Ishophloroglucin A Isolated from Ishige okamurae Suppresses Melanogenesis Induced by α-MSH: In Vitro and In Vivo
Abstract
1. Introduction
2. Results
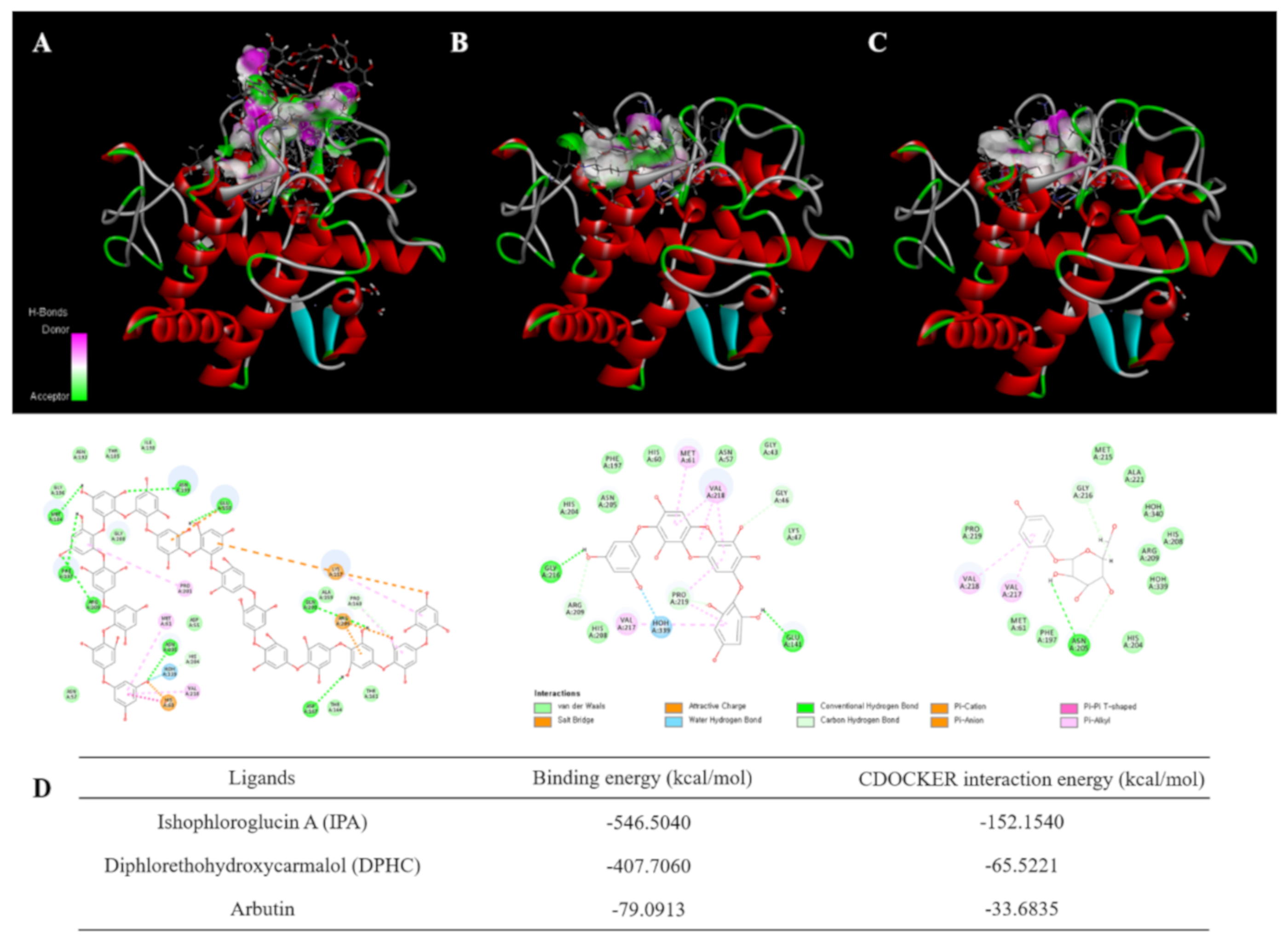
2.1. Molecular Docking Study
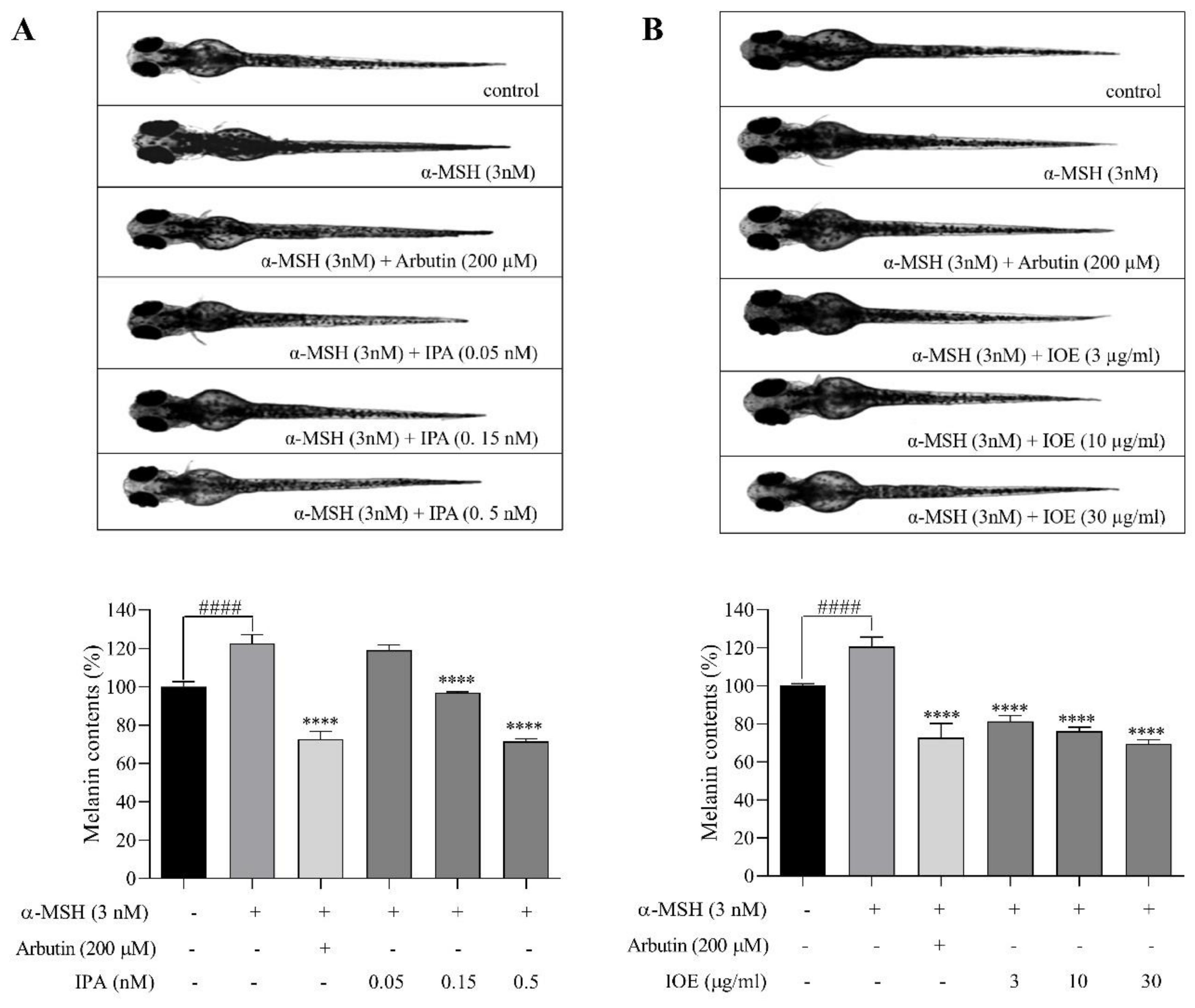
2.2. Effects of Melanogenic Inhibitors on Melanin Synthesis in Zebrafish Larvae
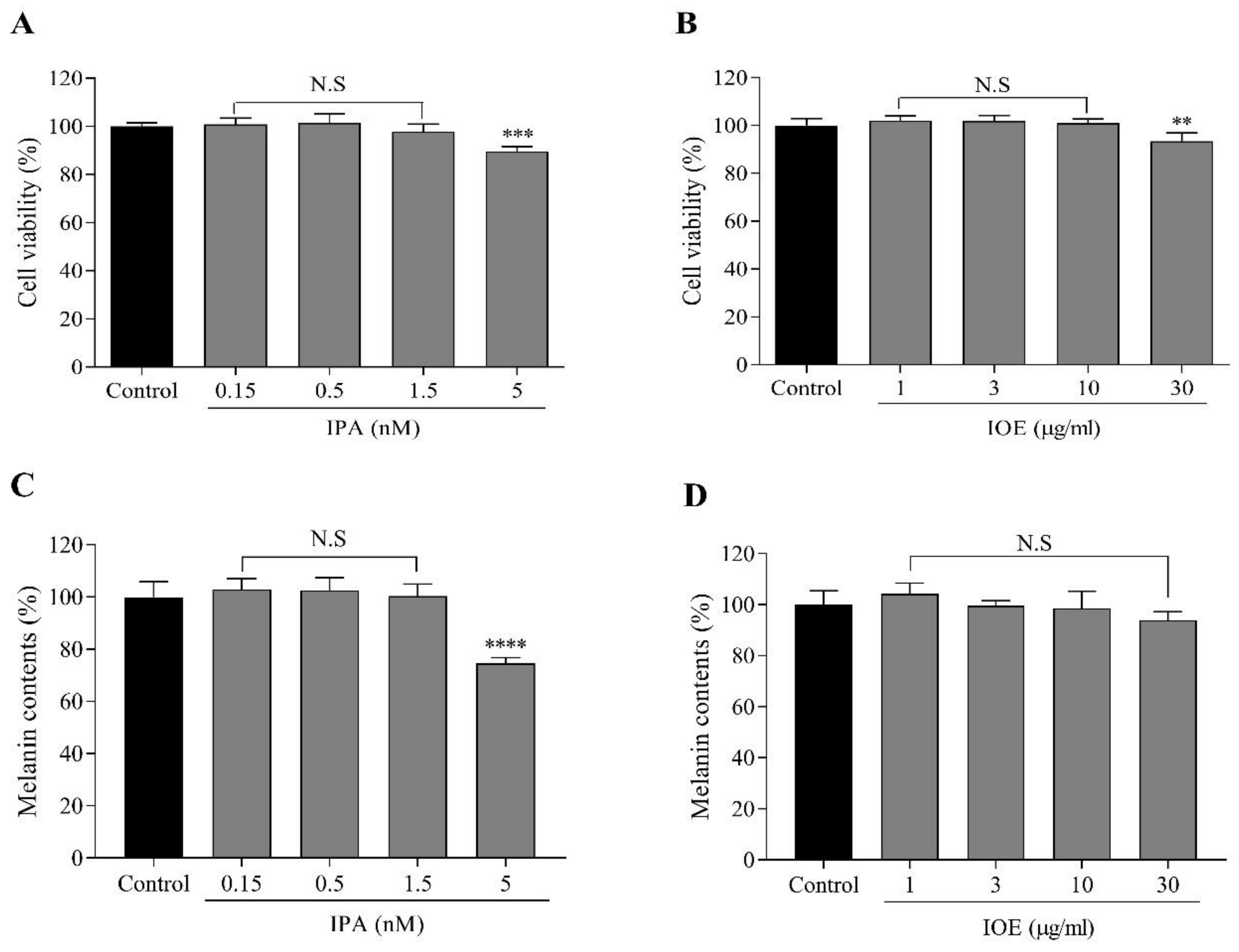
2.3. Effects of IPA and IOE on Melanin Synthesis in B16F10 Cells
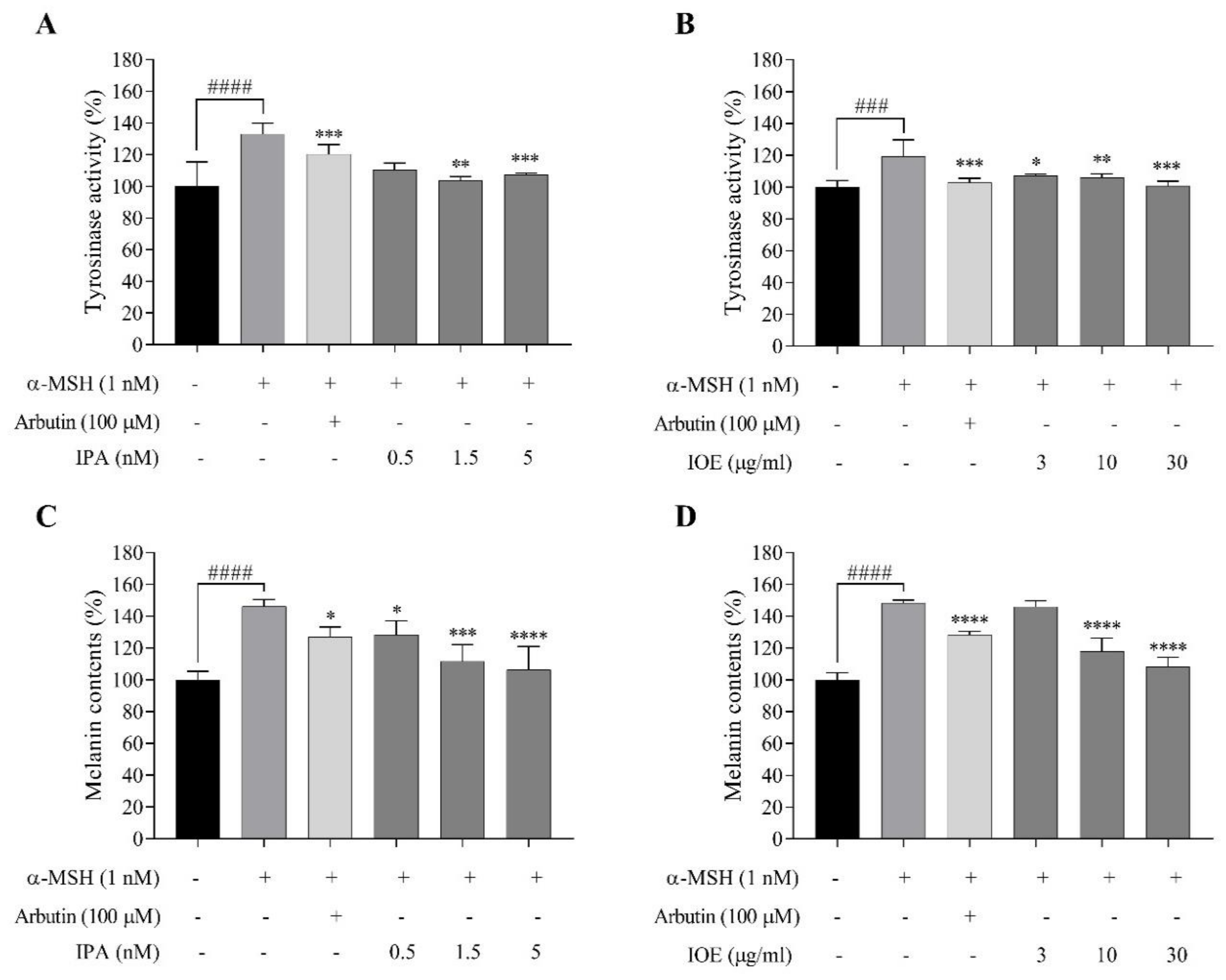
2.4. Effects of IPA and IOE on Tyrosinase Activity and Melanin Synthesis in α-MSH-Stimulated B16F10 Cells
2.5. Effects of IPA and IOE on the Molecular Mechanism in α-MSH-Stimulated B16F10 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Molecular Docking of Tyrosinase
4.3. Preparation of IOE and Isolation of IPA
4.4. Origin and Maintenance of Parental Zebrafish
4.5. Measurement of Melanin Content in Zebrafish Larvae
4.6. Cytoxicity of IPA and IOE in B16F10 Cells
4.7. Determination of Cellular Melanin Content
4.8. Tyrosinase Inhibition Activity and Melanin Content Induced by α-MSH
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Athukorala, Y.; Lee, K.; Kim, S.-K.; Jeon, Y. Anticoagulant activity of marine green and brown algae collected from Jeju Island in Korea. Bioresour. Technol. 2007, 98, 1711–1716. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Heo, S.-J.; Kim, K.-N.; Lee, S.-H.; Jeon, Y.-J. Isolation and identification of new compound, 2, 7 ″-phloroglucinol-6, 6′-bieckol from brown algae, Ecklonia cava and its antioxidant effect. J. Funct. Foods 2012, 4, 158–166. [Google Scholar] [CrossRef]
- Heo, S.-J.; Yoon, W.-J.; Kim, K.-N.; Ahn, G.-N.; Kang, S.-M.; Kang, D.H.; Affan, A.; Oh, C.; Jung, W.-K.; Jeon, Y.-J. Evaluation of anti-inflammatory effect of fucoxanthin isolated from brown algae in lipopolysaccharide-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2010, 48, 2045–2051. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.; Lee, J.-S.; Kim, W.-S.; Jeon, Y.-J.; Sanjeewa, K.K.A. The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef]
- Lee, S.-H.; Jeon, Y.-J. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef]
- Kazir, M.; AbuHassira, Y.; Robin, A.; Nahor, O.; Luo, J.; Israel, A.; Golberg, A.; Livney, Y.D. Extraction of proteins from two marine macroalgae, Ulva sp. and Gracilaria sp., for food application, and evaluating digestibility, amino acid composition and antioxidant properties of the protein concentrates. Food Hydrocoll. 2019, 87, 194–203. [Google Scholar] [CrossRef]
- Brunt, E.G.; Burgess, J.G. The promise of marine molecules as cosmetic active ingredients. Int. J. Cosmet. Sci. 2017, 40, 1–15. [Google Scholar] [CrossRef]
- Heo, S.-J.; Ko, S.-C.; Kang, S.-M.; Cha, S.-H.; Lee, S.-H.; Kang, D.-H.; Jung, W.-K.; Affan, A.; Oh, C.; Jeon, Y.-J. Inhibitory effect of diphlorethohydroxycarmalol on melanogenesis and its protective effect against UV-B radiation-induced cell damage. Food Chem. Toxicol. 2010, 48, 1355–1361. [Google Scholar] [CrossRef]
- Ryu, B.; Jiang, Y.; Kim, H.-S.; Hyun, J.-M.; Lim, S.-B.; Li, Y.; Jeon, Y.-J. Ishophloroglucin A, a Novel Phlorotannin for Standardizing the Anti-α-Glucosidase Activity of Ishige okamurae. Mar. Drugs 2018, 16, 436. [Google Scholar] [CrossRef]
- Morris, G.M.; Lim-Wilby, M. Molecular docking. In Molecular Modeling of Proteins; Springer: Berlin, Germany, 2008; pp. 365–382. [Google Scholar]
- Ewing, T.J.; Makino, S.; Skillman, A.G.; Kuntz, I.D. DOCK 4.0: Search strategies for automated molecular docking of flexible molecule databases. J. Comput. Mol. Des. 2001, 15, 411–428. [Google Scholar] [CrossRef]
- Shoichet, B.K.; Kuntz, I.D.; Bodian, D.L. Molecular docking using shape descriptors. J. Comput. Chem. 1992, 13, 380–397. [Google Scholar] [CrossRef]
- Anantharaman, A.; Hemachandran, H.; Priya, R.R.; Sankari, M.; Mohan, S.; Palanisami, N.; Siva, R. Inhibitory effect of apocarotenoids on the activity of tyrosinase: Multi-spectroscopic and docking studies. J. Biosci. Bioeng. 2016, 121, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ashraf, Z.; Kumar, N.; Rafiq, M.; Jabeen, F.; Park, J.H.; Choi, K.H.; Lee, S.; Seo, S.-Y.; Choi, E.; et al. Influence of plasma-activated compounds on melanogenesis and tyrosinase activity. Sci. Rep. 2016, 6, 21779. [Google Scholar] [CrossRef] [PubMed]
- Ando, H.; Kondoh, H.; Ichihashi, M.; Hearing, V.J. Approaches to Identify Inhibitors of Melanin Biosynthesis via the Quality Control of Tyrosinase. J. Investig. Dermatol. 2007, 127, 751–761. [Google Scholar] [CrossRef]
- Roulier, B.; Pérès, B.; Haudecoeur, R. Advances in the Design of Genuine Human Tyrosinase Inhibitors for Targeting Melanogenesis and Related Pigmentations. J. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Gilchrest, B.A.; Park, H.-Y.; Eller, M.S.; Yaar, M. Mechanisms of Ultraviolet Light-Induced Pigmentation. Photochem. Photobiol. 1996, 63, 1–10. [Google Scholar] [CrossRef]
- Agar, N.; Young, A.R. Melanogenesis: A photoprotective response to DNA damage? Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2005, 571, 121–132. [Google Scholar] [CrossRef]
- Lee, T.H.; Lee, M.S.; Lu, M.-Y. Effects of α-MSH on Melanogenesis and Tyrosinase of B-16 Melanoma. Endocrinology 1972, 91, 1180–1188. [Google Scholar] [CrossRef]
- Lamason, R.L.; Mohideen, M.-A.P.; Mest, J.R.; Wong, A.C.; Norton, H.L.; Aros, M.C.; Jurynec, M.J.; Mao, X.; Humphreville, V.R.; Humbert, J.E.; et al. SLC24A5, a Putative Cation Exchanger, Affects Pigmentation in Zebrafish and Humans. Science 2005, 310, 1782–1786. [Google Scholar] [CrossRef]
- Kelsh, R.; Harris, M.L.; Colanesi, S.; Erickson, C.A. Stripes and belly-spots—A review of pigment cell morphogenesis in vertebrates. Semin. Cell Dev. Biol. 2009, 20, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Colanesi, S.; Taylor, K.L.; Temperley, N.D.; Lundegaard, P.R.; Liu, D.; North, T.E.; Ishizaki, H.; Kelsh, R.; Patton, E.E. Small molecule screening identifies targetable zebrafish pigmentation pathways. Pigment. Cell Melanoma Res. 2012, 25, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Logan, D.W.; Burn, S.; Jackson, I. Regulation of pigmentation in zebrafish melanophores. Pigment. Cell Res. 2006, 19, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.-J.; Hwang, J.-Y.; Choi, J.-I.; Han, J.S.; Kim, H.-J.; Jeon, Y.-J. Diphlorethohydroxycarmalol isolated from Ishige okamurae, a brown algae, a potent α-glucosidase and α-amylase inhibitor, alleviates postprandial hyperglycemia in diabetic mice. Eur. J. Pharmacol. 2009, 615, 252–256. [Google Scholar] [CrossRef]
- Fernando, K.; Yang, H.-W.; Jiang, Y.; Jeon, Y.-J.; Ryu, B. Ishige okamurae Extract and Its Constituent Ishophloroglucin a Attenuated in Vitro and in Vivo High Glucose-Induced Angiogenesis. Int. J. Mol. Sci. 2019, 20, 5542. [Google Scholar] [CrossRef]
- Huang, H.-C.; Huang, W.-Y.; Tsai, T.-C.; Hsieh, W.-Y.; Ko, W.-P.; Chang, K.-J.; Chang, T.-M. Supercritical fluid extract of Lycium chinense Miller root inhibition of melanin production and its potential mechanisms of action. BMC Complement. Altern. Med. 2014, 14, 208. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Jeon, H.B.; Lim, H.; Shin, J.H.; Park, S.J.; Jo, Y.K.; Oh, W.; Yang, Y.S.; Cho, D.-H.; Kim, J.-Y. Conditioned Media from Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Inhibits Melanogenesis by Promoting Proteasomal Degradation of MITF. PLoS ONE 2015, 10, e0128078. [Google Scholar] [CrossRef]
- Chakraborty, A.; Chakraborty, D. The effect of tryptophan on dopa-oxidation by melanosomal tyrosinase. Int. J. Biochem. 1993, 25, 1277–1280. [Google Scholar] [CrossRef]
- Santi, M.D.; Peralta, M.A.; Puiatti, M.; Cabrera, J.L.; Ortega, M.G. Melanogenic inhibitory effects of Triangularin in B16F0 melanoma cells, in vitro and molecular docking studies. Bioorg. Med. Chem. 2019, 27, 3722–3728. [Google Scholar] [CrossRef]
- Kang, S.-M.; Heo, S.-J.; Kim, K.-N.; Lee, S.-H.; Yang, H.-M.; Kim, A.-D.; Jeon, Y.-J. Molecular docking studies of a phlorotannin, dieckol isolated from Ecklonia cava with tyrosinase inhibitory activity. Bioorg. Med. Chem. 2012, 20, 311–316. [Google Scholar] [CrossRef]
- Promden, W.; Viriyabancha, W.; Monthakantirat, O.; Umehara, K.; Noguchi, H.; De-Eknamkul, W. Correlation between the Potency of Flavonoids on Mushroom Tyrosinase Inhibitory Activity and Melanin Synthesis in Melanocytes. Molecules 2018, 23, 1403. [Google Scholar] [CrossRef] [PubMed]
- Cha, S.-H.; Ko, S.-C.; Kim, D.; Jeon, Y.-J. Screening of marine algae for potential tyrosinase inhibitor: Those inhibitors reduced tyrosinase activity and melanin synthesis in zebrafish. J. Dermatol. 2010, 38, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.S.; Wang, H.-M.D.; Wen, Y.-S.; Liu, W.; Li, P.-H.; Chiu, C.-C.; Chen, P.-C.; Huang, C.-Y.; Sheu, J.-H.; Wen, Z.-H. 4-(Phenylsulfanyl)butan-2-One Suppresses Melanin Synthesis and Melanosome Maturation In Vitro and In Vivo. Int. J. Mol. Sci. 2015, 16, 20240–20257. [Google Scholar] [CrossRef]
- Choi, T.-Y.; Kim, J.-H.; Ko, D.H.; Kim, C.-H.; Hwang, J.-S.; Ahn, S.; Kim, S.Y.; Kim, C.D.; Lee, J.-H.; Yoon, T.-J. Zebrafish as a new model for phenotype-based screening of melanogenic regulatory compounds. Pigment. Cell Res. 2007, 20, 120–127. [Google Scholar] [CrossRef]
- Körner, A.; Pawelek, J. Mammalian tyrosinase catalyzes three reactions in the biosynthesis of melanin. Science 1982, 217, 1163–1165. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.; Kim, S.Y.; Jung, J.M.; Won, C.H.; Choi, J.H.; Lee, M.W.; Chang, S.E. The antimycotic agent clotrimazole inhibits melanogenesis by accelerating ERK and PI3K-/Akt-mediated tyrosinase degradation. Exp. Dermatol. 2015, 24, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-Activated Protein Kinase Pathways Mediated by ERK, JNK, and p38 Protein Kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef]
- Su, B.; Karin, M. Mitogen-activated protein kinase cascades and regulation of gene expression. Curr. Opin. Immunol. 1996, 8, 402–411. [Google Scholar] [CrossRef]
- Roux, P.P.; Blenis, J. ERK and p38 MAPK-Activated Protein Kinases: A Family of Protein Kinases with Diverse Biological Functions. Microbiol. Mol. Biol. Rev. 2004, 68, 320–344. [Google Scholar] [CrossRef]
- Zhou, J.; Shang, J.; Ping, F.; Zhao, G. Alcohol extract from Vernonia anthelmintica (L.) willd seed enhances melanin synthesis through activation of the p38 MAPK signaling pathway in B16F10 cells and primary melanocytes. J. Ethnopharmacol. 2012, 143, 639–647. [Google Scholar] [CrossRef]
- Geng, J.; Yuan, P.; Shao, C.; Yu, S.-B.; Zhou, B.; Zhou, P.; Chen, X. Bacterial melanin interacts with double-stranded DNA with high affinity and may inhibit cell metabolism in vivo. Arch. Microbiol. 2010, 192, 321–329. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kang, S.-M.; Sok, C.H.; Hong, J.T.; Oh, J.-Y.; Jeon, Y.-J. Cellular activities and docking studies of eckol isolated from Ecklonia cava (Laminariales, Phaeophyceae) as potential tyrosinase inhibitor. Algae 2015, 30, 163–170. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yang, H.-W.; Jiang, Y.; Oh, J.-Y.; Jeon, Y.-J.; Ryu, B. Ishophloroglucin A Isolated from Ishige okamurae Suppresses Melanogenesis Induced by α-MSH: In Vitro and In Vivo. Mar. Drugs 2020, 18, 470. https://doi.org/10.3390/md18090470
Li X, Yang H-W, Jiang Y, Oh J-Y, Jeon Y-J, Ryu B. Ishophloroglucin A Isolated from Ishige okamurae Suppresses Melanogenesis Induced by α-MSH: In Vitro and In Vivo. Marine Drugs. 2020; 18(9):470. https://doi.org/10.3390/md18090470
Chicago/Turabian StyleLi, Xining, Hye-Won Yang, Yunfei Jiang, Jae-Young Oh, You-Jin Jeon, and Bomi Ryu. 2020. "Ishophloroglucin A Isolated from Ishige okamurae Suppresses Melanogenesis Induced by α-MSH: In Vitro and In Vivo" Marine Drugs 18, no. 9: 470. https://doi.org/10.3390/md18090470
APA StyleLi, X., Yang, H.-W., Jiang, Y., Oh, J.-Y., Jeon, Y.-J., & Ryu, B. (2020). Ishophloroglucin A Isolated from Ishige okamurae Suppresses Melanogenesis Induced by α-MSH: In Vitro and In Vivo. Marine Drugs, 18(9), 470. https://doi.org/10.3390/md18090470





