Potential Biomedical Applications of Collagen Filaments derived from the Marine Demosponges Ircinia oros (Schmidt, 1864) and Sarcotragus foetidus (Schmidt, 1862)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Sponge Collagen Filaments (SCFs) Characterisation
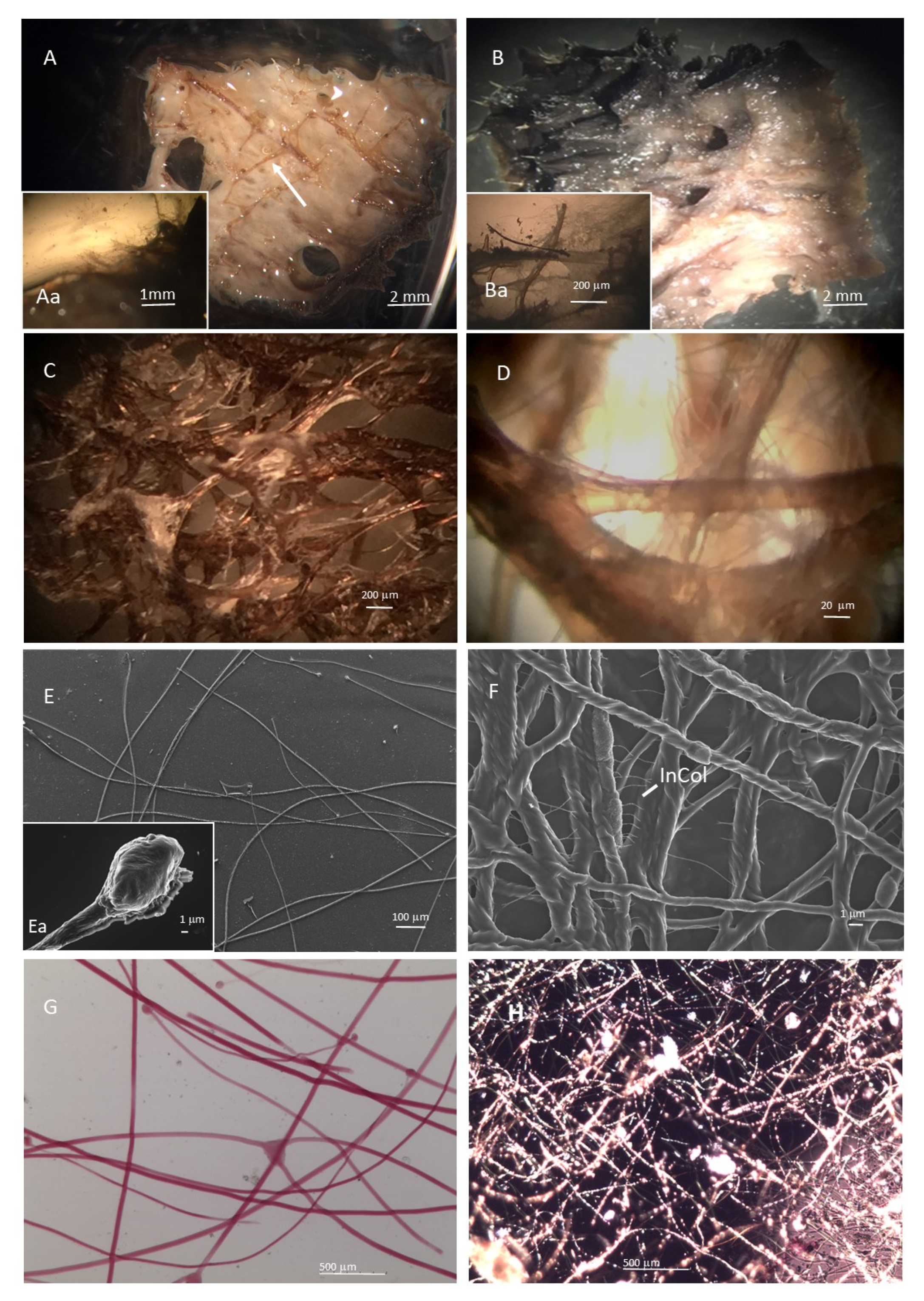
2.1.1. SCFs Microscopy Analysis
2.1.2. SCFs Biochemical Analysis
Amino Acid Composition
Amino Acids, Glycosaminoglycans (GAGs) and Iron Content
Sodium Dodecyl Sulfate Poly Acrylamide Gel Electrophoresis (SDS-PAGE)
2.2. Sponge Collagen Filament Membranes (SCFMs) Characterisation
2.2.1. SCFM Surface Morphologies
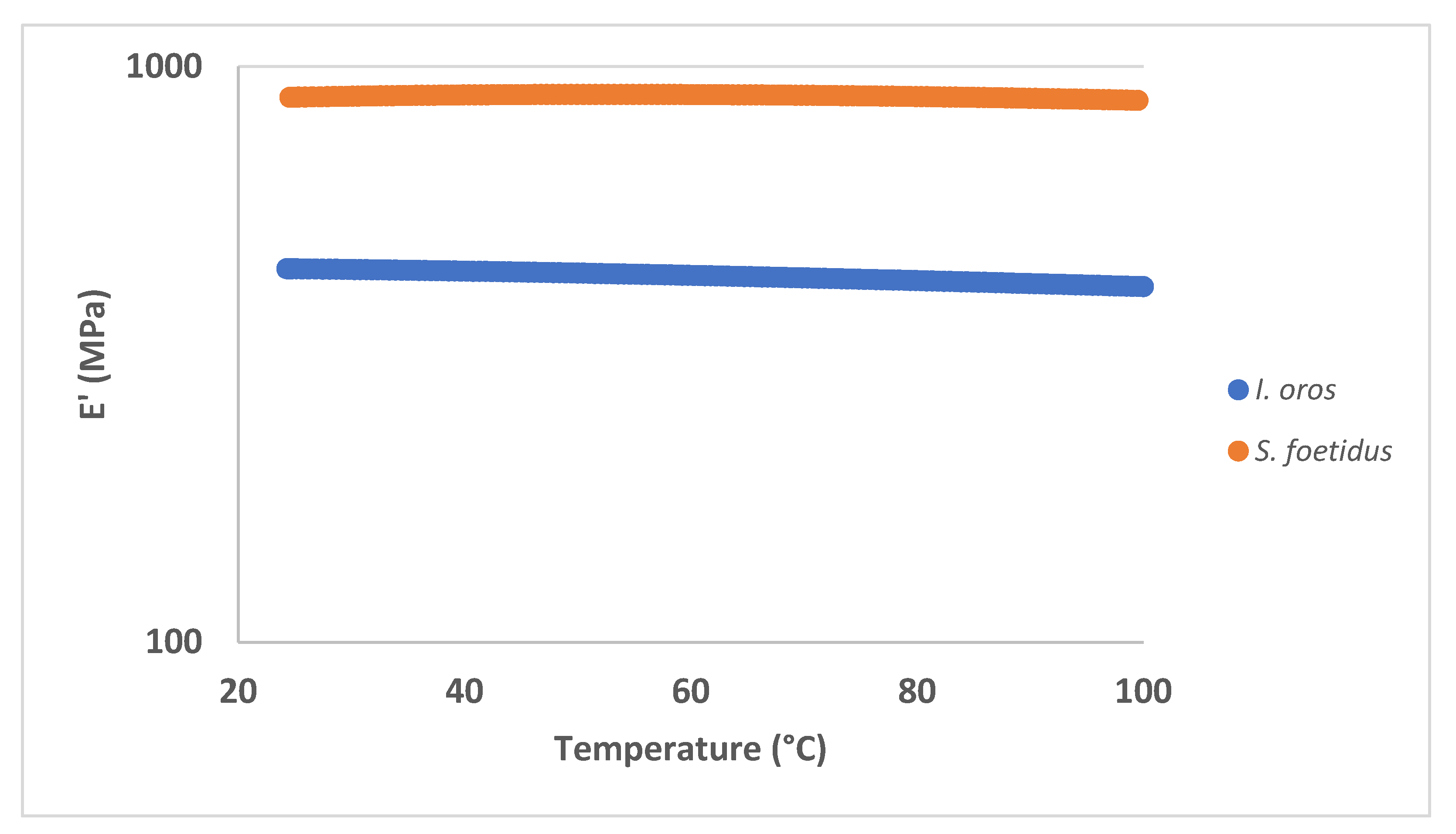
2.2.2. Thermal Properties
2.2.3. Mechanical Properties
2.2.4. In Vitro Degradation Evaluation
2.2.5. Swelling Test and Antioxidant Activity
2.3. SCFM Biocompatibility Evaluation
2.3.1. Cell Adhesion and Cell Proliferation
2.3.2. Fibroblast Gene Expression Analysis and Collagen Expression Level
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Sponge Sampling
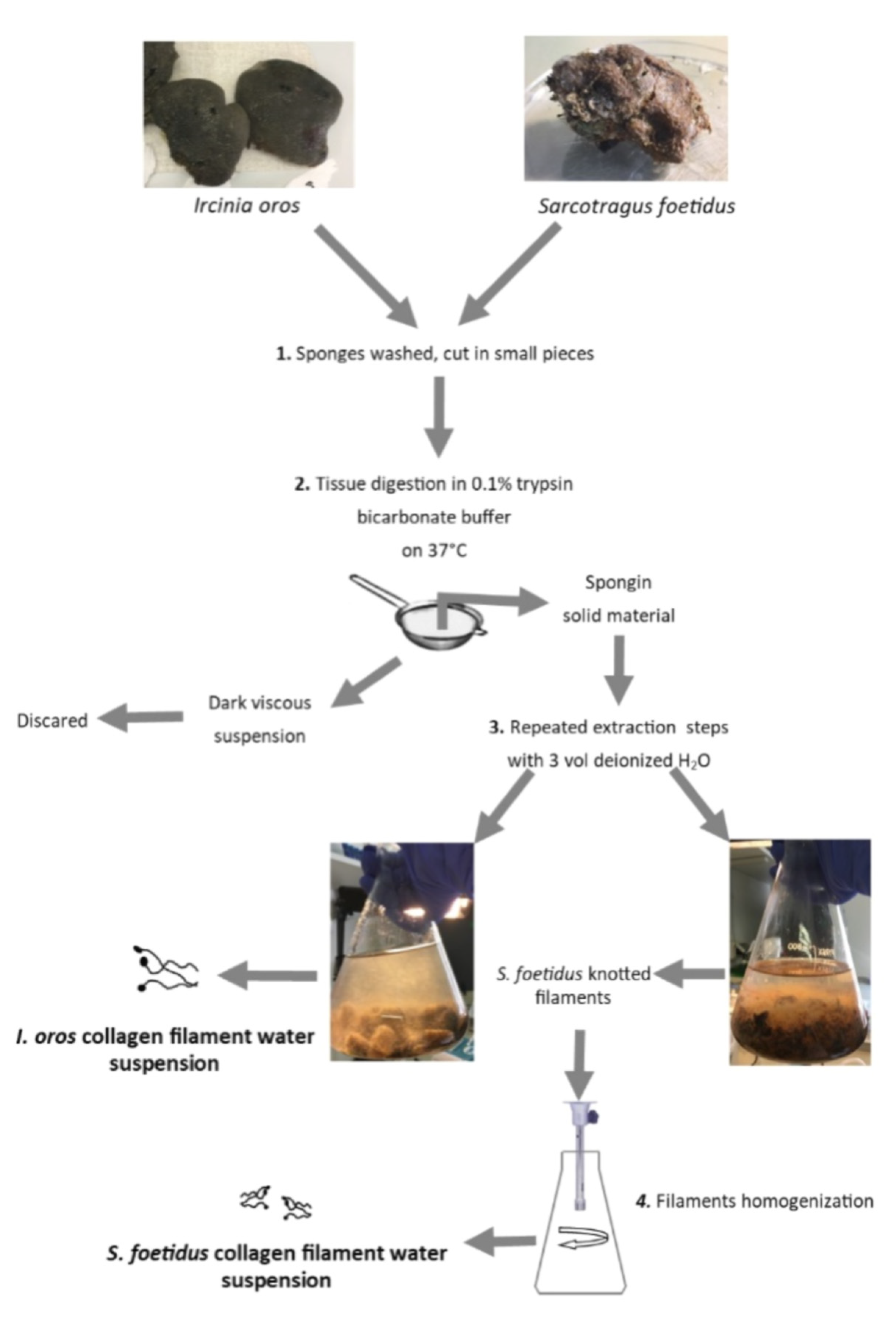
4.3. Sponge Collagenous Filaments and Intercellular Collagen Isolation
4.4. Light Microscopy and Environmental Scanning Electron Microscope (ESEM) Observation
4.5. SCFs Biochemical Characterisation
4.5.1. Amino Acid Composition
4.5.2. Glycosaminoglycans (GAGs) Quantification
4.5.3. Quantitative Analysis of Iron Content
4.5.4. Sodium Dodecyl Sulfate Poly Acrylamide Gel Electrophoresis (SDS-PAGE)
4.6. SCFMs Production
4.7. SCFMs Characterisation
4.7.1. Differential Scanning Calorimetry
4.7.2. DMA|DMTA
4.7.3. In Vitro Degradation Study
4.7.4. Swelling Test
4.7.5. DPPH Radical Scavenging Activity
4.8. SCM Biocompatibility Evaluation
4.8.1. Cell Cultures
4.8.2. Cell Growth and Cell Adhesion
4.8.3. L929 Fibroblast Gene Expression Analysis
4.8.4. L929 Fibroblast Collagen Synthesis Evaluation
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chevallay, B.; Herbage, D. Collagen-Based Biomaterials as 3D Scaffold for Cell Cultures: Applications for Tissue Engineering and Gene Therapy. Med. Biol. Eng. Comput. 2000, 38, 211–218. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef]
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; de Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, Y.-S.; Ok, Y.-J.; Hwang, S.-Y.; Kwak, J.-Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khrunyk, Y.; Lach, S.; Petrenko, I.; Ehrlich, H. Progress in Modern Marine Biomaterials Research. Mar. Drugs 2020, 18, 589. [Google Scholar] [CrossRef] [PubMed]
- Granito, R.N.; Custódio, M.R.; Rennó, A.C.M. Natural Marine Sponges for Bone Tissue Engineering: The State of Art and Future Perspectives. J Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 1717–1727. [Google Scholar] [CrossRef]
- Parisi, J.R.; Fernandes, K.R.; Avanzi, I.R.; Dorileo, B.P.; Santana, A.F.; Andrade, A.L.; Gabbai-Armelin, P.R.; Fortulan, C.A.; Trichês, E.S.; Granito, R.N.; et al. Incorporation of Collagen from Marine Sponges (Spongin) into Hydroxyapatite Samples: Characterization and In Vitro Biological Evaluation. Mar. Biotechnol. 2019, 21, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Parisi, J.R.; Fernandes, K.R.; de Almeida Cruz, M.; Avanzi, I.R.; de França Santana, A.; do Vale, G.C.A.; de Andrade, A.L.M.; de Góes, C.P.; Fortulan, C.A.; de Sousa Trichês, E.; et al. Evaluation of the In Vivo Biological Effects of Marine Collagen and Hydroxyapatite Composite in a Tibial Bone Defect Model in Rats. Mar. Biotechnol. 2020, 22, 357–366. [Google Scholar] [CrossRef]
- Fernandes, K.R.; Parisi, J.R.; Magri, A.M.P.; Kido, H.W.; Gabbai-Armelin, P.R.; Fortulan, C.A.; Zanotto, E.D.; Peitl, O.; Granito, R.N.; Renno, A.C.M. Influence of the Incorporation of Marine Spongin into a Biosilicate®: An in Vitro Study. J. Mater. Sci. Mater. Med. 2019, 30, 64. [Google Scholar] [CrossRef]
- Langasco, R.; Cadeddu, B.; Formato, M.; Lepedda, A.J.; Cossu, M.; Giunchedi, P.; Pronzato, R.; Rassu, G.; Manconi, R.; Gavini, E. Natural Collagenic Skeleton of Marine Sponges in Pharmaceutics: Innovative Biomaterial for Topical Drug Delivery. Mater. Sci. Eng. C 2017, 70, 710–720. [Google Scholar] [CrossRef]
- Pozzolini, M.; Scarfì, S.; Gallus, L.; Castellano, M.; Vicini, S.; Cortese, K.; Gagliani, M.C.; Bertolino, M.; Costa, G.; Giovine, M. Production, Characterization and Biocompatibility Evaluation of Collagen Membranes Derived from Marine Sponge Chondrosia Reniformis Nardo, 1847. Mar. Drugs 2018, 16, 111. [Google Scholar] [CrossRef] [Green Version]
- Green, D.; Howard, D.; Yang, X.; Kelly, M.; Oreffo, R.O.C. Natural Marine Sponge Fiber Skeleton: A Biomimetic Scaffold for Human Osteoprogenitor Cell Attachment, Growth, and Differentiation. Tissue Eng. 2003, 9, 1159–1166. [Google Scholar] [CrossRef]
- Pozzolini, M.; Bruzzone, F.; Berilli, V.; Mussino, F.; Cerrano, C.; Benatti, U.; Giovine, M. Molecular Characterization of a Nonfibrillar Collagen from the Marine Sponge Chondrosia Reniformis Nardo 1847 and Positive Effects of Soluble Silicates on Its Expression. Mar. Biotechnol. 2012, 14, 281–293. [Google Scholar] [CrossRef]
- Ehrlich, H.; Wysokowski, M.; Żółtowska-Aksamitowska, S.; Petrenko, I.; Jesionowski, T. Collagens of Poriferan Origin. Mar. Drugs 2018, 16, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozzolini, M.; Scarfì, S.; Mussino, F.; Ferrando, S.; Gallus, L.; Giovine, M. Molecular Cloning, Characterization, and Expression Analysis of a Prolyl 4-Hydroxylase from the Marine Sponge Chondrosia Reniformis. Mar. Biotechnol. 2015, 17, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Pozzolini, M.; Scarfì, S.; Gallus, L.; Ferrando, S.; Cerrano, C.; Giovine, M. Silica-Induced Fibrosis: An Ancient Response from the Early Metazoans. J. Exp. Biol. 2017, 220, 4007–4015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soest, R.W.M.V.; Boury-Esnault, N.; Vacelet, J.; Dohrmann, M.; Erpenbeck, D.; Voogd, N.J.D.; Santodomingo, N.; Vanhoorne, B.; Kelly, M.; Hooper, J.N.A. Global Diversity of Sponges (Porifera). PLoS ONE 2012, 7, e35105. [Google Scholar] [CrossRef]
- Gross, J.; Sokal, Z.; Rougvie, M. Structural and Chemical Studies on the Connective Tissue of Marine Sponges. J. Histochem. Cytochem. 1956, 4, 227–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jesionowski, T.; Norman, M.; Żółtowska-Aksamitowska, S.; Petrenko, I.; Joseph, Y.; Ehrlich, H. Marine Spongin: Naturally Prefabricated 3D Scaffold-Based Biomaterial. Mar. Drugs 2018, 16, 88. [Google Scholar] [CrossRef] [Green Version]
- Petrenko, I.; Summers, A.P.; Simon, P.; Żółtowska-Aksamitowska, S.; Motylenko, M.; Schimpf, C.; Rafaja, D.; Roth, F.; Kummer, K.; Brendler, E.; et al. Extreme Biomimetics: Preservation of Molecular Detail in Centimeter-Scale Samples of Biological Meshes Laid down by Sponges. Sci. Adv. 2019, 5, eaax2805. [Google Scholar] [CrossRef] [Green Version]
- Garrone, R.; Vacelet, J.; Junqua, S.; Robert, L.; Huca, X. Peculiar Collagen Formation-Filaments of Horny Sponges Ircinia-Ultrastructural, Physicochemical and Biochemical Study. J. Microsc. -Oxf. 1973, 17, 241–260. [Google Scholar]
- Junqua, S.; Robert, L.; Garrone, R.; Ceccatty, M.P.D.; Vacelet, J. Biochemical and Morphological Studies on Collagens of Horny Sponges. Ircinia Filaments Compared to Spongines. Connect. Tissue Res. 1974, 2, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Kölliker, A. Icones Histiologicae; Oder, Atlas Der Vergleichenden Gewebelehre, 1st ed.; Wilhel Engelmann: Leipzig, Germany, 1864; pp. 46–59. [Google Scholar]
- Towe, K.M.; Rützler, K. Lepidocrocite Iron Mineralization in Keratose Sponge Granules. Science 1968, 162, 268–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsurkan, D.; Simon, P.; Schimpf, C.; Motylenko, M.; Rafaja, D.; Roth, F.; Inosov, D.S.; Makarova, A.A.; Stepniak, I.; Petrenko, I.; et al. Extreme Biomimetics: Designing of the First Nanostructured 3D Spongin–Atacamite Composite and Its Application. Adv. Mater. 2021. [Google Scholar] [CrossRef] [PubMed]
- Manconi, R.; Pansini, M.; Pronzato, R. Fauna d’Italia Vol. XLVI - Porifera I - Calcarea, Demospongiae (partim), Hexactinellida, Homoscleromorpha, 1st ed.; Calderini: Bologna, Italy, 2011; pp. 297–308. [Google Scholar]
- Bonfrate, V.; Manno, D.; Serra, A.; Salvatore, L.; Sannino, A.; Buccolieri, A.; Serra, T.; Giancane, G. Enhanced Electrical Conductivity of Collagen Films through Long-Range Aligned Iron Oxide Nanoparticles. J. Colloid Interface Sci. 2017, 501, 185–191. [Google Scholar] [CrossRef]
- Zhuang, J.; Lin, S.; Dong, L.; Cheng, K.; Weng, W. Magnetically Assisted Electrodeposition of Aligned Collagen Coatings. ACS Biomater. Sci. Eng. 2018, 4, 1528–1535. [Google Scholar] [CrossRef]
- Mertens, M.E.; Hermann, A.; Bühren, A.; Olde-Damink, L.; Möckel, D.; Gremse, F.; Ehling, J.; Kiessling, F.; Lammers, T. Iron Oxide-Labeled Collagen Scaffolds for Non-Invasive MR Imaging in Tissue Engineering. Adv. Funct. Mater. 2014, 24, 754–762. [Google Scholar] [CrossRef]
- Burjanadze, T.V.; Veis, A. A Thermodynamic Analysis of the Contribution of Hydroxyproline to the Structural Stability of the Collagen Triple Helix. Connect. Tissue Res. 1997, 36, 347–365. [Google Scholar] [CrossRef]
- Ehrlich, H.; Deutzmann, R.; Brunner, E.; Cappellini, E.; Koon, H.; Solazzo, C.; Yang, Y.; Ashford, D.; Thomas-Oates, J.; Lubeck, M.; et al. Mineralization of the Metre-Long Biosilica Structures of Glass Sponges Is Templated on Hydroxylated Collagen. Nat. Chem 2010, 2, 1084–1088. [Google Scholar] [CrossRef]
- Yamauchi, M.; Sricholpech, M. Lysine Post-Translational Modifications of Collagen. Essays Biochem. 2012, 52, 113–133. [Google Scholar] [CrossRef] [Green Version]
- Garrone, R.; Huc, A.; Junqua, S. Fine Structure and Physicochemical Studies on the Collagen of the Marine Sponge Chondrosia Reniformis Nardo. J. Ultrastruct. Res. 1975, 52, 261–275. [Google Scholar] [CrossRef]
- Swatschek, D.; Schatton, W.; Kellermann, J.; Müller, W.E.G.; Kreuter, J. Marine Sponge Collagen: Isolation, Characterization and Effects on the Skin Parameters Surface-PH, Moisture and Sebum. Eur. J. Pharm. Biopharm. 2002, 53, 107–113. [Google Scholar] [CrossRef]
- Ghosh, A.; Grosvenor, A.J.; Dyer, J.M. Marine Spongia Collagens: Protein Characterization and Evaluation of Hydrogel Films. J. Appl. Polym. Sci. 2019, 136, 47996. [Google Scholar] [CrossRef]
- Carvalho, A.M.; Marques, A.P.; Silva, T.H.; Reis, R.L. Evaluation of the Potential of Collagen from Codfish Skin as a Biomaterial for Biomedical Applications. Mar. Drugs 2018, 16, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS Scavenging Activity, Photoprotective, and Wound-Healing Properties of Collagen-Derived Peptides from the Marine Sponge Chondrosia Reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef] [Green Version]
- Srivatsan, K.V.; Lakra, R.; Purna Sai, K.; Kiran, M.S. Effect of bimetallic iron:zinc nanoparticles on collagen stabilization. J. Mater. Chem. B 2016, 4, 1437–1447. [Google Scholar] [CrossRef]
- Desimone, M.F.; Hélary, C.; Rietveld, I.B.; Bataille, I.; Mosser, G.; Giraud-Guille, M.-M.; Livage, J.; Coradin, T. Silica–Collagen Bionanocomposites as Three-Dimensional Scaffolds for Fibroblast Immobilization. Acta Biomater. 2010, 6, 3998–4004. [Google Scholar] [CrossRef] [PubMed]
- Jang, D.; Idrobo, J.-C.; Laoui, T.; Karnik, R. Water and Solute Transport Governed by Tunable Pore Size Distributions in Nanoporous Graphene Membranes. ACS Nano 2017, 11, 10042–10052. [Google Scholar] [CrossRef]
- Dodero, A.; Scarfi, S.; Pozzolini, M.; Vicini, S.; Alloisio, M.; Castellano, M. Alginate-Based Electrospun Membranes Containing ZnO Nanoparticles as Potential Wound Healing Patches: Biological, Mechanical, and Physicochemical Characterization. Acs Appl. Mater. Interfaces 2020, 12, 3371–3381. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Grabska, S. Preparation and Characterization of 3D Collagen Materials with Magnetic Properties. Polym. Test. 2017, 62, 382–391. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Oxidative Stress in Normal and Impaired Wound Repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin Loaded Poly(ε-Caprolactone) Nanofibers: Diabetic Wound Dressing with Antioxidant and Anti-Inflammatory Properties. Clin. Exp. Pharm. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef] [Green Version]
- Mehbub, M.F.; Lei, J.; Franco, C.; Zhang, W. Marine Sponge Derived Natural Products between 2001 and 2010: Trends and Opportunities for Discovery of Bioactives. Mar. Drugs 2014, 12, 4539–4577. [Google Scholar] [CrossRef] [Green Version]
- Leal, M.C.; Puga, J.; Serôdio, J.; Gomes, N.C.M.; Calado, R. Trends in the Discovery of New Marine Natural Products from Invertebrates over the Last Two Decades – Where and What Are We Bioprospecting? PLoS ONE 2012, 7, e30580. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, A.R.; Gupta, A.; Kim, B.S. Ultrasound Assisted Green Synthesis of Silver and Iron Oxide Nanoparticles Using Fenugreek Seed Extract and Their Enhanced Antibacterial and Antioxidant Activities. BioMed Res. Int. 2019, 2019, 1714358. [Google Scholar] [CrossRef]
- Lin, Z.; Solomon, K.L.; Zhang, X.; Pavlos, N.J.; Abel, T.; Willers, C.; Dai, K.; Xu, J.; Zheng, Q.; Zheng, M. In Vitro Evaluation of Natural Marine Sponge Collagen as a Scaffold for Bone Tissue Engineering. Int. J. Biol. Sci. 2011, 7, 968–977. [Google Scholar] [CrossRef] [Green Version]
- Tal, H.; Moses, O.; Kozlovsky, A.; Nemcovsky, C. Bioresorbable Collagen Membranes for Guided Bone Regeneration. In Bone Regeneration; Tal, H., Ed.; InTech: Rijeka, Croatia, 2012; pp. 111–134. [Google Scholar]
- Rousselle, P.; Montmasson, M.; Garnier, C. Extracellular Matrix Contribution to Skin Wound Re-Epithelialization. Matrix Biol. 2019, 75–76, 12–26. [Google Scholar] [CrossRef]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound Repair and Regeneration: Mechanisms, Signaling, and Translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.A.; Prajapati, R.; McGrouther, D.A.; Yannas, I.V.; Eastwood, M. Tensional homeostasis in dermal fibroblasts: Mechanical responses to mechanical loading in three-dimensional substrates. J Cell Physiol 1998, 175, 323–332. [Google Scholar] [CrossRef]
- Pozzolini, M.; Ferrando, S.; Gallus, L.; Gambardella, C.; Ghignone, S.; Giovine, M. Aquaporin in Chondrosia Reniformis Nardo, 1847 and Its Possible Role in the Interaction Between Cells and Engulfed Siliceous Particles. Biol. Bull. 2016, 230, 220–232. [Google Scholar] [CrossRef]
- Tonelli, F.; Cotti, S.; Leoni, L.; Besio, R.; Gioia, R.; Marchese, L.; Giorgetti, S.; Villani, S.; Gistelinck, C.; Wagener, R.; et al. Crtap and P3h1 Knock out Zebrafish Support Defective Collagen Chaperoning as the Cause of Their Osteogenesis Imperfecta Phenotype. Matrix Biol. 2020, 90, 40–60. [Google Scholar] [CrossRef]
- Ardini, F.; Soggia, F.; Abelmoschi, M.L.; Magi, E.; Grotti, M. Ionomic profiling of Nicotiana langsdorffii wild-type and mutant genotypes exposed to abiotic stresses. Anal. Bioanal. Chem. 2013, 405, 665–677. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]









| Amino Acid | I.oros | S. foetidus | Rat * | Codfish * |
|---|---|---|---|---|
| Ala+Arg | 152 | 157 | 153.4 | 121.93 |
| Aspartic acid | 100.03 | 78.83 | 45.32 | 38.82 |
| Glutamic acid | 111.90 | 114.27 | 73.33 | 56.08 |
| Glycine | 359.00 | 385.46 | 333.18 | 266.12 |
| Histidine | 0.96 | 1.34 | 3.61 | 5.01 |
| Hydroxylysine | 13.91 | 14.26 | 9.33 | 6.65 |
| Hydroxyproline | 69.29 | 61.53 | 96.09 | 39.6 |
| Isoleucine | 8.57 | 5.62 | 7.48 | 5.61 |
| Leucine | 23.19 | 18.96 | 23.29 | 6.51 |
| Lysine | 13.80 | 12.00 | 27.07 | 19.62 |
| Methionine | 1.64 | 1.80 | 8.03 | 15.04 |
| Phenylalanine | 13.31 | 10.42 | 14.62 | 12.7 |
| Proline | 57.06 | 43.56 | 109.21 | 62.69 |
| Serine | 41.65 | 61.28 | 42.74 | 53.87 |
| Threonine | 18.53 | 18.66 | 18.79 | 16.89 |
| Tyrosine | 4.99 | 5.17 | 3.76 | 2.25 |
| Valine | 10.59 | 10.18 | 17.08 | 12.02 |
| - | Amino Acids (μg/mg) | GAGs (μg/mg) | Iron (μg/mg) |
|---|---|---|---|
| I. oros | 490.3 ± 3.4 | 12.28 ± 5.4 | 2.7 ± 0.32 |
| S. foetidus | 250.9 ± 2.9 | 28.49 ± 8.3 | 45.32 ± 2.8 |
| Sample | E’ @ 1 Hz, 25 °C (MPa) | St. Dev | E" @ 1 Hz, 25 °C (MPa) | St. Dev |
|---|---|---|---|---|
| I. oros | 447.15 | 9.49 | 18.52 | 0.86 |
| S. foetidus | 819.27 | 37.88 | 44.25 | 4.50 |
| Time (days) | I. oros | S. foetidus |
|---|---|---|
| μg/mL | μg/mL | |
| 7 | 3.64 ± 0.56 | <0.6 |
| 14 | 5.78 ± 0.28 | <0.6 |
| 21 | 6.03 ± 0.89 | 2.30 ± 0.37 |
| Sample | Swelling Index (%) | Antioxidant Activity (%) |
|---|---|---|
| I. oros | 1511.44 ± 149.23 | 4.64 ± 1.78 |
| S. foetidus | 827.94 ± 64.40 | 57.24 ± 8.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozzolini, M.; Tassara, E.; Dodero, A.; Castellano, M.; Vicini, S.; Ferrando, S.; Aicardi, S.; Cavallo, D.; Bertolino, M.; Petrenko, I.; et al. Potential Biomedical Applications of Collagen Filaments derived from the Marine Demosponges Ircinia oros (Schmidt, 1864) and Sarcotragus foetidus (Schmidt, 1862). Mar. Drugs 2021, 19, 563. https://doi.org/10.3390/md19100563
Pozzolini M, Tassara E, Dodero A, Castellano M, Vicini S, Ferrando S, Aicardi S, Cavallo D, Bertolino M, Petrenko I, et al. Potential Biomedical Applications of Collagen Filaments derived from the Marine Demosponges Ircinia oros (Schmidt, 1864) and Sarcotragus foetidus (Schmidt, 1862). Marine Drugs. 2021; 19(10):563. https://doi.org/10.3390/md19100563
Chicago/Turabian StylePozzolini, Marina, Eleonora Tassara, Andrea Dodero, Maila Castellano, Silvia Vicini, Sara Ferrando, Stefano Aicardi, Dario Cavallo, Marco Bertolino, Iaroslav Petrenko, and et al. 2021. "Potential Biomedical Applications of Collagen Filaments derived from the Marine Demosponges Ircinia oros (Schmidt, 1864) and Sarcotragus foetidus (Schmidt, 1862)" Marine Drugs 19, no. 10: 563. https://doi.org/10.3390/md19100563
APA StylePozzolini, M., Tassara, E., Dodero, A., Castellano, M., Vicini, S., Ferrando, S., Aicardi, S., Cavallo, D., Bertolino, M., Petrenko, I., Ehrlich, H., & Giovine, M. (2021). Potential Biomedical Applications of Collagen Filaments derived from the Marine Demosponges Ircinia oros (Schmidt, 1864) and Sarcotragus foetidus (Schmidt, 1862). Marine Drugs, 19(10), 563. https://doi.org/10.3390/md19100563













