Heterocornols from the Sponge-Derived Fungus Pestalotiopsis heterocornis with Anti-Inflammatory Activity
Abstract
:1. Introduction
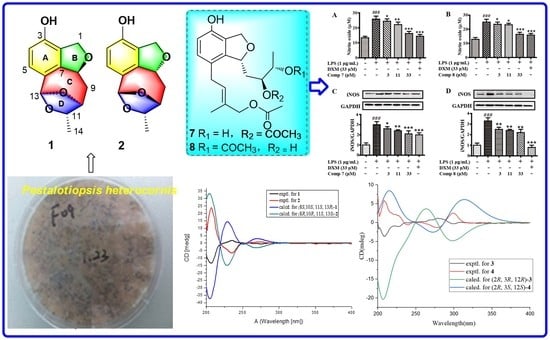
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Fungal Material
3.3. Fermentation, Extraction, and Isolation
3.4. ECD Calculations
3.5. Cell Culture and Cytotoxicity Assay
3.6. Antimicrobial Assay
3.7. Determination of Nitric Oxide Production
3.8. Western Blot Analysis
3.9. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yoon, V.; Nodwell, J.R. Activating secondary metabolism with stress and chemicals. J. Ind. Microbiol. Biotechnol. 2014, 41, 415–424. [Google Scholar] [CrossRef]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big effects from small changes: Possible ways to explore nature’s chemical diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef]
- Wang, W.J.; Li, D.Y.; Li, Y.C.; Hua, H.M.; Ma, E.L.; Li, Z.L. Caryophyllene sesquiterpenes from the marine-derived fungus Ascotricha sp. ZJ-M-5 by the one strain–many compounds strategy. J. Nat. Prod. 2014, 77, 1367–1371. [Google Scholar] [CrossRef]
- Rateb, M.E.; Houssen, W.E.; Harrison, W.T.A.; Deng, H.; Okoro, C.K.; Asenjo, J.A.; Andrews, B.A.; Bull, A.T.; Goodfellow, M.; Ebel, R.; et al. Diverse metabolic profiles of a streptomyces strain isolated from a hyper-arid environment. J. Nat. Prod. 2011, 74, 1965–1971. [Google Scholar] [CrossRef] [PubMed]
- Paranagama, P.A.; Wijeratne, E.M.K.; Gunatilaka, A.A.L. Uncovering biosynthetic potential of plant-associated fungi: Effect of culture conditions on metabolite production by paraphaeosphaeria quadriseptata and chaetomium chiversii (1). J. Nat. Prod. 2007, 70, 1939–1945. [Google Scholar] [CrossRef]
- Wang, X.R.; Filho, J.G.S.; Hoover, A.R.; King, J.B.; Ellis, T.K.; Powell, D.R.; Cichewicz, R.H. Chemical epigenetics alters the secondary metabolite composition of guttate excreted by an atlantic-forest-soil-derived Penicillium citreonigrum. J. Nat. Prod. 2010, 73, 942–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, K.L.; Zhu, G.L.; Hao, J.J.; Wang, Y.; Zhu, W.M. Chemical-epigenetic method to enhance the chemodiversity of the marine algicolous fungus, Aspergillus terreus OUCMDZ-2739. Tetrahedron 2018, 74, 83–87. [Google Scholar] [CrossRef]
- Akone, S.H.; Mandi, A.; Kurtan, T.; Hartmann, R.; Lin, W.H.; Daletos, G.; Proksch, P. Inducing secondary metabolite production by the endophytic fungus Chaetomium sp. through fungal–bacterial co-culture and epigenetic modification. Tetrahedron 2016, 72, 6340–6347. [Google Scholar] [CrossRef] [Green Version]
- Han, J.Y.; Zhang, J.Y.; Song, Z.J.; Zhu, G.L.; Liu, M.M.; Dai, H.Q.; Hsiang, T.; Liu, X.T.; Zhang, L.X.; Quinn, R.J.; et al. Genome-based mining of new antimicrobial meroterpenoids from the phytopathogenic fungus Bipolaris sorokiniana strain 11134. Appl. Microbiol. Biotechnol. 2020, 104, 3835–3846. [Google Scholar] [CrossRef]
- Challis, G.L. Genome mining for novel natural product discovery. J. Med. Chem. 2008, 51, 2618–2628. [Google Scholar] [CrossRef]
- Ziemert, N.; Alanjary, M.; Weber, T. The evolution of genome mining in microbes—A review. Nat. Prod. Rep. 2016, 33, 988–1005. [Google Scholar] [CrossRef] [Green Version]
- Helfrich, E.J.N.; Reite, S.; Piel, J. Recent advances in genome-based polyketide discovery. Curr. Opin. Biotechnol. 2014, 29, 107–115. [Google Scholar] [CrossRef]
- Nett, M.; Ikeda, H.; Moore, B.S. Genomic basis for natural product biosynthetic diversity in the actinomycetes. Nat. Prod. Rep. 2009, 26, 1362–1384. [Google Scholar] [CrossRef]
- Lei, H.; Lin, X.P.; Han, L.; Ma, J.; Dong, K.L.; Wang, X.B.; Mu, Y.; Liu, Y.H.; Huang, X.S. Polyketide derivatives from a marine-sponge-associated fungus Pestalotiopsis heterocornis. Phytochemistry 2017, 142, 51–59. [Google Scholar] [CrossRef]
- Lei, H.; Lei, J.; Zhou, X.F.; Hu, M.; Niu, H.; Song, C.; Chen, S.W.; Liu, Y.H.; Zhang, D. Cytotoxic polyketides from the marine sponge-derived fungus pestalotiopsis heterocornis XWS03F09. Molecules 2019, 24, 2655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, H.; Lin, X.P.; Han, L.; Ma, J.; Ma, Q.J.; Zhong, J.L.; Liu, Y.H.; Sun, T.M.; Wang, J.H.; Huang, X.S. New metabolites and bioactive chlorinated benzophenone derivatives produced by a marine-derived fungus Pestalotiopsis heterocornis. Mar. Drugs 2017, 15, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cueto, M.; Jensen, P.R.; Kauffman, C.; Fenical, W.; Lobkovsky, E.; Clardy, J. Pestalone, a new antibiotic produced by a marine fungus in response to bacterial challenge. J. Nat. Prod. 2001, 64, 1444–1446. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Jiang, L.H.; Guo, L.D.; Zhang, H.; Che, Y.S. Pestalachlorides A-C, antifungal metabolites from the plant endophytic fungus Pestalotiopsis adusta. Bioorg. Med. Chem. 2008, 16, 7894–7899. [Google Scholar] [CrossRef]
- Wang, J.F.; Liang, R.; Liao, S.R.; Yang, B.; Tu, Z.C.; Lin, X.P.; Wang, B.G.; Liu, Y.H. Vaccinols J–S, ten new salicyloid derivatives from the marine mangrove-derived endophytic fungus Pestalotiopsis vaccinii. Fitoterapia 2017, 120, 164–170. [Google Scholar] [CrossRef]
- Höller, U.; Gloer, J.B.; Wicklow, D.T. Biologically active polyketide metabolites from an undetermined fungicolous hyphomycete resembling Cladosporium. J. Nat. Prod. 2002, 65, 876–882. [Google Scholar] [CrossRef]
- Xing, Q.; Gan, L.S.; Mou, X.F.; Wang, W.; Wang, C.Y.; Wei, M.Y.; Shao, C.L. Isolation, resolution and biological evaluation of pestalachlorides E and F containing both point and axial chirality. RSC Adv. 2016, 6, 22653–22658. [Google Scholar] [CrossRef]
- Pescitelli, G.; Bruhn, T. Good computational practice in the assignment of absolute configurations by TDDFT calculations of ECD spectra. Chirality 2016, 28, 466–474. [Google Scholar] [CrossRef] [Green Version]
- Kurtan, T.; Antus, S.; Pescitelli, G. Comprehensive Chiroptical Spectroscopy; Berova, N., Polavarapu, P.L., Nakanishi, K., Woody, R.W., Eds.; Wiley: Hoboken, NJ, USA, 2012; Volume 2, pp. 73–114. [Google Scholar]
- Chen, X.W.; Li, C.W.; Cui, C.B.; Hua, W.; Zhu, T.J.; Gu, Q.Q. Nine new and five known polyketides derived from a deep sea-sourced Aspergillus sp. 16-02-1. Mar. Drugs 2014, 12, 3116–3137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, M.W.; Cui, C.B.; Li, C.W.; Wu, C.J. Three new and eleven known unusual C25 steroids: Activated production of silent metabolites in a marine-derived fungus by chemical mutagenesis strategy using diethyl sulphate. Mar. Drugs 2014, 12, 1545–1568. [Google Scholar] [CrossRef] [Green Version]
- Yaoita, Y.; Kohata, R.; Kakuda, R.; Machida, K.; Kikuchi, M. Ceramide constituents from five mushrooms. Chem. Pharm. Bull. 2002, 50, 681–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, M.O.; Rodriguez, A.D. Palominin, a novel furanosesterterpene from a Caribbean sponge Iricinia sp. Tetrahedron 1990, 46, 1119–1124. [Google Scholar] [CrossRef]
- Yaoita, Y.; Satoh, Y.; Kikuchi, M. A new ceramide from Ramaria botrytis (Pers.) Ricken. J. Nat. Med. 2007, 61, 205–207. [Google Scholar] [CrossRef]
- Kwon, H.C.; Kim, K.R.; Zee, S.D.; Cho, S.Y.; Lee, K.R. A new indolinepeptide from Paecilomyces sp. J300. Arch. Pharm. Res. 2004, 27, 604–609. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Jin, Y.; Aobulikasimu, N.; Zhang, Z.G.; Liu, C.B.; Cao, B.X.; Lin, B.; Guan, P.P.; Mu, Y.; Jiang, Y.; Han, L.; et al. Amycolasporins and dibenzoyls from lichen-associated Amycolatopsis hippodromi and their antibacterial and antiinflammatory activities. J. Nat. Prod. 2020, 83, 3545–3553. [Google Scholar] [CrossRef]
- Ding, N.; Jiang, Y.; Han, L.; Chen, X.; Ma, J.; Qu, X.; Mu, Y.; Liu, J.; Li, L.; Jiang, C.; et al. Bafilomycins and odoriferous sesquiterpenoids from Streptomyces albolongus isolated from Elephas maximus feces. J. Nat. Prod. 2016, 79, 799–805. [Google Scholar] [CrossRef]







| 1 | 2 | 3 | 4 | |||||
|---|---|---|---|---|---|---|---|---|
| No. | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) |
| 1 | 72.4, CH2 | 5.12, dd (12.1, 2.5) | 72.1, CH2 | 5.11, dd (12.0, 2.2) | 31.7, CH2 | 2.81, d (16.4) | 31.7, CH | 2.83, d (8.3) |
| 4.96, dd (12.1, 2.7) | 4.93, dd (12.0, 3.2) | 3.07, dd (16.4, 8.5) | 3.10, dd (16.3, 8.3) | |||||
| 2 | 127.4, C | 127.8, C | 55.6, CH | 2.05, m | 55.5, CH | 2.00, m | ||
| 3 | 153.4, C | 153.5, C | 40.8, CH | 3.75, dd (10.6, 2.3) | 40.9, CH | 3.76, dd (10.6, 2.8) | ||
| 4 | 113.8, CH | 6.58, d (7.9) | 113.7, CH | 6.58, d (7.9) | 147.4, C | 147.2, C | ||
| 5 | 127.1, CH | 7.01, d (7.9) | 126.8, CH | 7.01, d (7.9) | 119.1, C | 119.1, C | ||
| 6 | 129.4, C | 129.2, C | 154.8, C | 154.8, C | 6.63, d (8.0) | |||
| 7 | 143.7, C | 143.7, C | 114.0, CH | 6.63, d (8.1) | 114.0, CH | 6.94, d (8.0) | ||
| 8 | 82.6, CH | 5.50, dd (8.3, 2.5) | 82.5, CH | 5.71, ddt (10.9, 5.6, 2.6) | 124.2, CH | 6.93, d (8.1) | 124.2, CH | 2.79, m |
| 9 | 39.4, CH2 | 2.32, dt (13.5, 4.7) | 35.8, CH2 | 2.32, ddd (14.8, 5.6, 2.5) | 134.0, C | 134.0, C | 2.90, d (2.8) | |
| 1.94, ddd (13.5, 11.3, 2.8) | 1.97, ddd (14.8, 11.2, 4.5) | |||||||
| 10 | 77.3, CH | 4.61, q (6.0) | 77.3, CH | 4.40, q (3.9) | 44.2, CH2 | 2.74, dd (16.2, 10.7) | 43.9, CH2 | 2.79, m |
| 3.01, dd (16.2,2.4) | 2.90, d (2.8) | |||||||
| 11 | 81.4, CH | 4.34, t (2.5) | 78.5, CH | 4.28, dd (6.6, 4.4) | 208.1, C | 208.0, C | ||
| 12 | 74.7, CH | 5.08, q (7.2) | 75.1, CH | 5.06, q (7.1) | ||||
| 13 | 106.4, CH | 5.96, s | 105.3, CH | 5.82, s | 14.8, CH3 | 1.39, d (7.2) | 14.9, CH3 | 1.39, d (7.1) |
| 14 | 21.1, CH3 | 1.30, d (6.2) | 13.0, CH3 | 1.51, d (6.6) | 72.6, C | 72.5, C | ||
| 15 | 25.3, CH3 | 0.91, s | 25.3, CH3 | 0.91, s | ||||
| 16 | 25.7, CH3 | 1.05, s | 25.7, CH3 | 1.05, s | ||||
| 1′ | 64.3, CH2 | 4.48, d (10.6), | 64.3, CH2 | 4.48, d (10.6) | ||||
| 4.60, d (10.6) | 4.60, d (10.6) | |||||||
| 3′ | 65.5, CH2 | 3.57, d (7.0) | 65.4, CH2 | 3.56, dd (7.1, 3.5) | ||||
| 4′ | 14.1, CH3 | 1.20, d (7.0) | 14.1, CH3 | 1.19, d (6.7) | ||||
| AcO | 170.7, C | 170.7, C | ||||||
| 19.1, CH3 | 2.09, s | 19.2, CH3 | 2.10, s | |||||
| 5 a | 6 a | 7 a | 8 a | 9 b | |
|---|---|---|---|---|---|
| No. | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) | δH (J in Hz) |
| 1 | 5.06, dd (12.0, 2.7) | 5.04, dd (12.3,2.8) | 5.10, dd (12.0, 3.1) | 5.08, dd (12.1, 2.7) | 4.32, dt (8.1, 4.0) |
| 4.97, d (12.0) | 4.97, dd (12.3) | 4.94, d (12.0) | 4.98, d (12.1) | 4.19, dt (8.1, 3.8) | |
| 2 | 4.21, m | ||||
| 3 | 5.39, d (9.9) | 5.18, dd (10.0, 2.1) | 5.43, brd (9.0) | 5.55, brd (9.0) | 4.20, brs |
| 4 | 5.49, dd (15.4, 6.4) | ||||
| 5 | 6.67, d (7.7) | 6.67, d (7.8) | 5.77, dt (15.4, 6.4) | ||
| 6 | 7.11, t (7.7) | 7.11, t (7.8) | 6.88, d (8.1) | 6.87, d (8.1) | 2.08, m |
| 7 | 6.66, d (7.7) | 6.66, d (7.8) | 6.60, d (8.1) | 6.60, d (8.1) | |
| 8 | 5.08, d (6.4) | ||||
| 10 | 1.84, ddd (14.3, 10.3, 2.3) | 2.08, m | 1.84, dd (6.7, 4.3) | 2.13, m | 1.95, d (7.7) |
| 1.71, ddd (14.3, 10.0, 2.5) | 1.86, dt (4.5, 2.5) | ||||
| 11 | 3.93, ddd (10.3, 4.2, 2.5) | 3.93, ddd (10.2, 4.5, 2.3) | 5.03, ddd (10.0, 4.3, 2.0) | 5.04, ddd (10.0, 4.7, 2.6) | 1.24, m |
| 12 | 4.85, m | 5.05, m | 4.87, dd (6.5, 4.3) | 4.89, dd (6.4, 4.7) | 1.26, m |
| 13 | 1.21, d (6.5) | 1.13, d (6.5) | 1.12, s | 1.23, s | 1.32, m |
| 1 | 3.25, m | 3.36, m | |||
| 2 | 5.54, t (7.0) | 5.45, t (6.5) | 4.11, dt (8.1, 3.8) | ||
| 3 | 1.61, m, 1.81, m | ||||
| 4 | 2.03, s | 2.06, s | 1.42, m | ||
| 5 | 4.48, s | 4.69, s | 1.26, m | ||
| 11-OAc | 2.09, s | 1.61, s | 1.34, m | ||
| 12-OAc | 2.04, s | 1.62, s | |||
| 5-OAc | 2.02, s | 2.04, s | |||
| 1-OAc | 2.06, m | ||||
| 14~17 | 1.24~1.42 | ||||
| 18 | 0.88, t (7.0) | ||||
| 19 | 1.58, brs |
| 5 a | 6 a | 7 a | 8 a | 9 b | |
|---|---|---|---|---|---|
| No. | δC, Type | δC, Type | δC, Type | δC, Type | δC, Type |
| 1 | 71.4, CH2 | 71.6, CH2 | 72.8, CH2 | 70.0, CH2 | 62.9, CH2 |
| 2 | 53.0, CH | ||||
| 3 | 82.2, CH | 82.2, CH | 84.1, CH | 83.2, CH | 73.0, CH |
| 4 | 145.4, C | 145.1, C | 142.8, C | 141.8, C | 128.2, CH |
| 5 | 113.0, CH | 113.0, CH | 126.0, C | 126.0, C | 134.4, CH |
| 6 | 130.2, CH | 130.3, CH | 130.7, CH | 130.3, CH | 31.9, CH2 |
| 7 | 114.9, CH | 115.0, CH | 115.6, C | 115.2, CH | 27.5, CH2 |
| 8 | 152.9, C | 153.0, C | 151.3, C | 151.3, C | 123.1, CH |
| 9 | 126.1, C | 126.3, C | 126.1, C | 126.5, C | 136.2, C |
| 10 | 40.4, CH2 | 37.1, CH2 | 38.9, CH2 | 35.0, CH2 | 39.7, CH2 |
| 11 | 71.0, CH | 69.7, CH | 74.7, CH | 72.4, CH | 28.0, CH2 |
| 12 | 75.3, CH | 76.2, CH | 71.5, CH | 74.7, CH | 29.4, CH2 |
| 13 | 15.1, CH3 | 18.4, CH3 | 15.0, CH3 | 15.0, CH3 | 29.4, CH2 |
| 1 | 31.3, CH2 | 31.2, CH2 | 174.6, C | ||
| 2 | 128.7, CH | 129.7, CH | 72.2, CH | ||
| 3 | 132.5, C | 132.4, C | 34.9, CH2 | ||
| 4 | 21.2, CH3 | 21.6, CH3 | 25.0, CH2 | ||
| 5 | 70.9, CH2 | 64.7, CH3 | 28.0, CH2 | ||
| 6~15 | 29.7~31.9 | ||||
| 16 | 14.1, CH3 | ||||
| 11-AcO | 172.4, C | ||||
| 20.8, CH3 | |||||
| 12-AcO | 172.5, C | 172.7, C | 171.4, C | ||
| 21.2, CH3 | 21.1, CH3 | 20.8, CH3 | |||
| 5-AcO | 172.8, C | 172.8, C | |||
| 21.2, CH3 | 20.8, CH3 | ||||
| 1-AcO | 171.3, C | ||||
| 20.9, CH3 | |||||
| 14~17 | 22.7~31.9, CH2 | ||||
| 18 | 14.1, CH3 | ||||
| 19 | 16.0, CH3 |
| Strains | S. aureus | B. subtilis | E. coli | C. albicans |
|---|---|---|---|---|
| 12 | 50 | 25 | 25 | 100 |
| Positive | 0.25 a | 0.13 a | 0.13 a | 1.0 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, H.; Bi, X.; Lin, X.; She, J.; Luo, X.; Niu, H.; Zhang, D.; Yang, B. Heterocornols from the Sponge-Derived Fungus Pestalotiopsis heterocornis with Anti-Inflammatory Activity. Mar. Drugs 2021, 19, 585. https://doi.org/10.3390/md19110585
Lei H, Bi X, Lin X, She J, Luo X, Niu H, Zhang D, Yang B. Heterocornols from the Sponge-Derived Fungus Pestalotiopsis heterocornis with Anti-Inflammatory Activity. Marine Drugs. 2021; 19(11):585. https://doi.org/10.3390/md19110585
Chicago/Turabian StyleLei, Hui, Xiaoxu Bi, Xiuping Lin, Jianglian She, Xiaowei Luo, Hong Niu, Dan Zhang, and Bin Yang. 2021. "Heterocornols from the Sponge-Derived Fungus Pestalotiopsis heterocornis with Anti-Inflammatory Activity" Marine Drugs 19, no. 11: 585. https://doi.org/10.3390/md19110585
APA StyleLei, H., Bi, X., Lin, X., She, J., Luo, X., Niu, H., Zhang, D., & Yang, B. (2021). Heterocornols from the Sponge-Derived Fungus Pestalotiopsis heterocornis with Anti-Inflammatory Activity. Marine Drugs, 19(11), 585. https://doi.org/10.3390/md19110585