The Potential Protective Effect and Possible Mechanism of Peptides from Oyster (Crassostrea hongkongensis) Hydrolysate on Triptolide-Induced Testis Injury in Male Mice
Abstract
:1. Introduction
2. Results
2.1. Molecular Weight and Main Peptide Sequences of OPs
2.2. Amino Acid Composition of OPs
2.3. Effects of OPs on Sperm Parameters of TP-Induced Mice
2.4. Effects of OPs on Testicular Injury of TP-Induced Mice
2.5. Effects of OPs on TP-Induced Reproductive Hormone Level
2.6. OPs Increased Testicular Marker Enzyme Activity and Reduced Oxidative Stress Induced by TP
2.7. Effects of OPs on the Testicular Apoptotic Induced by TP
2.8. Effects of OPs on Related Proteins Expression in the Nrf2 and JNK Pathways
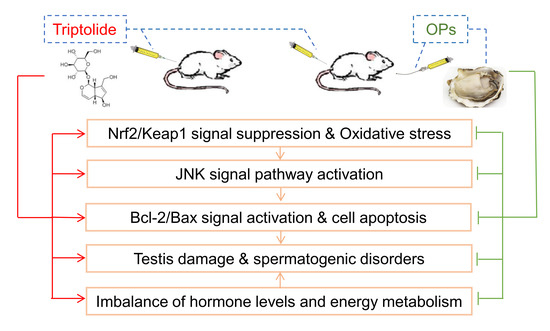
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of OPs
4.3. OPs Characterization by LC-ESI/MS/MS
4.4. Determination of Amino Acid Composition
4.5. Animals and Treatment
4.6. Sperm Analysis
4.7. Histopathological and Ultrastructural Assessment
4.8. Measurements of Enzyme and Hormone
4.9. TUNEL Apoptosis Assay
4.10. Western Blotting
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef] [Green Version]
- Jarow, J.P.; Sharlip, I.D.; Belker, A.M.; Lipshultz, L.I.; Sigman, M.; Thomas, A.J.; Schlegel, P.N.; Howards, S.S.; Nehra, A.; Damewood, M.D.; et al. Best practice policies for male infertility. J. Urol. 2002, 167, 2138–2144. [Google Scholar] [CrossRef]
- Karna, K.K.; Choi, B.R.; Kim, M.-J.; Kim, H.K.; Park, J.K. The Effect of Schisandra chinensis Baillon on Cross-Talk between Oxidative Stress, Endoplasmic Reticulum Stress, and Mitochondrial Signaling Pathway in Testes of Varicocele-Induced SD Rat. Int. J. Mol. Sci. 2019, 20, 5785. [Google Scholar] [CrossRef] [Green Version]
- Benoff, S.; Jacob, A.; Hurley, I.R. Male infertility and environmental exposure to lead and cadmium. Hum. Reprod. Update 2000, 6, 107–121. [Google Scholar] [CrossRef]
- Clavijo, R.I.; Hsiao, W. Update on male reproductive endocrinology. Transl. Androl. Urol. 2018, 7, S367–S372. [Google Scholar] [CrossRef] [PubMed]
- Ilacqua, A.; Izzo, G.; Emerenziani, G.P.; Baldari, C.; Aversa, A. Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. 2018, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Semet, M.; Paci, M.; Saïas-Magnan, J.; Metzler-Guillemain, C.; Boissier, R.; Lejeune, H.; Perrin, J. The impact of drugs on male fertility: A review. Andrology 2017, 5, 640–663. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, J.; Zhu, Z.; Bao, X.; Zhang, M.; Ren, C.; Zhang, Q. Aucubin, a natural iridoid glucoside, attenuates oxidative stress-induced testis injury by inhibiting JNK and CHOP activation via Nrf2 up-regulation. Phytomedicine 2019, 64, 153057. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.J.; Dong, W.Y.; Zhao, H.; Shi, X.H.; Zhang, Y.L. Effect of molybdenum on reproductive function of male mice treated with busulfan. Theriogenology 2019, 126, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Court, W.A.; Dailey, R.G., Jr.; Gilmore, C.J.; Bryan, R.F. Triptolide and tripdiolide, novel antileukemic diterpenoid triepoxides from Tripterygium wilfordii. J. Am. Chem. Soc. 1972, 94, 7194–7195. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ma, L.; Tao, X.; Lipsky, P.E. Triptolide, an active component of the Chinese herbal remedy Tripterygium wilfordii Hook F, Inhibits production of nitric oxide by decreasing inducible nitric oxide synthase gene transcription. Arthritis Rheum. 2004, 50, 2995–3003. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Bao, X.; Sun, T.; Xu, J.; Zheng, W.; Shen, P. Triptolide attenuate the oxidative stress induced by LPS/D-GalN in mice. J. Cell Biochem. 2012, 113, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Guo, S.H.; Shang, X.J.; Yu, L.S.; Zhu, J.W.; Zhao, A.; Zhou, Y.F.; An, G.H.; Zhang, Q.; Ma, B. Triptolide induces Sertoli cell apoptosis in mice via ROS/JNK-dependent activation of the mitochondrial pathway and inhibition of Nrf2-mediated antioxidant response. Acta Pharmacol. Sin. 2018, 39, 311–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, Y.; Watanabe, T. Effects of oyster extract on the reproductive function of zinc-deficient mice: Bioavailability of zinc contained in oyster extract. Congenit. Anom. 2003, 43, 271–279. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, X.; Gao, J.; Lin, H.; Zhang, C.; Huang, Y. Optimization of enzymatic hydrolysis from Crassostrea gigas and effects of its enzymatic hydrolysate on TM3 Leydig cells. J. Guangdong Ocean. Univ. 2019, 39, 96–102. [Google Scholar]
- Wang, Q.; Li, W.; He, Y.; Ren, D.; Kow, F.; Song, L.; Yu, X. Novel antioxidative peptides from the protein hydrolysate of oysters (Crassostrea talienwhanensis). Food Chem. 2014, 145, 991–996. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Zhang, C.H.; Cao, W.H.; Qin, X.M.; Gao, J.L.; Zheng, H.N. The purification and identification of immunoregulatory peptides from oyster (Crassostrea hongkongensis) enzymatic hydrolysate. Rsc. Adv. 2019, 9, 32854–32863. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.Y.; Dong, S.Y.; Xu, J.; Zeng, M.Y.; Song, H.X.; Zhao, Y.H. Production of cysteine-rich antimicrobial peptide by digestion of oyster (Crassostrea gigas) with alcalase and bromelin. Food Control 2008, 19, 231–235. [Google Scholar] [CrossRef]
- Umayaparvathi, S.; Arumugam, M.; Meenakshi, S.; Drager, G.; Kirschning, A.; Balasubramanian, T. Purification and Characterization of Antioxidant Peptides from Oyster (Saccostrea cucullata) Hydrolysate and the Anticancer Activity of Hydrolysate on Human Colon Cancer Cell Lines. Int. J. Pept. Res. Ther. 2014, 20, 231–243. [Google Scholar] [CrossRef]
- Miao, J.; Liao, W.; Kang, M.; Jia, Y.; Wang, Q.; Duan, S.; Xiao, S.; Cao, Y.; Ji, H. Anti-fatigue and anti-oxidant activities of oyster (Ostrea rivularis) hydrolysate prepared by compound protease. Food Funct. 2018, 9, 6577–6585. [Google Scholar] [CrossRef]
- Byun, J.H.; Choi, Y.J.; Choung, S.Y. Protective effect of Oyster hydrolysate peptide in alcohol induced alcoholic fatty liver in SD-rats. Planta Med. 2016, 82, 571. [Google Scholar] [CrossRef]
- Li, S.J.; Song, Z.Y.; Liu, T.T.; Liang, J.; Yuan, J.; Xu, Z.C.; Sun, Z.H.; Lai, X.P.; Xiong, Q.P.; Zhang, D.Y. Polysaccharide from Ostrea rivularis attenuates reproductive oxidative stress damage via activating Keap1-Nrf2/ARE pathway. Carbohyd. Polym. 2018, 186, 321–331. [Google Scholar] [CrossRef]
- Li, Y.; Qiu, W.; Zhang, Z.; Han, X.; Bu, G.; Meng, F.; Kong, F.; Cao, X.; Huang, A.; Feng, Z.; et al. Oral oyster polypeptides protect ovary against d-galactose-induced premature ovarian failure in C57BL/6 mice. J. Sci. Food Agric. 2020, 100, 92–101. [Google Scholar] [CrossRef]
- Bahadorani, M.; Tavalaee, M.; Abedpoor, N.; Ghaedi, K.; Nazem, M.N.; Nasr-Esfahani, M.H. Effects of branched-chain amino acid supplementation and/or aerobic exercise on mouse sperm quality and testosterone production. Andrologia 2019, 51, e13183. [Google Scholar] [CrossRef]
- Wu, G. Dietary protein intake and human health. Food Funct. 2016, 7, 1251–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leahy, T.; Gadella, B.M. Sperm surface changes and physiological consequences induced by sperm handling and storage. Reproduction 2011, 142, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.-J.; Wu, D.; Xu, S.-Y.; Li, Q.; Fang, Z.-F.; Che, L.-Q.; Wu, C.-M.; Xu, X.-Y.; Lin, Y. Effect of dietary supplementation with amino acids on boar sperm quality and fertility. Anim. Reprod. Sci. 2016, 172, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Neto, F.T.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef]
- Huynh, P.N.; Hikim, A.P.; Wang, C.; Stefonovic, K.; Lue, Y.H.; Leung, A.; Atienza, V.; Baravarian, S.; Reutrakul, V.; Swerdloff, R.S. Long-term effects of triptolide on spermatogenesis, epididymal sperm function, and fertility in male rats. J. Androl. 2000, 21, 689–699. [Google Scholar]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef]
- Zhang, J.; Bao, X.; Zhang, M.; Zhu, Z.; Zhou, L.; Chen, Q.; Zhang, Q.; Ma, B. MitoQ ameliorates testis injury from oxidative attack by repairing mitochondria and promoting the Keap1-Nrf2 pathway. Toxicol. Appl. Pharmacol. 2019, 370, 78–92. [Google Scholar] [CrossRef]
- El-Demerdash, F.M.; Yousef, M.I.; Kedwany, F.S.; Baghdadi, H.H. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: Protective role of vitamin E and beta-carotene. Food Chem. Toxicol. 2004, 42, 1563–1571. [Google Scholar] [CrossRef]
- Morimoto, H.; Iwata, K.; Ogonuki, N.; Inoue, K.; Atsuo, O.; Kanatsu-Shinohara, M.; Morimoto, T.; Yabe-Nishimura, C.; Shinohara, T. ROS are required for mouse spermatogonial stem cell self-renewal. Cell Stem. Cell 2013, 12, 774–786. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Hu, J.; Ma, H.; Yagoub, A.E.; Yu, X.; Owusu, J.; Ma, H.; Qin, X. Antioxidant peptides from corn gluten meal: Orthogonal design evaluation. Food Chem. 2015, 187, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Wajda, A.; Lapczuk, J.; Grabowska, M.; Slojewski, M.; Laszczynska, M.; Urasinska, E.; Drozdzik, M. Nuclear factor E2-related factor-2 (Nrf2) expression and regulation in male reproductive tract. Pharmacol. Rep. 2016, 68, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Griswold, M.D. The central role of Sertoli cells in spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, X.; Shao, H.; Zhang, Z.H.; Ng, J.C.; Peng, C. Malathion-induced testicular toxicity is associated with spermatogenic apoptosis and alterations in testicular enzymes and hormone levels in male Wistar rats. Environ. Toxicol. Phar. 2015, 39, 659–667. [Google Scholar] [CrossRef] [Green Version]
- Sadik, N.A. Effects of diallyl sulfide and zinc on testicular steroidogenesis in cadmium-treated male rats. J. Biochem. Mol. Toxicol. 2008, 22, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Qin, X.M.; Lin, H.S.; Cao, W.H.; Zheng, H.N.; Gao, J.L.; Zhang, C.H. Protective effect of hydrolyzed ultrafiltration fractions from the Oyster (Crassostrea hongkongensis) on oxidative damage of TM4 Sertoli cells. South China Fish. Sci. 2021, 17, 118–125. [Google Scholar]
- Jiang, X.; Zhu, C.; Li, X.; Sun, J.; Tian, L.; Bai, W. Cyanidin-3- O-glucoside at Low Doses Protected against 3-Chloro-1,2-propanediol Induced Testis Injury and Improved Spermatogenesis in Male Rats. J. Agric. Food Chem. 2018, 66, 12675–12684. [Google Scholar] [CrossRef] [PubMed]
- Bonde, J.P.; Flachs, E.M.; Rimborg, S.; Glazer, C.H.; Giwercman, A.; Ramlau-Hansen, C.H.; Hougaard, K.S.; Hoyer, B.B.; Haervig, K.K.; Petersen, S.B.; et al. The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: A systematic review and meta-analysis. Hum. Reprod Update 2016, 23, 104–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitteloud, N.; Dwyer, A.A.; DeCruz, S.; Lee, H.; Boepple, P.A.; Crowley, W.F.; Hayes, F.J. Inhibition of luteinizing hormone secretion by testosterone in men requires aromatization for its pituitary but not its hypothalamic effects: Evidence from the tandem study of normal and gonadotropin-releasing hormone-deficient men. J. Clin. Endocr. Metab. 2008, 93, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhou, M.; Wei, Y.; Jia, F.; Yan, Y.; Zhang, R.; Cai, M.; Gu, R. The beneficial effect of oyster peptides and oyster powder on cyclophosphamide-induced reproductive impairment in male rats: A comparative study. J. Food Biochem. 2020, 44, e13468. [Google Scholar] [CrossRef]
- Qian, Z.J.; Jung, W.K.; Byun, H.G.; Kim, S.K. Protective effect of an antioxidative peptide purified from gastrointestinal digests of oyster, Crassostrea gigas against free radical induced DNA damage. Bioresour. Technol. 2008, 99, 3365–3371. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Chen, B.; Zheng, Q.; Zhu, G.; Cao, W.; Qin, X.; Zhang, C. Ameliorative Effects of Peptides from the Oyster (Crassostrea hongkongensis) Protein Hydrolysates against UVB-Induced Skin Photodamage in Mice. Mar. Drugs 2020, 18, 288. [Google Scholar] [CrossRef]
- Hamza, R.Z.; Al-Harbi, M.S.; El-Shenawy, N.S. Ameliorative effect of vitamin E and selenium against oxidative stress induced by sodium azide in liver, kidney, testis and heart of male mice. Biomed. Pharm. 2017, 91, 602–610. [Google Scholar] [CrossRef]
- Qiu, C.; Cheng, Y. Effect of Astragalus membranaceus polysaccharide on the serum cytokine levels and spermatogenesis of mice. Int. J. Biol. Macromol. 2019, 140, 771–774. [Google Scholar] [CrossRef]
- Oghbaei, H.; Hamidian, G.; Alipour, M.R.; Alipour, S.; Keyhanmanesh, R. The effect of prolonged dietary sodium nitrate treatment on the hypothalamus-pituitary-gonadal axis and testicular structure and function in streptozotocin-induced diabetic male rats. Food Funct. 2020, 11, 2451–2465. [Google Scholar] [CrossRef]









| No. | Peptide Sequence | Theoretical Mass (Mr) | Observed Mass (m/z) | Scores |
|---|---|---|---|---|
| 1 | LAGPQSIIGRTM | 1242.68 | 622.34 | 56.04 |
| 2 | IIDAPGHRDF | 1139.57 | 380.87 | 62.28 |
| 3 | YDNEFGYSFR | 1296.55 | 649.28 | 56.59 |
| 4 | RVPVPDVSVVDL | 1293.73 | 647.87 | 48.44 |
| 5 | AFRVPVPDVSVVDL | 1511.84 | 756.93 | 37.93 |
| 6 | GIVLDSGDGVSH | 1154.56 | 578.28 | 57.46 |
| 7 | LDLAGRDLTD | 1087.56 | 544.78 | 36.34 |
| 8 | PDGQVITI | 841.46 | 421.73 | 35.7 |
| 9 | KSYELPDGQVIT | 1348.69 | 675.35 | 33.95 |
| 10 | KSYELPDGQVITIG | 1518.79 | 760.4 | 33.88 |
| 11 | IAQDFKTDLR | 1205.65 | 402.89 | 37.72 |
| 12 | GLALLVP | 681.45 | 341.73 | 32.1 |
| 13 | LLQALD | 671.39 | 336.7 | 35.92 |
| 14 | GIVLDSGDGVTH | 1168.58 | 585.3 | 42.1 |
| 15 | LDLAGRDLTD | 1087.55 | 544.78 | 36.34 |
| Amino Acid | Contents of OPs |
|---|---|
| Thr | 2.81 |
| Val | 3.34 |
| Met | 1.41 |
| Ile | 3.02 |
| Leu | 4.56 |
| Phe | 2.27 |
| Lys | 4.78 |
| His | 1.03 |
| Arg | 4.23 |
| Asp | 5.56 |
| Ser | 2.88 |
| Glu | 8.41 |
| Pro | 2.30 |
| Gly | 3.25 |
| Ala | 2.69 |
| Tyr | 2.21 |
| Cys | ND e |
| TAA a | 54.75 |
| EAA b | 22.19 |
| HAA c | 17.29 |
| BCAA d | 10.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Peng, Z.; Zheng, H.; Zhang, C.; Lin, H.; Qin, X. The Potential Protective Effect and Possible Mechanism of Peptides from Oyster (Crassostrea hongkongensis) Hydrolysate on Triptolide-Induced Testis Injury in Male Mice. Mar. Drugs 2021, 19, 566. https://doi.org/10.3390/md19100566
Zhang X, Peng Z, Zheng H, Zhang C, Lin H, Qin X. The Potential Protective Effect and Possible Mechanism of Peptides from Oyster (Crassostrea hongkongensis) Hydrolysate on Triptolide-Induced Testis Injury in Male Mice. Marine Drugs. 2021; 19(10):566. https://doi.org/10.3390/md19100566
Chicago/Turabian StyleZhang, Xueyan, Zhilan Peng, Huina Zheng, Chaohua Zhang, Haisheng Lin, and Xiaoming Qin. 2021. "The Potential Protective Effect and Possible Mechanism of Peptides from Oyster (Crassostrea hongkongensis) Hydrolysate on Triptolide-Induced Testis Injury in Male Mice" Marine Drugs 19, no. 10: 566. https://doi.org/10.3390/md19100566
APA StyleZhang, X., Peng, Z., Zheng, H., Zhang, C., Lin, H., & Qin, X. (2021). The Potential Protective Effect and Possible Mechanism of Peptides from Oyster (Crassostrea hongkongensis) Hydrolysate on Triptolide-Induced Testis Injury in Male Mice. Marine Drugs, 19(10), 566. https://doi.org/10.3390/md19100566