Efficacy of Chondroprotective Food Supplements Based on Collagen Hydrolysate and Compounds Isolated from Marine Organisms †
Abstract
:1. Introduction
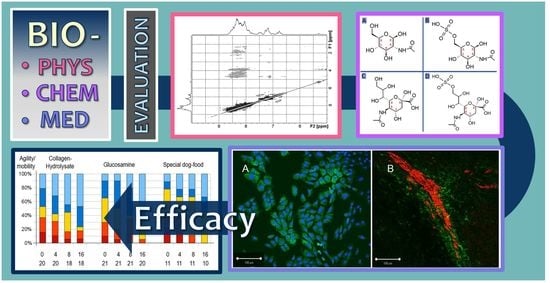
2. Results
2.1. Drug Administration and Sta†istical Analysis od Dog Treatment
2.2. X-ray and Statistics
2.3. Cell Biology Tests and Blood Parameters
2.4. NMR Analysis of Fortigel Collagen Hydrolysate
2.5. Molecular Modeling
3. Discussion
4. Materials and Methods
4.1. Dog Osteoarthritis and Drug Administration
4.2. Cell Biology Tests and Blood Parameters Determination
4.3. Statistical Analysis
4.4. Nuclear Magnetic Resonance (NMR) Spectroscopy
4.5. Molecular Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tacke, S.; Gollwitzer, A.; Grammel, L.; Henke, J. Pain therapy in small pets. Tierarztliche Praxis. Ausgabe K Kleintiere/Heimtiere 2017, 45, 53–60. [Google Scholar] [CrossRef]
- Bockstahler, B.; Levine, D.; Millis, D.L.; Wandrey, S.O.N. Essential Facts of Physiotherapy in Dogs and Cats; BE VetVerlag: Babenhausen, Germany, 2004. [Google Scholar]
- Benavente, M.; Arias, S.; Moreno, L.; Martínez, J. Production of Glucosamine Hydrochloride from Crustacean Shell. J. Pharm. Pharmacol. 2015, 3, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Siebert, H.C.; Burg-Roderfeld, M.; Eckert, T.; Stötzel, S.; Kirch, U.; Diercks, T.; Humphries, M.J.; Frank, M.; Wechselberger, R.; Tajkhorshid, E.; et al. Interaction of the alpha2A domain of integrin with small collagen fragments. Protein Cell 2010, 1, 393–405. [Google Scholar] [CrossRef]
- Krylov, V.B.; Grachev, A.A.; Ustyuzhanina, N.E.; Ushakova, N.A.; Preobrazhenskaya, M.E.; Kozlova, N.I.; Portsel, M.N.; Konovalova, I.N.; Novikov, V.Y.; Siebert, H.C.; et al. Preliminary structural characterization, anti-inflammatory and anticoagulant activities of chondroitin sulfates from marine fish cartilage. Russian Chem. Bull. 2011, 60, 746–753. [Google Scholar] [CrossRef]
- Burg-Roderfeld, M.; Eckert, T.; Siebert, H.C. Bioaktive Kollagenfragmente. Neue struktur-biologische Studien an Kollagen-Integrin-Komplexen belegen Justus Liebigs wegweisende Ideen. Spiegel der Forschung 2011, 28, 36–43. [Google Scholar]
- Stötzel, S.; Schurink, M.; Wienk, H.; Siebler, U.; Burg-Roderfeld, M.; Eckert, T.; Kulik, B.; Wechselberger, R.; Sewing, J.; Steinmeyer, J.; et al. Molecular organization of various collagen fragments as revealed by atomic force microscopy and diffusion-ordered NMR spectroscopy. ChemPhysChem 2012, 13, 3117–3125. [Google Scholar] [CrossRef]
- Schadow, S.; Simons, V.S.; Lochnit, G.; Kordelle, J.; Gazova, Z.; Siebert, H.C.; Steinmeyer, J. Metabolic Response of Human Osteoarthritic Cartilage to Biochemically Characterized Collagen Hydrolysates. Int. J. Mol. Sci. 2017, 18, 207. [Google Scholar] [CrossRef]
- Schadow, S.; Siebert, H.C.; Lochnit, G.; Kordelle, J.; Rickert, M.; Steinmeyer, J. Collagen metabolism of human osteoarthritic articular cartilage as modulated by bovine collagen hydrolysates. PLoS ONE 2013, 8, e53955. [Google Scholar] [CrossRef] [Green Version]
- Raabe, O.; Reich, C.; Wenisch, S.; Hild, A.; Burg-Roderfeld, M.; Siebert, H.C.; Arnhold, S. Hydrolyzed fish collagen induced chondrogenic differentiation of equine adipose tissue-derived stromal cells. Histochem Cell Biol. 2010, 134, 545–554. [Google Scholar] [CrossRef]
- Bertini, I.; Calderone, V.; Cosenza, M.; Fragai, M.; Lee, Y.M.; Luchinat, C.; Mangani, S.; Terni, B.; Turano, P. Conformational variability of matrix metalloproteinases: Beyond a single 3D structure. Proc. Natl. Acad. Sci. USA 2005, 102, 5334–5339. [Google Scholar] [CrossRef] [Green Version]
- Mosyak, L.; Georgiadis, K.; Shane, T.; Svenson, K.; Hebert, T.; McDonagh, T.; Mackie, S.; Olland, S.; Lin, L.; Zhong, X.; et al. Crystal structures of the two major aggrecan degrading enzymes, ADAMTS4 and ADAMTS-5. Protein Sci. Publ. Protein Soc. 2008, 17, 16–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcaraz, L.A.; Banci, L.; Bertini, I.; Cantini, F.; Donaire, A.; Gonnelli, L. Matrix metalloproteinase-inhibitor interaction: The solution structure of the catalytic domain of human matrix metalloproteinase-3 with different inhibitors. J. Biol. Inorg. Chem. JBIC Publ. Soc. Biol. Inorg. Chem. 2007, 12, 1197–1206. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, T.; Steinmeyer, J. Effects of tetracyclines on the production of matrix metalloproteinases and plasminogen activators as well as of their natural inhibitors, tissue inhibitor of metalloproteinases-1 and plasminogen activator inhibitor-1. Inflamm. Res. 2001, 50, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Masuyer, G.; Schwager, S.L.; Sturrock, E.D.; Isaac, R.E.; Acharya, K.R. Molecular recognition and regulation of human angiotensin-I converting enzyme (ACE) activity by natural inhibitory peptides. Sci. Rep. 2012, 2, 717. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, Y.; Matsuo, K.; Murata, M.; Yudoh, K.; Nakamura, H.; Shimizu, H.; Beppu, M.; Inaba, Y.; Saito, T.; Kato, T.; et al. Expression of Angiotensin II Receptor-1 in Human Articular Chondrocytes. Arthritis 2012, 2012, 648537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, F.; Tsukamoto, I.; Inoue, S.; Hashimoto, K.; Akagi, M. Cyclic compressive loading activates angiotensin II type 1 receptor in articular chondrocytes and stimulates hypertrophic differentiation through a G-protein-dependent pathway. FEBS Open Bio 2018, 8, 962–973. [Google Scholar] [CrossRef]
- Kouguchi, T.; Ohmori, T.; Shimizu, M.; Takahata, Y.; Maeyama, Y.; Suzuki, T.; Morimatsu, F.; Tanabe, S. Effects of a chicken collagen hydrolysate on the circulation system in subjects with mild hypertension or high-normal blood pressure. Biosci. Biotechnol. Biochem. 2013, 77, 691–696. [Google Scholar] [CrossRef] [Green Version]
- Kosinska, M.K.; Ludwig, T.E.; Liebisch, G.; Zhang, R.; Siebert, H.C.; Wilhelm, J.; Kaesser, U.; Dettmeyer, R.B.; Klein, H.; Ishaque, B.; et al. Articular Joint Lubricants during Osteoarthritis and Rheumatoid Arthritis Display Altered Levels and Molecular Species. PLoS ONE 2015, 10, e0125192. [Google Scholar] [CrossRef]
- Oke, S.; Aghazadeh-Habashi, A.; Weese, J.S.; Jamali, F. Evaluation of glucosamine levels in commercial equine oral supplements for joints. Equine Vet. J. 2006, 38, 93–95. [Google Scholar] [CrossRef]
- Eckert, T.; Stötzel, S.; Burg-Roderfeld, M.; Sewing, J.; Lütteke, T.; Nifantiev, N.E.; Vliegenthart, J.F.G.; Siebert, H.-C. In silico Study on Sulfated and Non-Sulfated Carbohydrate Chains from Proteoglycans in Cnidaria and Interaction with Collagen. Open J. Phys. Chem. 2012, 2, 123–133. [Google Scholar] [CrossRef] [Green Version]
- Bhunia, A.; Vivekanandan, S.; Eckert, T.; Burg-Roderfeld, M.; Wechselberger, R.; Romanuka, J.; Bächle, D.; Kornilov, A.V.; von der Lieth, C.-W.; Jiménez-Barbero, J.S.; et al. Why Structurally Different Cyclic Peptides Can Be Glycomimetics of the HNK-1 Carbohydrate Antigen. J. Am. Chem. Soc. 2010, 132, 96–105. [Google Scholar] [CrossRef]
- Tsvetkov, Y.E.; Burg-Roderfeld, M.; Loers, G.; Arda, A.; Sukhova, E.V.; Khatuntseva, E.A.; Grachev, A.A.; Chizhov, A.O.; Siebert, H.C.; Schachner, M.; et al. Synthesis and molecular recognition studies of the HNK-1 trisaccharide and related oligosaccharides. The specificity of monoclonal anti-HNK-1 antibodies as assessed by surface plasmon resonance and STD NMR. J. Am. Chem. Soc. 2012, 134, 426–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toegel, S.; Pabst, M.; Wu, S.Q.; Grass, J.; Goldring, M.B.; Chiari, C.; Kolb, A.; Altmann, F.; Viernstein, H.; Unger, F.M. Phenotype-related differential alpha-2,6- or alpha-2,3-sialylation of glycoprotein N-glycans in human chondrocytes. Osteoarthr. Cartil. 2010, 18, 240–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schauer, R.; Kamerling, J.P. Exploration of the Sialic Acid World. Adv. Carb. Chem. Biochem. 2018, 75, 1–213. [Google Scholar] [CrossRef]
- Zhang, R.; Loers, G.; Schachner, M.; Boelens, R.; Wienk, H.; Siebert, S.; Eckert, T.; Kraan, S.; Rojas-Macias, M.A.; Lütteke, T.; et al. Molecular Basis of the Receptor Interactions of Polysialic Acid (polySia), polySia Mimetics, and Sulfated Polysaccharides. ChemMedChem 2016, 11, 990–1002. [Google Scholar] [CrossRef]
- Siebert, H.-C.; Scheidig, A.; Eckert, T.; Wienk, H.; Boelens, R.; Mahvash, M.; Petridis, A.K.; Schauer, R. Interaction studies of sialic acids with model receptors contribute to nanomedical therapies. J. Neurol. Disord. 2015, 3, 1–6. [Google Scholar] [CrossRef]
- Siebert, H.-C.; Lu, S.-Y.; Wechselberger, R.; Born, K.; Eckert, T.; Liang, S.; Lieth, C.-W.v.d.; Jiménez-Barbero, J.; Schauer, R.; Vliegenthart, J.F.G.; et al. A lectin from the Chinese bird-hunting spider binds sialic acids. Carbohydr. Res. 2010, 344, 1515–1525. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Wu, L.; Eckert, T.; Burg-Roderfeld, M.; Rojas-Macias, M.A.; Lütteke, T.; Krylov, V.B.; Argunov, D.A.; Datta, A.; Markart, P.; et al. Lysozyme’s lectin-like characteristics facilitates its immune defense function. Q. Rev. Biophys. 2017, 50, e9. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Eckert, T.; Lütteke, T.; Hanstein, S.; Scheidig, A.; Bonvin, A.M.; Nifantiev, N.E.; Kozar, T.; Schauer, R.; Enani, M.A.; et al. Structure-Function Relationships of Antimicrobial Peptides and Proteins with Respect to Contact Molecules on Pathogen Surfaces. Curr. Top. Med. Chem. 2016, 16, 89–98. [Google Scholar] [CrossRef] [Green Version]
- Kar, R.K.; Gazova, Z.; Bednarikova, Z.; Mroue, K.H.; Ghosh, A.; Zhang, R.; Ulicna, K.; Siebert, H.C.; Nifantiev, N.E.; Bhunia, A. Evidence for Inhibition of Lysozyme Amyloid Fibrillization by Peptide Fragments from Human Lysozyme: A Combined Spectroscopy, Microscopy, and Docking Study. Biomacromolecules 2016, 17, 1998–2009. [Google Scholar] [CrossRef]
- Oesser, S.; Adam, M.; Babel, W.; Seifert, J. Oral administration of (14)C labeled gelatin hydrolysate leads to an accumulation of radioactivity in cartilage of mice (C57/BL). J. Nutr. 1999, 129, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- Oesser, S.; Seifert, J. Stimulation of type II collagen biosynthesis and secretion in bovine chondrocytes cultured with degraded collagen. Cell Tissue Res. 2003, 311, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Schunck, M.; Louton, H.; Oesser, S. The Effectiveness of Specific Collagen Peptides on Osteoarthritis in Dogs-Impact on Metabolic Processes in Canine Chondrocytes. Open J. Anim. Sci. 2017, 07, 254–266. [Google Scholar] [CrossRef] [Green Version]
- Dobenecker, B.; Reese, S.; Jahn, W.; Schunck, M.; Hugenberg, J.; Louton, H.; Oesser, S. Specific bioactive collagen peptides (PETAGILE((R))) as supplement for horses with osteoarthritis: A two-centred study. J. Anim. Physiol. Anim. Nutr. 2018, 102 (Suppl. 1), 16–23. [Google Scholar] [CrossRef]
- Simons, V.S.; Lochnit, G.; Wilhelm, J.; Ishaque, B.; Rickert, M.; Steinmeyer, J. Comparative Analysis of Peptide Composition and Bioactivity of Different Collagen Hydrolysate Batches on Human Osteoarthritic Synoviocytes. Sci. Rep. 2018, 8, 17733. [Google Scholar] [CrossRef]
- Porfírio, E.; Fanaro, G.B. Collagen supplementation as a complementary therapy for the prevention and treatment of osteoporosis and osteoarthritis: A systematic review. Revista Brasileira de Geriatria e Gerontologia 2016, 19, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Qiao, S.; Zhang, G. Analysis of angiotensin-converting enzyme 2 (ACE2) from different species sheds some light on cross-species receptor usage of a novel coronavirus 2019-nCoV. J. Infect. 2020, 80, 469–496. [Google Scholar] [CrossRef] [Green Version]
- Saponaro, F.; Rutigliano, G.; Sestito, S.; Bandini, L.; Storti, B.; Bizzarri, R.; Zucchi, R. ACE2 in the Era of SARS-CoV-2: Controversies and Novel Perspectives. Front. Mol. Biosci. 2020, 7. [Google Scholar] [CrossRef]
- Lam, S.D.; Bordin, N.; Waman, V.P.; Scholes, H.M.; Ashford, P.; Sen, N.; van Dorp, L.; Rauer, C.; Dawson, N.L.; Pang, C.S.M.; et al. SARS-CoV-2 spike protein predicted to form complexes with host receptor protein orthologues from a broad range of mammals. Sci. Rep. 2020, 10, 16471. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, C.; Zhang, R.; Jin, L.; Yin, X.; Zheng, X.; Siebert, H.C.; Li, Y.; Wang, Z.; Loers, G.; et al. Amelioration of clinical course and demyelination in the cuprizone mouse model in relation to ketogenic diet. Food Funct. 2020, 11, 5647–5663. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Liu, C.; Jin, L.; Zhang, R.; Siebert, H.C.; Wang, Z.; Prakash, S.; Yin, X.; Li, J.; Hou, D.; et al. Influence of Long-Chain/Medium-Chain Triglycerides and Whey Protein/Tween 80 Ratio on the Stability of Phosphatidylserine Emulsions (O/W). ACS Omega 2020, 5, 7792–7801. [Google Scholar] [CrossRef]
- Zhang, R.; Jin, L.; Zhang, N.; Petridis, A.K.; Eckert, T.; Scheiner-Bobis, G.; Bergmann, M.; Scheidig, A.; Schauer, R.; Yan, M.; et al. The Sialic Acid-Dependent Nematocyst Discharge Process in Relation to Its Physical-Chemical Properties Is A Role Model for Nanomedical Diagnostic and Therapeutic Tools. Mar. Drugs 2019, 17, 469. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Zhang, N.; Mohri, M.; Wu, L.; Eckert, T.; Krylov, V.B.; Antosova, A.; Ponikova, S.; Bednarikova, Z.; Markart, P.; et al. Nanomedical Relevance of the Intermolecular Interaction Dynamics-Examples from Lysozymes and Insulins. ACS Omega 2019, 4, 4206–4220. [Google Scholar] [CrossRef]
- Mele, E. Epidemiologie der Osteoarthritis-Osteoarthrose(OA). Vet. Focus 2007, 17, 4–10. [Google Scholar] [CrossRef]
- Belshaw, Z.; Asher, L.; Harvey, N.D.; Dean, R.S. Quality of life assessment in domestic dogs: An evidence-based rapid review. Vet. J. 2015, 206, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Hansen, B.D. Assessment of pain in dogs: Veterinary clinical studies. ILAR J. 2003, 44, 197–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hielm-Björkman, A.K.; Kuusela, E.; Liman, A.; Markkola, A.; Saarto, E.; Huttunen, P.; Leppäluoto, J.; Tulamo, R.-M.; Raekallio, M. Evaluation of methods for assessment of painassociated with chronic osteoarthritis in dogs. JAVMA 2003, 11, 1552–1558. [Google Scholar] [CrossRef] [PubMed]
- Weide, N. The Application of Gelatinehydrolysate in Clinical Orthopedic Healthy Dogs and Dogs with Chronic Defects on the Locomotor System; Tierärztliche Hochschule: Hannover, Germany, 2004. [Google Scholar]
- Hielm-Björkman, A. Assessment of Chronic Pain and Evaluation of Three Complementary Therapies (Gold Implants, Green Lipped Mussel and a Homeopathic Combination Preparation) for Canine Osteoarthritis, Using Ran Domized, Controlled, Double-Blind Study Designs; Ubiversity of Helsinki: Helsinki, Finland, 2007. [Google Scholar]
- Sastravaha, A.; Suwanna, N.; Sinthusingha, C.; Noosud, J.; Roongsitthicha, A. Ameliorative Effects of Omega-3 Concentrate in Managing Coxofemoral Osteoarthritic Pain in Dogs. Thai. J. Vet. Med. 2016, 46, 305–311. [Google Scholar]
- Seales, E.C.; Jurado, G.A.; Singhal, A.; Bellis, S.L. Ras oncogene directs expression of a differentially sialylated, functionally altered beta1 integrin. Oncogene 2003, 22, 7137–7145. [Google Scholar] [CrossRef] [PubMed]
- Petridis, A.K.; Nikolopoulos, S.N.; El-Maarouf, A. Physical and functional cooperation of neural cell adhesion molecule and beta1-integrin in neurite outgrowth induction. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2011, 18, 1109–1113. [Google Scholar] [CrossRef]
- Luo, Y.; Sinkeviciute, D.; He, Y.; Karsdal, M.; Henrotin, Y.; Mobasheri, A.; Onnerfjord, P.; Bay-Jensen, A. The minor collagens in articular cartilage. Protein Cell 2017, 8, 560–572. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, M.; Tortorella, M.; Nagase, H.; Brew, K. TIMP-3 is a potent inhibitor of aggrecanase 1 (ADAM-TS4) and aggrecanase 2 (ADAM-TS5). J. Biol. Chem. 2001, 276, 12501–12504. [Google Scholar] [CrossRef] [Green Version]
- Parkkonen, O.; Nieminen, M.T.; Vesterinen, P.; Tervahartiala, T.; Perola, M.; Salomaa, V.; Jousilahti, P.; Sorsa, T.; Pussinen, P.J.; Sinisalo, J. Low MMP-8/TIMP-1 reflects left ventricle impairment in takotsubo cardiomyopathy and high TIMP-1 may help to differentiate it from acute coronary syndrome. PLoS ONE 2017, 12, e0173371. [Google Scholar] [CrossRef] [PubMed]
- Ertunc, N.; Sato, C.; Kitajima, K. Sialic acid sulfation is induced by the antibiotic treatment in mammalian cells. Biosci. Biotechnol. Biochem. 2020, 84, 2311–2318. [Google Scholar] [CrossRef]
- Dixon, W.J. BMDP Statistical Software Manual; University of California Press: Berkeley, CA, USA, 1993. [Google Scholar]
- Cytel Studio StatXact; Cytel Software Corporation: Cambridge, MA, USA, 2010.
- Schrödinger. Maestro, v. 12.3.013; Schrödinger, LLC: New York, NY, USA, 2020. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Bella, J.; Liu, J.; Kramer, R.; Brodsky, B.; Berman, H.M. Conformational effects of Gly-X-Gly interruptions in the collagen triple helix. J. Mol. Biol. 2006, 362, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Halgren, T. New method for fast and accurate binding-site identification and analysis. Chem. Biol. Drug Des. 2007, 69, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger. SiteMap, Version 3.9; Schrödinger, LLC: New York, NY, USA, 2013. [Google Scholar]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [Green Version]
- Schrödinger. Glide, Version 6.1; Schrödinger, LLC: New York, NY, USA, 2013. [Google Scholar]
- Ritchie, D.W. Evaluation of protein docking predictions using Hex 3.1 in CAPRI rounds 1 and 2. Proteins 2003, 52, 98–106. [Google Scholar] [CrossRef]
- Bowers, K.J.; Sacerdoti, F.D.; Salmon, J.K.; Shan, Y.; Shaw, D.E.; Chow, E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; et al. Molecular dynamics—Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the 2006 ACM/IEEE Conference on Supercomputing—SC ’06, Tampa, FL, USA, 11–17 November 2006. [Google Scholar]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. Potential energy functions for atomic-level simulations of water and organic and biomolecular systems. Proc. Natl. Acad. Sci. USA 2005, 102, 6665–6670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The Missing Term in Effective Pair Potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef] [PubMed]











| Group 1: Collagen hydrolysate | |||||||
|---|---|---|---|---|---|---|---|
| Dog Nr. | Race | Age | Gender | Weight | Disease | DL | BCS |
| 2 | Golden Retriever | 10 | m | 43.7 | CA | 2 | 8 |
| 4 | Cairn Terrier | 8 | m | 11 | CA | stiff movement | 5 |
| 6 | Boxer mix | 13 | fc | 30.5 | CA | 1 | 5 |
| 14 | Boerboel | 3 | f | 45 | GA | 2 | 4 |
| 15 | Magyar Vizsla | 6 | fc | 24 | CA | 2 | 6 |
| 19 | Leonberger | 4 | f | 51 | CA | 0 | 7 |
| 20 | Mixed breed | 8 | mc | 16 | GA | 1 | 8 |
| 21 | Bernese Mountain Dog | 9 | fc | 38.6 | CA | 2 | 6 |
| 25 | German Wirehaired Pointer | 5 | m | 46 | CA | 0 | 7 |
| 26 | Shepherd mix | 13 | fc | 34.9 | GA | 2 | 6 |
| 27 | Labrador | 3 | m | 40 | CA | 0 | 5 |
| 33 | Shepherd mix | 11 | mc | 38 | CA | 3 | 7 |
| 34 | German Shepherd | 2 | f | 26 | CA | 0 | 4 |
| 35 | Gordon Setter | 10 | m | 29.4 | CA | 1 | 5 |
| 37 | Poodle | 13 | fc | 9.4 | CA | 3 | 7 |
| 40 | Appenzeller | 8 | fc | 37.8 | CA | 1 | 7 |
| 45 | Samoyed | 4 | fc | 24.8 | * | 1 | 4 |
| 49 | German Shepherd mix | 2 | fc | 23.4 | CA | 1 | 5 |
| 51 | Mixed breed | 14 | mc | 29.7 | CA | 1 | 5 |
| 52 | Border collie mix | 2 | fc | 23.1 | GA | 0 | 3 |
| Summary: | Ø 7.35 | m: 5 mc: 3 f:3 fc: 9 | Ø 31.1 | CA: 15 GA: 4 | Ø 1.23 | Ø 5.7 | |
| Group 2: Glucosamine | |||||||
| Dog Nr. | Race | Age | Gender | Weight | Disease | DL | BCS |
| 1 | Schnauzer mix | 5 | mc | 26.5 | CA | 2 to 3 | 6 |
| 3 | Newfoundland Dog | 7 | fc | 50 | GA | 2 | 6 |
| 5 | Mixed breed | 12 | f | 28 | CA | 3 | 7 |
| 7 | Mixed breed | 12 | mc | 28 | CA/GA | 3 | 5 |
| 8 | Bernese Mountain Dog | 6 | m | 41 | CA | 2 | 6 |
| 10 | Mixed breed | 8 | m | 40 | CA | 1 | 6 |
| 12 | Mixed breed | 7 | mc | 17 | CA/GA | 1 | 7 |
| 13 | Cairn Terrier | 8 | mc | 11 | CA | stiff movement | 5 |
| 16 | Beagle | 4 | mc | 16.8 | CA | 1 | 6 |
| 18 | Swiss Mountain Dog | 4 | m | 55 | CA | stiff movement | 5 |
| 22 | Labrador | 10 | mc | 32 | CA | 2 | 6 |
| 23 | Newfoundland Dog | 10 | m | 59 | CA | 2 | 3 |
| 28 | Boxer | 3 | f | 30 | CA | 2 | 5 |
| 29 | Kangal | 2.5 | fc | 50 | CA | 0 | 6 |
| 36 | Yorkshire Terrier | 6 | fc | 6.4 | CA | 2 | 5 |
| 39 | German Shepherd | 9 | m | 45.6 | CA | 1 | 7 |
| 41 | Shepherd mix | 6 | m | 32.2 | CA | 1 | 5 |
| 42 | Belgian Shepherd Dog | 10 | f | 36 | CA | 2 | 4 |
| 44 | Ridgeback | 1 | fc | 27.5 | CA | 2 | 3 |
| 48 | Mixed breed | 4 | mc | 29.4 | CA | 1 | 3 |
| 50 | Labrador | 8 | fc | 24.8 | CA | 0 | 3 |
| Summary: | Ø 6,8 | m: 6 mc: 7 f: 3 fc: 5 | Ø 32.7 | CA: 18 GA: 1 GA/CA: 2 | Ø 1.45 | Ø 5.2 | |
| GROUP 3: commercial joint diet | |||||||
| Dog Nr. | Race | Age | Gender | Weight | Disease | DL | BCS |
| 9 | Shepherd mix | 8 | fc | 42 | GA | 1 | 5 |
| 11 | Shepherd mix | 7 | f | 35 | GA | 2 | 5 |
| 17 | German Shepherd | 10 | fc | 34 | CA | 2 | 6 |
| 24 | Mixed breed | 9 | m | 23 | CA | 1 | 4 |
| 30 | German Shepherd–Sennendog-Mix | 5 | mc | 37 | CA | 1 | 7 |
| 31 | Mixed breed | 5 | fc | 33 | CA | 0 | 6 |
| 32 | German Shepherd–Husky-Mix | 13 | fc | 40.5 | CA | 2 | 7 |
| 38 | Pomeranian | 8 | mc | 8 | CA | 2 | 8 |
| 43 | Mixed breed | 4 | mc | 38 | GA | 2 | 5 |
| 46 | Golden Retriever | 5 | mc | 29 | CA | 1 | 5 |
| 47 | Mixed breed | 10 | fc | 26 | CA | 1 | 6 |
| Summary: | Ø 7.6 | m: 1 mc: 4 f: 1 fc: 5 | Ø 31.4 | CA: 8 GA: 3 | Ø 1.32 | Ø 5.8 | |
| Behavior | Collagen Hydrolysate | Glucosamine | Special Dog-Food | |||
|---|---|---|---|---|---|---|
| Start | 16 Weeks | Start | 16 Weeks | Start | 16 Weeks | |
| Mind and mood | 0.5 | 0.26 | 0.55 | 0.35 | 0.78 | 0.67 |
| Vocalization | 0.36 | 0.43 | 1 | 0.88 | 0 | 0 |
| Joy of playing | 1.31 | 1 | 1.72 | 1.35 | 1.25 | 1 |
| Joy of running | 1.5 | 0.76 | 1.83 | 0.94 | 1.78 | 1 |
| Will to trot | 1.56 | 1 | 1.89 | 1.47 | 1.33 | 0.89 |
| Will to gallop | 2.06 | 1.5 | 2.33 | 1.82 | 2.22 | 2 |
| Jumping | 2.25 | 1.71 | 2.72 | 2 | 2.22 | 2.11 |
| Laying down | 1.44 | 1.29 | 1.56 | 1.29 | 1.78 | 1.67 |
| Standing up | 2.13 | 1.79 | 2.61 | 2 | 2.33 | 2.22 |
| Difficulty moving after a long break | 2.38 | 2.14 | 2.61 | 1.94 | 2.22 | 2 |
| Difficulty moving after exercise | 2.07 | 1.58 | 2.94 | 2.5 | 2 | 2 |
| Dog Name (#Number) | Treatment | Breed | Age (years) | Weight (kg) |
|---|---|---|---|---|
| Buddy (#1) | glucosamine | Schnauzer-mix | 5 | 26.5 |
| Cora (#3) | glucosamine | Newfoundland Dog | 7 | 50 |
| Sarabi (#14) | collagen hydrolysate | Boerboel | 3 | 45 |
| Tobi (#20) | collagen hydrolysate | Mixed-breed | 8 | 16 |
| Emma | special dog-food with lipids and vitamins | German shepherd-mix | 7 | 35 |
| Docking Score (kcal/mol) | ||||||
|---|---|---|---|---|---|---|
| Protein | PDB Code | Binding Site | GlcNAc | GlcNAc-sulf | Neu5Ac | Neu5Ac-sulf |
| MMP-3 | 2JT6 | BS1 | −8.16 | −8.23 | −11.48 * | −8.76 |
| BS2 | −9.20 | −9.12 | −8.68 | −9.43 * | ||
| BS3 | −5.24 | −6.27 | −6.88 | −7.42 * | ||
| ADAMTS-5 | 2RJQ | BS1 | −6.28 | −6.96 | −7.61 | −8.09* |
| BS2 | −7.05 | −7.94 | −9.00 | −9.22* | ||
| BS3 | −5.94 | −5.45 | −5.87 | −5.78 | ||
| BS4 | −7.25 | −9.16 | −8.61 | −9.35* | ||
| BS5 | −6.99 | −5.73 | −8.61 | −7.73 | ||
| BS6 | −4.47 | -4.89 | ||||
| BS7 | −7.07 | −7.31 | −8.35 | −8.88 | ||
| BS8 | −6.07 | −5.85 | −8.86 | −8.19 | ||
| BS9 | −6.68 | −6.81 | −8.82 | −7.93 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckert, T.; Jährling-Butkus, M.; Louton, H.; Burg-Roderfeld, M.; Zhang, R.; Zhang, N.; Hesse, K.; Petridis, A.K.; Kožár, T.; Steinmeyer, J.; et al. Efficacy of Chondroprotective Food Supplements Based on Collagen Hydrolysate and Compounds Isolated from Marine Organisms. Mar. Drugs 2021, 19, 542. https://doi.org/10.3390/md19100542
Eckert T, Jährling-Butkus M, Louton H, Burg-Roderfeld M, Zhang R, Zhang N, Hesse K, Petridis AK, Kožár T, Steinmeyer J, et al. Efficacy of Chondroprotective Food Supplements Based on Collagen Hydrolysate and Compounds Isolated from Marine Organisms. Marine Drugs. 2021; 19(10):542. https://doi.org/10.3390/md19100542
Chicago/Turabian StyleEckert, Thomas, Mahena Jährling-Butkus, Helen Louton, Monika Burg-Roderfeld, Ruiyan Zhang, Ning Zhang, Karsten Hesse, Athanasios K. Petridis, Tibor Kožár, Jürgen Steinmeyer, and et al. 2021. "Efficacy of Chondroprotective Food Supplements Based on Collagen Hydrolysate and Compounds Isolated from Marine Organisms" Marine Drugs 19, no. 10: 542. https://doi.org/10.3390/md19100542
APA StyleEckert, T., Jährling-Butkus, M., Louton, H., Burg-Roderfeld, M., Zhang, R., Zhang, N., Hesse, K., Petridis, A. K., Kožár, T., Steinmeyer, J., Schauer, R., Engelhard, P., Kozarova, A., Hudson, J. W., & Siebert, H.-C. (2021). Efficacy of Chondroprotective Food Supplements Based on Collagen Hydrolysate and Compounds Isolated from Marine Organisms. Marine Drugs, 19(10), 542. https://doi.org/10.3390/md19100542