Deep-Water Fish Are Potential Vectors of Ciguatera Poisoning in the Gambier Islands, French Polynesia
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ciguatoxin-like Activity as Assessed by MBA, rRBA, and CBA-N2a
2.2. Ciguatoxin Profiles as Assessed by LC–MS/MS
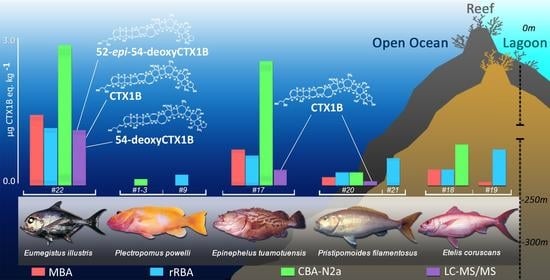
2.3. Comparisons of CTX-like Contents in Deep-Water Fish Using Four Detection Methods
2.4. Contribution of Deep-Water Fish to the Trophic Chain of Ciguatera
3. Materials and Methods
3.1. Study Site
3.2. Fish Sample Collection
3.3. Ciguatoxin Standard
3.4. Biological Methods
3.4.1. Radioactive Receptor Binding Assay (rRBA)
3.4.2. Mouse Bioassay (MBA)
3.4.3. Neuroblastoma Cell-Based Assay (CBA-N2a)
3.5. Chemical Methods—Liquid Chromatography Tandem Mass Spectrometry (LC–MS/MS)
3.6. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponsonnet, C. Les Poissons Profonds Démersaux de Polynésie Française: Les Paru; IFREMER, IRD, SHOM, UPF: Paris, France; DAF, SRM, STEM: Papeete, French Polynesia, 2002; p. 45. [Google Scholar]
- Newman, S.J.; Williams, A.J.; Wakefield, C.B.; Nicol, S.J.; Taylor, B.M.; O’Malley, J.M. Review of the life history characteristics, ecology and fisheries for deep-water tropical demersal fish in the Indo-Pacific region. Rev. Fish Biol. Fish. 2016, 26, 537–562. [Google Scholar] [CrossRef]
- Uehara, M.; Ebisawa, A.; Ohta, I. Comparative age-specific demography of four commercially important deep-water snappers: Implication for fishery management of a long-lived lutjanid. J. Fish Biol. 2020, 97, 121–136. [Google Scholar] [CrossRef] [PubMed]
- Oyafuso, Z.S.; Drazen, J.C.; Moore, C.H.; Franklin, E.C. Habitat-based species distribution modelling of the Hawaiian deepwater snapper-grouper complex. Fish. Res. 2017, 195, 19–27. [Google Scholar] [CrossRef]
- Luers, M.A.; DeMartini, E.E.; Humphreys, R.L. Seasonality, sex ratio, spawning frequency and sexual maturity of the opakapaka Pristipomoides filamentosus (Perciformes: Lutjanidae) from the main Hawaiian Islands: Fundamental input to size-at-retention regulations. Mar. Freshw. Res. 2018, 69, 325–335. [Google Scholar] [CrossRef]
- Uehara, M.; Ebisawa, A.; Ohta, I.; Aonuma, Y. Effectiveness of deepwater marine protected areas: Implication for Okinawan demersal fisheries management. Fish. Res. 2019, 215, 123–130. [Google Scholar] [CrossRef]
- Andrews, A.H.; Brodziak, J.; DeMartini, E.E.; Cruz, E. Long-lived life history for onaga Etelis coruscans in the Hawaiian Islands. Mar. Freshw. Res. 2020, 72, 848–859. [Google Scholar] [CrossRef]
- Wakefield, C.B.; O’Malley, J.M.; Williams, A.J.; Taylor, B.M.; Nichols, R.S.; Halafihi, T.; Humphreys Jr, R.L.; Kaltavara, J.; Nicol, S.J.; Newman, S.J. Ageing bias and precision for deep-water snappers: Evaluating nascent otolith preparation methods using novel multivariate comparisons among readers and growth parameter estimates. ICES J. Mar. Sci. 2017, 74, 193–203. [Google Scholar] [CrossRef]
- Newman, S.J.; Wakefield, C.B.; Williams, A.J.; O’Malley, J.M.; Taylor, B.M.; Nicol, S.J.; Nichols, R.S.; Hesp, S.A.; Hall, N.G.; Hill, N.; et al. International workshop on advancing methods to overcome challenges associated with life history and stock assessments of data-poor deep-water snappers and groupers. Mar. Policy 2017, 79, 78–83. [Google Scholar] [CrossRef]
- Andrews, A.H.; DeMartini, E.E.; Brodziak, J.; Nichols, R.S.; Humphreys, R.L. A long-lived life history for a tropical, deepwater snapper (Pristipomoides filamentosus): Bomb radiocarbon and lead–radium dating as extensions of daily increment analyses in otoliths. Can. J. Fish. Aquat. Sci. 2012, 69, 1850–1869. [Google Scholar] [CrossRef] [Green Version]
- Uehara, M.; Ebisawa, A.; Ohta, I. Reproductive traits of deep-sea snappers (Lutjanidae): Implication for Okinawan bottomfish fisheries management. Reg. Stud. Mar. Sci. 2018, 17, 112–126. [Google Scholar] [CrossRef]
- Randall, J.E. A review of ciguatera, tropical fish poisoning, with a tentative explanation of its cause. Bull. Mar. Sci. 1958, 8, 236–267. [Google Scholar]
- Scheuer, P.J.; Takahashi, W.; Tsutsumi, J.; Yoshida, T. Ciguatoxin: Isolation and Chemical Nature. Science 1967, 155, 1267–1268. [Google Scholar] [CrossRef] [PubMed]
- Murata, M.; Legrand, A.M.; Yasumoto, T. A probable partial structure of ciguatoxin isolated from the moray eel Gymnothorax javanicus. Tetrahedron Lett. 1989, 30, 3793–3796. [Google Scholar] [CrossRef]
- Murata, M.; Legrand, A.M.; Ishibashi, Y.; Fukui, M.; Yasumoto, T. Structures and configurations of ciguatoxin from the moray eel Gymnothorax javanicus and its likely precursor from the dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1990, 112, 4380–4386. [Google Scholar] [CrossRef]
- Satake, M.; Ishibashi, Y.; Legrand, A.-M.; Yasumoto, T. Isolation and structure of ciguatoxin-4A, a New ciguatoxin precursor, from cultures of dinoflagellate Gambierdiscus toxicus and parrotfish Scarus gibbus. Biosci. Biotechnol. Biochem. 1996, 60, 2103–2105. [Google Scholar] [CrossRef] [Green Version]
- Chinain, M.; Gatti, C.M.; Roué, M.; Darius, H.T. Ciguatera-causing dinoflagellates in the genera Gambierdiscus and Fukuyoa: Distribution, ecophysiology and toxicology. In Ciguatera-Poisoning Causing Dinoflagellates; Subba Rao, D.V., Ed.; Nova Science Publishers, Inc.: New York, NY, USA, 2020; pp. 405–457. [Google Scholar]
- Yasumoto, T.; Satake, M. Chemistry, Etiology and Determination Methods of Ciguatera Toxins. J. Toxicol. Toxin Rev. 1996, 15, 91–107. [Google Scholar] [CrossRef]
- Yasumoto, T. Chemistry, etiology, and food chain dynamics of marine toxins. Proc. Jpn. Acad. Ser. B 2005, 81, 43–51. [Google Scholar] [CrossRef] [Green Version]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Chateau-Degat, M.L.; Huin-Blondey, M.O.; Chinain, M.; Darius, H.T.; Legrand, A.M.; Nguyen, N.L.; Laudon, F.; Chansin, R.; Dewailly, E. Prevalence of chronic symptoms of ciguatera disease in French Polynesian adults. Am. J. Trop. Med. Hyg. 2007, 77, 842–846. [Google Scholar] [CrossRef] [Green Version]
- Friedman, M.A.; Fleming, L.E.; Fernandez, M.; Bienfang, P.; Schrank, K.; Dickey, R.; Bottein, M.Y.; Backer, L.; Ayyar, R.; Weisman, R.; et al. Ciguatera fish poisoning: Treatment, prevention and management. Mar. Drugs 2008, 6, 456–479. [Google Scholar] [CrossRef]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An updated review of ciguatera fish poisoning: Clinical, epidemiological, environmental, and public health management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef]
- Halstead, B.W. Poisonous and Venomous Marine Animals of the World; The Darwin Press: Princeton, NJ, USA, 1978; p. 283. [Google Scholar]
- Chinain, M.; Gatti, C.M.; Darius, H.T.; Quod, J.P.; Tester, P.A. Ciguatera poisonings: A global review of occurrences and trends. Harmful Algae 2021, 102, 101873. [Google Scholar] [CrossRef]
- Gatti, C.M.; Lonati, D.; Darius, H.T.; Zancan, A.; Roué, M.; Schicchi, A.; Locatelli, C.A.; Chinain, M. Tectus niloticus (Tegulidae, Gastropod) as a novel vector of Ciguatera Poisoning: Clinical characterization and follow-up of a mass poisoning event in Nuku Hiva Island (French Polynesia). Toxins 2018, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Lewis, N.D. Ciguatera-implications for nutrition and marine ressource development in the Pacific Islands. J. Soc. Océanistes 1983, 39, 89–104. [Google Scholar] [CrossRef]
- Kumar-Roine, S.; Matsui, M.; Reybier, K.; Darius, H.T.; Chinain, M.; Pauillac, S.; Laurent, D. Ability of certain plant extracts traditionally used to treat ciguatera fish poisoning to inhibit nitric oxide production in RAW 264.7 macrophages. J. Ethnopharmacol. 2009, 123, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Roiné, S.; Darius, H.T.; Matsui, M.; Fabre, N.; Haddad, M.; Chinain, M.; Pauillac, S.; Laurent, D. A review of traditional remedies of ciguatera fish poisoning in the Pacific. Phytother. Res. 2011, 25, 947–958. [Google Scholar] [CrossRef]
- Rossi, F.; Jullian, V.; Pawlowiez, R.; Kumar-Roiné, S.; Haddad, M.; Darius, H.T.; Gaertner-Mazouni, N.; Chinain, M.; Laurent, D. Protective effect of Heliotropium foertherianum (Boraginaceae) folk remedy and its active compound, rosmarinic acid, against a Pacific ciguatoxin. J. Ethnopharmacol. 2012, 143, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lewis, N.D. Disease and development: Ciguatera fish poisoning. Soc. Sci. Med. 1986, 23, 983–993. [Google Scholar] [CrossRef]
- Darius, H.T.; Drescher, O.; Ponton, D.; Pawlowiez, R.; Laurent, D.; Dewailly, E.; Chinain, M. Use of folk tests to detect ciguateric fish: A scientific evaluation of their effectiveness in Raivavae Island (Australes, French Polynesia). Food Addit. Contam. Part A 2013, 30, 550–566. [Google Scholar] [CrossRef]
- Daneshian, M.; Botana, L.M.; Dechraoui Bottein, M.Y.; Buckland, G.; Campàs, M.; Dennison, N.; Dickey, R.W.; Diogène, J.; Fessard, V.; Hartung, T.; et al. A roadmap for hazard monitoring and risk assessment of marine biotoxins on the basis of chemical and biological test systems. ALTEX 2013, 30, 487–545. [Google Scholar] [CrossRef] [Green Version]
- Nicolas, J.; Hendriksen, P.J.M.; Gerssen, A.; Bovee, T.F.H.; Rietjenso, I.M.C. Marine neurotoxins: State of the art, bottlenecks, and perspectives for mode of action based methods of detection in seafood. Mol. Nutr. Food Res. 2014, 58, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ha, D.V.; Uesugi, A.; Uchida, H. Analytical challenges to ciguatoxins. Curr. Opin. Food Sci. 2017, 18, 37–42. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Appendix 5—FDA and EPA safety levels in regulations and guidance. In Fish and Fishery Products Hazards and Control Guidance, 4th ed.; University of Florida: Gainesville, FL, USA, 2020. Available online: https://www.fda.gov/media/80400/downloadpp (accessed on 10 March 2021).
- Caillaud, A.; de la Iglesia, P.; Darius, H.T.; Pauillac, S.; Aligizaki, K.; Fraga, S.; Chinain, M.; Diogène, J. Update on methodologies available for ciguatoxin determination: Perspectives to confront the onset of ciguatera fish poisoning in europe. Mar. Drugs 2010, 8, 1838–1907. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Hoogenboom, R.L.A.P.; Hendriksen, P.J.M.; Bodero, M.; Bovee, T.F.H.; Rietjens, I.M.C.M.; Gerssen, A. Marine biotoxins and associated outbreaks following seafood consumption: Prevention and surveillance in the 21st century. Glob. Food Secur. 2017, 15, 11–21. [Google Scholar] [CrossRef]
- Leonardo, S.; Gaiani, G.; Tsumuraya, T.; Hirama, M.; Turquet, J.; Sagristà, N.; Rambla-Alegre, M.; Flores, C.; Caixach, J.; Diogène, J.; et al. Addressing the analytical challenges for the detection of ciguatoxins using an electrochemical biosensor. Anal. Chem. 2020, 92, 4858–4865. [Google Scholar] [CrossRef]
- Pasinszki, T.; Lako, J.; Dennis, T.E. Advances in Detecting Ciguatoxins in Fish. Toxins 2020, 12, 494. [Google Scholar] [CrossRef]
- Lewis, R.J.; Endean, R. Occurrence of a ciguatoxin-like substance in the Spanish mackerel (Scomberomorus commersoni). Toxicon 1983, 21, 19–24. [Google Scholar] [CrossRef]
- Lewis, R.J.; Sellin, M. Multiple ciguatoxins in the flesh of fish. Toxicon 1992, 30, 915–919. [Google Scholar] [CrossRef]
- Oshiro, N.; Yogi, K.; Asato, S.; Sasaki, T.; Tamanaha, K.; Hirama, M.; Yasumoto, T.; Inafuku, Y. Ciguatera incidence and fish toxicity in Okinawa, Japan. Toxicon 2010, 56, 656–661. [Google Scholar] [CrossRef]
- Wong, C.K.; Hung, P.; Lo, J.Y.C. Ciguatera fish poisoning in Hong Kong—A 10-year perspective on the class of ciguatoxins. Toxicon 2014, 86, 96–106. [Google Scholar] [CrossRef]
- Ha, D.; Uesugi, A.; Uchida, H.; Ky, P.; Minh, D.; Watanabe, R.; Matsushima, R.; Oikawa, H.; Nagai, S.; Iwataki, M.; et al. Identification of causative ciguatoxins in red snappers Lutjanus bohar implicated in ciguatera fish poisonings in Vietnam. Toxins 2018, 10, 420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, R.J.; Sellin, M.; Poli, M.A.; Norton, R.S.; MacLeod, J.K.; Sheil, M.M. Purification and characterization of ciguatoxins from moray eel (Lycodontis javanicus, Muraenidae). Toxicon 1991, 29, 1115–1127. [Google Scholar] [CrossRef]
- Dechraoui, M.Y.; Naar, J.; Pauillac, S.; Legrand, A.M. Ciguatoxins and brevetoxins, neurotoxic polyether compounds active on sodium channels. Toxicon 1999, 37, 125–143. [Google Scholar] [CrossRef]
- Darius, H.T.; Ponton, D.; Revel, T.; Cruchet, P.; Ung, A.; Tchou Fouc, M.; Chinain, M. Ciguatera risk assessment in two toxic sites of French Polynesia using the receptor-binding assay. Toxicon 2007, 50, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Chinain, M.; Darius, H.T.; Ung, A.; Tchou Fouc, M.; Revel, T.; Cruchet, P.; Pauillac, S.; Laurent, D. Ciguatera risk management in French Polynesia: The case study of Raivavae Island (Australes Archipelago). Toxicon 2010, 56, 674–690. [Google Scholar] [CrossRef]
- Gaboriau, M.; Ponton, D.; Darius, H.T.; Chinain, M. Ciguatera fish toxicity in French Polynesia: Size does not always matter. Toxicon 2014, 84, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Chinain, M.; Gatti, C.M.; Martin-Yken, H.; Roué, M.; Darius, H.T. Ciguatera poisoning: An increasing burden for Pacific Islands communities in light of climate change? In Climate Change and Marine and Freshwater Toxins, 2nd ed.; Botana, L.M., Louzao, M.C., Vilariño, N., Eds.; De Gruyter: Berlin, Germany, 2021; pp. 369–428. [Google Scholar] [CrossRef]
- Viallon, J.; Chinain, M.; Darius, H.T. Revisiting the neuroblastoma cell-based assay (CBA-N2a) for the improved detection of marine toxins active on voltage gated sodium channels (VGSCs). Toxins 2020, 12, 281. [Google Scholar] [CrossRef]
- Chinain, M.; Gatti, C.M.; Ung, A.; Cruchet, P.; Revel, T.; Viallon, J.; Sibat, M.; Varney, P.; Laurent, V.; Hess, P.; et al. Evidence for the range expansion of ciguatera in French Polynesia: A revisit of the 2009 mass-poisoning outbreak in Rapa Island (Australes Archipelago). Toxins 2020, 12, 759. [Google Scholar] [CrossRef]
- Díaz-Asencio, L.; Clausing, R.J.; Vandersea, M.W.; Chamero-Lago, D.; Gómez-Batista, M.; Hernández-Albernas, J.I.; Chomérat, N.; Rojas-Abrahantes, G.; Litaker, R.W.; Tester, P.; et al. Ciguatoxin occurrence in food-web components of a Cuban coral reef ecosystem: Risk-assessment implications. Toxins 2019, 11, 722. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Henao, A.; García-Álvarez, N.; Silva Sergent, F.; Estévez, P.; Gago-Martínez, A.; Martín, F.; Ramos-Sosa, M.; Fernández, A.; Diogène, J.; Real, F. Presence of CTXs in moray eels and dusky groupers in the marine environment of the Canary Islands. Aquat. Toxicol. 2020, 221, 105427. [Google Scholar] [CrossRef]
- Yasumoto, T.; Igarashi, T.; Legrand, A.M.; Cruchet, P.; Chinain, M.; Fujita, T.; Naoki, H. Structural elucidation of ciguatoxin congeners by fast-atom bombardment tandem mass spectroscopy. J. Am. Chem. Soc. 2000, 122, 4988–4989. [Google Scholar] [CrossRef]
- Lewis, R.J.; Yang, A.; Jones, A. Rapid extraction combined with LC-tandem mass spectrometry (CREM-LC/MS/MS) for the determination of ciguatoxins in ciguateric fish flesh. Toxicon 2009, 54, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.; Whittle, N.; Shaw, G.; Eaglesham, G.; Moore, M.R.; Lewis, R.J. Human fatality associated with Pacific ciguatoxin contaminated fish. Toxicon 2010, 56, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Yogi, K.; Oshiro, N.; Inafuku, Y.; Hirama, M.; Yasumoto, T. Detailed LC-MS/MS analysis of ciguatoxins revealing distinct regional and species characteristics in fish and causative alga from the Pacific. Anal. Chem. 2011, 83, 8886–8891. [Google Scholar] [CrossRef]
- Wu, J.J.; Mak, Y.L.; Murphy, M.B.; Lam, J.C.W.; Chan, W.C.; Wang, M.; Chan, L.L.; Lam, P.K.S. Validation of an accelerated solvent extraction liquid chromatography tandem mass spectrometry method for Pacific ciguatoxin-1 in fish flesh and comparison with the mouse neuroblastoma assay. Anal. Bioanal. Chem. 2011, 400, 3165–3175. [Google Scholar] [CrossRef]
- Mak, Y.L.; Wai, T.-C.; Murphy, M.B.; Chan, W.H.; Wu, J.J.; Lam, J.C.W.; Chan, L.L.; Lam, P.K.S. Pacific ciguatoxins in food web components of coral reef systems in the Republic of Kiribati. Environ. Sci. Technol. 2013, 47, 14070–14079. [Google Scholar] [CrossRef]
- Yogi, K.; Sakugawa, S.; Oshiro, N.; Ikehara, T.; Sugiyama, K.; Yasumoto, T. Determination of toxins involved in ciguatera fish poisoning in the Pacific by LC/MS. J. AOAC Int. 2014, 97, 398–402. [Google Scholar] [CrossRef]
- Murray, J.S.; Boundy, M.J.; Selwood, A.I.; Harwood, D.T. Development of an LC–MS/MS method to simultaneously monitor maitotoxins and selected ciguatoxins in algal cultures and P-CTX-1B in fish. Harmful Algae 2018, 80, 80–87. [Google Scholar] [CrossRef]
- Oshiro, N.; Nagasawa, H.; Kuniyoshi, K.; Kobayashi, N.; Sugita-Konishi, Y.; Asakura, H.; Yasumoto, T. Characteristic distribution of ciguatoxins in the edible parts of a grouper, Variola louti. Toxins 2021, 13, 218. [Google Scholar] [CrossRef]
- Hamilton, B.; Hurbungs, M.; Vernoux, J.P.; Jones, A.; Lewis, R.J. Isolation and characterisation of Indian Ocean ciguatoxin. Toxicon 2002, 40, 685–693. [Google Scholar] [CrossRef]
- Bottein Dechraoui, M.Y.; Tiedeken, J.A.; Persad, R.; Wang, Z.; Granade, H.R.; Dickey, R.W.; Ramsdell, J.S. Use of two detection methods to discriminate ciguatoxins from brevetoxins: Application to great barracuda from Florida Keys. Toxicon 2005, 46, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Hardison, D.R.; Holland, W.C.; McCall, J.R.; Bourdelais, A.J.; Baden, D.G.; Darius, H.T.; Chinain, M.; Tester, P.A.; Shea, D.; Flores Quintana, H.A.; et al. Fluorescent receptor binding assay for detecting ciguatoxins in fish. PLoS ONE 2016, 11, e0153348. [Google Scholar] [CrossRef] [PubMed]
- Hardison, D.R.; Holland, W.C.; Darius, H.T.; Chinain, M.; Tester, P.A.; Shea, D.; Bogdanoff, A.K.; Morris, J.A., Jr.; Flores Quintana, H.A.; Loeffler, C.R.; et al. Investigation of ciguatoxins in invasive lionfish from the greater Caribbean region: Implications for fishery development. PLoS ONE 2018, 13, e0198358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, P.R.; Estévez, P.; Soliño, L.; Castro, D.; Rodrigues, S.M.; Timoteo, V.; Leao-Martins, J.M.; Santos, C.; Gouveia, N.; Diogène, J.; et al. An update on ciguatoxins and CTX-like toxicity in fish from different trophic levels of the Selvagens Islands (NE Atlantic, Madeira, Portugal). Toxins 2021, 13, 580. [Google Scholar] [CrossRef] [PubMed]
- Kohli, G.S.; Haslauer, K.; Sarowar, C.; Kretzschmar, A.L.; Boulter, M.; Harwood, D.T.; Laczka, O.; Murray, S.A. Qualitative and quantitative assessment of the presence of ciguatoxin, P-CTX-1B, in Spanish Mackerel (Scomberomorus commerson) from waters in New South Wales (Australia). Toxicol. Rep. 2017, 4, 328–334. [Google Scholar] [CrossRef]
- Harwood, D.T.; Murray, S.; Boundy, M.J. Chapter Three—Sample preparation prior to marine toxin analysis. In Comprehensive Analytical Chemistry; Diogène, J., Campàs, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 78, pp. 89–136. [Google Scholar]
- Estevez, P.; Castro, D.; Leao, J.M.; Yasumoto, T.; Dickey, R.; Gago-Martinez, A. Implementation of liquid chromatography tandem mass spectrometry for the analysis of ciguatera fish poisoning in contaminated fish samples from Atlantic coasts. Food Chem. 2019, 280, 8–14. [Google Scholar] [CrossRef]
- Pottier, I.; Hamilton, B.; Jones, A.; Lewis, R.J.; Vernoux, J.P. Identification of slow and fast-acting toxins in a highly ciguatoxic barracuda (Sphyraena barracuda) by HPLC/MS and radiolabelled ligand binding. Toxicon 2003, 42, 663–672. [Google Scholar] [CrossRef]
- Darius, H.T.; Roué, M.; Sibat, M.; Viallon, J.; Gatti, C.M.; Vandersea, M.W.; Tester, P.A.; Litaker, R.W.; Amzil, Z.; Hess, P.; et al. Tectus niloticus (Tegulidae, Gastropod) as a novel vector of ciguatera poisoning: Detection of Pacific ciguatoxins in toxic samples from Nuku Hiva Island (French Polynesia). Toxins 2018, 10, 2. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Scientific opinion on marine biotoxins in shellfish—Emerging toxins: Ciguatoxin group. EFSA J. 2010, 8, 1627. [Google Scholar] [CrossRef]
- Taylor, F.; Gustavson, M. An underwater survey of the organism chiefly responsible for “ciguatera” fish poisoning in the eastern Caribbean region: The benthic dinoflagellate Gambierdiscus toxicus. In Proceedings of the 7th International Diving Science Symposium, Padova, Italy, 15–18 September 1983; CMAS, University of Padua: Padua, Italy, 1983; pp. 95–111. [Google Scholar]
- Grzebyk, D.; Berland, B.; Thomassin, B.A.; Bosi, C.; Arnoux, A. Ecology of ciguateric dinoflagellates in the coral reef complex of Mayotte Island (S.W. Indian Ocean). J. Exp. Mar. Biol. Ecol. 1994, 178, 51–66. [Google Scholar] [CrossRef]
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Chinain, M.; Holmes, M.J.; Holland, W.C.; Tester, P.A. Taxonomy of Gambierdiscus including four new species, Gambierdiscus caribaeus, Gambierdiscus carolinianus, Gambierdiscus carpenteri and Gambierdiscus ruetzleri (Gonyaulacales, Dinophyceae). Phycologia 2009, 48, 344–390. [Google Scholar] [CrossRef]
- Tester, P.A.; Vandersea, M.W.; Buckel, C.A.; Kibler, S.R.; Holland, W.C.; Davenport, E.D.; Clark, R.D.; Edwards, K.F.; Taylor, J.C.; Pluym, J.L.V.; et al. Gambierdiscus (Dinophyceae) species diversity in the Flower Garden Banks National Marine Sanctuary, Northern Gulf of Mexico, USA. Harmful Algae 2013, 29, 1–9. [Google Scholar] [CrossRef]
- Gray, A.E. Fine Scale Movement of the Lustrous Pomfret (Eumegistus illustris) at Cross Seamount. Master’s Thesis, University of Hawai’i at Mānoa, Mānoa, HI, USA, 2016. [Google Scholar]
- A Global Information System on Fishes. Available online: https://www.fishbase.in/home.htm (accessed on 11 March 2021).
- Rongo, T.; van Woesik, R. Ciguatera poisoning in Rarotonga, Southern Cook Islands. Harmful Algae 2011, 10, 345–355. [Google Scholar] [CrossRef]
- Ikehara, T.; Kuniyoshi, K.; Oshiro, N.; Yasumoto, T. Biooxidation of ciguatoxins leads to species-specific toxin profiles. Toxins 2017, 9, 205. [Google Scholar] [CrossRef] [Green Version]
- Yasumoto, T.; Inoue, A.; Ochi, T.; Fujimoto, K.; Oshima, Y.; Fukuyo, Y.; Adachi, R.; Bagnis, R. Environmental studies on a toxic dinoflagellate responsible for ciguatera. Nippon Suisan Gakkaishi 1980, 46, 1397–1404. [Google Scholar] [CrossRef] [Green Version]
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Nau, A.W.; Holland, W.C.; Chinain, M.; Holmes, M.J.; Tester, P.A. Global distribution of ciguatera causing dinoflagellates in the genus Gambierdiscus. Toxicon 2010, 56, 711–730. [Google Scholar] [CrossRef]
- Darius, H.T.; Roué, M.; Ung, A.; Cruchet, P.; Revel, T.; Viallon, J.; Suhas, E.; Gatti, C.M.; Chinain, M. Ecotoxicological characterization of 3 different lagoons of French Polynesia: Tikehau, Kaukura and Mangareva. In Proceedings of the 16th International Conference on Harmful Algae, Wellington, New Zealand, 27–31 October 2014; p. 207. [Google Scholar]
- Chinain, M.; Darius, H.T.; Gatti, C.M.; Roué, M. Update on ciguatera research in French Polynesia. SPC Fish. 2016, 150, 42–51. [Google Scholar]
- Longo, S.; Sibat, M.; Viallon, J.; Darius, H.T.; Hess, P.; Chinain, M. Intraspecific variability in the toxin production and toxin profiles of in vitro cultures of Gambierdiscus polynesiensis (Dinophyceae) from French polynesia. Toxins 2019, 11, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, S.; Sibat, M.; Darius, H.T.; Hess, P.; Chinain, M. Effects of pH and nutrients (nitrogen) on growth and toxin profile of the ciguatera-causing dinoflagellate Gambierdiscus polynesiensis (Dinophyceae). Toxins 2020, 12, 767. [Google Scholar] [CrossRef]
- Skinner, M.P.; Brewer, T.D.; Johnstone, R.; Fleming, L.E.; Lewis, R.J. Ciguatera fish poisoning in the Pacific Islands (1998 to 2008). PLoS Negl. Trop. Dis. 2011, 5, e1416. [Google Scholar] [CrossRef]
- Chinain, M.; Gatti, C.M.; Roué, M.; Darius, H.T. Ciguatera poisoning in French Polynesia: Insights into the novel trends of an ancient disease. New Microbes New Infect. 2019, 31, 100565. [Google Scholar] [CrossRef] [PubMed]
- Annual Reports of CP in French Polynesia. Available online: https://www.ciguatera.pf/index.php/fr/la-ciguatera/surveillance-et-statistiques (accessed on 10 March 2021).
- Halstead, B.W.; Auerbach, P.S.; Campbell, D.R. A Colour Atlas of Dangerous Marine Animals; Wolfe Medical Publications Ltd., W.S. Cowell Ltd.: Ipswich, UK, 1990; p. 192. [Google Scholar]
- Bagnis, R. Account of the ciguateric endemicity in the Gambier Islands. Cah. Poc. 1974, 18, 585–599. [Google Scholar]
- Yasumoto, T.; Inoue, A.; Bagnis, R. Ecological survey of a toxic dinoflagellate associated with ciguatera. In Toxic Dinoflagellate Blooms; Taylor, D.L., Seliger, H.H., Eds.; Elsevier: New York, NY, USA, 1979; pp. 221–224. [Google Scholar]
- Ma, K.Y.; Craig, M.T. An inconvenient monophyly: An update on the taxonomy of the groupers (Epinephelidae). Copeia 2018, 106, 443–456. [Google Scholar] [CrossRef]
- Legrand, A.M.; Litaudon, M.; Genthon, J.N.; Bagnis, R.; Yasumoto, T. Isolation and some properties of ciguatoxin. J. Appl. Phycol. 1989, 1, 183–188. [Google Scholar] [CrossRef]
- Schlumberger, S.; Mattei, C.; Molgó, J.; Benoit, E. Dual action of a dinoflagellate-derived precursor of Pacific ciguatoxins (P-CTX-4B) on voltage-dependent K+ and Na+ channels of single myelinated axons. Toxicon 2010, 56, 768–775. [Google Scholar] [CrossRef]
- Mattei, C.; Molgó, J.; Benoit, E. Involvement of both sodium influx and potassium efflux in ciguatoxin-induced nodal swelling of frog myelinated axons. Neuropharmacology 2014, 85, 417–426. [Google Scholar] [CrossRef]
- Roué, M.; Darius, H.T.; Picot, S.; Ung, A.; Viallon, J.; Gaertner-Mazouni, N.; Sibat, M.; Amzil, Z.; Chinain, M. Evidence of the bioaccumulation of ciguatoxins in giant clams (Tridacna maxima) exposed to Gambierdiscus spp. cells. Harmful Algae 2016, 57, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Clausing, R.J.; Losen, B.; Oberhaensli, F.R.; Darius, H.T.; Sibat, M.; Hess, P.; Swarzenski, P.W.; Chinain, M.; Dechraoui Bottein, M.-Y. Experimental evidence of dietary ciguatoxin accumulation in an herbivorous coral reef fish. Aquat. Toxicol. 2018, 200, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Darius, H.T.; Roué, M.; Sibat, M.; Viallon, J.; Gatti, C.M.; Vandersea, M.W.; Tester, P.A.; Litaker, R.W.; Amzil, Z.; Hess, P.; et al. Toxicological investigations on the sea urchin Tripneustes gratilla (Toxopneustidae, Echinoid) from Anaho Bay (Nuku Hiva, French Polynesia): Evidence for the presence of Pacific ciguatoxins. Mar. Drugs 2018, 16, 122. [Google Scholar] [CrossRef] [Green Version]
- Sibat, M.; Herrenknecht, C.; Darius, H.T.; Roué, M.; Chinain, M.; Hess, P. Detection of Pacific ciguatoxins using liquid chromatography coupled to either low or high resolution mass spectrometry (LC-MS/MS). J. Chromatogr. A 2018, 1571, 16–28. [Google Scholar] [CrossRef]
- Poli, M.A.; Mende, T.J.; Baden, D.G. Brevetoxins: Unique activators of voltage-sensitive sodium channels, bind to specific sites in rat brain synaptosomes. Mol. Pharmacol. 1986, 30, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Lombet, A.; Bidard, J.N.; Lazdunski, M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS Lett. 1987, 219, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Laurent, D.; Kerbrat, A.S.; Darius, H.T.; Rossi, F.; Yeeting, B.; Haddad, M.; Golubic, S.; Pauillac, S.; Chinain, M. Ciguatera Shellfish Poisoning (CSP): A new ecotoxicological phenomenon from cyanobacteria to humans via giant clams. In Food Chains: New Research; Jensen, M.A., Muller, D.W., Eds.; Nova Science Publishers, Inc.: NewYork, NY, USA, 2012; Volume 1, pp. 1–43. [Google Scholar]
- Yasumoto, T. The chemistry and biological function of natural marine toxins. Chem. Rec. 2001, 1, 228–242. [Google Scholar] [CrossRef] [PubMed]
- Vernoux, J.P.; Lahlou, N.; Abbad el Andaloussi, S.; Riyeche, N.; Magras, L.P. A study of the distribution of ciguatoxin in individual Caribbean fish. Acta Trop. 1985, 42, 225–233. [Google Scholar] [PubMed]
- Loeffler, C.R.; Robertson, A.; Flores Quintana, H.A.; Silander, M.C.; Smith, T.B.; Olsen, D. Ciguatoxin prevalence in 4 commercial fish species along an oceanic exposure gradient in the US Virgin Islands. Environ. Toxicol. Chem. 2018, 37, 1852–1863. [Google Scholar] [CrossRef] [PubMed]
- Vial, J.; Jardy, A. Experimental comparison of the different approaches to estimate LOD and LOQ of an HPLC method. Anal. Chem. 1999, 71, 2672–2677. [Google Scholar] [CrossRef]







| Species | Site | Sample | Flesh Equivalent Injected (g) | Survival Time of Mice c | Symptoms | Total Fish Flesh Toxicity (MU g−1) d | CTX-like Content (µg CTX1B eq. kg−1) e |
|---|---|---|---|---|---|---|---|
| Saloptia powelli | Unknown a | 1–3 b | 200 | ≥24 h | No symptoms | <0.005 | <LOD f |
| Tauna | 4–5 b | 200 | <0.005 | <LOD | |||
| East Tepapuri | 6 | 200 | <0.005 | <LOD | |||
| North Tepapuri | 7 | 200 | <0.005 | <LOD | |||
| Gaioio | 8 | 200 | <0.005 | <LOD | |||
| Tenoko | 9 | 200 | <0.005 | <LOD | |||
| Tekava | 10–11 b | 200 | <0.005 | <LOD | |||
| Totegegie | 12–13 b | 100 | <0.01 | <LOD | |||
| Gaioio | 14–15 b | 100 | <0.01 | <LOD | |||
| Epinephelus tuamotuensis | Gaioio | 16 | 100 | ≥24 h | No symptoms | <0.01 | <LOD |
| Tokorua | 17 | 10 | Death after 5 h/death in the night | Diarrhea, dyspnea, cyanosis | 0.10 | 0.70 | |
| Etelis coruscans | Unknown | 18 | 25 | ≥24 h/death in the night | Diarrhea | 0.04 | 0.28 |
| Tokorua | 19 | 200 | Death after 5 h/6 h | Diarrhea, dyspnea, flickering walk ataxia, jump before death | 0.005 | 0.04 | |
| Pristipomoides filamentosus | Unknown | 20 | 50 | Death after 3 h 15/4 h 30 | Diarrhea, dyspnea, ataxia | 0.02 | 0.14 |
| Tokorua | 21 | 100 | ≥24 h | No symptoms | <0.01 | <LOD | |
| Eumegistus illustris | Tokorua | 22 | 5 | Death after 2 h 50/3 h 50 | Diarrhea, dyspnea, priapism, cyanosis | 0.20 | 1.40 |
| Species | Site | Sample | IC50 b | CTX-like Content d |
|---|---|---|---|---|
| (mg Flesh eq. mL−1) | (µg CTX1B eq. kg−1) | |||
| Saloptia powelli | Unknown a | 1 | ND c | <LOD e |
| 2 | ND | <LOD | ||
| 3 | ND | <LOD | ||
| Tauna | 4 | ND | <LOD | |
| 5 | ND | <LOD | ||
| East Tepapuri | 6 | ND | <LOD | |
| North Tepapuri | 7 | ND | <LOD | |
| Gaioio | 8 | ND | <LOD | |
| Tenoko | 9 | 1260 ± 245 | 0.21 ± 0.04 | |
| Tekava | 10 | ND | <LOD | |
| 11 | ND | <LOD | ||
| Totegegie | 12 | ND | <LOD | |
| 13 | ND | <LOD | ||
| Gaioio | 14 | ND | <LOD | |
| 15 | ND | <LOD | ||
| Epinephelus tuamotuensis | Gaioio | 16 | ND | <LOD |
| Tokorua | 17 | 435 ± 36.3 | 0.60 ± 0.05 | |
| Etelis coruscans | Unknown | 18 | 967 ± 117 | 0.27 ± 0.03 |
| Tokorua | 19 | 384 ± 121 | 0.72 ± 0.21 | |
| Pristipomoides filamentosus | Unknown | 20 | 1076 ± 215 | 0.25 ± 0.06 |
| Tokorua | 21 | 473 ± 12.7 | 0.55 ± 0.01 | |
| Eumegistus illustris | Tokorua | 22 | 231 ± 45.0 | 1.15 ± 0.22 |
| Species | Site | Sample | Viability Percentages c | EC50 e ng Dry Extract mL−1 | EC50 e mg Flesh eq. mL−1 | CTX-like Content (µg CTX1B eq. kg−1) |
|---|---|---|---|---|---|---|
| Saloptia powelli | Unknown a | 1–3 b | 53.5% | 9139 ± 652 | 17.9 ± 1.28 | 0.10 ± 0.01 b |
| Est Tepapuri | 6 | 102.8% | ND f | ND f | <LOD h | |
| North Tepapuri | 7 | 94.0% | ND | ND | <LOD | |
| Gaioio | 8 | 100.5% | ND | ND | <LOD | |
| Tenoko | 9 | 116.4% | ND | ND | <LOD | |
| Tekava | 10–11 b | 116.4% | ND | ND | <LOD | |
| Epinephelus tuamotuensis | Tokorua | 17 | 1.1% | 1391 ± 91 | 0.67 ± 0.04 | 2.68 ± 0.14 |
| Etelis coruscans | Unknown | 18 | 5.7% | 4494 ± 444 | 2.39 ± 0.24 | 0.74 ± 0.04 |
| Pristipomoides filamentosus | Unknown | 20 | 1.3% | 1080 ± 136 | 7.20 ± 0.91 | 0.25 ± 0.02 |
| Eumegistus illustris | Tokorua | 22 | 0.7% | 818 ± 109 | 0.63 ± 0.08 | 2.83 ± 0.23 |
| (LF100) | 65.3% d | 19,924 ± 1728 g | 15.3 ± 1.33 | 0.12 ± 0.01 |
| Fish Species | Site | Sample | CTX Content per Analog (µg CTX1B kg−1) | Total CTX Content | ||
|---|---|---|---|---|---|---|
| CTX1B | 52-epi-54-deoxyCTX1B | 54-deoxyCTX1B | (µg kg−1) | |||
| Saloptia powelli | Unknown a | 1–3 b | <LOD c | <LOD | <LOD | <LOD |
| Tekava | 10–11 b | <LOD | <LOD | <LOD | <LOD | |
| Epinephelus tuamotuensis | Tokorua | 17 | 0.28 | <LOD | <LOD | 0.28 |
| Etelis coruscans | Unknown | 18 | <LOD | <LOD | <LOD | <LOD |
| Pristipomoides filamentosus | Unknown | 20 | 0.08 | <LOD | <LOD | 0.08 |
| Eumegistus illustris | Tokorua | 22 | 0.36 | 0.49 | 0.26 | 1.11 |
| Species | Site | Sample | rRBA | MBA | CBA-N2a | LC–MS/MS |
|---|---|---|---|---|---|---|
| Saloptia powelli | Unknown a | 1 | <LOD | <LOD b | 0.10 ± 0.01 b | <LOD b |
| 2 | <LOD | |||||
| 3 | <LOD | |||||
| Tauna | 4 | <LOD | <LOD | |||
| 5 | <LOD | |||||
| East Tepapuri | 6 | <LOD | <LOD | <LOD | ||
| North Tepapuri | 7 | <LOD | <LOD | <LOD | ||
| Gaioio | 8 | <LOD | <LOD | <LOD | ||
| Tenoko | 9 | 0.21 ± 0.04 | <LOD | <LOD | ||
| Tekava | 10 | <LOD | <LOD b | <LOD b | <LOD b | |
| 11 | <LOD | |||||
| Totegegie | 12 | <LOD | <LOD b | |||
| 13 | <LOD | |||||
| Gaioio | 14 | <LOD | <LOD b | |||
| 15 | <LOD | |||||
| Epinephelus tuamotuensis | Gaioio | 16 | <LOD | <LOD | <LOD | |
| Tokorua | 17 | 0.60 ± 0.05 | 0.7 | 2.68 ± 0.14 | 0.28 | |
| Etelis coruscans | Unknown | 18 | 0.27 ± 0.03 | 0.28 | 0.74 ± 0.04 | <LOD |
| Tokorua | 19 | 0.72 ± 0.21 | 0.04 | |||
| Pristipomoides filamentosus | Unknown | 20 | 0.25 ± 0.06 | 0.14 | 0.25 ± 0.02 | 0.08 |
| Tokorua | 21 | 0.55 ± 0.01 | <LOD | |||
| Eumegistus illustris | Tokorua | 22 | 1.15 ± 0.22 | 1.4 | 2.83 ± 0.23 | 1.11 |
| (LF100 c) | 0.12 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darius, H.T.; Revel, T.; Cruchet, P.; Viallon, J.; Gatti, C.M.i.; Sibat, M.; Hess, P.; Chinain, M. Deep-Water Fish Are Potential Vectors of Ciguatera Poisoning in the Gambier Islands, French Polynesia. Mar. Drugs 2021, 19, 644. https://doi.org/10.3390/md19110644
Darius HT, Revel T, Cruchet P, Viallon J, Gatti CMi, Sibat M, Hess P, Chinain M. Deep-Water Fish Are Potential Vectors of Ciguatera Poisoning in the Gambier Islands, French Polynesia. Marine Drugs. 2021; 19(11):644. https://doi.org/10.3390/md19110644
Chicago/Turabian StyleDarius, Hélène Taiana, Taina Revel, Philippe Cruchet, Jérôme Viallon, Clémence Mahana iti Gatti, Manoëlla Sibat, Philipp Hess, and Mireille Chinain. 2021. "Deep-Water Fish Are Potential Vectors of Ciguatera Poisoning in the Gambier Islands, French Polynesia" Marine Drugs 19, no. 11: 644. https://doi.org/10.3390/md19110644
APA StyleDarius, H. T., Revel, T., Cruchet, P., Viallon, J., Gatti, C. M. i., Sibat, M., Hess, P., & Chinain, M. (2021). Deep-Water Fish Are Potential Vectors of Ciguatera Poisoning in the Gambier Islands, French Polynesia. Marine Drugs, 19(11), 644. https://doi.org/10.3390/md19110644