Fucoidan Structure and Its Impact on Glucose Metabolism: Implications for Diabetes and Cancer Therapy
Abstract
:1. Introduction
2. Fucoidan Structure
2.1. Fucoidan Backbone & Monosaccharide Composition
2.2. Sulphate Content and Position
2.3. Molecular Weight
3. Overview of Glucose Metabolism
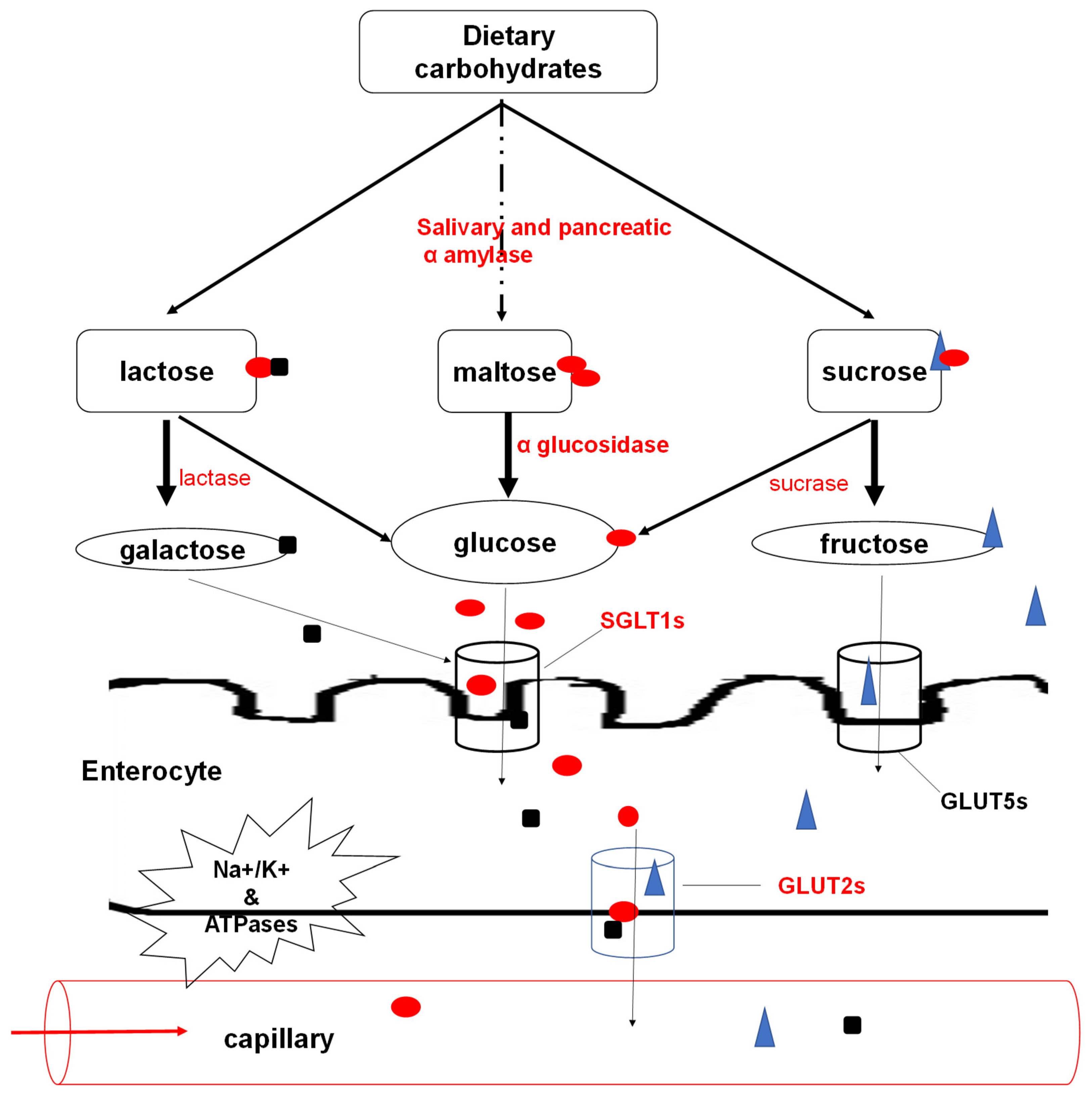
3.1. Carbohydrate Digestion and Absorption
3.2. Regulation of Glucose Metabolism
3.3. Glucose Metabolism in Cells
3.3.1. Glucose Uptake
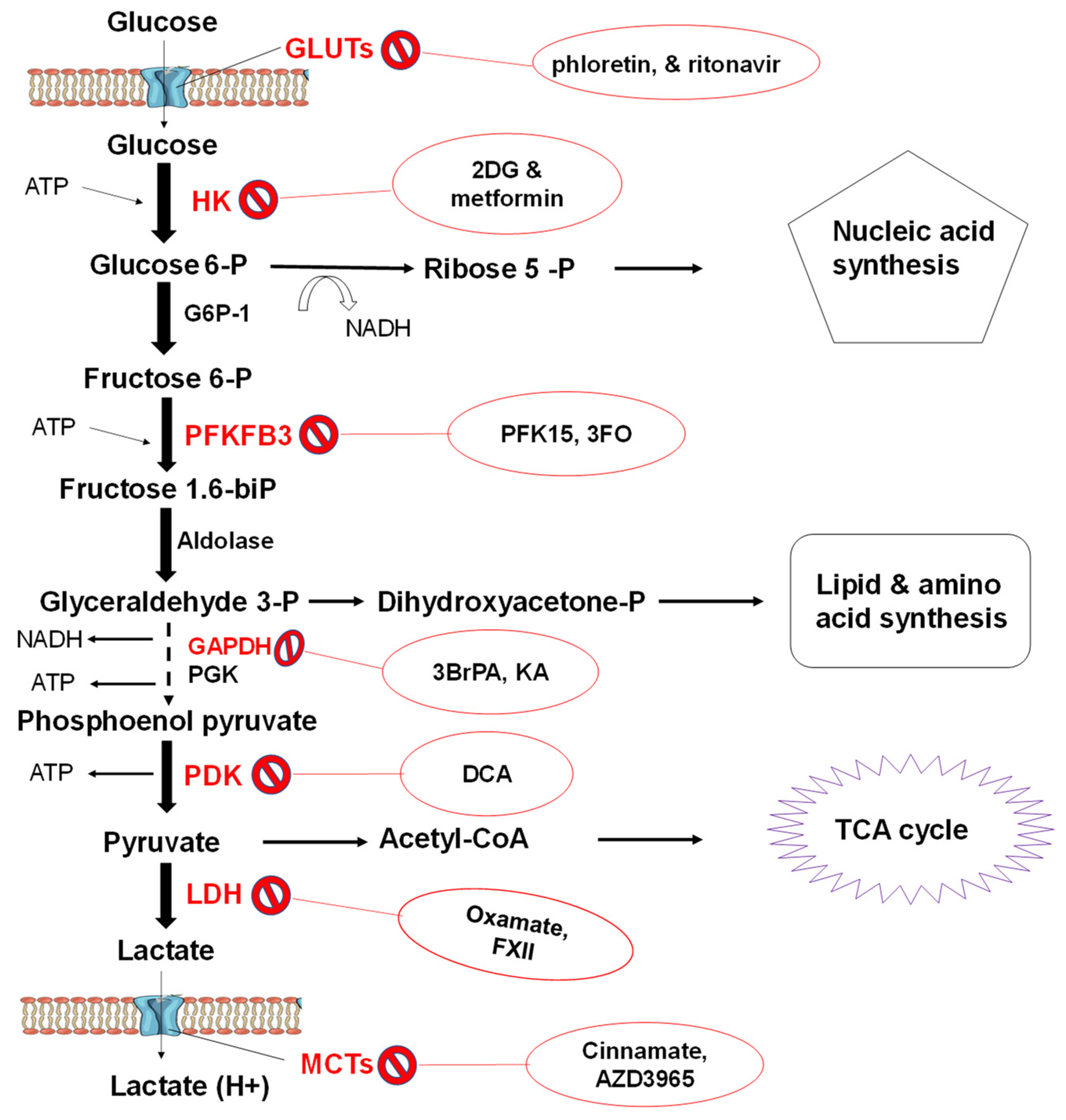
3.3.2. Glycolytic Flux
3.3.3. Pathophysiology of Glucose Metabolism
Relevance to Diabetes
Relevance to Cancer
4. Therapeutic Roles of Fucoidan
4.1. Significance of Fucoidan as an Anti-Diabetic Agent
4.2. Significance of Fucoidan as an Anticancer Agent
5. Potential Double Impact of Fucoidan on Diabetes and Cancer
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-DG | 2-Deoxy-D-glucose |
| Acetyl-CoA | Acetyl coenzyme A |
| DCA | Dichloroacetate |
| F-1, 6-bisP | Fructose 1.6 phosphate |
| F-6-P | Fructose 6 phosphate |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GLP-1 | Glucagon-like peptide-1 |
| G-6-P | Glucose 6 phosphates |
| GLUT | Glucose transporter |
| GIP | Glucose-dependent insulinotropic polypeptide |
| HGP | Hepatic glucose production |
| HK | Hexokinases |
| HMWF | High molecular weight fucoidan |
| IGF | Insulin-like growth factor |
| IO | Iodoacetate |
| KO | Koningic acid |
| LDH | Lactate dehydrogenase |
| LMWF | Low molecular weight fucoidan |
| MCTs | Monocarboxylate transporters |
| Na+ | Sodium ion |
| ROS | Reactive oxygen species |
| SGLT | Na-glucose transporter |
| OXID-P | Oxidative phosphorylation |
| PFK1 | Phosphofructokinase |
| T2DM | Type 2 diabetes mellitus |
References
- Cho, M.; Han, J.H.; You, S. Inhibitory Effects of Fucan Sulfates on Enzymatic Hydrolysis of Starch. LWT 2011, 44, 1164–1171. [Google Scholar] [CrossRef]
- Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S.L. Molecular Weight and Sulfate Content Modulate the Inhibition of α-amylase by Fucoidan Relevant for Type 2 Diabetes Management. PharmaNutrition 2015, 3, 108–114. [Google Scholar] [CrossRef]
- Shan, X.; Liu, X.; Hao, J.; Cai, C.; Fan, F.; Dun, Y.; Zhao, X.; Liu, X.; Li, C.; Yu, G. In Vitro and in Vivo Hypoglycemic Effects of Brown Algal Fucoidans. Int. J. Biol. Macromol. 2016, 82, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-T.; Rioux, L.-E.; Turgeon, S.L. Alpha-Amylase and Alpha-Glucosidase Inhibition is Differentially Modulated by Fucoidan Obtained from Fucus Vesiculosus and Ascophyllum Nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef]
- Skriptsova, A.V.; Shevchenko, N.M.; Zvyagintseva, T.N.; Imbs, T.I. Monthly Changes in the Content and Monosaccharide Composition of Fucoidan from Undaria Pinnatifida (Laminariales, Phaeophyta). Environ. Boil. Fishes 2010, 22, 79–86. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and Bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef] [Green Version]
- Van Weelden, G.; Bobiński, M.; Okła, K.; Van Weelden, W.J.; Romano, A.; Pijnenborg, J. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The Anti-Cancer Effects of Fucoidan:A Review of both in Vivo and in Vitro Investigations. Cancer Cell Int. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- Daub, C.D.; Mabate, B.; Malgas, S.; Pletschke, B. Fucoidan from Ecklonia Maxima is a Powerful Inhibitor of the Diabetes-Related Enzyme, α-Glucosidase. Int. J. Biol. Macromol. 2020, 151, 412–420. [Google Scholar] [CrossRef]
- Tudzarova, S.; Osman, M. The Double Trouble of Metabolic Diseases: The Diabetes–Cancer Link. Mol. Biol. Cell 2015, 26, 3129–3139. [Google Scholar] [CrossRef]
- Ale, M.T.; Meyer, A.S. Fucoidans from Brown Seaweeds: An Update on Structures, Extraction Techniques and use of Enzymes as Tools for Structural Elucidation. RSC Adv. 2013, 3, 8131–8141. [Google Scholar] [CrossRef] [Green Version]
- Senthilkumar, K.; Manivasagan, P.; Venkatesan, J.; Kim, S.-K. Brown Seaweed Fucoidan: Biological Activity and Apoptosis, Growth Signaling Mechanism in Cancer. Int. J. Biol. Macromol. 2013, 60, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Lakshmanasenthil, S.; VinothKumar, T.; Geetharamani, D.; Maruthupandi, T. Screening of Seaweeds Collected from South-East Coastal Area of India for Alpha Amylase Inhibitory Activity, Antioxidant Activity and Biocompatibility. Int. J. Phar. Phar. Sc. 2013, 5, 240–244. [Google Scholar]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J. The Seasonal Variation of Fucoidan Within Three Species of Brown Macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Pragasam, V.; Badrinathan, S.; Shiju, T.M.; Christa, A.S.S.; Arya, R. Purification and Structural Characterization of Sulfated Polysaccharide from Sargassum Myriocystum and its Efficacy in Scavenging Free Radicals. Indian J. Pharm. Sci. 2012, 74, 549–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological Activities of Fucoidan and the Factors Mediating Its Therapeutic Effects: A Review of Recent Studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [Green Version]
- Suprunchuk, V. Low-Molecular-Weight Fucoidan: Chemical Modification, Synthesis of its Oligomeric Fragments and Mimetics. Carbohydr. Res. 2019, 485, 107806. [Google Scholar] [CrossRef]
- Zayed, A.; Muffler, K.; Hahn, T.; Rupp, S.; Finkelmeier, D.; Burger-Kentischer, A.; Ulber, R. Physicochemical and Biological Characterisation of Fucoidan from Fucus Vesiculosus Purified by Dye Affinity Chromatography. Mar. Drugs 2016, 14, 79. [Google Scholar] [CrossRef] [Green Version]
- Foley, S.; Szegezdi, E.; Mulloy, B.; Samali, A.; Tuohy, M.G. An Unfractionated Fucoidan from Ascophyllum Nodosum: Extraction, Characterisation, and Apoptotic Effects in Vitro. J. Nat. Prod. 2011, 74, 1851–1861. [Google Scholar] [CrossRef]
- Kumar, T.V.; Lakshmanasenthil, S.; Geetharamani, D.; Marudhupandi, T.; Suja, G.; Suganya, P. Fucoidan – A α-d-glucosidase Inhibitor from Sargassum Wightii with Relevance to Type 2 Diabetes Mellitus Therapy. Int. J. Biol. Macromol. 2015, 72, 1044–1047. [Google Scholar] [CrossRef]
- January, G.; Naidoo, R.; Kirby-McCullough, B.; Bauer, R. Assessing Methodologies for Fucoidan Extraction from South African Brown Algae. Algal Res. 2019, 40, 101517. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [Green Version]
- Aronoff, S.L.; Berkowitz, K.; Shreiner, B.; Want, L. Glucose Metabolism and Regulation: Beyond Insulin and Glucagon. Diabetes Spectr. 2004, 17, 183–190. [Google Scholar] [CrossRef] [Green Version]
- Molnar, C.; Gair, J. Digestive System Processes. In Concepts of Biology, 1st ed.; Pressbooks: Victoria, Canada, 2019. [Google Scholar]
- Devlin, T.M. Textbook of Biochemistry with Clinical Correlations; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Goodman, B.E. Insights into Digestion and Absorption of Major Nutrients in Humans. Adv. Physiol. Educ. 2010, 34, 44–53. [Google Scholar] [CrossRef]
- Chen, L.; Tuo, B.; Dong, H. Regulation of Intestinal Glucose Absorption by Ion Channels and Transporters. Nutrients 2016, 8, 43. [Google Scholar] [CrossRef] [Green Version]
- Longpré, J.-P.; Lapointe, J.-Y. Determination of the Na+/Glucose Cotransporter (SGLT1) Turnover Rate Using the Ion-Trap Technique. Biophys. J. 2011, 100, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Aronson, P.S.; Boron, W.F.; Boulpaep, E.L. Physiology of Membranes. In Medical Physiology: A Cellular and Molecular Approach; Saunders: Philadelphia, PA, USA, 2003; pp. 66–67. [Google Scholar]
- Röder, P.V.; Geillinger, K.E.; Zietek, T.S.; Thorens, B.; Koepsell, H.; Daniel, H. The Role of SGLT1 and GLUT2 in Intestinal Glucose Transport and Sensing. PLoS ONE 2014, 9, e89977. [Google Scholar] [CrossRef]
- Sharabi, K.; Tavares, C.D.J.; Rines, A.K.; Puigserver, P. Molecular Pathophysiology of Hepatic Glucose Production. Mol. Asp. Med. 2015, 46, 21–33. [Google Scholar] [CrossRef] [Green Version]
- Kowalski, G.M.; Bruce, C.R. The Regulation of Glucose Metabolism: Implications and Considerations for the Assessment of Glucose Homeostasis in Rodents. Am. J. Physiol. Metab. 2014, 307, E859–E871. [Google Scholar] [CrossRef] [Green Version]
- Baron, A.D.; Brechtel, G.; Wallace, P.; Edelman, S.V. Rates and Tissue Sites of Non-Insulin- and Insulin-Mediated Glucose Uptake in Humans. Am. J. Physiol. Metab. 1988, 255, E769–E774. [Google Scholar] [CrossRef]
- Kelley, D.; Mitrakou, A.; Marsh, H.; Schwenk, F.; Benn, J.; Sonnenberg, G.; Arcangeli, M.; Aoki, T.; Sorensen, J.; Berger, M. Skeletal Muscle Glycolysis, Oxidation, and Storage of an Oral Glucose Load. J. Clin. Investig. 1988, 81, 1563–1571. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, G. Insulin and Insulin Resistance. Clin Biochem Rev. 2005, 26, 19–39. Available online: https://www.ncbi.nlm.nih.gov/pubmed/16278749 (accessed on 15 September 2020).
- Nauck, M.A.; Meier, J.J. The Incretin Effect in Healthy Individuals and Those with Type 2 Diabetes: Physiology, Pathophysiology, and Response to Therapeutic Interventions. Lancet Diabetes Endocrinol. 2016, 4, 525–536. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. GIP and GLP-1: Stepsiblings Rather Than Monozygotic Twins Within the Incretin Family. Diabetes 2019, 68, 897–900. [Google Scholar] [CrossRef] [Green Version]
- Smith, U. Impaired (‘diabetic’) Insulin Signaling and Action occur in Fat Cells Long Before Glucose Intolerance—Is Insulin Resistance Initiated in the Adipose Tissue? Int. J. Obes. 2002, 26, 897–904. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, D.H.; Kang, L.; Ayala, J.E.; Fueger, P.T.; Lee-Young, R.S. The Physiological Regulation of Glucose Flux into Muscle in Vivo. J. Exp. Biol. 2010, 214, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Tanner, L.B.; Goglia, A.G.; Wei, M.H.; Sehgal, T.; Parsons, L.R.; Park, J.O.; White, E.; Toettcher, J.E.; Rabinowitz, J.D. Four Key Steps Control Glycolytic Flux in Mammalian Cells. Cell Syst. 2018, 7, 49–62.e8. [Google Scholar] [CrossRef]
- Melkonian, E.A.; Asuka, E.; Schury, M.P. StatPearls. 2020. Available online: www.ncbi.nlm.nih.gov/books/NBK541119 (accessed on 15 September 2020).
- Buck, M.D.; Sowell, R.T.; Kaech, S.M.; Pearce, E.L. Metabolic Instruction of Immunity. Cell 2017, 169, 570–586. [Google Scholar] [CrossRef]
- Nakrani, M.N.; Wineland, R.H.; Anjum, F. StatPearls. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK560599/ (accessed on 16 September 2020).
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Skyler, J.S.; Bakris, G.L.; McElvaine, A.T.; Palmer, J.P.; Pugliese, A.; Schatz, D.A.; Sosenko, J.M.; Wilding, J.; Ratner, R.E.; Bonifacio, E.; et al. Differentiation of Diabetes by Pathophysiology, Natural History, and Prognosis. Diabetes 2017, 66, 241–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsimihodimos, V.; Florentin, M. Hypertension and Dyslipidemia in Patients with Pre-Diabetes: Dietary and Other Therapies: Implications for Cardiovascular Disease. In Glucose Intake and Utilisation in Pre-Diabetes and Diabetes; Watson, R.R., Dokken, B.B., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 157–179. [Google Scholar]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, L.; Chen, X.; Sun, X.; Wang, L.; Chen, S. The Glycolytic Switch in Tumors: How Many Players Are Involved? J. Cancer 2017, 8, 3430–3440. [Google Scholar] [CrossRef] [PubMed]
- Nolop, K.B.; Rhodes, C.G.; Brudin, L.H.; Beaney, R.P.; Krausz, T.; Jones, T.; Hughes, J.M. Glucose Utilisation In Vivo by Human Pulmonary Neoplasms. Cancer 1987, 60, 2682–2689. [Google Scholar] [CrossRef]
- Gottschalk, S.; Anderson, N.; Hainz, C.; Eckhardt, S.G.; Serkova, N.J. Imatinib (STI571)-Mediated Changes in Glucose Metabolism in Human Leukemia BCR-ABL-Positive Cells. Clin. Cancer Res. 2004, 10, 6661–6668. [Google Scholar] [CrossRef] [Green Version]
- Warburg, O.; Posener, K.; Negelein, E. Über Den Stoffwechsel Der Carcinomzelle. Biochem. Zeitschr. 1924, 152, 309–344. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, J.M. Altered Energy Metabolism in Cancer; A Unique Opportunity for Therapeutic Intervention. Cancer Biol Ther. 2013, 14, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Cros, M.; Hemmerlin, C.; Hofmann, F.; Ferretti, S.; Zhang, J.; Gounarides, J.S.; Yin, H.; Muller, A.; Haberkorn, A.; Chene, P.; et al. M2 Isoform of Pyruvate Kinase is Dispensable for Tumor Maintenance and Growth. Proc. Natl. Acad. Sci. USA 2012, 110, 489–494. [Google Scholar] [CrossRef] [Green Version]
- Nielson, T.C.; Le, H.V. Inhibition of Glycolysis and Glutaminolysis: An Emerging Drug Discovery Approach to Combat Cancer. Curr. Top. Med. Chem. 2018, 18, 494–504. [Google Scholar] [CrossRef]
- Bomanji, J.B.; Costa, D.C.; Ell, P.J. Clinical Role of Positron Emission Tomography in Oncology. Lancet Oncol. 2001, 2, 157–164. [Google Scholar] [CrossRef]
- Weiler-Sagie, M.; Bushelev, O.; Epelbaum, R.; Dann, E.J.; Haim, N.; Avivi, I.; Ben-Barak, A.; Ben-Arie, Y.; Bar-Shalom, R.; Israel, O. 18F-FDG Avidity in Lymphoma Readdressed: A Study of 766 Patients. J. Nucl. Med. 2009, 51, 25–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Zhang, B.; Zheng, J.; Yu, M.; Zhou, T.; Zhao, K.; Jia, Y.; Gao, X.; Chen, C.; Wei, T. The Inhibition of Migration and Invasion of Cancer Cells by Graphene via the Impairment of Mitochondrial Respiration. Biomaterials 2014, 35, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Roesch, A.; Vultur, A.; Krause, E.; Pätzold, S.; Villanueva, J.; Krepler, C.; Fukunaga-Kalabis, M.; Hoth, M.; Bastian, B.C.; Vogt, T.; et al. Overcoming Intrinsic Multidrug Resistance in Melanoma by Blocking the Mitochondrial Respiratory Chain of Slow-Cycling JARID1Bhigh Cells. Cancer Cell 2013, 23, 811–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Wahab, A.F.; Mahmoud, W.; Al-Harizy, R.M. Targeting Glucose Metabolism to Suppress Cancer Progression: Prospective of Anti-Glycolytic Cancer Therapy. Pharmacol. Res. 2019, 150, 104511. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do Cancers Have High Aerobic Glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Jiang, B. Aerobic Glycolysis and High Level of Lactate in Cancer Metabolism and Microenvironment. Genes Dis. 2017, 4, 25–27. [Google Scholar] [CrossRef]
- Hu, X.; Chao, M.; Wu, H. Central Role of Lactate and Proton in Cancer Cell Resistance to Glucose Deprivation and its Clinical Translation. Signal Transduct. Target. Ther. 2017, 2, 16047. [Google Scholar] [CrossRef] [Green Version]
- Lu, W.; Hu, Y.; Liu, J.; McKeehan, W.; Wang, H.; Luo, Y.; Huang, P.; Chen, G.; Chen, Z.; Zhang, H.; et al. Novel Role of NOX in Supporting Aerobic Glycolysis in Cancer Cells with Mitochondrial Dysfunction and as a Potential Target for Cancer Therapy. PLoS Biol. 2012, 10, e1001326. [Google Scholar] [CrossRef]
- Lu, C.-L.; Qin, L.; Liu, H.-C.; Candas, D.; Fan, M.; Chung-Ling, L. Tumor Cells Switch to Mitochondrial Oxidative Phosphorylation under Radiation via mTOR-Mediated Hexokinase II Inhibition—A Warburg-Reversing Effect. PLoS ONE 2015, 10, e0121046. [Google Scholar] [CrossRef]
- De Saedeleer, C.J.; Copetti, T.; Porporato, P.E.; Verrax, J.; Feron, O.; Sonveaux, P. Lactate activates HIF-1 in Oxidative but not in Warburg-Phenotype Human Tumor Cells. PLoS ONE 2012, 7, e46571. [Google Scholar] [CrossRef] [Green Version]
- Jia, D.; Park, J.H.; Jung, K.H.; Levine, H.; Kaipparettu, B.A. Elucidating the Metabolic Plasticity of Cancer: Mitochondrial Reprogramming and Hybrid Metabolic States. Cells 2018, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Maiuri, M.C.; Kroemer, G. Essential Role for Oxidative Phosphorylation in Cancer Progression. Cell Metab. 2015, 21, 11–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalva-Aydemir, S.; Bajpai, R.; Shanmugam, M.; Martinez, M.; Adekola, K.U.; Kandela, I.; Wei, C.; Singhal, S.; Koblinski, J.E.; Raje, N.S.; et al. Targeting the Metabolic Plasticity of Multiple Myeloma with FDA-Approved Ritonavir and Metformin. Clin. Cancer Res. 2015, 21, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.-T.; Tu, S.-H.; Yang, P.-S.; Hsu, S.-P.; Lee, W.-H.; Ho, C.-T.; Wu, C.-H.; Lai, Y.-H.; Chen, M.-Y.; Chen, L.-C. Apple Polyphenol Phloretin Inhibits Colorectal Cancer Cell Growth via Inhibition of the Type 2 Glucose Transporter and Activation of p53-Mediated Signaling. J. Agric. Food Chem. 2016, 64, 6826–6837. [Google Scholar] [CrossRef] [PubMed]
- Biasutti, M.A. Comparative Analysis of Forms and Wikis as Tools for Online Collaborative Learning. Comput. Educ. 2017, 107, 158–171. [Google Scholar] [CrossRef]
- Robey, R.B.; Hay, N. Mitochondrial Hexokinases, Novel Mediators of the Antiapoptotic Effects of Growth Factors and Akt. Oncogene 2006, 25, 4683–4696. [Google Scholar] [CrossRef] [Green Version]
- Jae, H.J.; Chung, J.W.; Park, H.S.; Lee, M.J.; Lee, K.C.; Kim, H.-C.; Yoon, J.H.; Chung, H.; Park, J.H. The Antitumor Effect and Hepatotoxicity of a Hexokinase II Inhibitor 3-Bromopyruvate: In Vivo Investigation of Intraarterial Administration in a Rabbit VX2 Hepatoma Model. Korean J. Radiol. 2009, 10, 596–603. [Google Scholar] [CrossRef] [Green Version]
- Marini, C.; Salani, B.; Massara, C.; Boccardo, S.; Fabbi, M.; Campi, C.; Ravera, S.; Angelini, G.; Morbelli, S.; Cilli, M.; et al. Direct Inhibition of Hexokinase Activity by Metformin at Least Partially Impairs Glucose Metabolism and Tumor Growth in Experimental Breast Cancer. Cell Cycle 2013, 12, 3490–3499. [Google Scholar] [CrossRef] [Green Version]
- Raez, L.E.; Papadopoulos, K.; Kroll, S.; Jung, D.T.; Kurtoglu, M.; Rosenblatt, J.; Lampidis, T.J.; Ricart, A.D.; Chiorean, E.G.; DiPaola, R.S.; et al. A Phase I Dose-Escalation Trial of 2-deoxy-d-glucose Alone or Combined with Docetaxel in Patients with Advanced Solid Tumors. Cancer Chemother. Pharmacol. 2013, 71, 523–530. [Google Scholar] [CrossRef]
- Colombo, S.L.; Palacios-Callender, M.; Frakich, N.; De Leon, J.; Schmitt, C.A.; Boorn, L.; Davis, N.; Moncada, S. Anaphase-Promoting Complex/Cyclosome-Cdh1 Coordinates Glycolysis and Glutaminolysis with Transition to S Phase in Human T Lymphocytes. Proc. Natl. Acad. Sci. USA 2010, 107, 18868–18873. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Ye, L.; Zhang, J.; Yu, P.; Wang, H.; Ye, Z.; Tian, J. PFK15, a Small Molecule Inhibitor of PFKFB3, Induces Cell Cycle Arrest, Apoptosis and Inhibits Invasion in Gastric Cancer. PLoS ONE 2016, 11, e0163768. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy-Kanniappan, S. Evolution of GAPDH as a Druggable Target of Tumor Glycolysis? Expert Opin. Ther. Targets 2018, 22, 295–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunbar, E.M.; Coats, B.S.; Shroads, A.L.; Langaee, T.; Lew, A.; Forder, J.R.; Shuster, J.J.; Wagner, D.A.; Stacpoole, P.W. Phase 1 Trial of Dichloroacetate (DCA) in Adults with Recurrent Malignant Brain Tumors. Investig. New Drugs 2014, 32, 452–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, A.; Cooper, C.R.; Gouw, A.M.; Dinavahi, R.; Maitra, A.; Deck, L.M.; Royer, R.E.; Jagt, D.L.V.; Semenza, G.L.; Dang, C.V. Inhibition of Lactate Dehydrogenase A Induces Oxidative Stress and Inhibits Tumor Progression. Proc. Natl. Acad. Sci. USA 2010, 107, 2037–2042. [Google Scholar] [CrossRef] [Green Version]
- Marchiq, I.; Pouysségur, J. Hypoxia, Cancer Metabolism and the Therapeutic Benefit of Targeting Lactate/H+ Symporters. J. Mol. Med. 2016, 94, 155–171. [Google Scholar] [CrossRef] [Green Version]
- Halestrap, A.P. The Monocarboxylate Transporter Family-Structure and Functional Characterisation. IUBMB Life 2011, 64, 1–9. [Google Scholar] [CrossRef]
- Locasale, J.W.; Cantley, L.C. Altered Metabolism in Cancer. BMC Biol. 2010, 8, 88. [Google Scholar] [CrossRef] [Green Version]
- DeBerardinis, R.J.; Sayed, N.; Ditsworth, D.; Thompson, C.B. Brick by Brick: Metabolism and Tumor Cell Growth. Curr. Opin. Genet. Dev. 2008, 18, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Du, S.; Du, Y.; Ren, J.; Ying, G.; Yan, Z. Glutathione Reductase Mediates Drug Resistance in Glioblastoma Cells by Regulating Redox Homeostasis. J. Neurochem. 2017, 144, 93–104. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, C.; Longatto-Filho, A.; Azevedo-Silva, J.; Casal, M.; Schmitt, F.C.; Baltazar, F. Role of Monocarboxylate Transporters in Human Cancers: State of the Art. J. Bioenerg. Biomembr. 2012, 44, 127–139. [Google Scholar] [CrossRef]
- Hussien, R.; Brooks, G.A. Mitochondrial and Plasma Membrane Lactate Transporter and Lactate Dehydrogenase Isoform Expression in Breast Cancer Cell Lines. Physiol. Genom. 2011, 43, 255–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feron, O. Pyruvate into Lactate and Back: From the Warburg Effect to Symbiotic Energy Fuel Exchange in Cancer Cells. Radiother. Oncol. 2009, 92, 329–333. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Health Organisation. Diabetes. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes. (accessed on 23 September 2020).
- Rena, G.; Hardie, D.G.; Pearson, E.R. The Mechanisms of Action of Metformin. Diabetologia 2017, 60, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Felman, A. Medical News Today. 2020. Available online: https://www.medicalnewstoday.com/articles/323716. (accessed on 23 September 2020).
- Mao, X.-M.; He, K.; Shi, J.-C. Safety and Efficacy of Acarbose in the Treatment of Diabetes in Chinese Patients. Ther. Clin. Risk Manag. 2014, 10, 505–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FDA. U.S Food & Drug Administration. 2020. Available online: https://www.fda.gov/news-events/press-announcements/fda-alerts-patients-and-health-care-professionals-nitrosamine-impurity-findings-certain-metformin (accessed on 23 September 2020).
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus vesiculosus, L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Havale, S.H.; Pal, M. Medicinal Chemistry Approaches to the Inhibition of Dipeptidyl peptidase-4 for the Treatment of Type 2 Diabetes. Bioorganic Med. Chem. 2009, 17, 1783–1802. [Google Scholar] [CrossRef]
- Sakai, C.; Abe, S.; Kouzuki, M.; Shimohiro, H.; Ota, Y.; Sakinada, H.; Takeuchi, T.; Okura, T.; Kasagi, T.; Hanaki, K. A Randomized Placebo-Controlled Trial of an Oral Preparation of High Molecular Weight Fucoidan in Patients with Type 2 Diabetes with Evaluation of Taste Sensitivity. Yonago Acta Medica 2018, 62, 014–023. [Google Scholar] [CrossRef] [Green Version]
- Unnikrishnan, P.S.; Suthindhiran, K.; Jayasri, M.A. Inhibitory Potential of Turbinaria Ornataagainst Key Metabolic Enzymes Linked to Diabetes. BioMed Res. Int. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.M.; Camara, R.B.G.; Monte, J.F.S.; Viana, R.L.S.; Melo, K.R.T.; Queiroz, M.F.; Filgueira, L.G.A.; Oyama, L.M.; Rocha, H.A.D.O. Commercial Fucoidans from Fucus Vesiculosus Can Be Grouped into Antiadipogenic and Adipogenic Agents. Mar. Drugs 2018, 16, 193. [Google Scholar] [CrossRef] [Green Version]
- Unnikrishnan, P.; Suthindhiran, K.; Jayasri, M.A. Antidiabetic Potential of Marine Algae by Inhibiting Key Metabolic Enzymes. Front. Life Sci. 2015, 8, 148–159. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhao, Y.; Hu, S.; Shi, D.; Xue, C. Fucoidan from Sea Cucumber Cucumaria Frondosa Exhibits Anti-Hyperglycemic Effects in Insulin Resistant Mice via Activating the PI3K/PKB Pathway and GLUT4. J. Biosci. Bioeng. 2016, 121, 36–42. [Google Scholar] [CrossRef]
- Gabbia, D.; Dall’Acqua, S.; De Martin, S.; Di Gangi, I.M.; Bogialli, S.; Caputi, V.; Albertoni, L.; Marsilio, I.; Paccagnella, N.; Carrara, M.; et al. The Phytocomplex from Fucus Vesiculosus and Ascophyllum Nodosum Controls Postprandial Plasma Glucose Levels: An In Vitro and In Vivo Study in a Mouse Model of NASH. Mar. Drugs 2017, 15, 41. [Google Scholar] [CrossRef]
- Sim, S.-Y.; Shin, Y.-E.; Kim, H.-K. Fucoidan from Undaria Pinnatifida has Anti-Diabetic Effects by Stimulation of Glucose Uptake and Reduction of Basal Lipolysis in 3T3-L1 Adipocytes. Nutr. Res. 2019, 65, 54–62. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structural Dependence of Sulfated Polysaccharide for Diabetes Management: Fucoidan From Undaria Pinnatifida Inhibiting α-Glucosidase More Strongly Than α-Amylase and Amyloglucosidase. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Hu, S.; Xia, G.; Wang, J.; Wang, Y.; Li, Z.; Xue, C. Fucoidan from Sea Cucumber Protects Against High-Fat High-Sucrose Diet-Induced Hyperglycaemia and Insulin Resistance in Mice. J. Funct. Foods 2014, 10, 128–138. [Google Scholar] [CrossRef]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and Cancer: A Multifunctional Molecule with Anti-Tumor Potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [Green Version]
- Moreau, D.; Thomas-Guyon, H.; Jacquot, C.; Juge, M.; Culioli, G.; Ortalo-Magné, A.; Piovetti, L.; Roussakis, C. An Extract from the Brown alga Bifurcaria Bifurcata Induces Irreversible Arrest of Cell Proliferation in a non-Small-Cell Bronchopulmonary Carcinoma Line. Environ. Boil. Fishes 2006, 18, 87–93. [Google Scholar] [CrossRef]
- Malyarenko, O.; Ermakova, S.; Zvyagintseva, T.N. Sulfated Polysaccharides from Brown Seaweeds Saccharina Japonica and Undaria Pinnatifida: Isolation, Structural Characteristics, and Antitumor Activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef]
- Miyamoto-Yamasaki, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan Induces Apoptosis through Activation of Caspase-8 on Human Breast Cancer MCF-7 Cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef]
- Teruya, T.; Konishi, T.; Uechi, S.; Tamaki, H.; Tako, M. Anti-Proliferative Activity of Oversulfated Fucoidan from Commercially Cultured Cladosiphon Okamuranus TOKIDA in U937 Cells. Int. J. Biol. Macromol. 2007, 41, 221–226. [Google Scholar] [CrossRef]
- Han, Y.-S.; Lee, J.H.; Lee, S.H. Antitumor Effects of Fucoidan on Human Colon Cancer Cells via Activation of Akt Signaling. Biomol. Ther. 2015, 23, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Cho, T.-M.; Kim, W.-J.; Moon, S.-K. AKT Signaling is Involved in Fucoidan-Induced Inhibition of Growth and Migration of Human Bladder Cancer Cells. Food Chem. Toxicol. 2014, 64, 344–352. [Google Scholar] [CrossRef]
- Boo, H.-J.; Hyun, J.-H.; Kim, S.-C.; Kang, J.-I.; Kim, M.-K.; Kim, S.-Y.; Cho, H.; Yoo, E.-S.; Kang, H.-K. Fucoidan from Undaria Pinnatifida Induces Apoptosis in A549 Human Lung Carcinoma Cells. Phytotherapy Res. 2011, 25, 1082–1086. [Google Scholar] [CrossRef]
- Yan, M.-D.; Yao, C.-J.; Chow, J.-M.; Chang, C.-L.; Hwang, P.-A.; Chuang, S.-E.; Whang-Peng, J.; Yao, C.-J. Fucoidan Elevates MicroRNA-29b to Regulate DNMT3B-MTSS1 Axis and Inhibit EMT in Human Hepatocellular Carcinoma Cells. Mar. Drugs 2015, 13, 6099–6116. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-C.; Hsu, W.-L.; Hwang, P.-A.; Chou, T.-C. Low Molecular Weight Fucoidan Inhibits Tumor Angiogenesis through Downregulation of HIF-1/VEGF Signaling under Hypoxia. Mar. Drugs 2015, 13, 4436–4451. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds Inhibit Proliferation of Melanoma Cells and Induce Apoptosis by Activation of Caspase-3 in Vitro. Mar. Drugs 2011, 9, 2605–2621. [Google Scholar] [CrossRef] [Green Version]
- Ermakova, S.; Sokolova, R.; Kim, S.M.; Um, B.H.; Isakov, V.; Zvyagintseva, T. Fucoidans from Brown Seaweeds Sargassum Hornery, Eclonia Cava, Costaria Costata: Structural Characteristics and Anti-Cancer Activity. Appl. Biochem. Biotechnol. 2011, 164, 841–850. [Google Scholar] [CrossRef]
- Pádua, D.; Rocha, E.; Gargiulo, D.; Ramos, A.A. Bioactive Compounds from Brown Seaweeds: Phloroglucinol, Fucoxanthin and Fucoidan as Promising Therapeutic Agents Against Breast Cancer. Phytochem. Lett. 2015, 14, 91–98. [Google Scholar] [CrossRef]
- Alekseyenko, T.V.; Zhanayeva, S.Y.; Venediktova, A.A.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Besednova, N.N.; Korolenko, T.A. Antitumor and Antimetastatic Activity of Fucoidan, a Sulfated Polysaccharide Isolated from the Okhotsk Sea Fucus Evanescens Brown Alga. Bull. Exp. Biol. Med. 2007, 143, 730–732. [Google Scholar] [CrossRef]
- Giovannucci, E.L.; Harlan, D.M.; Archer, M.C.; Bergenstal, R.M.; Gapstur, S.M.; Habel, L.A.; Pollak, M.; Regensteiner, J.G.; Yee, D. Diabetes and Cancer: A Consensus Report. Diabetes Care 2010, 33, 1674–1685. [Google Scholar] [CrossRef] [Green Version]
- Noto, H. Unfolding Link Between Diabetes and Cancer. J. Diabetes Investig. 2017, 9, 473–474. [Google Scholar] [CrossRef]
- Han, Y.-S.; Lee, J.H.; Lee, S.H. Fucoidan Inhibits the Migration and Proliferation of HT-29 Human Colon Cancer Cells via the Phosphoinositide-3 Kinase/Akt/Mechanistic Target of Rapamycin Pathways. Mol. Med. Rep. 2015, 12, 3446–3452. [Google Scholar] [CrossRef]

| Source | Mw (kDa) | Sulphate Content (w/w) | Fucose Content (w/w) | Monosaccharide Composition (w/w) | Polyphenol Content (w/w) | References |
|---|---|---|---|---|---|---|
| Fucus vesiculosus | 98 | 15.5 ± 1.1% | 94.8% | 2.3% xylose, 1.9% galactose | ND | [4,5,19] |
| Ascophyllum nodosum | 420 | 20.6 ± 0.3% | 80.1% | 14.3% xylose, 5.6% galactose | ND | [3,4,20] |
| Sargassum wightii | 637 | 36 ± 0.60% | 53 ± 0.52% | ND | ND | [21] |
| Sargassum honeri | ND | ND | 32.5% | 23.2% mannose, 27.6% galactose, 4.2% xylose | ND | [4] |
| Ecklonia maxima | 470 | 6.01 ± 0.53% | 4.45 ± 0.25% | 12.78% fructose, 1.44% galactose, 26.55% glucose, 4.3% mannose, 0.78% xylose | 0% | [10,22] |
| Turbinaria ornata | ND | 33 ± 0.42% | 59 ± 0.69% | ND | ND | [14] |
| Undaria pinnatifida | 378 | 15.02% | 39.24% | 28.85% xylose, 26.4% galactose, 5.04% mannose, 0.95% glucose | ND | [23] |
| Fucoidan Source | Harvest Location | MW (kDa) | Sulphate w/w (%) | α-Amylase (IC50) | α-Glucosidase (IC50) | Other | Test System | Reference |
|---|---|---|---|---|---|---|---|---|
| Turbinaria ornata | India | ND | 33 ± 0.42 | 36.6 μg/mL | ND | Not cytotoxic to normal cells | In vitro | [14] |
| ND | ND | 250 μg/mL | 535.6 μg/mL | Inhibited dipeptidyl peptidase IV (IC50 = 55.2 μg/mL) | In vitro | [97] | ||
| Ascophyllum nodosum | Canada | 420 | ND | 0.12–4.64 mg/mL | 13–47 μg/mL | In vitro | [5,20] | |
| Fucus vesiculosus | Canada | 98 | 23.7 ± 0.04 | No activity | 49 μg/mL | In vitro | [5,19,98] | |
| Russia | 735 | 27 | ND | ND | Inhibited dipeptidyl peptidase IV (IC50 = 11.1 μg/mL) | In vitro | [94] | |
| Sargassum wightii | India | 637 | 36 ± 0.60% | ND | 139 μg/mL | In vitro | [21] | |
| ND | ND | 378.3 μg/mL | 314.8 μg/mL | Inhibited dipeptidyl peptidase IV (IC50 38.27 μg/mL) | In vitro | [99] | ||
| Cucumaria frondosa | China | ND | ND | ND | ND | -Activates the PI3K/PKB pathway which regulates insulin production -Activates GLUT4 translocation | In vivo | [100] |
| Algal extract mixture (F. vesiculosus & A. nodosum) | Commercial (Italy) | ND | ND | 1.49 ± 0.32 μg/mL | 0.604 ± 0.004 μg/mL | -Significantly reduced postprandial glucose -Implicated in preventing progression of in non-alcoholic steatohepatitis to T2DM | In vitro & in vivo | [101] |
| Undaria pinnatifida | Commercial (Sigma Aldrich) | ND | ND | -Reduced blood glucose levels and improve insulin sensitivity in mice -Reduction of basal lipolysis in 3T3-L1 adipocytes | In vivo | [102] | ||
| New Zealand | ND | 15.02 | 0.190 ± 0.005 mg/mL | 0.137 ± 0.012 mg/mL | -Non-competitive inhibitor of α amylase -Competitive inhibitor of α glucosidase | In vitro | [23,103] | |
| Acaudina molpadioides | China | 1614.1 | 26.3 ± 2.7 | ND | ND | -Acutely reduced blood glucose levels and improves insulin resistance -Inhibition of glucose metabolism-related enzyme (hexokinase, pyruvate kinase) activities and up-regulation of the PKB/GLUT4 pathway. | In vivo | [104] |
| Ecklonia maxima | South Africa | 470 | 6.01 ± 0.97 | No activity | 0.29 mg/mL | Mixed inhibitor of α glucosidase | In vitro | [10,22] |
| Fucoidan Source | Harvest Location | MW (kDa) | Sulphate w/w (%) | Cell Line | Mechanism of Action | Test System | Reference |
|---|---|---|---|---|---|---|---|
| Bifurcaria bifurcata | France | ND | ND | NSCLC-N6 cell line | Cell cycle arrest (G1 arrest) | in vitro | [106] |
| Saccharina japonica, Undaria pinnatifida | Japan | ND | 0–29% | T-47D and SK-MEL-28 cells | Inhibited cell proliferation and colony formation | in vitro | [107] |
| NPO organisation fucoidan laboratory | ND | ND | ND | ER+ breast cancer cell line (MCF-7) | Inhibited cell proliferation, induced apoptosis (Caspase 8 activation) | in vitro | [108] |
| Cladosiphon okamuranus | Okinawa Island | ND | Native 13.5% and over-sulphated 32.8% | Human leukaemia cell line (U937 cell) | Native; very weak anti-proliferative activity Over sulphated; Induced apoptosis via caspase-3 and -7 activation-dependent pathways | in vitro | [109] |
| Fucus vesiculosus (Sigma) | ND | ND | ND | HT29 colon cancer cells | Induced G1 cell cycle arrest (induced p21WAF1 expression; suppressed cyclin and cyclin-dependent kinase expression); induction of apoptosis and angiogenesis | in vitro & in vivo | [110] |
| Fucoidan (Sigma) | ND | ND | ND | Human bladder carcinoma cell lines (5637 and T-24) | Cell growth inhibition via p21WAF1-mediated G1-phase cell-cycle arrest by activation of AKT | in vitro | [111] |
| Undaria pinnatifida | ND | ND | ND | Human lung cancer A549 cells | Apoptosis induction (activation of ERK1/2 MAPK pathways; downregulation of p38, PI3K/Akt signalling) | in vitro | [112] |
| Sargassum hemiphyllum (Hi-Q Marine International Ltd.) | ND | ND | ND | Human hepatocellular carcinoma cells | Inhibits angiogenesis and metastasis of tumour cells (regulation of miR-29b-DNMT3B-MTSS1; inhibition of TGF-β receptor and Smad signalling) | in vitro | [113] |
| S. hemiphyllum, (Hi-Q Marine International Ltd.) | ND | LMWF | ND | Hypoxic human bladder cancer cells (T24) cells; Female athymic nude mice (BALB/c) | Inhibits angiogenesis and tumour growth (inhibition of HIF-1/VEGF-regulated signalling pathway) | in vitro & in vivo | [114] |
| F. vesiculosus (Sigma) Sargassum sp | ND | ND | 34.2% 38.4% | Lewis Lung Carcinoma cells (LCC); Melanoma B16 cells | Induced apoptosis by fragmentation and condensation of chromatin | in vitro | [115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mabate, B.; Daub, C.D.; Malgas, S.; Edkins, A.L.; Pletschke, B.I. Fucoidan Structure and Its Impact on Glucose Metabolism: Implications for Diabetes and Cancer Therapy. Mar. Drugs 2021, 19, 30. https://doi.org/10.3390/md19010030
Mabate B, Daub CD, Malgas S, Edkins AL, Pletschke BI. Fucoidan Structure and Its Impact on Glucose Metabolism: Implications for Diabetes and Cancer Therapy. Marine Drugs. 2021; 19(1):30. https://doi.org/10.3390/md19010030
Chicago/Turabian StyleMabate, Blessing, Chantal Désirée Daub, Samkelo Malgas, Adrienne Lesley Edkins, and Brett Ivan Pletschke. 2021. "Fucoidan Structure and Its Impact on Glucose Metabolism: Implications for Diabetes and Cancer Therapy" Marine Drugs 19, no. 1: 30. https://doi.org/10.3390/md19010030
APA StyleMabate, B., Daub, C. D., Malgas, S., Edkins, A. L., & Pletschke, B. I. (2021). Fucoidan Structure and Its Impact on Glucose Metabolism: Implications for Diabetes and Cancer Therapy. Marine Drugs, 19(1), 30. https://doi.org/10.3390/md19010030









