.; Ashabi, G.; Kiaei, M.; Khodagholi, F. Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 2011, 32, 5438–5458. [Google Scholar] [CrossRef]
- Idota, Y.; Kogure, Y.; Kato, T.; Ogawa, M.; Kobayashi, S.; Kakinuma, C.; Yano, K.; Arakawa, H.; Miyajima, C.; Kasahara, F.; et al. Cholesterol-lowering effect of calcium alginate in Rats. Biol. Pharm. Bull. 2016, 39, 62–67. [Google Scholar] [CrossRef]
- Lin, C.Z.; Guan, H.S.; Li, H.H.; Yu, G.L.; Gu, C.X.; Li, G.Q. The influence of molecular mass of sulfated propylene glycol ester of low-molecular-weight alginate on anticoagulant activities. Eur. Polym. J. 2007, 43, 3009–3015. [Google Scholar] [CrossRef]
- Iwamoto, M.; Kurachi, M.; Nakashima, T.; Kim, D.; Yamaguchi, K.; Oda, T.; Iwamoto, Y.; Muramatsu, T. Structure-activity relationship of alginate oligosaccharides in the induction of cytokine production from RAW264.7 cells. FEBS Lett. 2005, 579, 4423–4429. [Google Scholar] [CrossRef]
- Kawada, A.; Hiura, N.; Tajima, S.; Takahara, H. Alginate oligosaccharides stimulate VEGF-mediated growth and migration of human endothelial cells. Arch. Dermatol. Res. 1999, 291, 542–547. [Google Scholar] [CrossRef]
- Suzuki, T.; Nakai, K.; Yoshie, Y.; Shirai, T.; Hirano, T. Effect of sodium alginates rich in guluronic and mannuronic acids on cholesterol levels and digestive organs of high-cholesterol-fed Rats. NIPPON SUISAN GAKKAISHI 1993, 59, 545–551. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Liu, F.; Gao, Y.; Xue, C.H.; Li, R.W.; Tang, Q.J. Transcriptome analysis revealed anti-obesity effects of the Sodium Alginate in high-fat diet -induced obese mice. Int. J. Biol. Macromol. 2018, 115, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Marounek, M.; Volek, Z.; Skřivanová, E.; Taubner, T.; Pebriansyah, A.; Dušková, D. Comparative study of the hypocholesterolemic and hypolipidemic activity of alginate and amidated alginate in rats. Int. J. Biol. Macromol. 2017, 105, 620–624. [Google Scholar] [CrossRef]
- Rudling, M.; Angelin, B. Growth hormone reduces plasma cholesterol in LDL receptor-deficient mice. FASEB J. 2001, 15, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Bang, M.A.; Jang, C.H.; Jo, G.H.; Jung, S.K.; Ki, S.H. Alginate oligosaccharide enhances LDL uptake via regulation of LDLR and PCSK9 expression. J. Nutr. Biochem. 2015, 26, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Abifadel, M.; Varret, M.; Rabès, J.P.; Allard, D.; Ouguerram, K.; Devillers, M.; Cruaud, C.; Benjannet, S.; Wickham, L.; Erlich, D.; et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat. Genet. 2003, 34, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, X.; Zhang, J.; Duan, Y.; Wang, X.; Zhang, Q. Synthesis and antihyperlipidemic activity of acetylated derivative of ulvan from Ulva pertusa. Int. J. Biol. Macromol. 2012, 50, 270–272. [Google Scholar] [CrossRef]
- Yaich, H.; Ben Amira, A.; Abbes, F.; Bouaziz, M.; Besbes, S.; Richel, A.; Blecker, C.; Attia, H.; Garna, H. Effect of extraction procedures on structural, thermal and antioxidant properties of ulvan from Ulva lactuca collected in Monastir coast. Int. J. Biol. Macromol. 2017, 105, 1430–1439. [Google Scholar] [CrossRef]
- Lahaye, M. NMR spectroscopic characterisation of oligosaccharides from two Ulva rigida ulvan samples (Ulvales, Chlorophyta) degraded by a lyase. Carbohydr. Res. 1998, 314, 1–12. [Google Scholar] [CrossRef]
- Lahaye, M.; Cimadevilla, E.A.-C.; Kuhlenkamp, R.; Quéméner, B.; Lognoné, V.; Dion, P. Chemical composition and 13C NMR spectroscopic characterisation of ulvans from Ulva (Ulvales, Chlorophyta). Environ. Biol. Fishes 1999, 11, 1–7. [Google Scholar] [CrossRef]
- Godard, M.; Décordé, K.; Ventura, E.; Soteras, G.; Baccou, J.C.; Cristol, J.P.; Rouanet, J.M. Polysaccharides from the green alga Ulva rigida improve the antioxidant status and prevent fatty streak lesions in the high cholesterol fed hamster, an animal model of nutritionally-induced atherosclerosis. Food Chem. 2009, 115, 176–180. [Google Scholar] [CrossRef]
- Hassan, S.; El-Twab, S.A.; Hetta, M.H.; Mahmoud, B. Improvement of lipid profile and antioxidant of hypercholesterolemic albino rats by polysaccharides extracted from the green alga Ulva lactuca Linnaeus. Saudi J. Biol. Sci. 2011, 18, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.Z.; Zhang, Q.B.; Li, N.; Xu, Z.H.; Wang, Y.M.; Li, Z.E. Polysaccharides from Ulva pertusa (Chlorophyta) and preliminary studies on their antihyperlipidemia activity. J. Appl. Phycol. 2003, 15, 21–27. [Google Scholar] [CrossRef]
- Qi, H.; Huang, L.; Liu, X.; Liu, D.; Zhang, Q.; Liu, S. Antihyperlipidemic activity of high sulfate content derivative of polysaccharide extracted from Ulva pertusa (Chlorophyta). Carbohydr. Polym. 2012, 87, 1637–1640. [Google Scholar] [CrossRef]
- Qi, H.; Sheng, J. The antihyperlipidemic mechanism of high sulfate content ulvan in rats. Mar. Drugs 2015, 13, 3407–3421. [Google Scholar] [CrossRef] [PubMed]
- Tair, Z.I.; Bensalah, F.; Boukortt, F. Effect of green alga Ulva lactuca polysaccharides supplementation on blood pressure and on atherogenic risk factors, in rats fed a high fat diet. Ann. Cardiol. Angeiol. 2018, 67, 133–140. [Google Scholar] [CrossRef]
- Pengzhan, Y.; Ning, L.; Xiguang, L.; Gefei, Z.; Quanbin, Z.; Pengcheng, L. Antihyperlipidemic effects of different molecular weight sulfated polysaccharides from Ulva pertusa (Chlorophyta). Pharmacol. Res. 2003, 48, 543–549. [Google Scholar] [CrossRef]
- Qi, H.; Zhao, T.; Zhang, Q.; Li, Z.; Zhao, Z.; Xing, R. Antioxidant activity of different molecular weight sulfated polysaccharides from Ulva pertusa Kjellm (Chlorophyta). Environ. Biol. Fishes 2005, 17, 527–534. [Google Scholar] [CrossRef]
- Tang, Z.; Gao, H.; Wang, S.; Wen, S.; Qin, S. Hypolipidemic and antioxidant properties of a polysaccharide fraction from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 58, 186–189. [Google Scholar] [CrossRef]
- Cho, M.; Yang, C.; Kim, S.M.; You, S. Molecular characterization and biological activities of watersoluble sulfated polysaccharides from Enteromorpha prolifera. Food Sci. Biotechnol. 2010, 19, 525–533. [Google Scholar] [CrossRef]
- Yu, Y.; Li, Y.; Du, C.; Mou, H.; Wang, P. Compositional and structural characteristics of sulfated polysaccharide from Enteromorpha prolifera. Carbohydr. Polym. 2017, 165, 221–228. [Google Scholar] [CrossRef]
- Teng, Z.; Qian, L.; Zhou, Y. Hypolipidemic activity of the polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2013, 62, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Gong, J.; Zhao, Y.; Zhuang, X.; Ye, Y.; Lin, W. Sulfated polysaccharides from Enteromorpha prolifera suppress SREBP-2 and HMG-CoA reductase expression and attenuate non-alcoholic fatty liver disease induced by a high-fat diet. Food Funct. 2017, 8, 1899–1904. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Yang, Z.; Zhao, A.; Huang, Y.; Lin, S.; Gong, J.; Chen, J.; Zhu, P.; Huang, F.; Lin, W. Sulfated polysaccharide from Enteromorpha prolifera increases hydrogen sulfide production and attenuates non-alcoholic fatty liver disease in high-fat diet rats. Food Funct. 2018, 9, 4376–4383. [Google Scholar] [CrossRef] [PubMed]
- Frishman, W. Biologic markers as predictors of cardiovascular disease. Am. J. Med. 1998, 104, 18S–27S. [Google Scholar] [CrossRef]
- Jiang, C.; Xiong, Q.; Gan, D.; Jiao, Y.; Liu, J.; Ma, L.; Zeng, X. Antioxidant activity and potential hepatoprotective effect of polysaccharides from Cyclina sinensis. Carbohydr. Polym. 2013, 91, 262–268. [Google Scholar] [CrossRef]
- Wang, Z. Research progress of bioactives from porphyra yezoensis. Food Res. Dev. 2017, 10, 215–218. [Google Scholar]
- Ao-qiong, Z.; Yi-feng, R.; Rui-jin, Y. Inhibitory effect of polysaccharide from Porphyra yezoensis on α-amylase. Biologicals 2019, 32, 396–402. [Google Scholar]
- Zhang, Q.; Li, N.; Liu, X.; Zhao, Z.; Li, Z.; Xu, Z. The structure of a sulfated galactan from Porphyra haitanensis and its in vivo antioxidant activity. Carbohydr. Res. 2004, 339, 105–111. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Xiao, L.; Liu, C.; Qi, H.; Zhang, Z. In vivo antihyperlipidemic and antioxidant activity of porphyran in hyperlipidemic mice. Carbohydr. Polym. 2017, 174, 417–420. [Google Scholar] [CrossRef]
- Qian, L.; Zhou, Y.; Ma, J.X. Hypolipidemic effect of the polysaccharides from Porphyra yezoensis. Int. J. Biol. Macromol. 2014, 68, 48–49. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, N.; Zhou, G.; Lu, X.; Xu, Z.; Li, Z. In vivo antioxidant activity of polysaccharide fraction from Porphyra haitanesis (Rhodephyta) in aging mice. Pharmacol. Res. 2003, 48, 151–155. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, S.; Qiao, M.; Jiao, R.; Li, J.; Song, P.; Zhang, X.; Huang, H. Synthesis of structurally defined chondroitin sulfate: Paving the way to the structure-activity relationship studies. Carbohydr. Polym. 2020, 248. [Google Scholar] [CrossRef]
- Higashi, K.; Takeuchi, Y.; Mukuno, A.; Tomitori, H.; Miya, M.; Linhardt, R.J.; Toida, T. Composition of Glycosaminoglycans in Elasmobranchs including Several Deep-Sea Sharks: Identification of Chondroitin/Dermatan Sulfate from the Dried Fins of Isurus oxyrinchus and Prionace glauca. PLoS ONE 2015, 10, e0120860. [Google Scholar] [CrossRef] [PubMed]
- Tamura, J.I.; Arima, K.; Imazu, A.; Tsutsumishita, N.; Fujita, H.; Yamane, M.; Matsumi, Y. Sulfation patterns and the amounts of chondroitin sulfate in the diamond squid, thysanoteuthis rhombus. Biosci. Biotechnol. Biochem. 2009, 73, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xue, C.; Yin, L.; Tang, Q.; Yu, G.; Chai, W. Comparison of structures and anticoagulant activities of fucosylated chondroitin sulfates from different sea cucumbers. Carbohydr. Polym. 2011, 83, 688–696. [Google Scholar] [CrossRef]
- Maccari, F.; Galeotti, F.; Volpi, N. Isolation and structural characterization of chondroitin sulfate from bony fishes. Carbohydr. Polym. 2015, 129, 143–147. [Google Scholar] [CrossRef]
- Volpi, N. Analytical aspects of pharmaceutical grade chondroitin sulfates. J. Pharm. Sci. 2007, 96, 3168–3180. [Google Scholar] [CrossRef]
- Melgar-Lesmes, P.; Sanchez-Herrero, A.; Lozano-Juan, F.; de la Torre Hernandez, J.M.; Montell, E.; Jimenez, W.; Edelman, E.R.; Balcells, M. Chondroitin sulfate attenuates atherosclerosis in ApoE knockout mice involving cellular regulation of the inflammatory response. Thromb. Haemost. 2018, 118, 1329–1339. [Google Scholar]
- Roman, M.J.; Devereux, R.B.; Schwartz, J.; Lockshin, M.D.; Paget, S.A.; Davis, A.; Crow, M.K.; Sammaritano, L.; Levine, D.M.; Shankar, B.A.; et al. Arterial stiffness in chronic inflammatory diseases. Hypertension 2005, 46, 194–199. [Google Scholar] [CrossRef]
- Rincón, I.; Williams, K.; Stern, M.; Freeman, G.; Escalante, A. High incidence of CV events in a RA cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001, 44, 2737–2745. [Google Scholar] [CrossRef]
- Martinez-Calatrava, M.J.; Largo, R.; Herrero-Beaumont, G. Improvement of experimental accelerated atherosclerosis by chondroitin sulfate. Osteoarthr. Cartil. 2010, 18 (Suppl. 1), S12–S16. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Beaumont, G.; Marcos, M.E.; Sanchez-Pernaute, O.; Granados, R.; Ortega, L.; Montell, E.; Verges, J.; Egido, J.; Largo, R. Effect of chondroitin sulfate in a rabbit model of atherosclerosis aggravated by chronic arthritis. Br. J. Pharmacol. 2008, 154, 843–851. [Google Scholar] [CrossRef]
- Morrison, L.M.; Murata, K.; Quilligan, J.J.; Schjeide, O.A.; Freeman, L. Prevention of atherosclerosis in sub-human primates by chondroitin sulfate A. Circ. Res. 1966, 19, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhang, Y.; Ye, X.; Hu, Y.; Ding, T.; Chen, S. Sulfation pattern of fucose branches affects the anti-hyperlipidemic activities of fucosylated chondroitin sulfate. Carbohydr. Polym. 2016, 147, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Largo, R.; Alvarez-Soria, M.A.; Sanchez-Pernaute, O.; Calvo, E.; Egido, J.; Herrero-Beaumont, G. Differential anticatabolic profile of glucosamine sulfate versus other anti-osteoarthritic drugs on human osteoarthritic chondrocytes and synovial fibroblast in culture. Osteoporosis Int. 2007, 18, S57. [Google Scholar]
- Du Souich, P.; Garcia, A.G.; Verges, J.; Montell, E. Immunomodulatory and anti-inflammatory effects of chondroitin sulfate. J. Cell Mol. Med. 2009, 13, 1451–1463. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Muzzarelli, R.A.; Ilari, P.; Petrarulo, M. Solubility and structure of. Int. J. Biol. Macromol. 1994, 16, 177–180. [Google Scholar] [CrossRef]
- Ormrod, D.J.; Holmes, C.C.; Miller, T.E. Dietary chitosan inhibits hypercholesterolemia and atherogenesis in the apolipoprotein E-deficient mouse model of atherosclerosis. Atherosclerosis 1998, 138, 329–334. [Google Scholar] [CrossRef]
- Sugano, M.; Fujikawa, T.; Hiratsuji, Y.; Nakashima, K.; Fukuda, N.; Hasegawa, Y. A novel use of chitosan as a hypocholesterolemic agent in rats. Am. J. Clin. Nutr. 1980, 33, 787–793. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, T.; Liu, S.; Song, G.H.; Han, J.J.; Wang, Y.; Yao, S.T.; Feng, L.; Qin, S.C. Chitosan oligosaccharides attenuate atherosclerosis and decrease non-HDL in ApoE-/- Mice. J. Atheroscler. Thromb. 2015, 22, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Zong, C.; Li, L.; Wang, X.; Luo, T.; Yu, Y.; Song, G.; Qin, S. Chitosan oligosaccharides promote reverse cholesterol transport and expression of scavenger receptor BI and CYP7A1 in mice. Exp. Biol. Med. 2012, 237, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.P.; Yuan, X.B.; Meng, X.P.; Jiao, S.M.; Feng, C.; Yu-Guang, D.U.; Liu, H.T.J.J.o.S.A.U. Chitosan oligosaccharides inhibit the occurrence of atherosclerosis in ApoE(-/-) mice. Journal of Shenyang Agricultural University. 2018, 49, 150–157. [Google Scholar] [CrossRef]
- Liu, H.T.; Li, W.M.; Huang, P.; Chen, W.J.; Liu, Q.S.; Bai, X.F.; Yu, C.; Du, Y.G. Chitosan oligosaccharides inhibit TNF-α-induced VCAM-1 and ICAM-1 expression in human umbilical vein endothelial cells by blocking p38 and ERK1/2 signaling pathways. Carbohydr. Polym. 2010, 81, 49–56. [Google Scholar] [CrossRef]
- Ye, M.; Zhou, J.; Zhong, Y.; Xu, J.; Hou, J.; Wang, X.; Wang, Z.; Guo, D. SR-A-targeted phase-transition nanoparticles for the detection and treatment of atherosclerotic vulnerable plaques. ACS Appl. Mater. Interfaces 2019, 11, 9702–9715. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Q.; Li, D.; An, Y.; Kutryk, M.B.J. Hydroxybutyl chitosan polymer-mediated CD133 antibody coating of metallic stents to reduce restenosis in a porcine model of atherosclerosis. J. Cardiovasc. Pharmacol. Ther. 2014, 20, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, A.; Shulmin, A.; Sharkova, A.; Patlataya, N.; Bolshakov, I. Morphological reconstruction of main arteries by perivascular implantation of sulfated chitosan in experimental atherosclerosis. Sovrem. Teh. Med. 2017, 9, 115. [Google Scholar] [CrossRef]
- Ma, L.; Chen, H.; Zhang, Y.; Zhang, N.; Fu, L. Chemical modification and antioxidant activities of polysaccharide from mushroom Inonotus obliquus. Carbohydr. Polym. 2012, 89, 371–378. [Google Scholar] [CrossRef]
- Wang, Y.; Yong-Chang, S.U.; Jing-Na, W.U.; Liu, S.J.; Cheng-Ye, W.U. Study on the hypolipidemic effects and antioxidative activity of porphyra peptide. Fish. Res. Inst. 2013, 16, 71. [Google Scholar]
- Rodríguez, E.; González, B.; Caride, M.A.; Lamas, A.; Taboada, M.A.C. Nutritional value ofHolothuria forskaliprotein and effects on serum lipid profile in rats. Biochemistry 2000, 56, 39–43. [Google Scholar]
- Lifeng, B.; Jinshuang, L.; Peigen, Z. Effects of the scallop skirt peptide extract in regulating blood lipid and anti-oxidation of rats with hyperlipidemia. J. Anhui Agric. Univ. 2008, 35, 615–618. [Google Scholar]
- Ding, J.F.; Xiu-Rong, S.U.; Yan-Yan, L.I.; Gao, X.; Yue, F.P. The hypolipidemic and antioxidative effects of jellyfish collagen peptide. Development 2012, 24, 362–365. [Google Scholar]
- Wang, J.F.; Pang, L.; Wang, Y.M.; Gao, S.; Xue, C.H. Studies on the treatment effects of pearsonothuria graeffei and apostichopus japonicus on hyperlipidemia rats. J. Ocean Univ. Chin. 2007, 4, 597–600. [Google Scholar]
- Vik, R.; Parolina, C.; Bjørndal, B.; Busnelli, A.; Holm, S.; Brattelid, T.; Manzini, S.; Ganzetti, G.; Halvorsen, B.; Aukrust, P.; et al. A salmon protein hydrolysate excerts lipid-independent anti-atherosclerotic activity in apoe-deficient mice. Atherosclerosis 2014, 235, e188. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, Y.; Liu, B.; Nawroth, P.; Dierichs, R. Endothelial cells injured by oxidized low density lipoprotein. Am. J. Hematol. 1995, 49, 250–252. [Google Scholar] [CrossRef]
- Qin, Z.; Dong, L.; Liu, H.M. Comparative study on the protective effects of collagen polypeptides from two species of sea cucumber on vascular endothelial cells. Chin. J. Mar. Drugs 2016, 35, 50–56. [Google Scholar]
- Zehua, C.; Huiyun, H.; Ying, C. Research progress of omega-3 polyunsaturated fatty acids in regulating blood lipid and atherosclerosis. Herald Med. 2018, 37, 1334–1338. [Google Scholar]
- McLaughlin, J.B.; Middaugh, J.; Boudreau, D.; Malcom, G.; Parry, S.; Tracy, R.; Newman, W. Adipose tissue triglyceride fatty acids and atherosclerosis in Alaska Natives and non-Natives. Atherosclerosis 2005, 181, 353–362. [Google Scholar] [CrossRef]
- Niki, T.; Wakatsuki, T.; Yamaguchi, K.; Taketani, Y.; Oeduka, H.; Kusunose, K.; Ise, T.; Iwase, T.; Yamada, H.; Soeki, T.; et al. Effects of the addition of eicosapentaenoic acid to strong statin therapy on inflammatory cytokines and coronary plaque components assessed by integrated backscatter intravascular ultrasound. Circ. J. 2016, 80, 450–460. [Google Scholar] [CrossRef]
- Thiès, F.; Mc Garry, J.; Yaqoob, P.; Rerkasem, K.; Williams, J.; Shearman, C.P.; Gallagher, P.J.; Calder, P.C.; Grimble, R.F. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet 2003, 361, 477–485. [Google Scholar] [CrossRef]
- Zampelas, A. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis 2010, 212, 34–35. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, E.; Flammer, J.; Lerman, L.O.; Elízaga, J.; Lerman, A.; Fernández-Avilés, F. Endothelial dysfunction over the course of coronary artery disease. Eur. Heart J. 2013, 34, 3175–3181. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.H.; Hung, T.M.; Wei, J.; Chiang, A.N. Fish oil increases antioxidant enzyme activities in macrophages and reduces atherosclerotic lesions in apoE-knockout mice. Cardiovasc. Res. 2004, 61, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Chisaki, K.; Okuda, Y.; Suzuki, S.; Miyauchi, T.; Soma, M.; Ohkoshi, N.; Sone, H.; Yamada, N.; Nakajima, T. Eicosapentaenoic acid suppresses basal and insulin-stimulated endothelin-1 production in human endothelial cells. Hypertens. Res. 2003, 26, 655–661. [Google Scholar] [CrossRef]
- Wu, S.Y.; Mayneris-Perxachs, J.; Lovegrove, J.A.; Todd, S.; Yaqoob, P. Fish-oil supplementation alters numbers of circulating endothelial progenitor cells and microparticles independently of eNOS genotype. Am. J. Clin. Nutr. 2014, 100, 1232–1243. [Google Scholar] [CrossRef]
- Lopez, D.; Orta, X.; Casós, K.; Sáiz, M.P.; Puig-Parellada, P.; Farriol, M.; Mitjavila, M.T. Upregulation of endothelial nitric oxide synthase in rat aorta after ingestion of fish oil-rich diet. Am. J. Physiol. Circ. Physiol. 2004, 287, H567–H572. [Google Scholar] [CrossRef]
- Yaqoob, P. The nutritional significance of lipid rafts. Annu. Rev. Nutr. 2009, 29, 257–282. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Wang, M.; Liu, F.; Zhao, S.; Ma, J.; Luo, N.; Li, N.; Li, Y.; Xu, G.; et al. Docosahexaenoic acid affects endothelial nitric oxide synthase in caveolae. Arch. Biochem. Biophys. 2007, 466, 250–259. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Wang, M.; Zhao, S.; Ma, J.; Luo, N.; Li, N.; Li, Y.; Xu, G.; Li, J. Eicosapentaenoic acid modifies lipid composition in caveolae and induces translocation of endothelial nitric oxide synthase. Biochim. Biophys. Acta 2007, 89, 169–177. [Google Scholar] [CrossRef]
- Backes, J.M.; Anzalone, D.; Hilleman, D.; Catini, J. The clinical relevance of omega-3 fatty acids in the management of hypertriglyceridemia. Lipids Health Dis. 2016, 15, 118. [Google Scholar] [CrossRef]
- King, A.B.; Armstrong, D.U.; Chinnapongse, S. Comparison of glycemic and lipid response to pioglitazone treatment in Mexican-Americans and non-Hispanic Caucasians with type 2 diabetes. Diabetes Care 2003, 26, 245–246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guichardant, M.; Calzada, C.; Bernoud-Hubac, N.; Lagarde, M.; Véricel, E. Omega-3 polyunsaturated fatty acids and oxygenated metabolism in atherothrombosis. Biochim. Biophys. Acta 2015, 1851, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.A.; Burke, V.; Puddey, I.B.; Watts, G.F.; O’Neal, D.N.; Best, J.; Beilin, L.J. Purified eicosapentaenoic and docosahexaenoic acids have differential effects on serum lipids and lipoproteins, LDL particle size, glucose and insulin in mildly hyperlipidemic men. Am. J. Clin. Nutr. 2000, 71, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. The role of marine omega-3 (n–3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol. Nutr. Food Res. 2012, 56, 1073–1080. [Google Scholar] [CrossRef]
- Jump, D.B. The biochemistry of n-3 polyunsaturated fatty acids. J. Biol. Chem. 2002, 277, 8755–8758. [Google Scholar] [CrossRef]
- Yoshihara, T.; Shimada, K.; Fukao, K.; Sai, E.; Sato-Okabayashi, Y.; Matsumori, R.; Shiozawa, T.; Alshahi, H.; Miyazaki, T.; Tada, N.; et al. Omega 3 polyunsaturated fatty acids suppress the development of aortic aneurysms through the inhibition of macrophage-mediated inflammation. Circ. J. 2015, 79, 1470–1478. [Google Scholar] [CrossRef]
- Calviello, G.; Su, H.M.; Weylandt, K.H.; Fasano, E.; Serini, S.; Cittadini, A. Experimental evidence of omega-3 polyunsaturated fatty acid modulation of inflammatory cytokines and bioactive lipid mediators: Their potential role in inflammatory, neurodegenerative and neoplastic diseases. Biomed. Res. Int. 2013, 2013, 743171. [Google Scholar] [CrossRef]
- Egert, S.; Baxheinrich, A.; Lee-Barkey, Y.H.; Tschoepe, D.; Wahrburg, U.; Stratmann, B. Effects of an energy-restricted diet rich in plant-derived α-linolenic acid on systemic inflammation and endothelial function in overweight-to-obese patients with metabolic syndrome traits. Br. J. Nutr. 2014, 112, 1315–1322. [Google Scholar] [CrossRef]
- Zhang, L.; Geng, Y.; Yin, M.; Mao, L.; Zhang, S.; Pan, J. Low omega-6/omega-3 polyunsaturated fatty acid ratios reduce hepatic C-reactive protein expression in apolipoprotein E-null mice. Nutrition 2010, 26, 829–834. [Google Scholar] [CrossRef]
- Waldo, S.W.; Li, Y.; Buono, C.; Zhao, B.; Billings, E.M.; Chang, J.; Kruth, H.S. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am. J. Pathol. 2008, 172, 1112–1126. [Google Scholar] [CrossRef]
- Gladine, C.; Zmojdzian, M.; Joumard-Cubizolles, L.; Verny, M.A.; Comte, B.; Mazur, A. The omega-3 fatty acid docosahexaenoic acid favorably modulates the inflammatory pathways and macrophage polarization within aorta of LDLR(-/-) mice. Genes Nutr. 2014, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Hussein, G.; Sankawa, U.; Goto, H.; Matsumoto, A.K.; Watanabe, H. Astaxanthin, a carotenoid with potential in human health and nutrition. J. Nat. Prod. 2006, 69, 443–449. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Yoshida, H.; Kondo, K. Potential anti-atherosclerotic properties of astaxanthin. Mar. Drugs 2016, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Armenta, R.E.; Guerrero-Legarreta, I.J.J.o.A.; Chemistry, F. Stability studies on astaxanthin extracted from fermented shrimp byproducts. J. Agric. Food Chem. 2009, 57, 6095–6100. [Google Scholar]
- Kumar, R.; Salwe, K.J.; Kumarappan, M. Evaluation of antioxidant, hypolipidemic and antiatherogenic property of lycopene and astaxanthin in atherosclerosis-induced rats. Pharmacogn. Res. 2017, 9, 161–167. [Google Scholar] [CrossRef]
- Li, W.; Hellsten, A.; Jacobsson, L.S.; Blomqvist, H.M.; Olsson, A.G.; Yuan, X.M. Alpha-tocopherol and astaxanthin decrease macrophage infiltration, apoptosis and vulnerability in atheroma of hyperlipidaemic rabbits. J. Mol. Cell. Cardiol. 2004, 37, 969–978. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.K.; King, T.J.; Fujioka, K.; Pattison, J.; Pashkow, F.J.; Tsimikas, S. Effect of an oral astaxanthin prodrug (CDX-085) on lipoprotein levels and progression of atherosclerosis in LDLR(-/-) and ApoE(-/-) mice. Atherosclerosis 2012, 222, 99–105. [Google Scholar] [CrossRef]
- Yang, Y.; Seo, J.M.; Nguyen, A.; Pham, T.X.; Park, H.J.; Park, Y.; Kim, B.; Bruno, R.S.; Lee, J. Astaxanthin-rich extract from the green alga haematococcus pluvialis lowers plasma lipid concentrations and enhances antioxidant defense in apolipoprotein E knockout mice. J. Nutr. 2011, 141, 1611–1617. [Google Scholar] [CrossRef]
- Zou, T.B.; Zhu, S.-S.; Luo, F.; Li, W.Q.; Sun, X.R.; Wu, H. Effects of astaxanthin on reverse cholesterol transport and atherosclerosis in mice. BioMed Res. Int. 2017, 2017, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Tian, D.; Wang, F.; Zhang, M.; Fan, C.; Chen, W.; Wang, M.; Fu, X.; Ma, J.-K. Astaxanthin inhibits homocysteine-induced endothelial cell dysfunction via the regulation of the reactive oxygen species-dependent VEGF-VEGFR2-FAK signaling pathway. Mol. Med. Rep. 2019, 19, 4753–4760. [Google Scholar] [CrossRef]
- Fan, C.D.; Sun, J.Y.; Fu, X.T.; Hou, Y.J.; Li, Y.; Yang, M.F.; Sun, B.L.; Fu, X.Y. Astaxanthin attenuates homocysteine-induced cardiotoxicity in vitro and in vivo by inhibiting mitochondrial dysfunction and oxidative damage. Front. Physiol. 2017, 8, 1041. [Google Scholar] [CrossRef] [PubMed]
- Augusti, P.R.; Conterato, G.M.M.; Somacal, S.; Sobieski, R.; Quatrin, A.; Maurer, L.; Rocha, M.P.; DeNardin, I.T.; Da Cruz, I.B.M. Astaxanthin reduces oxidative stress but not aortic damage in atherosclerotic rabbits. J. Cardiovasc. Pharmacol. Ther. 2009, 14, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.W.; Cai, W.; Lin, Y.L.; Lin, Q.F.; Jiang, Q.; Lin, Z.; Chen, L.L. Ameliorative effect of astaxanthin on endothelial dysfunction in streptozotocin-induced diabetes in male rats. Arzneimittelforschung 2011, 61, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Kogure, K.; Abe, K.; Kimata, Y.; Kitahama, K.; Yamashita, E.; Terada, H. Efficient radical trapping at the surface and inside the phospholipid membrane is responsible for highly potent antiperoxidative activity of the carotenoid astaxanthin. Biochim. Biophys. Acta 2001, 1512, 251–258. [Google Scholar] [CrossRef]
- Kishimoto, Y.; Tani, M.; Uto-Kondo, H.; Iizuka, M.; Saita, E.; Sone, H.; Kurata, H.; Kondo, K. Astaxanthin suppresses scavenger receptor expression and matrix metalloproteinase activity in macrophages. Eur. J. Nutr. 2009, 49, 119–126. [Google Scholar] [CrossRef]
- Lee, S.J.; Bai, S.-K.; Lee, K.S.; Namkoong, S.; Na, H.J.; Ha, K.S.; Han, J.A.; Yim, S.V.; Chang, K.; Kwon, Y.-G.; et al. Astaxanthin inhibits nitric oxide production and inflammatory gene expression by suppressing I(kappa)B kinase-dependent NF-kappaB activation. Mol. Cells 2003, 16, 97–105. [Google Scholar]
- Macedo, R.C.; Bolin, A.P.; Marin, D.P.; Otton, R. Astaxanthin addition improves human neutrophils function: In vitro study. Eur. J. Nutr. 2010, 49, 447–457. [Google Scholar] [CrossRef]
- Arai, T.; Wang, N.; Bezouevski, M.; Welch, C.; Tall, A.R. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J. Biol. Chem. 1999, 274, 2366–2371. [Google Scholar] [CrossRef]
- Acton, S.L.; Kozarsky, K.F.; Rigotti, A. The HDL receptor SR-BI: A new therapeutic target for atherosclerosis? Mol. Med. Today 1999, 5, 518–524. [Google Scholar] [CrossRef]
- Ahmed, R.A.M.; Murao, K.; Imachi, H.; Yu, X.; Li, J.; Wong, N.C.; Ishida, T. Human scavenger receptor class b type 1 is regulated by activators of peroxisome proliferators-activated receptor-γ in hepatocytes. J. Endocrine. 2009, 35, 233–242. [Google Scholar] [CrossRef]
- Wahab, H.A.; Pham, N.B.; Muhammad, T.S.T.; Hooper, J.N.A.; Quinn, R.J. Merosesquiterpene congeners from the australian sponge hyrtios digitatus as potential drug leads for atherosclerosis disease. Mar. Drugs 2016, 15, 6. [Google Scholar] [CrossRef]
- Mohamad, H.; Rosmiati Muhammad, T.S.T.; Andriani, Y.; Bakar, K.; Ismail, N.; Saidin, J.; Latip, J.; Musa, N.; Parenrengi, A. Potential secondary metabolites from marine sponge aaptos aaptos for atherosclerosis and vibriosis treatments. Nat. Prod. Commun. 2017, 12, 1227–1230. [Google Scholar] [CrossRef]
- Malerød, L.; Sporstøl, M.; Juvet, L.K.; Mousavi, A.; Gjøen, T.; Berg, T.O. Hepatic scavenger receptor class B, type I is stimulated by peroxisome proliferator-activated receptor γ and hepatocyte nuclear factor 4α. Biochem. Biophys. Res. Commun. 2003, 305, 557–565. [Google Scholar] [CrossRef]
- Dang, N.H.; Van Thanh, N.; Van Kiem, P.; Huong, L.M.; Van Minh, C.; Kim, Y.H. Two new triterpene glycosides from the Vietnamese sea cucumber Holothuria scabra. Arch. Pharmacal Res. 2007, 30, 1387–1391. [Google Scholar] [CrossRef]
- Kerr, R.G.; Chen, Z. In vivo and in vitro biosynthesis of saponins in sea cucumbers. J. Nat. Prod. 1995, 58, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Caulier, G.; Mezali, K.; Soualili, D.L.; DeCroo, C.; Demeyer, M.; Eeckhaut, I.; Gerbaux, P.; Flammang, P. Chemical characterization of saponins contained in the body wall and the Cuvierian tubules of the sea cucumber Holothuria (Platyperona) sanctori (Delle Chiaje, 1823). Biochem. Syst. Ecol. 2016, 68, 119–127. [Google Scholar] [CrossRef]
- Bahrami, Y.; Zhang, W.; Chataway, T.; Franco, C. Structure elucidation of five novel isomeric saponins from the viscera of the sea cucumber Holothuria lessoni. Mar. Drugs 2014, 12, 4439–4473. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Xue, C.H.; Zhang, T.T.; Wang, Y. Saponins from sea cucumber and their biological activities. J. Agric. Food Chem. 2018, 66, 7222–7237. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, T.; Che, H.X.; Zhang, L.Y.; Xue, C.; Chang, Y.; Wang, Y. Saponins of sea cucumber attenuate atherosclerosis in ApoE−/− mice via lipid-lowering and anti-inflammatory properties. J. Funct. Foods 2018, 48, 490–497. [Google Scholar] [CrossRef]
- Han, Q.A.; Jia, S.; Li, K.; Sui, Y.; Hong, H.; Dong, X.; Luo, Y.; Zhu, B.W. Thelenota ananas saponin extracts attenuate the atherosclerosis in apoE−/− mice by modulating lipid metabolism. J. Funct. Foods 2019, 58, 238–247. [Google Scholar] [CrossRef]
- Han, Q.A.; Li, K.; Dong, X.; Luo, Y.; Zhu, B.W. Function of Thelenota ananas saponin desulfated holothurin A in modulating cholesterol metabolism. Sci. Rep. 2018, 8, 9506. [Google Scholar] [CrossRef] [PubMed]
- Extracts exhibit an antiobesity effect through inhibition of pancreatic lipase activity and upregulation of LXR-beta signaling. Pharm. Biol. 2016, 54, 1312–1325. [CrossRef] [PubMed]
- Wang, D.; Ding, L.; Dong, P.; Xue, Y.; Xue, C.H.; Wang, Y.M. Regulation of cholesterol metabolism by sea cucumber saponins in rats with fatty liver. Acta Nutr. Sin. 2016, 38, 67–70. [Google Scholar]
- Chen, C.; Han, X.; Dong, P.; Li, Z.J.; Yanagita, T.; Xue, C.; Zhang, T.; Wang, Y. Sea cucumber saponin liposomes ameliorate obesity-induced inflammation and insulin resistance in high-fat-diet-fed mice. Food Funct. 2018, 9, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.S.; Jeong, G.S.; Li, B.; Lee, S.U.; Oh, H.; Kim, Y.C. Asperlin from the marine-derived fungus Aspergillus sp. SF-5044 exerts anti-inflammatory effects through heme oxygenase-1 expression in murine macrophages. J. Pharmacol. Sci. 2011, 116, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, R.; Liu, D.; Wu, C.; Guo, P.; Lin, W. Asperlin inhibits LPS-evoked foam cell formation and prevents atherosclerosis in ApoE(-/-) Mice. Mar. Drugs 2017, 15, 358. [Google Scholar] [CrossRef]
- He, L.; Nan, M.H.; Oh, H.C.; Kim, Y.H.; Jang, J.H.; Erikson, R.L.; Ahn, J.S.; Kim, B.Y. Asperlin induces G(2)/M arrest through ROS generation and ATM pathway in human cervical carcinoma cells. Biochem. Biophys. Res. Commun. 2011, 409, 489–493. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Qi, G.; Liu, D.; Cao, X.; Yu, J.; Zhang, R.; Lin, W.; Guo, P. Asperlin stimulates energy expenditure and modulates gut microbiota in HFD-Fed mice. Mar. Drugs 2019, 17, 38. [Google Scholar] [CrossRef]
- Lin, X.; Huang, Y.; Fang, M.; Wang, J.; Zheng, Z.; Su, W. Cytotoxic and antimicrobial metabolites from marine lignicolous fungi, Diaporthesp. FEMS Microbiol. Lett. 2005, 251, 53–58. [Google Scholar] [CrossRef]
- Cai, P.; McPhail, A.T.; Krainer, E.; Katz, B.; Pearce, C.; Boros, C.; Caceres, B.; Smith, D.; Houck, D.R. ChemInform abstract: Mycoepoxydiene represents a novel class of fungal metabolites. Tetrahedron Lett. 2010, 30, 1479–1482. [Google Scholar] [CrossRef]
- Takao, K.I.; Watanabe, G.; Yasui, H.; Tadano, K.I. Total synthesis of (±)-mycoepoxydiene, a novel fungal metabolite having an oxygen-bridged cyclooctadiene skeleton. Organ. Lett. 2002, 4, 2941–2943. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, T.; Li, W.; Zhang, W.; Zhu, J.; Li, Y.; Huang, Y.; Shen, Y.; Yu, C. Correction: Mycoepoxydiene inhibits lipopolysaccharide-induced inflammatory responses through the suppression of TRAF6 polyubiquitination. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, B.; Zhang, W.; Wu, X.; Wang, R.; Huang, Y.; Chen, D.; Park, K.; Weimer, B.C.; Shen, Y. Mycoepoxydiene, a fungal polyketide, induces cell cycle arrest at the G2/M phase and apoptosis in HeLa cells. Bioorg. Med. Chem. Lett. 2010, 20, 7054–7058. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.C.; Chen, Q.; Liu, K.; Mo, P.L.; Zhu, J.W.; Zhuang, M.Q.; Shen, Y.M.; Yu, C. Mycoepoxydiene inhibits antigen-stimulated activation of mast cells and suppresses IgE-mediated anaphylaxis in mice. Int. Immunopharmacol. 2013, 17, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Li, Y.; Su, Q.; Huang, Z.; Shen, Y.M.; Li, W.; Yu, C. Inhibitory effects of Mycoepoxydiene on macrophage foam cell formation and atherosclerosis in ApoE-deficient mice. Cell Biosci. 2015, 5, 23. [Google Scholar] [CrossRef]
- Aoyama, T.; Fujiwara, H.; Masaki, T.; Sawamura, T. Induction of lectin-like oxidized LDL receptor by oxidized LDL and lysophosphatidylcholine in cultured endothelial cells. J. Mol. Cell. Cardiol. 1999, 31, 2101–2114. [Google Scholar] [CrossRef]
- Mehta, J.L.; Sanada, N.; Hu, C.; Chen, J.; Dandapat, A.; Sugawara, F.; Satoh, H.; Inoue, K.; Kawase, Y.; Jishage, K.I.; et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ. Res. 2007, 100, 1634–1642. [Google Scholar] [CrossRef]
- Wilson, P.D.; Pettigrew, J.D. Synthesis of Xyloketal A,B,C,D,and G Analogues. Chemistry 2006, 71, 1620–1625. [Google Scholar] [CrossRef]
- Zhao, J.; Li, L.; Ling, C.; Li, J.; Pang, J.Y.; Lin, Y.C.; Huang, R.; Wang, G.L.; Pei, Z.; Zeng, J.; et al. Marine compound Xyloketal B protects PC12 cells against OGD-induced cell damage. Brain Res. 2009, 1302, 240–247. [Google Scholar] [CrossRef]
- Chen, W.L.; Qian, Y.; Meng, W.F.; Pang, J.Y.; Lin, Y.C.; Guan, Y.Y.; Chen, S.P.; Liu, J.; Pei, Z.; Wang, G.-L. A novel marine compound xyloketal B protects against oxidized LDL-induced cell injury in vitro. Biochem. Pharmacol. 2009, 78, 941–950. [Google Scholar] [CrossRef]
- Xu, Z.; Li, Y.; Xiang, Q.; Pei, Z.; Liu, X.; Lu, B.; Chen, L.; Wang, G.; Pang, J.; Lin, Y. Design and synthesis of novel xyloketal derivatives and their vasorelaxing activities in rat thoracic aorta and angiogenic activities in zebrafish angiogenesis screen. J. Med. Chem. 2010, 53, 4642–4653. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.Y.; Li, J.; Yuan, F.; Li, M.; Zhang, Q.; Pang, J.Y.; Zhang, B.; Sun, F.Y.; Sun, H.S.; Li, Q.; et al. Xyloketal B Attenuates atherosclerotic plaque formation and endothelial dysfunction in apolipoprotein e deficient mice. Mar. Drugs 2015, 13, 2306–2326. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, C.Z.; Pang, Y.Y.; Lin, Y.C.; Wang, G.L.; Jie, Y. Application of Xyloketal B in preparing antiatherosclerotic medicaments. Patent CN102018699B, 26 March 2014. [Google Scholar]
- Yamashita, E. Let astaxanthin be thy medicine. PharmaNutrition 2015, 3, 115–122. [Google Scholar] [CrossRef]








| Activities | Sources | Composition | Indices Level (↑: Up-Regulation; ↓: Down-Regulation) | Reference |
|---|---|---|---|---|
| Lipid-lowering | S. japonica | molecular weight = 8177Da, fucose 35.07%, sulfate 36.85% and uronic acid 0.039%. | ↓ TC*, TG*, LDL-C*, ox-LDL* ↑ HDL-C* | [13] |
| S. japonica | molecular weight = 8177Da, fucose 35.07%, sulfate 36.85% and uronic acid 0.039% | ↓ TG, ox-LDL ↑ HDL-C | [14] | |
| Cladosiphono Kamuranus Tokida | glucuronic acid 6.7% ± 0.5%, fucose 36.2% ± 3.5%, galactose 1.0% ± 0.3%, mannose 1.0% ± 0.4%, glucose 0.5% ± 0.08% and xylose 1.9% ± 0.7% | ↓ TC, TG, LDL-C ↑ HDL-C, LPL* | [15] | |
| F. vesiculosus (Sigma-Aldrich) | - | ↓ TC, TG, LDL-C ↑ HDL-C | [17] | |
| A. nodosum | molecular weight = 361.4 kDa, carbohydrate 68.0%, sulfate 16.6% and protein 2.8% | ↓TC, TG, LDL-C, ApoB* ↑ApoA1*, LPL | [22] | |
| L. japonica | molecular weight = 189kDa, total sugar 48%, fucose 29% and sulfate 28% | ↓ TC, TG, LDL-C ↑ HDL-C | [25] | |
| L. japonica | molecular weight = 76 kDa, fucose 59.85%, arabinose 7.89% and rhamnose 0.14% | ↓ TC, TG, LDL-C ↑ HDL-C, LPL, HL*, LCAT* | [19] | |
| A. nodosum | molecular weight =207.2kDa, carbohydrate 60.4%and sulfate 16.6% | ↓ TC, TG ↑ HDL-C | [23] | |
| Anti-inflammatory | S. japonica | molecular weight = 8177Da, fucose 35.07%, sulfate 36.85% and uronic acid 0.039% | ↓ IL*-6, IL-10, p-SAPK* | [13] |
| S. japonica | molecular weight = 8177Da, fucose 35.07%, sulfate 36.85% and uronic acid 0.039% | ↓ IL-6, IL-10, p-JNK*, cyclin D1, | [14] | |
| L. japonica | molecular weight = 189kDa, total sugar 48%, fucose 29% and sulfate 28% | ↓ IL-1β, IL-6, TNF-α*, ICAM-1*, VCAM-1* | [25] | |
| Antioxidant | F. vesiculosus | molecular weight = 160 kDa, fucose 88.4%, galactose 6.0% and xylose 1.8% | ↓ MDA*, DC* | [18] |
| L. japonica | molecular weight = 189kDa, total sugar 48%, fucose 29% and sulfate 28% | ↓NOX-2*, NOX-4*, eNOS*, SOD1* | [25] |
| Activities | Sources | Composition | Indices Level (↑: Up-Regulation; ↓: Down-Regulation) | Reference |
|---|---|---|---|---|
| Lipid-lowering | Ulva lactuca | rhamnose, galactose, glucose, arabinose, xylose, mannose glucuronic acid and galacturonic acid | ↓Total lipids, TC, TG, LDL-C, VLDL-C ↑ HDL-C | [71] |
| Ulva pertusa (Chlorophyta) | ronic acids, rhamnose, xylose, glucose and sulfate comprised their main composition, with smaller amounts of mannose, arabinose and galactose. basic repeating units of the polysaccharides were (β-d-GlcpA-(1→4)-α-l-Rhap 3S) and (α-l-IdopA-(1→4)-α-l-Rhap 3S) | ↓ TC, TG, LDL-C ↑ HDL-C | [72] | |
| Ulva pertusa (Chlorophyta) | Ulvan molecular weight = 151.6 kDa, total sugar 47.6%, sulfate 17.1% and uronic acid 23.2% | ↓ TC, LDL-C | [76] | |
| U1 molecular weight = 64.5 kDa, total sugar 47.8%, sulfate 16.8% and uronic acid 22.7% | ↓ TG ↑ HDL-C | |||
| U2 molecular weight = 28.2 kDa, total sugar 48.1%, sulfate 17.4% and uronic acid 23.0% | ↓ TG ↑ HDL-C | |||
| Antioxidant | F. vesiculosus | molecular weight = 160 kDa, fucose 88.4%, galactose 6.0% and xylose 1.8% | ↓ TBARS* ↑ CAT*, GSH-Px*, SOD*, GSH*, T. thiol* | [71] |
| Ulva pertusa Kjellm (Chlorophyta) | Ulvan molecular weight = 151.7 kDa, neutral sugar 25.6%, sulfate 19.9% and uronic acid 19.2% | Hydroxyl radical scavenging activities U3 > U1(U) > U2 | [77] | |
| U1 molecular weight = 64.5 kDa, neutral sugar 24.8%, sulfate 20.4% and uronic acid 18.9% | ||||
| U2 molecular weight = 58.0 kDa, neutral sugar 26.3%, sulfate 19.1% and uronic acid 20.1% | ||||
| U3 molecular weight = 28.2 kDa, neutral sugar 25.1%, sulfate 19.4% and uronic acid 19.0% |
| Activities | Sources | Composition | Indices Level (↑: Up-Regulation; ↓: Down-Regulation) | Reference |
|---|---|---|---|---|
| Lipid-lowering | E. prolifera | EPF2* molecular weight = 103.51 kDa, carbohydrates 53.2%, proteins 11.5%, sulfate group 18.6% and uronic acid 12.4%; rhamnose, xylose, mannose, galactose and glucose in a molar ratio of 3.64:1.08:0.21:0.75:0.27. | ↓ TC, TG, LDL-C ↑ HDL-C | [78] |
| E. prolifera | EPsmolecular weight = 134.07 kDa, total sugar 54.6%, protein 10.1%, uronic acid 12.4% and sulfate contents 17.9%; rhamnose, xylose, mannose, galactose and Glucose | ↓ TC, TG, LDL-C ↑ HDL-C | [81] | |
| E. prolifera | rhamnose, glucuronic acid, arabinose, fucose, xylose and glucose in a molar ratio of 5.12:1.32:3.38:1.62:1:1.03. | ↓ TG, HMGCR, SREBP-2 | [82] | |
| E. prolifera | rhamnose, glucuronic acid, arabinose, fucose, xylose and glucose in a molar ratio of 5.12:1.32:3.38:1.62:1:1.03. | ↓ TG, ACC, SREBP-1c | [83] | |
| Antioxidant | E. prolifera | EPF2 molecular weight = 103.51 kDa, carbohydrates 53.2%, proteins 11.5%, sulfate group 18.6% and uronic acid 12.4%; rhamnose, xylose, mannose, galactose and glucose in a molar ratio of 3.64:1.08:0.21:0.75:0.27. | ↓ MDA ↑ CAT, GSH-Px, SOD | [78] |
| Activities | Sources | Indices Level (↑: Up-regulation; ↓: Down-regulation) | Reference |
|---|---|---|---|
| Lipid-lowering | Porphyra peptide | ↓ TC, TG, LDL-C, LDL-C/HDL-C ↑ HDL-C | [119] |
| Holothuria forskali protein | ↓ TG ↑ HDL-C | [120] | |
| Scallop skirt peptide | ↓ TC, TG, LDL-C ↑ HDL-C | [121] | |
| Jellyfish collagen peptide | ↓ TC, TG, LDL-C, LDL-C/HDL-C ↑ HDL-C, HDL-C/TC | [122] | |
| Pearsonothuria graef feihomogenate | ↓ TC, LDL-C, LDL-C/HDL-C ↑ HDL-C | [123] | |
| Apostichopus japonicus feihomogenate | ↓ TC, LDL-C, LDL-C/HDL-C ↑ HDL-C | ||
| Anti-inflammatory | Salmon protein hydrolysat | ↓ IL-1β, IL-6, TNF-α, *GM-CSF, *G-CSF | [124] |
| Antioxidant | Porphyra peptide | ↓ MDA ↑ GSH-Px, SOD | [119] |
| Scallop skirt peptide | ↓ MDA ↑ GSH-Px, SOD | [121] | |
| Jellyfish collagen peptide | ↓ MDA ↑ GSH-Px, SOD | [122] | |
| Pearsonothuria graef feihomogenate | ↓ MDA ↑ GSH-Px | [123] | |
| Apostichopus japonicus feihomogenate | ↓ MDA ↑ GSH-Px, SOD |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Q.; Zhao, J.; Xing, M.; Xiao, H.; Zhang, Q.; Liang, H.; Ji, A.; Song, S. Current Research Landscape of Marine-Derived Anti-Atherosclerotic Substances. Mar. Drugs 2020, 18, 440. https://doi.org/10.3390/md18090440
Cao Q, Zhao J, Xing M, Xiao H, Zhang Q, Liang H, Ji A, Song S. Current Research Landscape of Marine-Derived Anti-Atherosclerotic Substances. Marine Drugs. 2020; 18(9):440. https://doi.org/10.3390/md18090440
Chicago/Turabian StyleCao, Qi, Jiarui Zhao, Maochen Xing, Han Xiao, Qian Zhang, Hao Liang, Aiguo Ji, and Shuliang Song. 2020. "Current Research Landscape of Marine-Derived Anti-Atherosclerotic Substances" Marine Drugs 18, no. 9: 440. https://doi.org/10.3390/md18090440
APA StyleCao, Q., Zhao, J., Xing, M., Xiao, H., Zhang, Q., Liang, H., Ji, A., & Song, S. (2020). Current Research Landscape of Marine-Derived Anti-Atherosclerotic Substances. Marine Drugs, 18(9), 440. https://doi.org/10.3390/md18090440





