Metabolities from Marine Sponges of the Genus Callyspongia: Occurrence, Biological Activity, and NMR Data
Abstract
:1. Introduction
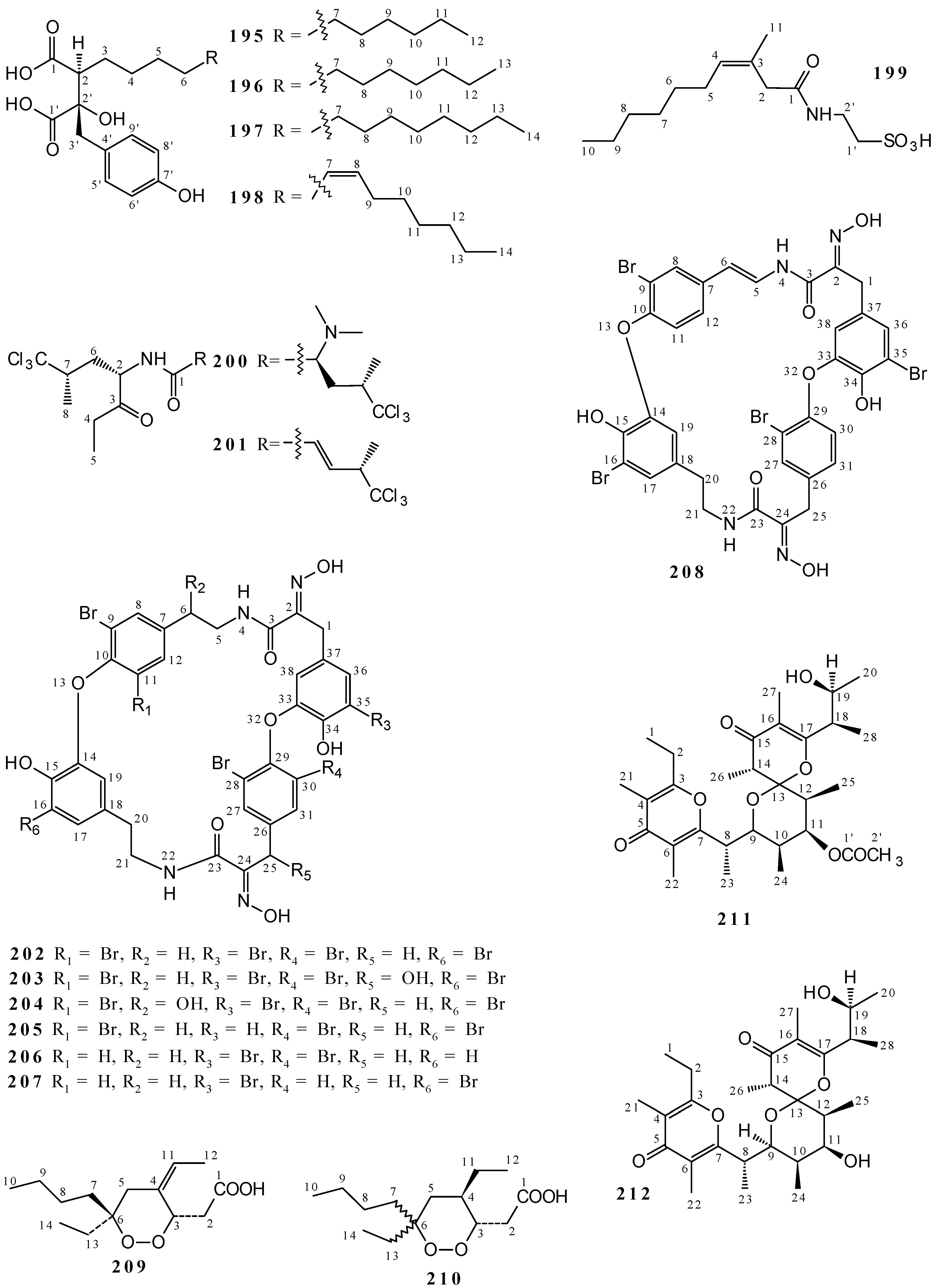
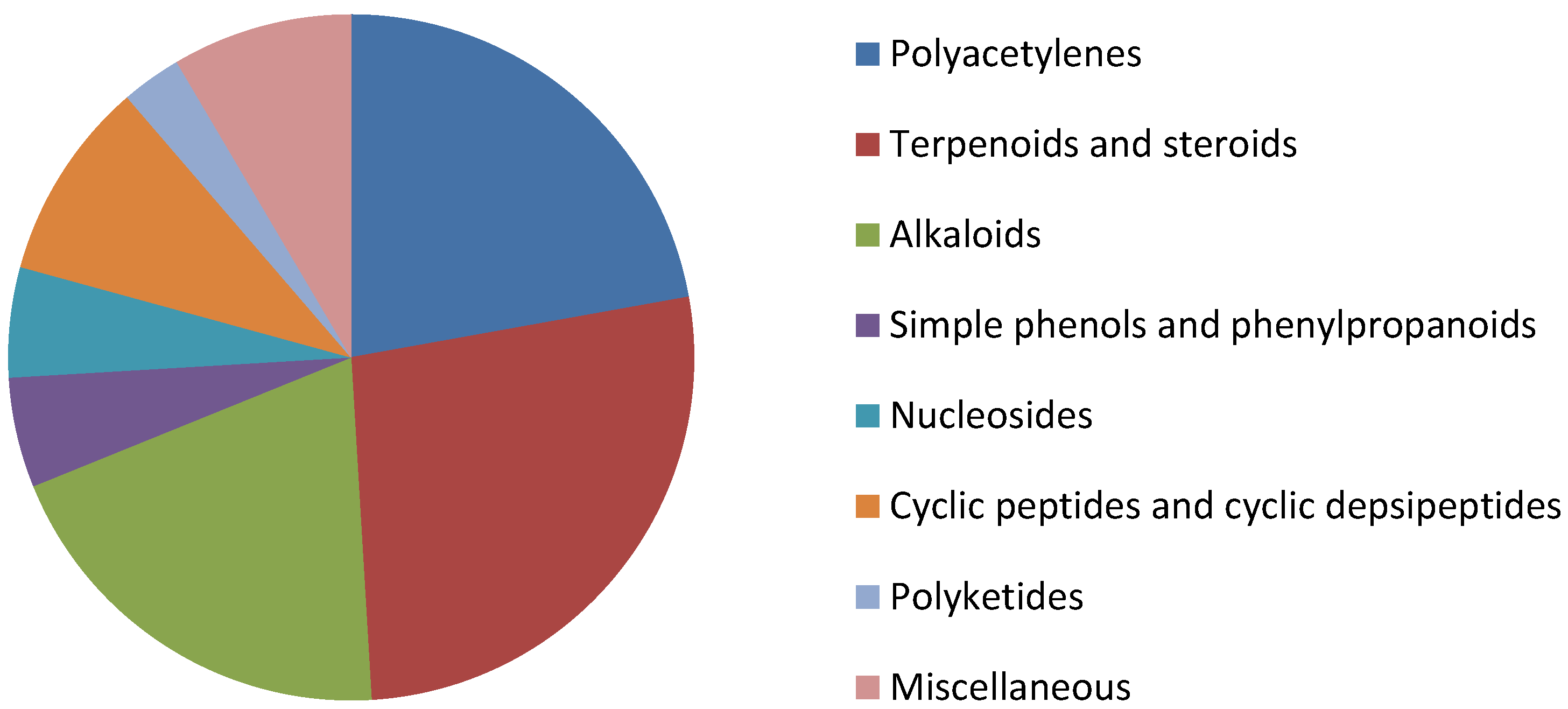
2. Chemical Aspects of Callyspongia species
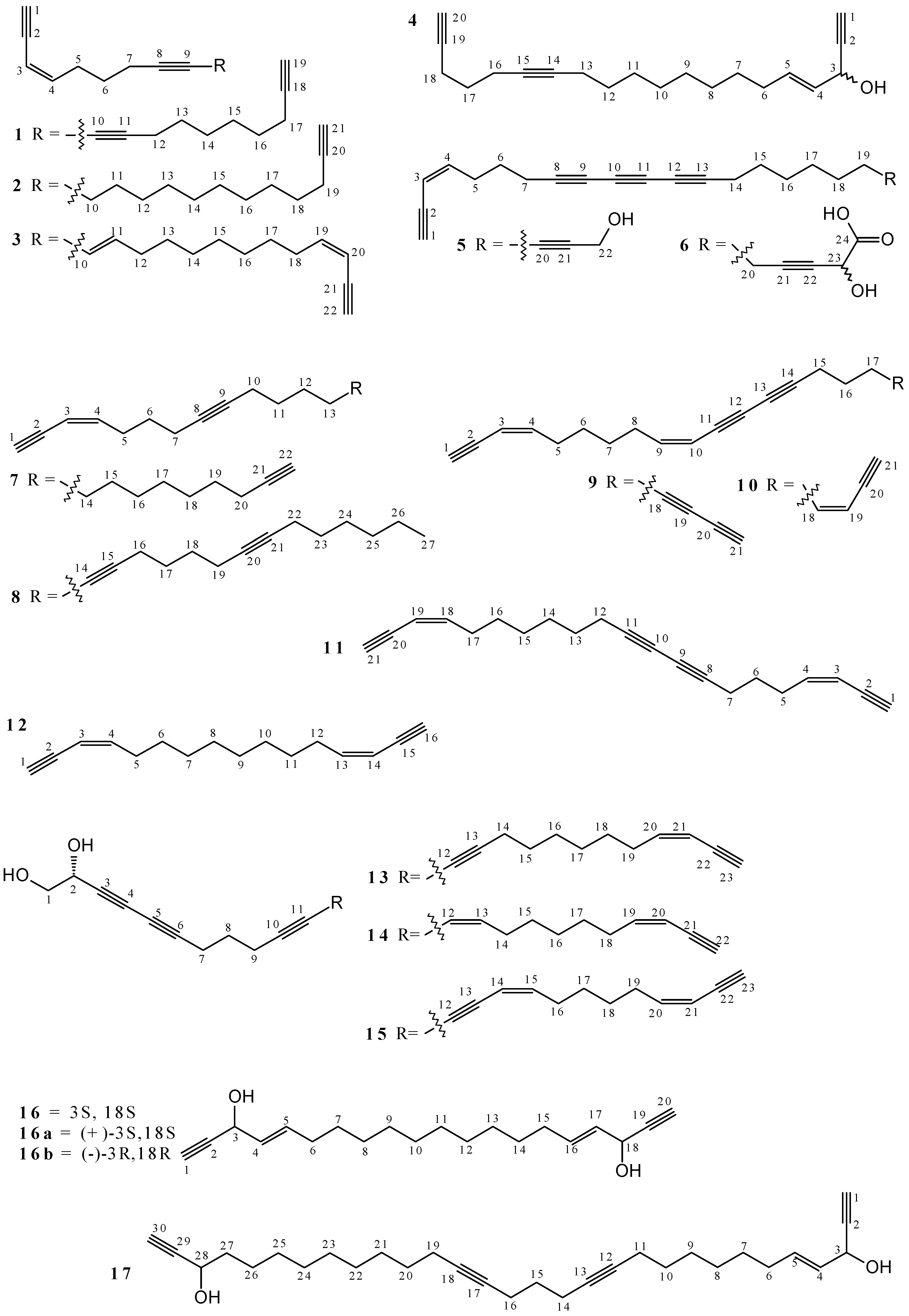
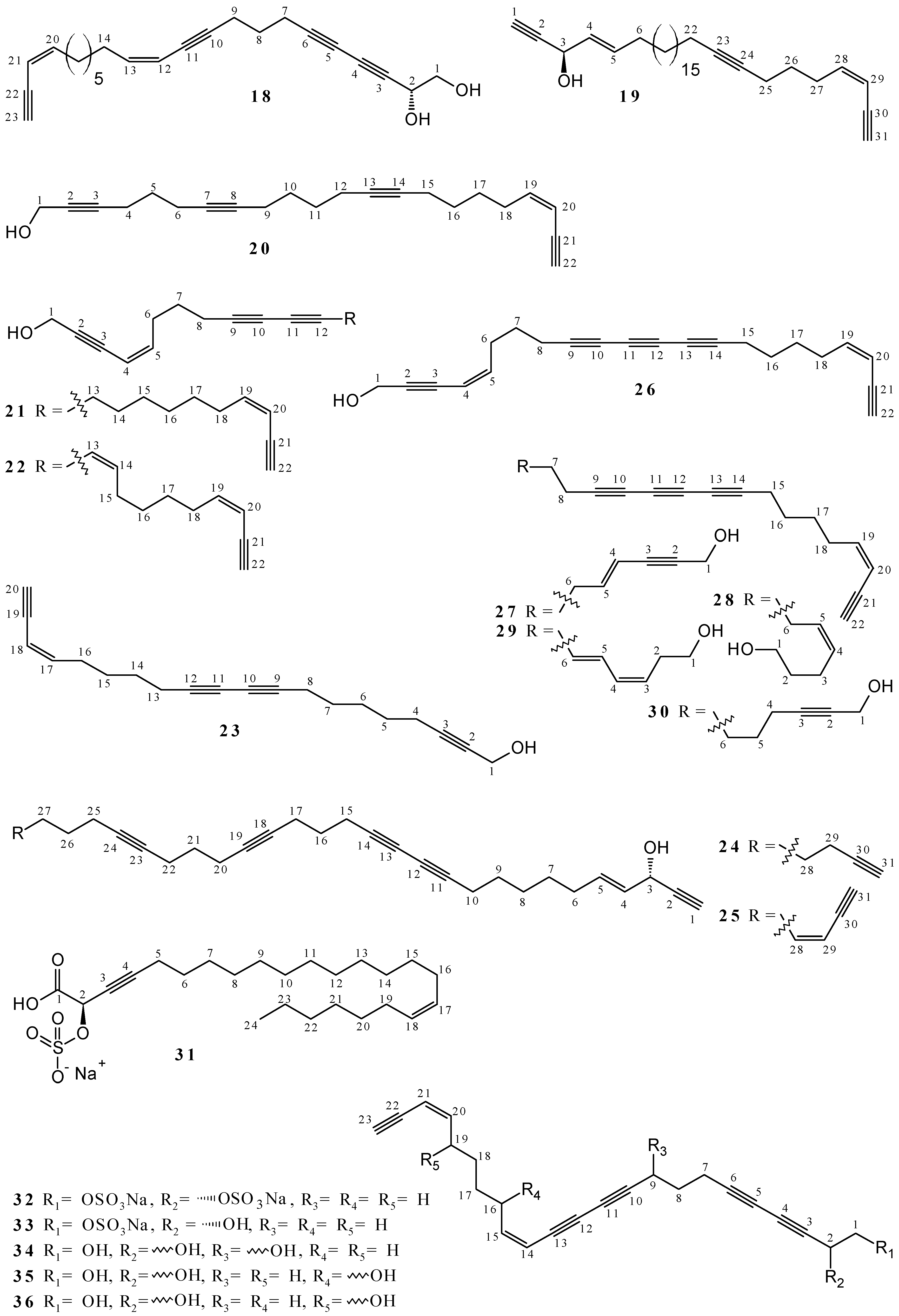
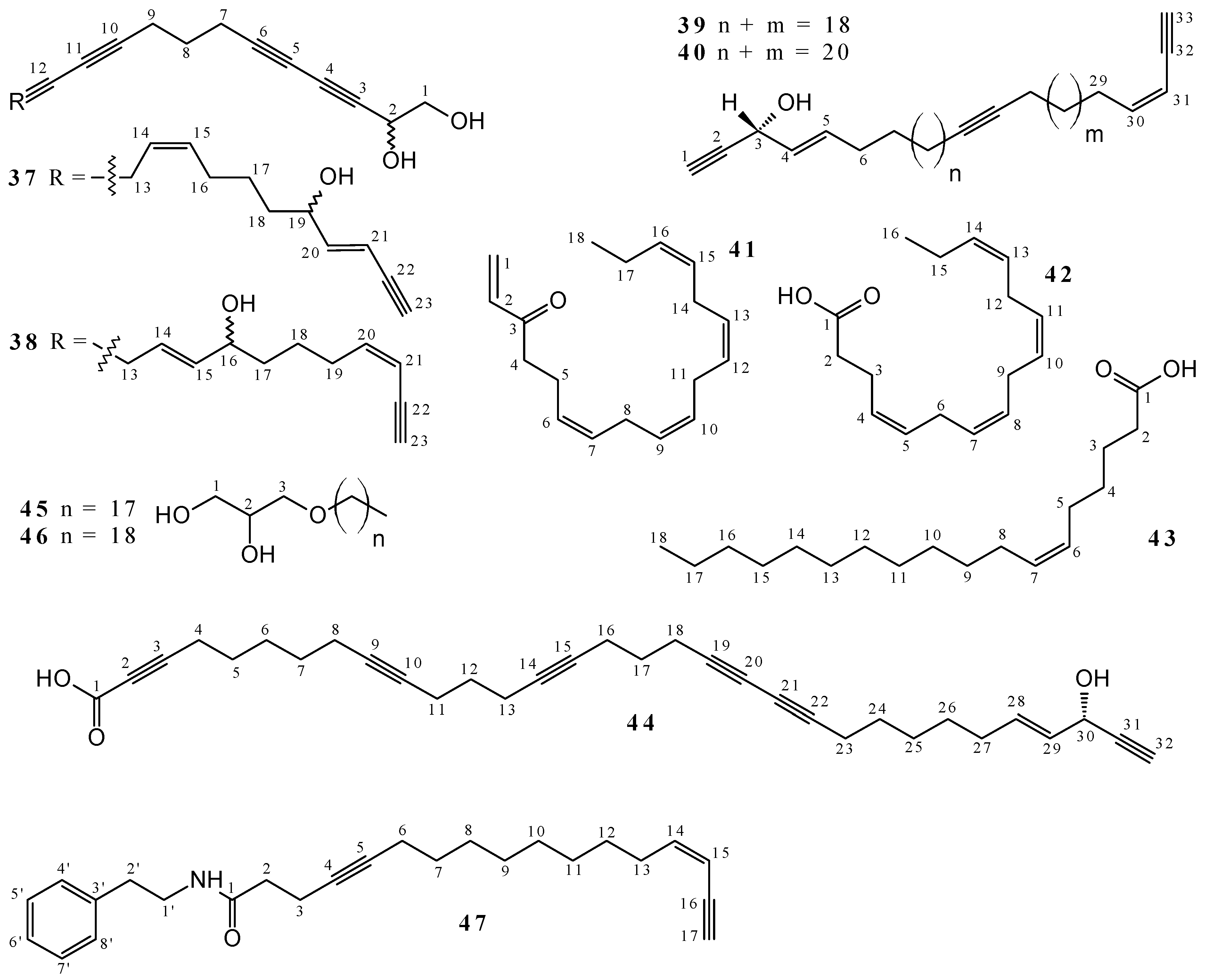
2.1. Polyacetylenes
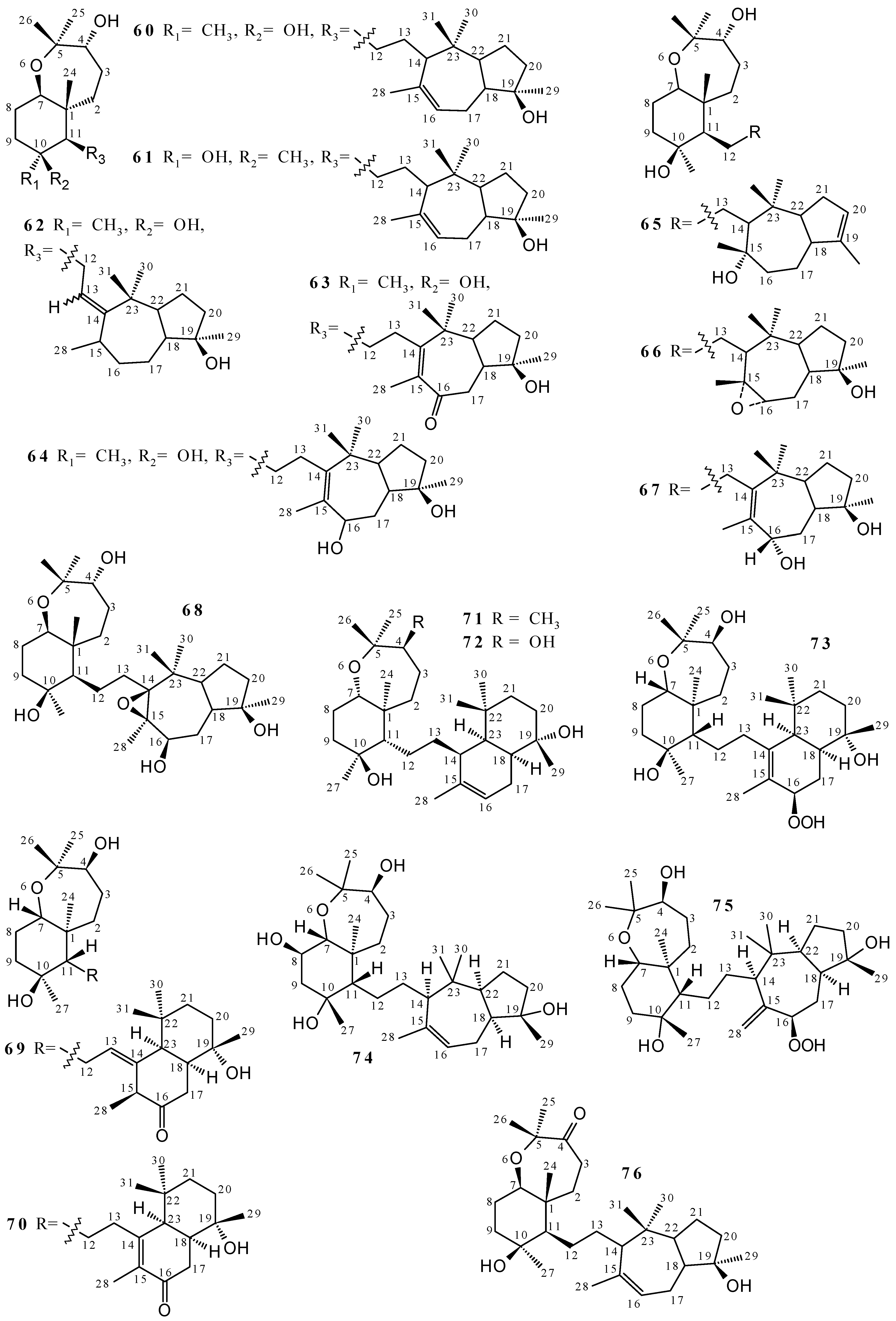
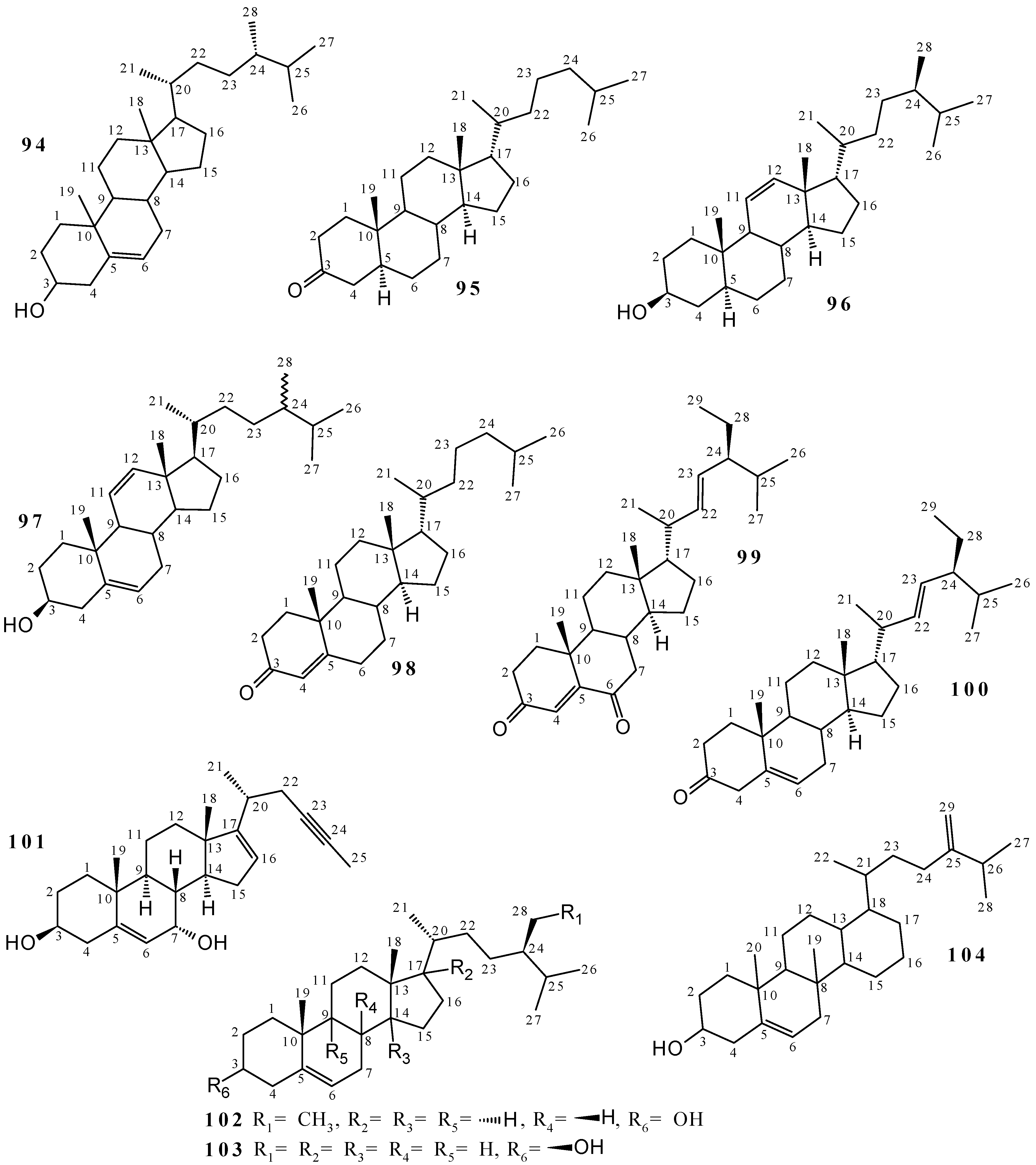
2.2. Terpenoids and Steroids
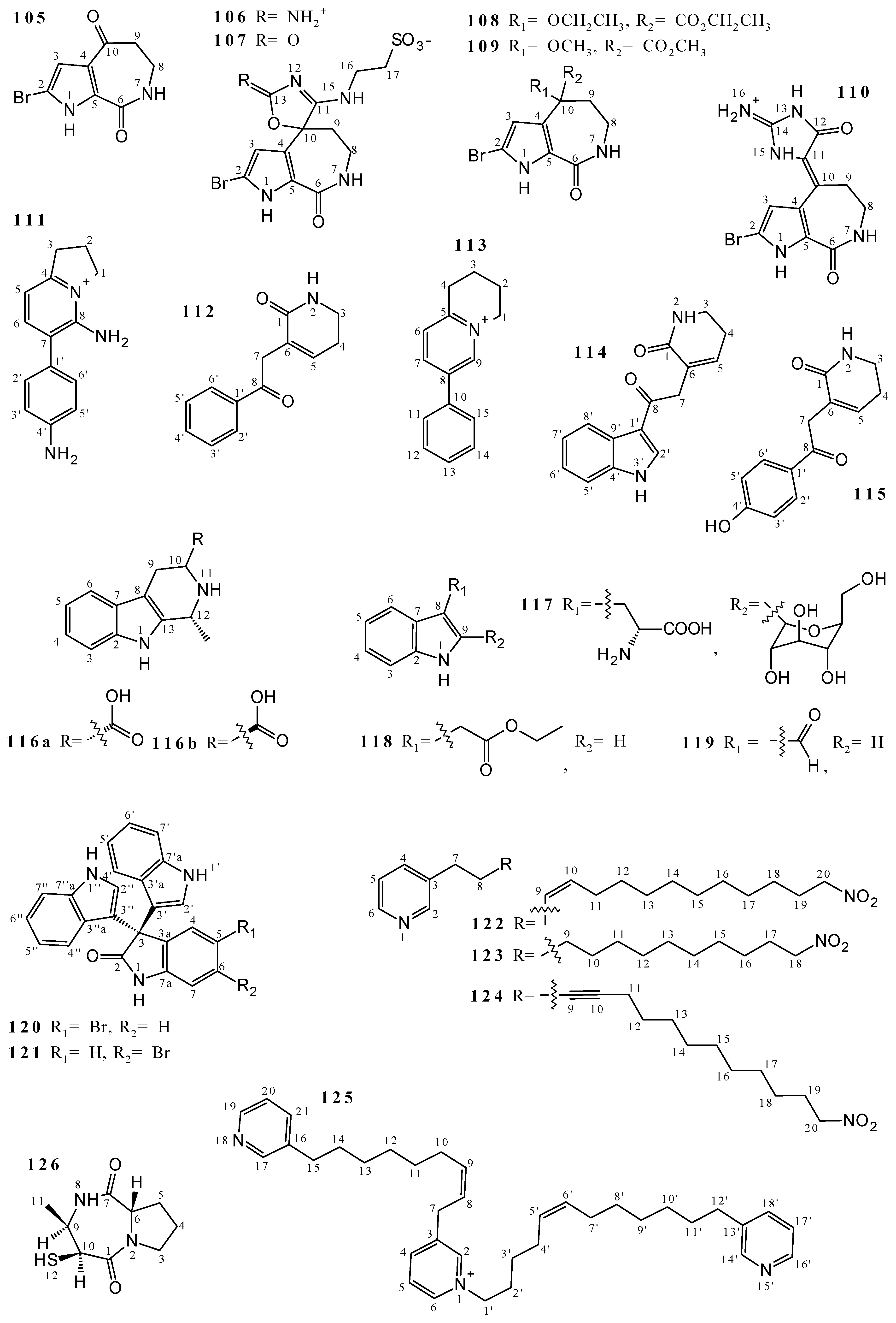
2.3. Alkaloids
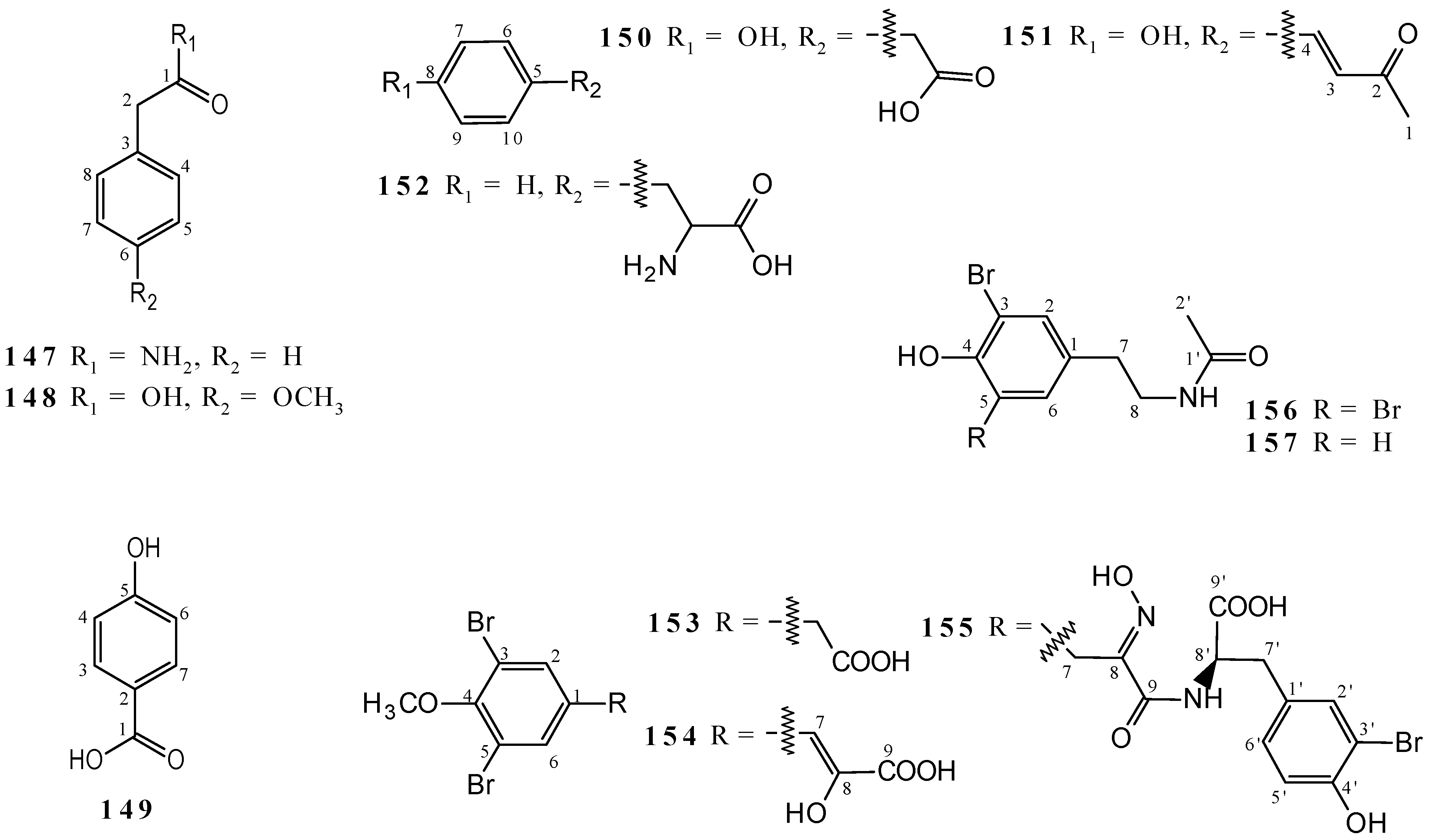
2.4. Simple Phenols and Phenylpropanoids
2.5. Nucleosides
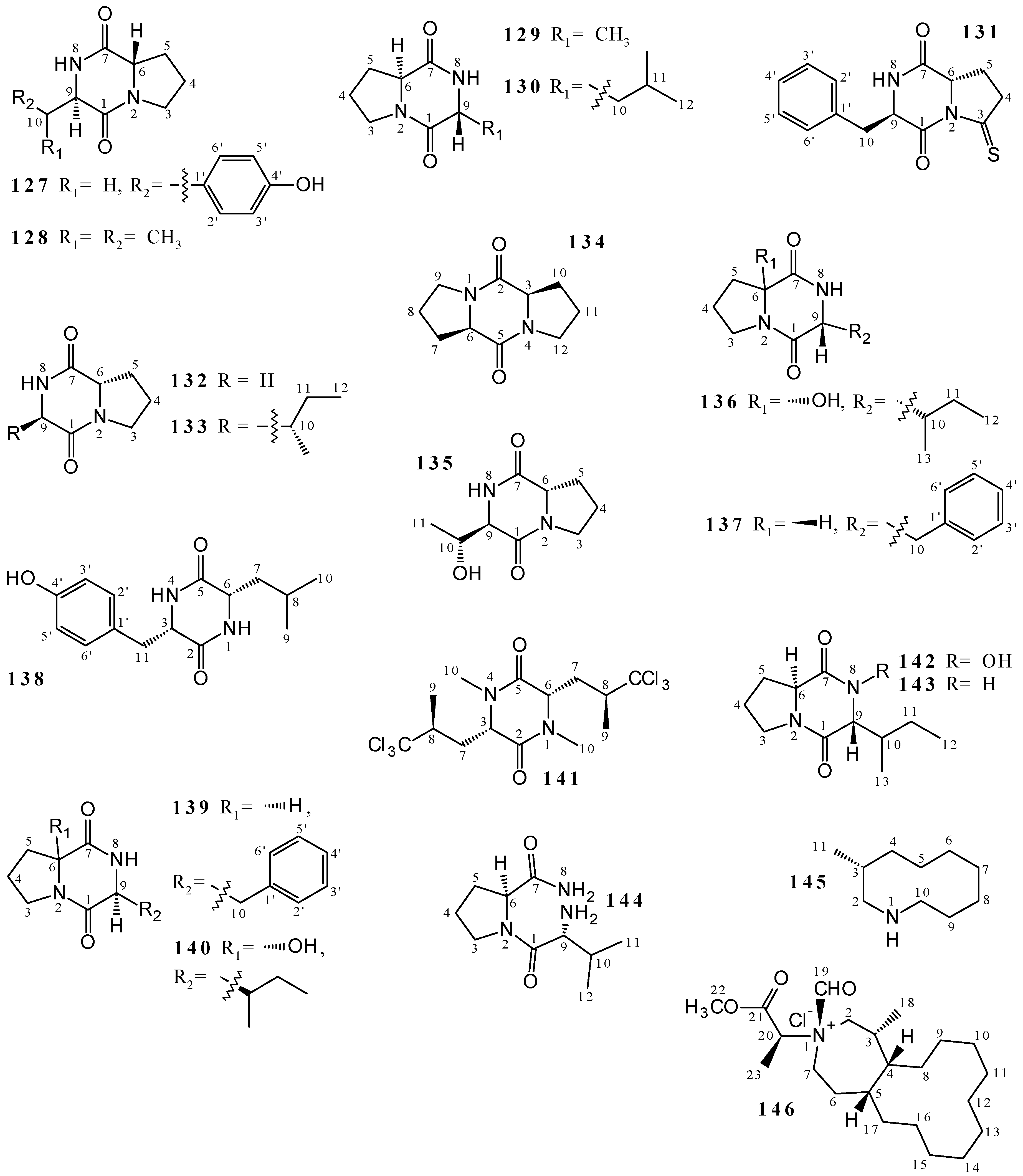
2.6. Cyclic Peptides and Cyclic Depsipeptides
2.7. Polyketides
2.8. Miscellanous
3. Biological Aspects of Metabolites Isolated in Callyspongia species
3.1. Polyacetylenes
3.2. Terpenoids and Steroids
3.3. Alkaloids
3.4. Simple Phenols and Phenyl Propanoids
3.5. Nucleosides
3.6. Cyclic Peptides and Cyclic Depsipeptides
3.7. Polyketides
3.8. Miscellanous
| Metabolite Name | Biological Activity | Ref. |
|---|---|---|
| Aikupikanyne E (5) | Cytotoxicity {(P-388, ATCC: CCL 46), (A-549, ATCC: CL 8) and (HT-29, ATCC: HTB 38)} | [27] |
| Aikupikanyne F (6) | Cytotoxicity {(P-388, ATCC: CCL 46), (A-549, ATCC: CL 8) and (HT-29, ATCC: HTB 38)} | [27] |
| Callyberyne A (Callypentayne) (9) | Metamorphosis-inducing (Ascidian Halocynthia roretzi larvae) | [29] |
| Callyberyne C (Callytetrayne) (11) | Metamorphosis-inducing (Ascidian Halocynthia roretzi larvae) | [29] |
| 14,15-Dihydrosiphonodiol (Dihydrosiphonodiol) (13) | Antiproliferative activity (HL-60 and HCT-15 cell lines) | [31] |
| Inhibitory activity (gastric H,K-ATPase) | [32,96] | |
| Callyspongidiol (14) | Antiproliferative activity (HL-60 and HCT-15 cell lines) | [31] |
| Siphonodiol (15) | Metamorphosis-inducing (Ascidian Halocynthia roretzi larvae) | [29] |
| Antifouling activity (Barnacle Balanus Amphitrite larvae) | [29] | |
| Antiproliferative activity (HL-60 and HCT-15 cell lines) | [31] | |
| Antibacterial (Staphylococcus aureus and Streptococcus pyogenes) | [33,96] | |
| Antifungal (Trichophyton asteroides) | [33,96] | |
| Inhibitory activity (gastric H,K-ATPase) | [32,96] | |
| (+)-(4E,16E)-icosa-4,16-diene-1,19-diyne-3,18-diol (16a) | Cytotoxic (TR-LE and HeLa cell lines) | [35] |
| (−)-(4E,16E)-icosa-4,16-diene-1,19-diyne-3,18-diol (16b) | Cytotoxic (TR-LE and HeLa cell lines) | [35] |
| Callyspongendiol (17) | Cytotoxicity (HCT-166 and MCF-7 cell lines) | [8,36] |
| Tetrahydrosiphonodiol (18) | Inhibitory activity (gastric H,K-ATPase) | [29,96] |
| (3R,4E,28Z)-Hentriacont-4,28-diene-1,23,30-triyn-3-ol (19) | Cytotoxicity (NBT-II cell line) | [37] |
| Callyspongenol A (20) | Cytotoxicity (P388 and HeLa cell lines) | [38] |
| Callyspongenol B (21) | Cytotoxicity (P388 and HeLa cell lines) | [38] |
| Callyspongenol C (22) | Cytotoxicity (P388 and HeLa cell lines) | [38] |
| Callyspongenol D (23) | Cytotoxicity (MCF-7 and HCT-116 cell lines) | [8,36] |
| Callysponyne A (24) | Cytotoxicity (MOLT-4, K-562, T-47D and HCT 116 cell lines) | [39] |
| Callysponyne B (25) | Cytotoxicity (MOLT-4, K-562, MDA-MB-231 and HCT 116 cell lines) | [39] |
| Dehydroisophonochalynol (Dehydrosiphonochalynol) (26) | Cytotoxicity (P388, HeLa, MCF-7 and A549 cell lines) | [36,38,40] |
| Siphonellanol A (27) | Cytotoxicity (HeLa, MCF-7 and A549 cell lines) | [40] |
| Siphonellanol B (28) | Cytotoxicity (HeLa, MCF-7 and A549 cell lines) | [40] |
| Siphonellanol C (29) | Cytotoxicity (HeLa, MCF-7 and A549 cell lines) | [40] |
| Siphonchalynol (30) | Cytotoxicity (HeLa, MCF-7 and A549 cell lines) | [40] |
| Callysponginol sulfate A (31) | Inhibitor of MT1-MMP | [41] |
| Callyspongin A (Siphonodiol disulfate) (32) | Inhibitor of fertilization of starfish gametes | [42] |
| Metamorphosis-inducing (Ascidian Halocynthia roretzi larvae) | [29] | |
| Antifouling activity (Barnacle Balanus Amphitrite larvae) | [29] | |
| Callyspongin B (Siphonodiol sulfate) (33) | Inhibitor of fertilization of starfish gametes | [42] |
| Metamorphosis-inducing (Ascidian Halocynthia roretzi larvae) | [29] | |
| Antifouling activity (Barnacle Balanus Amphitrite larvae) | [29] | |
| Callytriol A (34) | Metamorphosis-inducing (Ascidian Halocynthia roretzi larvae) | [29] |
| Antifouling activity (Barnacle Balanus Amphitrite larvae) | ||
| Callytriol B (35) | Metamorphosis-inducing (Ascidian Halocynthia roretzi larvae) | [29] |
| Antifouling activity (Barnacle Balanus Amphitrite larvae) | ||
| Callytriol C (36) | Metamorphosis-inducing (Ascidian Halocynthia roretzi larvae) | [29] |
| Antifouling activity (Barnacle Balanus Amphitrite larvae) | ||
| Callytriol D (37) | Metamorphosis-inducing (Ascidian Halocynthia roretzi larvae) | [29] |
| Antifouling activity (Barnacle Balanus Amphitrite larvae) | ||
| Callytriol E (38) | Metamorphosis-inducing (Ascidian Halocynthia roretzi larvae) | [29] |
| Antifouling activity (Barnacle Balanus Amphitrite larvae) | ||
| Callyspongynic Acid (44) | α-glucosidase inhibitor | [44] |
| Batyl alcohol (46) | Biofilm inhibition (Alteromona macleodii, Ochrobactrum pseudogrignonense, Vibrio harveyi and Staphylococcus aureus) | [97] |
| Callyspongamide A (47) | Cytotoxicity (HeLa cell lines) | [46] |
| Isocopalanol (49) | Cytotoxicity (PANC-1 cell line) | [50] |
| Akaterpin (50) | Enzyme Inhibitor (PI-PLC and neural sphingomyelinase) | [51] |
| Ilhabelanol (51) | Inhibitor of L-APRT | [11] |
| Ilhabrene (52) | Inhibitor of L-APRT | [11] |
| Isoakaterpin (53) | Inhibitor of L-APRT | [11] |
| (2S,4aS,5S,6R,8aS)-5-(2-((1S,3aS,5R,8aS,Z)-1-hydroxy-1,4,4,6-tetramethyl-1,2,3,3a,4,5,8,8a-octahydroazulen-5-yl)-ethyl)-4a,6-dimethyloctahydro-2H-chromene-2,6-diol (54) | Cytotoxicity (KB-3-1 and KB-C2) | [52] |
| Neviotine A (56) | Inhibitory activity (RANKL induced osteoclastogenesis) | [57] |
| Cytotoxicity (PC-3, A549, MCF-7 and HepG-2 cell lines) | [55,56] | |
| Antibacterial activity (Staphylococcus aureus, Bacillis subtilis and Escherichia coli) | [56] | |
| Antiviral activity (HAV-10) | [56] | |
| Neviotine C (58) | Cytotoxicity (PC-3 and A549 cell lines) | [55] |
| Neviotine D (59) | Inhibitory activity (RANKL induced osteoclastogenesis) | [57] |
| Sipholenol A (15-sipholen-4,10,19-triol) (60) | Cytotoxicity (KB-3-1, KB-C2, HepG-2, PC-3, A549, MCF-7 and HCT-116 cell lines) | [8,36,52,55,56,58,59] |
| Inhibitor of P-gp | [98] | |
| Antiproliferative activity (+SA mouse mammary epithelial cells) | [58] | |
| Antiviral (HAV-10) | [56] | |
| Sipholenol L (71) | Cytotoxicity (MCF-7 and HepG-2 cell lines) | [56] |
| Antibacterial activity (Staphylococcus aureus and Bacillis subtilis) | [56] | |
| Antiviral (HAV-10 and HSV-1) | [56] | |
| Sipholenol L (72) | Cytotoxicity (HCT-116, KB-3-1 and KB-C2 cell lines) | [8,52] |
| Sipholenol M (73) | Cytotoxicity (KB-3-1 and KB-C2 cell lines) | [52] |
| Sipholenone A (15-sipholen-10,19-diol-4-one) (76) | Cytotoxicity (HCT-116, PC-3, A549, MCF-7 and HepG-2 cell lines) | [8,55,56,58] |
| Antibacterial activity (Staphylococcus aureus, Bacillis subtilis and Escherichia coli) | [56] | |
| Reversal effects for KB-C2 | [59] | |
| Antiproliferative activity (+SA mouse mammary epithelial cells) | [58] | |
| Anti-angiogenic activity (CAM assay) | [58] | |
| Sipholenone E (80) | Cytotoxicity (KB-3-1 and KB-C2 cell lines) | [52] |
| Siphonellinol C (85) | Reversal effects for KB-C2 | [59] |
| Siphonellinol D (87) | Cytotoxicity (KB-3-1 and KB-C2 cell lines) | [52] |
| Siphonellinol E (88) | P-gp modulatory activity | [52] |
| 24S-24-methyl-cholestane-3β,5α,6β,25-tetraol-25-mono acetate (89) | Antimalarial (Plasmodium falciparum) | [23] |
| 24S-24-methyl chelestane-3β,5α,6β,12β,25-pentaol-25-O-acetate (90) | Antimalarial (Plasmodium falciparum) | [23] |
| 24S-24-methyl cholest-25-ene-3β,5α,6β,12β-tetrol (91) | Antimalarial (Plasmodium falciparum) | [23] |
| 24S-24-methyl cholestane-3β,6β,25-triol-25-O-acetate (92) | Antimalarial (Plasmodium falciparum) | [23] |
| Callysterol (ergosta-5,11-dien-3β-ol) (97) | Anti-inflammatory | [19] |
| Cholestenone (4-cholesten-3-one) (98) | Anti-metastasis of lung adenocarcinoma | [99] |
| Gelliusterol E (101) | Antichlamydial (Chlamydia trachomatis) | [28] |
| β-sitosterol (102) | Analgesic | [100,106] |
| Angiogenic | [101] | |
| Anthelminthic | [100] | |
| Antibacterial (Bacillus subtilis, Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella typhii, Corynebacterium diphtheria and Klebsiella pneumoniae) | [102,103,104] | |
| Antidiabetic | [102,105] | |
| Antifungal (Fusarium spp.) | [104] | |
| Anti-inflammatory | [100,106,107,108] | |
| Antimutagenic | [100] | |
| Antipyretic | [107] | |
| Cytotoxicity (MCF-7, HT-29, U937, MDA-MB-231, SGC-7901 and LNCaP) | [108,109,110,111,112,113,114] | |
| Hypocholesterolemic | [115] | |
| Immunomodulatory (pigs imune) | [116] | |
| Siphonocholin (103) | Anti-QS (inhibit the production of violacein) | [63] |
| Anti-biofilm (Paracoccus sp., Pseudomonas aeruginosa, Pseudoalteromonas sp. and Bacillus sp.) | [63] | |
| Ergosta-5,24(28)-dien-3β-ol (104) | Cytotoxicity (HCT-116 cell line) | [8] |
| 2-bromoaldisine (105) | Anti-HIV-1 | [117] |
| Inhibitory (Raf/MEK-1/MAPK cascade) | [118] | |
| Inhibitory (GSK-3, DYRK1A, CK-1) | [119] | |
| Hymenialdisine (110) | Cytotoxicity (SW620 and KB-3-1 cell lines) | [65] |
| Kinase inhibitor (CK1, CDK5 and GSK-3β) | [65,120] | |
| 3-(2-(4-hydroxyphenyl)-2-oxoethyl)-5,6-dihydropyridin-2(1H)-one (115) | Anti-allergic | [121] |
| (1R,3R)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid (116a) | Anti-oxidant | [122] |
| (1R,3S)-1-methyl-2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-3-carboxylic acid (116b) | Anti-oxidant | [122] |
| 1H-indole-3-carbaldehyde (119) | Inhibitor (Tyrosinase) | [123] |
| 5-bromo trisindoline (120) | Antibacterial (Staphylococcus aureus and Bacillus subtilis) | [7] |
| Biofilm inhibitory (Pseudomonas aeruginosa) | [7] | |
| Antitrypanosomal | [7] | |
| Cytotoxicity (HT-29, OVCAR-3 and MM.1S) | [7] | |
| 6-bromo trisindoline (121) | Antibacterial (Staphylococcus aureus and Bacillus subtilis) | [7] |
| Biofilm inhibitory (Pseudomonas aeruginosa) | [7] | |
| Antitrypanosomal | [7] | |
| Cytotoxicity (HT-29, OVCAR-3 and MM.1S) | [7] | |
| Untenine A (122) | Anti-microfouling | [68] |
| Untenine B (123) | Anti-microfouling | [68] |
| Untenine C (124) | Anti-microfouling | [68] |
| Niphatoxin C (125) | Cytotoxicity (THP-1 cell line) | [69] |
| Cyclo-(S-Pro-R-Ala) (129) | Antifouling (Cyprid larvae of the barnacle) | [66] |
| Cyclo-(S-Pro-R-Leu) (Cyclo-((S)-Pro-(R)-Leu)) (130) | Antifouling (Cyprid larvae of the barnacle) | [66] |
| Dysamide A (141) | Inhibitor of the SOAT1 and SOAT2 isozymes | [6] |
| (3R)-methylazacyclodecane (145) | Cytotoxic (K562 and A549 cell lines) | [5] |
| Callyazepin (146) | Cytotoxic (K562 and A549 cell lines) | [5] |
| 2-phenylacetamide (147) | Estrogenic activities | [124] |
| Inhibitory effect to the growth (rice, lettuce, barnyard millet and rape) | [125] | |
| 4-hydroxybenzoic acid (149) | Antimicrobial Activity (Staphylococcus aureus, Staphylococcus epidermidis, Bacillus subtilis, Lactobacillus plantarum, Leuconostoc mesenteroides, Escherichia coli, Salmonella typhimurium, Pseudomonas aeruginosa, Pseudomonas. Syringae, Pseudomonas. syringae pv. Tobaci, Ewinia carotovora subsp. carotovora, Xanthomonas campestri and Agrobacterium) | [126] |
| Fungitoxicity (inhibited the growth of Ganoderma boninense) | [127] | |
| Hypoglycemic activity | [128] | |
| 3,5-dibromo-4-methoxyphenylpyruvic acid (154) | ApoE modulatory (CCF-STTG1 cell line) | [80] |
| 2’-Deoxyadenosine (165) | Inhibitor of keratinocyte proliferation | [129] |
| Toxic to E3 embryos | [130] | |
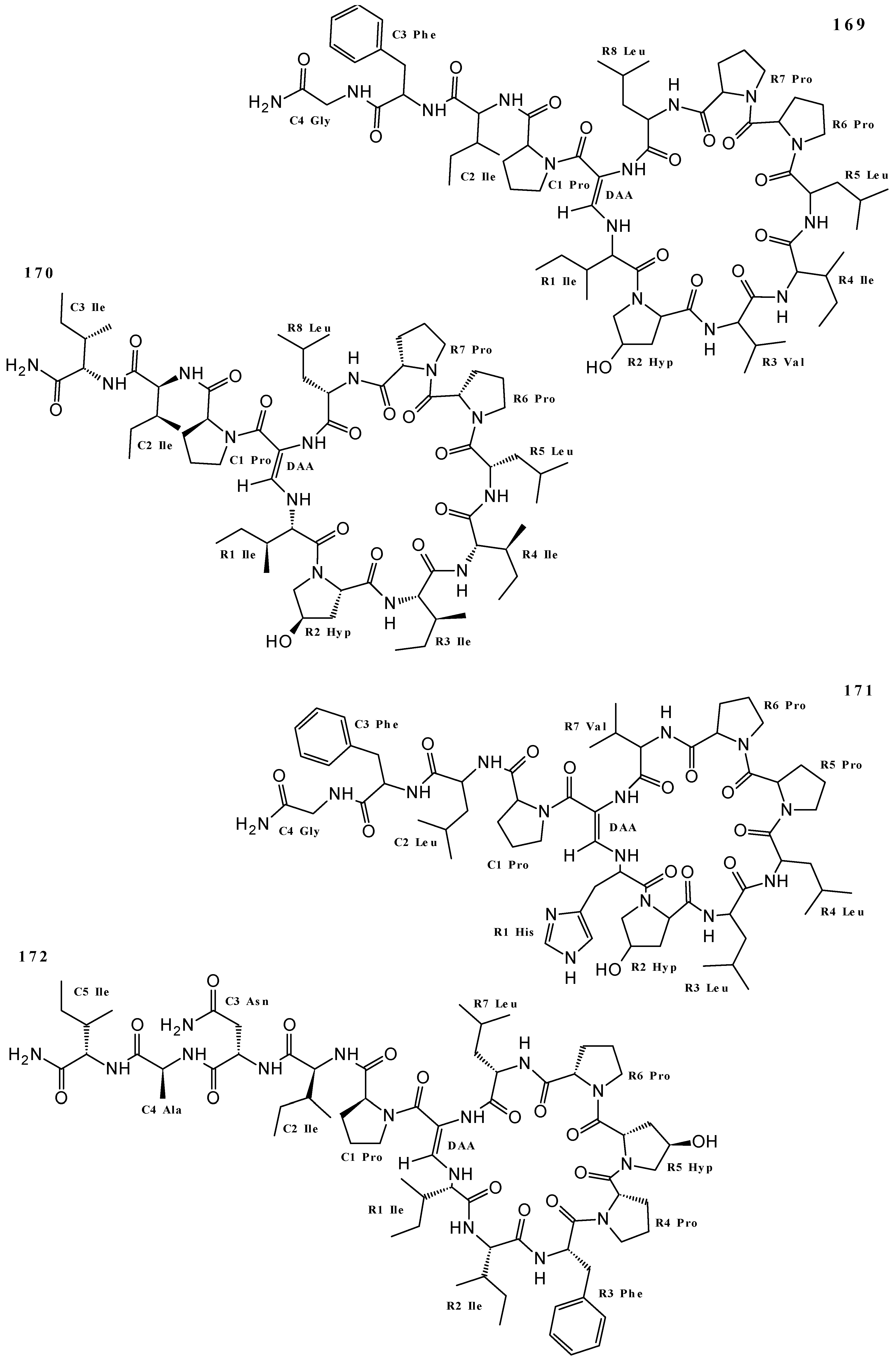
| Callyaerin A (169) | Anti-Tuberculosis | [22,131] |
| Antibacterial (Escherichia coli and Staphylococcus aureus) | [4] | |
| Antifungal (Candida albicans) | [4] | |
| Cytotoxicity (L5178Y cell line) | [4] | |
| Callyaerin B (170) | Anti-Tuberculosis | [22] |
| Antibacterial (Escherichia coli and Staphylococcus aureus) | [4] | |
| Antifungal (Candida albicans) | [4] | |
| Cytotoxicity (L5178Y, THP-1 and MRC-5 cell lines) | [4,22] | |
| Callyaerin C (171) | Cytotoxicity (L5178Y cell line) | [4] |
| Callyaerin D (172) | Cytotoxicity (L5178Y cell line) | [4] |
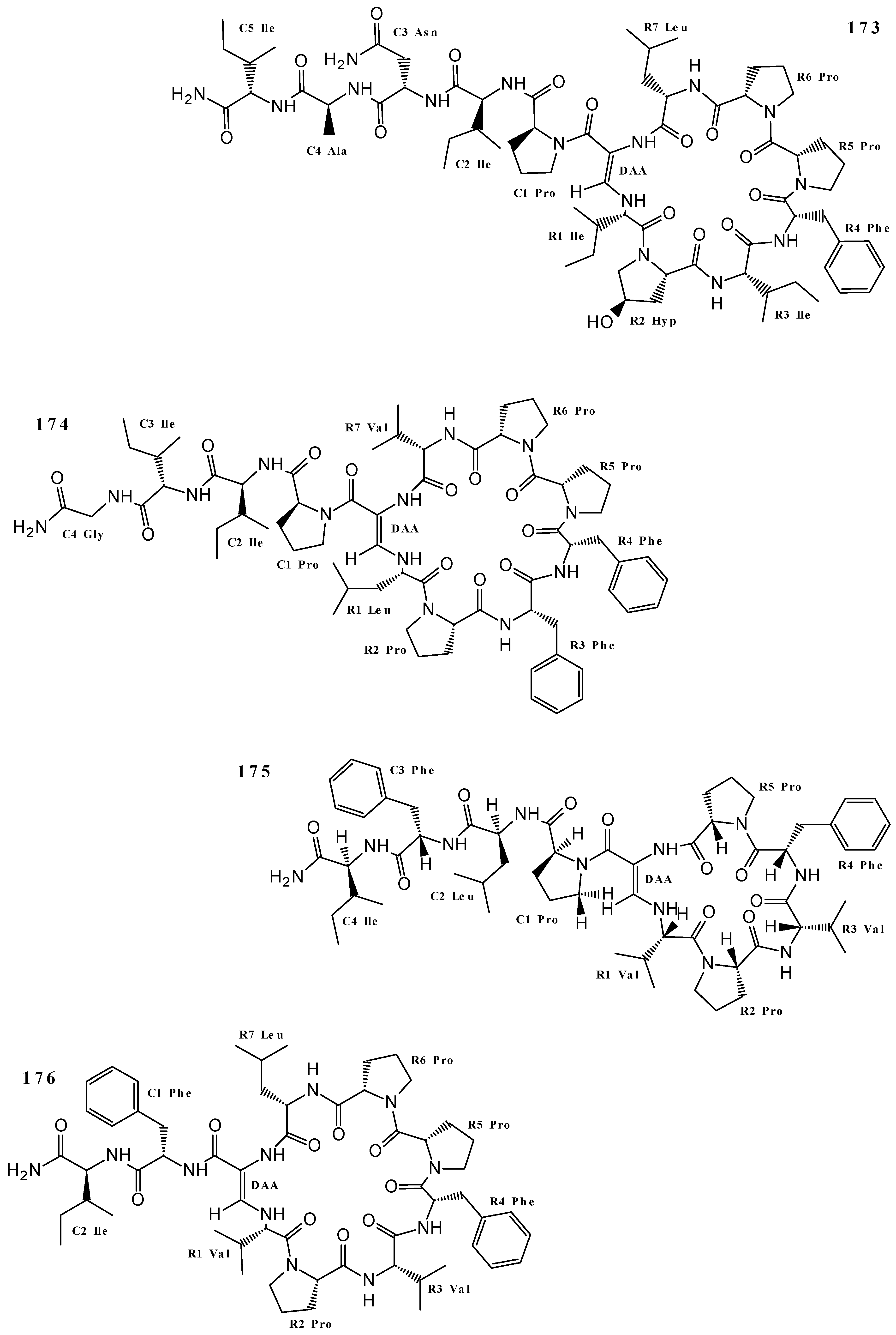
| Callyaerin E (174) | Cytotoxicity (L5178Y cell line) | [4] |
| Antimicrobial (Escherichia coli, Staphylococcus aureus Candida albicans and Bacilus subtilis) | [4] | |
| Callyaerin F (175) | Cytotoxicity (L5178Y cell line) | [4] |
| Callyaerin G (178) | Cytotoxicity (L5178Y and HeLa cell lines) | [4,82] |
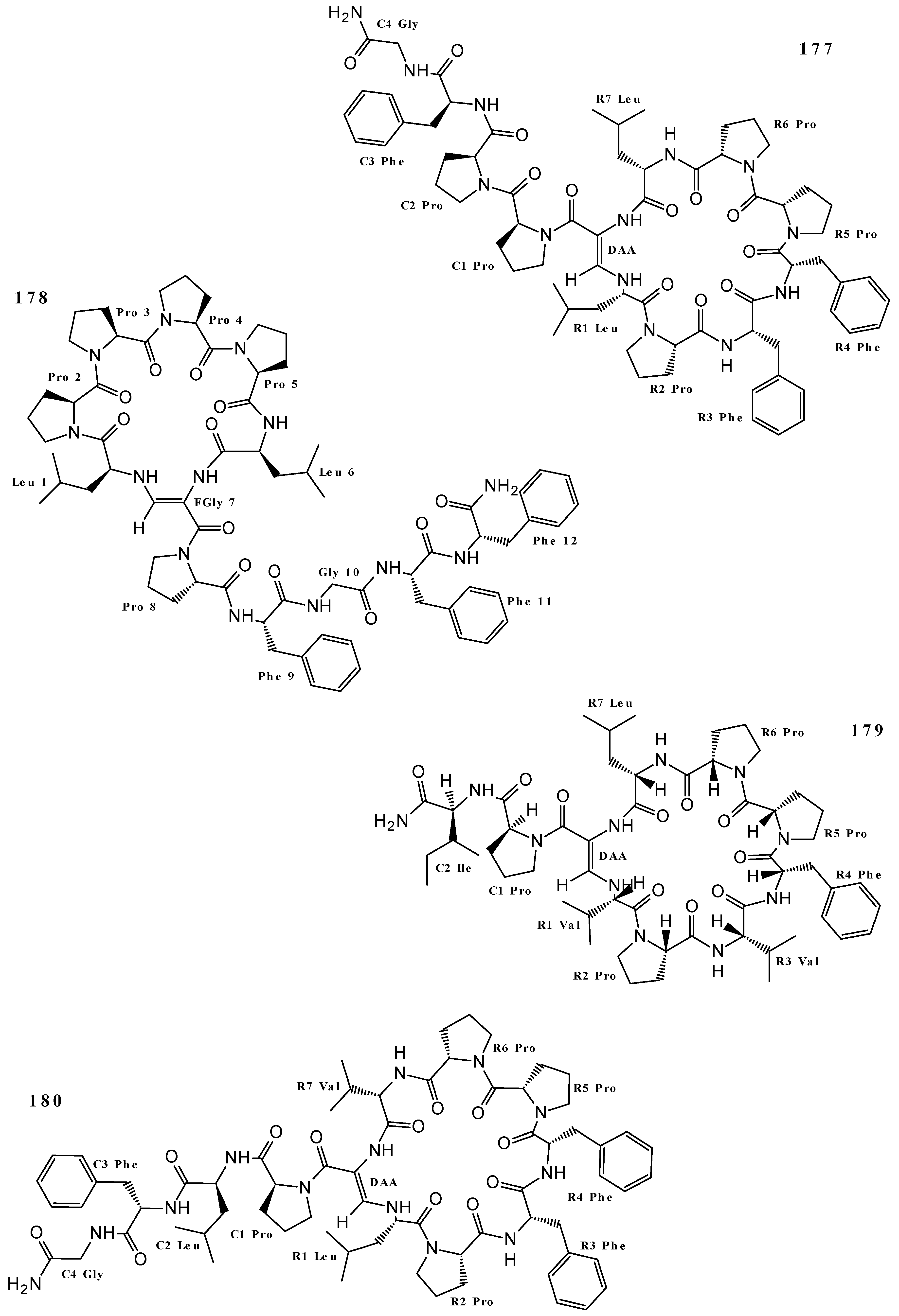
| Callyaerin H (179) | Cytotoxicity (L5178Y cell line) | [4] |
| Callyptide A (186) | Cytotoxicity {MDA-MB-231; ATCC: HTB 38, A549 (ATCC: CCL-185) and HT-29 (ATCC: HTB 38) cell lines} | [84] |
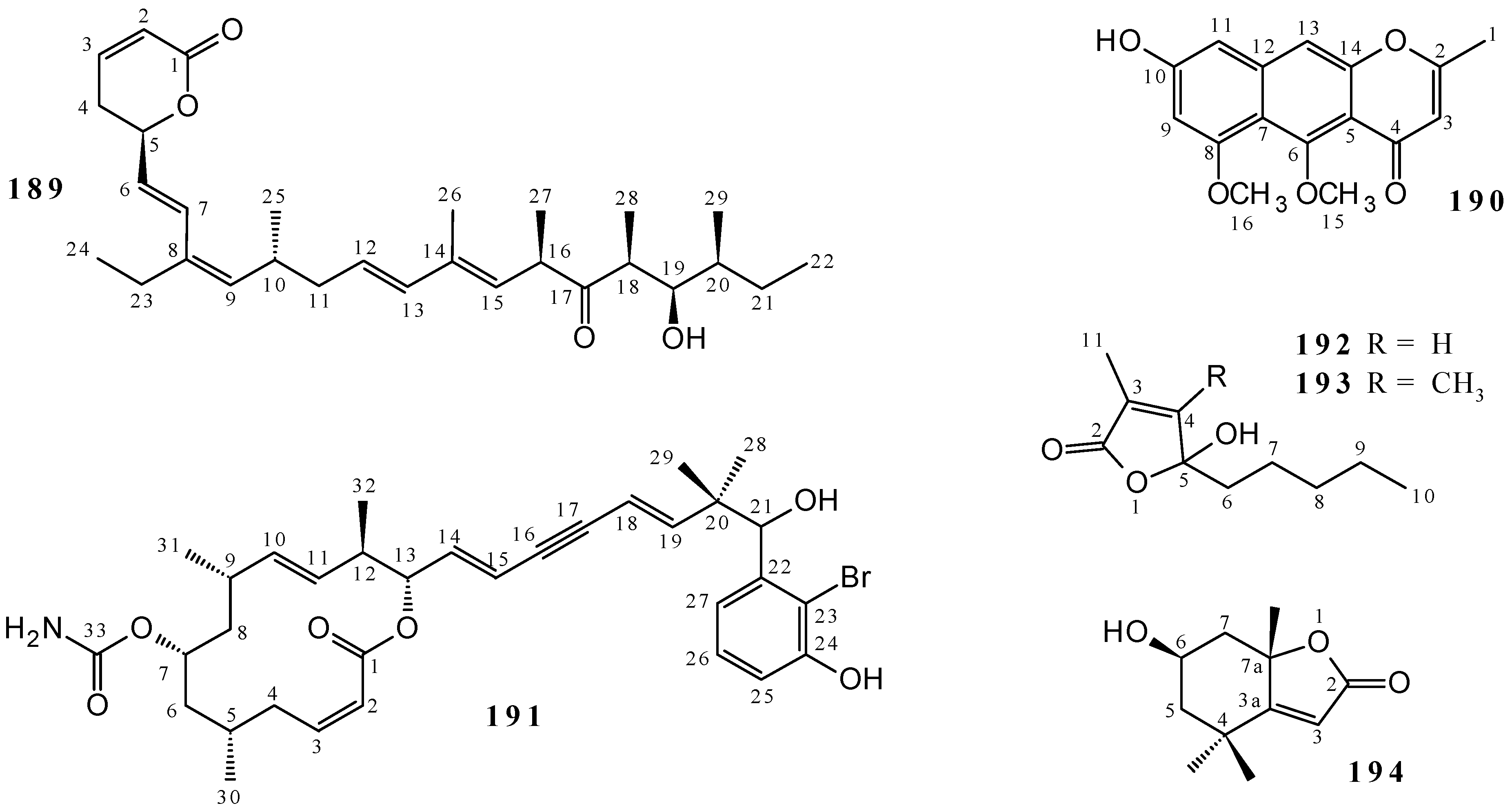
| Callystatin A (189) | Cytotoxicity (KB cell line) | [86,87] |
| Callyspongiolide (191) | Cytotoxicity (L5178Y cell line and Jurkat J16 T and Ramos B lymphocytes) | [88] |
| Inhibitor (Vacuolar ATPase) | [132] | |
| Hydroxydihydrobovolide (193) | Anti-HIV | [67,133] |
| Cytotoxicity (SH-SY5Y cell line) | [134] | |
| Plant growth inhibitor | [135] | |
| (–)-loliolide (194) | Antibacterial (Bacillus subtilis, Neisseria gonorrhoeae, Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus, Staphylococcus epidermidis, Enterobacter cloacae and Klebsiella pneumoniae) | [136,137,138] |
| Antidepressant | [138,139] | |
| Antifungal (Candida albicans and Aspergillus niger) | [137,138] | |
| Antimutagen | [138,140] | |
| Antioxidant (DPPH, H2O2 radicals and intercellular ROS) | [138,141] | |
| Cytotoxicity (L5187Y cell line) | [136,138] | |
| Germination inhibitor (lettuce and alfalfa seeds) | [138,142] | |
| Repellent for ants (Atta cephalotes) | [67,138] | |
| Callyspongidic acid C13:0 (196) | Cytotoxicity (A2058 cell line) | [12] |
| Callyspongiamide A (200) | Inhibitors of the SOAT1 and SOAT2 isozymes | [6] |
| Callyspongiamide B (201) | Inhibitors of the SOAT1 and SOAT2 isozymes | [6] |
| Bastadin 6 (202) | Anti-angiogenic activity (inhibit VEGF and bFGF of HUVECs) | [143] |
| Cytostatic and/or cytotoxic effects (L5178Y, MCF-7, A549, Hs683, U373, B16F10 and SKMEL 28) | [144,145] | |
| Bastadin 7 (203) | Cytotoxicity (L5178Y) | [145] |
| Inhibitor (the serum + hEGF-induced tubular formation of HUVEC) | [94] | |
| Bastadin 8 (204) | Inhibitor (IMPDH) | [95] |
| Bastadin 9 (205) | Cytostatic and/or cytotoxic effects (MCF-7, A549, Hs683, U373, B16F10 and SKMEL 28) | [144] |
| Bastadin 16 (206) | Cytostatic and/or cytotoxic effects (L5178Y, MCF-7, A549, Hs683, U373, B16F10 and SKMEL 28) | [144,145] |
| Bastadin 24 (208) | Cytotoxicity (CNXF SF268, LXFA 629L, MAXF 401NL, MEXF 276L and PRXF 22RV1) | [94] |
| [(3S,4Z,6S)-6-butyl-6-ethyl-4-ethylidene-1,2-dioxan-3-yl]acetic acid (209) | Cytotoxicity (P-388 cell line) | [92] |
| [(3S,4R)-6-butyl-4,6-diethyl-1,2dioxan-3-yl]acetic acid (210) | Cytotoxicity (P-388 cell line) | [92] |
| Callypyrone A (211) | Antihypertensive | [26] |
| Antioxidant | [26] | |
| Callypyrone B (212) | Antihypertensive | [26] |
| Antioxidant | [26] |
4. Discussion
5. Materials and Methods
6. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- WoRMS Editorial Board. Word Register of Marine Species. 2020. Available online: http://www.marinespecies.org (accessed on 26 November 2020).
- Desqueyroux-Faúndez, R.; Valentine, C. Family Callyspongiidae de Laubenfels, 1936. In Systema Porifera; Springer: Boston, MA, USA, 2002; pp. 835–851. [Google Scholar]
- Busutil, L.; García-Hernández, M.R.; Díaz, M.C.; Pomponi, S.A. Mesophotic sponges of the genus Callyspongia (Demospongiae, Haplosclerida) from Cuba, with the description of two new species. Zootaxa 2018, 4466, 78–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, S.R.M.; Min, C.C.; Teuscher, F.; Ebel, R.; Kakoschke, C.; Lin, W.; Wray, V.; Edrada-Ebel, R.; Proksch, P. Callyaerins A-F and H, new cytotoxic cyclic peptides from the Indonesian marine sponge Callyspongia aerizusa. Bioorg. Med. Chem. 2010, 18, 4947–4956. [Google Scholar] [CrossRef]
- Kim, C.K.; Woo, J.K.; Lee, Y.J.; Lee, H.S.; Sim, C.J.; Oh, D.C.; Oh, K.B.; Shin, J. Callyazepin and (3R)-methylazacyclodecane, nitrogenous macrocycles from a Callyspongia sp. sponge. J. Nat. Prod. 2016, 79, 1179–1183. [Google Scholar] [CrossRef] [PubMed]
- Kapojos, M.M.; Abdjul, D.B.; Yamazaki, H.; Ohshiro, T.; Rotinsulu, H.; Wewengkang, D.S.; Sumilat, D.A.; Tomoda, H.; Namikoshi, M.; Uchida, R. Callyspongiamides A and B, sterol O-acyltransferase inhibitors, from the Indonesian marine sponge Callyspongia sp. Bioorg. Med. Chem. Lett. 2018, 28, 1911–1914. [Google Scholar] [CrossRef] [PubMed]
- El-Hawary, S.S.; Sayed, A.M.; Mohammed, R.; Hassan, H.M.; Rateb, M.E.; Amin, E.; Mohammed, T.A.; El-Mesery, M.; Muhsinah, A.B.; Alsayari, A.; et al. Bioactive brominated oxindole alkaloids from the red sea sponge Callyspongia siphonella. Mar. Drugs 2019, 17, 465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobahi, T.R.A.; Ayyad, S.E.N.; Abdel-Lateff, A.; Algandaby, M.M.; Alorfi, H.S.; Abdel-Naim, A.B. Cytotoxic metabolites from Callyspongia siphonella display antiproliferative activity by inducing apoptosis in HCT-116 cells. Pharmacogn. Mag. 2017, 13, 37–40. [Google Scholar]
- Layne, T.H.; Tinto, W.F. A butenolide from the marine sponge Callyspongia vaginalis. Heterocycles 2006, 68, 2161–2164. [Google Scholar] [CrossRef]
- Araújo, R.D.; Caridade, T.N.S.; Araújo, R.M. Sulfated polysaccharide from the marine sponge Callyspongia vaginalis. Rev. Virtual Quim. 2018, 10, 1446–1454. [Google Scholar] [CrossRef]
- Gray, C.A.; Lira, S.P.; Silva, M.; Pimenta, E.F.; Thiemann, O.H.; Oliva, G.; Hajdu, E.; Andersen, R.J.; Berlinck, R.G.S. Sulfated meroterpenoids from the brazilian sponge Callyspongia sp. are inhibitors of the antileishmaniasis target adenosine phosphoribosyl transferase. J. Org. Chem. 2006, 71, 8685–8690. [Google Scholar] [CrossRef] [PubMed]
- Calabro, K.; Chalén, B.E.; Genta-Jouve, G.; Jaramillo, K.B.; Domínguez, C.; Cruz, M.; Cautain, B.; Reyes, F.; Thomas, O.P.; Rodríguez, J. Callyspongidic acids: Amphiphilic diacids from the tropical eastern pacific sponge Callyspongia cf. californica. J. Nat. Prod. 2018, 81, 2301–2305. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, R.W.M. Marine sponges from Curaçao and other Caribbean localities Part II. Haplosclerida. Stud. Fauna Curaçao Other Caribb. Isl. 1980, 62, 1–173. [Google Scholar]
- Lane, A.L.; Moore, B.S. A sea of biosynthesis: Marine natural products meet the molecular age. Nat. Prod. Rep. 2011, 28, 411–428. [Google Scholar] [CrossRef] [Green Version]
- Conte, M.; Fontana, E.; Nebbioso, A.; Altucci, L. Marine-derived secondary metabolites as promising epigenetic bio-compounds for anticancer therapy. Mar. Drugs 2021, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, W.F.; Mazzola, E.P. Nuclear magnetic resonance in the structural elucidation of natural products. In Progress in the Chemistry of Organic Natural Products; Springer: Cham, Switzerland, 2014; Volume 100, pp. 223–309. [Google Scholar]
- Urban, S.; Capon, R.J. A New lipid from an australian marine sponge, Callyspongia sp. Lipids 1997, 32, 675–677. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Tao, H.; Zhou, X.; Lin, X.P.; Liu, Y. Two new alkaloids from marine sponge Callyspongia sp. Nat. Prod. Res. 2012, 27, 1–5. [Google Scholar]
- Youssef, D.T.A.; Ibrahim, A.K.; Khalifa, S.I.; Mesbah, M.K.; Mayer, A.M.S.; Soest, R.W.M. New Anti-inflammatory sterols from the red sea sponges Scalarispongia aqabaensis and Callyspongia siphonella. Nat. Prod. Commun. 2010, 5, 27–31. [Google Scholar] [PubMed]
- Huang, R.; Zhou, X.; Peng, Y.; Yang, X.; Xu, T.; Liu, Y. Nucleosides from the marine sponge Callyspongia sp. Chem. Nat. Compd. 2011, 46, 1010–1011. [Google Scholar] [CrossRef]
- Umeyama, A.; Nagano, C.; Arihara, S. Three novel C21 polyacetylenes from the marine sponge Callyspongia sp. J. Nat. Prod. 1997, 60, 131–133. [Google Scholar] [CrossRef]
- Daletos, G.; Kalscheuer, R.; Koliwer-Brandl, H.; Hartmann, R.; Voogd, N.J.; Wray, V.; Lin, W.; Proksch, P. Callyaerins from the sarine sponge Callyspongia aerizusa: Cyclic peptides with antitubercular activity. J. Nat. Prod. 2015, 78, 1910–1925. [Google Scholar] [CrossRef]
- Rao, T.S.P.; Sarma, N.S.; Murthy, Y.L.N.; Kantamreddi, V.S.S.N.; Wright, C.W.; Parameswaran, P.S. New polyhydroxy sterols from the marine sponge Callyspongia fibrosa (Ridley & Dendly). Tetrahedron Lett. 2010, 51, 3583–3586. [Google Scholar]
- Azcuna, M.; Tun, J.O.; Yap, H.T.; Concepcion, G.P. Callyspongia samarensis (Porifera) extracts exhibit anticancer activity and induce bleaching in Porites cylindrica (Scleractinia). Chem. Ecol. 2018, 34, 397–411. [Google Scholar] [CrossRef]
- Shmueli, U.; Carmely, S.; Groweiss, A.; Kashman, Y. Sipholenol and Sipholenone, two new triterpenes from the marine sponge Siphonochalina Siphonella. Tetrahedron Lett. 1981, 22, 709–712. [Google Scholar] [CrossRef]
- Chakraborty, K.; Francis, P. Callypyrones from marine Callyspongiidae sponge Callyspongia diffusa: Antihypertensive bis-γ-pyrone polypropionates attenuate angiotensin-converting enzyme. Nat. Prod. Res. 2020, 1–12. [Google Scholar] [CrossRef]
- Youssef, D.T.A.; Yoshida, W.Y.; Kelly, M.; Scheuer, P.J. Polyacetylenes from a red sea sponge Callyspongia species. J. Nat. Prod. 2000, 63, 1406–1410. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, U.R.; Cheng, C.; Reimer, A.; Kozjak-Pavlovic, V.; Ibrahim, A.K.; Rudel, T.; Hentschel, U.; Edrada-Ebel, R.; Ahmed, S.A. Antichlamydial sterol from the red sea sponge Callyspongia aff implexa. Planta Med. 2015, 81, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, S.; Kato, H.; Hirota, H.; Fusetani, N. Seven new polyacetylene derivatives, showing both potent metamorphosis-inducing activity in ascidian larvae and antifouling activity against barnacle larvae, from the marine Sponge Callyspongia truncata. J. Nat. Prod. 1997, 60, 126–130. [Google Scholar] [CrossRef]
- Miao, S.; Andersen, R.J. Callydiyne, a new diacetylenic hydrocarbon from the sponge Callyspongla flammea. J. Nat. Prod. 1991, 54, 1433–1434. [Google Scholar] [CrossRef]
- Umeyama, A.; Matsuoka, N.; Mine, R.; Nakata, A.; Arimoto, E.; Matsui, M.; Shoji, N.; Arihara, S.; Takei, M.; Hashimoto, T. Polyacetylene diols with antiproliferative and driving Th1 polarization effects from the marine sponge Callyspongia sp. J. Nat. Med. 2010, 64, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Fusetani, N.; Sugano, M.; Matsunaga, S.; Hashimoto, K. H,K-atpase inhibitors from the marine sponge Siphonochalina truncata: Absolute configuration of siphonodiol and two related metabolites. Tetrahedron Lett. 1987, 28, 4311–4312. [Google Scholar] [CrossRef]
- Tada, H.; Yasuda, F. Siphonodiol, a new polyacetylenic metabolite from the sponge Siphonochalina truncate. Chem. Lett. 1984, 13, 779–780. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Devijver, C.; Dubut, D.; Soest, R.W.M. A new C-20 polyacetylene from the sponge Callyspongia pseudoreticulata. J. Nat. Prod. 2003, 66, 871–872. [Google Scholar] [CrossRef] [PubMed]
- Shirouzu, T.; Watari, K.; Ono, M.; Koizumi, K.; Saiki, I.; Tanaka, C.; Soest, R.W.M.; Miyamoto, T. Structure, synthesis, and biological activity of a C-20 bisacetylenic alcohol from a marine sponge Callyspongia sp. J. Nat. Prod. 2013, 76, 1337–1342. [Google Scholar] [CrossRef] [PubMed]
- Ayyad, S.E.N.; Angawy, R.; Alarif, W.M.; Saqer, E.A.; Badria, F.A. Cytotoxic polyacetylenes from the red sea sponge Siphonochalina siphonella. Z. Nat. C 2014, 69, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Balansa, W.; Trianto, A.; Voogd, N.J.; Tanaka, J. A new cytotoxic polyacetylenic alcohol from a sponge Callyspongia sp. Nat. Prod. Commun. 2017, 12, 1909–1911. [Google Scholar] [CrossRef] [Green Version]
- Youssef, D.T.A.; Soest, R.W.M.; Fusetani, N. Callyspongenols A-C, new cytotoxic C22-polyacetylenic alcohols from a red sea sponge, Callyspongia species. J. Nat. Prod. 2003, 66, 679–681. [Google Scholar] [CrossRef]
- Chiu, C.W.; Su, H.J.; Lu, M.C.; Wang, W.H.; Sheu, J.H.; Su, J.H. Cytotoxic polyacetylenes from a formosan marine sponge Callyspongia sp. Bull. Chem. Soc. Jpn. 2014, 87, 1231–1234. [Google Scholar] [CrossRef]
- Ki, D.W.; El-Desoky, A.H.; Wong, C.P.; Abdel-Ghani, M.; El-Beih, A.A.; Mizuguchi, M.; Morita, H. New cytotoxic polyacetylene alcohols from the egyptian marine sponge Siphonochalina siphonella. J. Nat. Med. 2020, 74, 409–414. [Google Scholar] [CrossRef]
- Fujita, M.; Nakao, Y.; Matsunaga, S.; Soest, R.W.M.; Itoh, Y.; Seiki, M.; Fusetani, N. Callysponginol sulfate A, an MT1-MMP inhibitor isolated from the marine sponge Callyspongia truncata. J. Nat. Prod. 2003, 66, 569–571. [Google Scholar] [CrossRef] [PubMed]
- Uno, M.; Ohta, S.; Ohta, E.; Ikegami, S. Callyspongins A and B: Novel polyacetylene sulfates from the marine sponge Callyspongia truncata that inhibit fertilization of starfish gametes. J. Nat. Prod. 1996, 59, 1146–1148. [Google Scholar] [CrossRef]
- Rooney, F.; Capon, R.J. Callyspongynes A and B: New polyacetylenic lipids from a southern Australian marine sponge, Callyspongia sp. Lipids 1998, 33, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Uehara, T.; Matunaga, S.; Fusetani, N.; Soest, R.W.M. Callyspongynic acid, a polyacetylenic acid which inhibits α-glucosidase, from the marine sponge Callyspongia truncata. J. Nat. Prod. 2002, 65, 922–924. [Google Scholar] [CrossRef]
- Xiao-Jian, L.; Shi-Hai, X.; Qi-Chang, H.; Dong-Hong, H. Studies on chemical constituents from Callyspongia fibrosa. Chin. J. Spectrosc. Lab. 2005, 22, 281–283. [Google Scholar]
- Youssef, D.T.A.; Soest, R.W.M.; Fusetani, N. Callyspongamide A, a new cytotoxic polyacetylenic amide from the red sea sponge Callyspongia fistularis. J. Nat. Prod. 2003, 66, 861–862. [Google Scholar] [CrossRef] [PubMed]
- Rotem, M.; Kashman, Y. New polyacetylenes from the sponge Siphonochalina sp. Tetrahedron Lett. 1979, 20, 3193–3196. [Google Scholar] [CrossRef]
- Delbeke, E.I.P.; Everaert, J.; Uitterhaegen, E.; Verweire, S.; Verlee, A.; Talou, T.; Soetaert, W.; Bogaert, I.N.A.; Stevens, C.V. Petroselinic acid purification and its use for the fermentation of new sophorolipids. AMB Express 2016, 6, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, H.S.; Agraval, S. Callyspinol, a new diterpene from sponge Callyspongia spinossima. Tetrahedron Lett. 1995, 36, 9035–9038. [Google Scholar] [CrossRef]
- Kurnianda, V.; Faradilla, S.; Karina, S.; Agustina, S.; Ulfah, M.; Octavina, C.; Syahliza, F.; Ramadhan, M.R.; Purnawan, S.; Musman, M. Polyoxygenated diterpene produced by the indonesian marine sponge Callyspongia sp. as an inhibitor of the human pancreatic cancer cells. Microbiol. Indones. 2019, 13, 70–74. [Google Scholar] [CrossRef]
- Fukami, A.; Ikeda, Y.; Kondo, S.; Naganawa, H.; Takeuchi, T.; Furuya, S.; Hirabayashi, Y.; Shimoike, K.; Hosaka, S.; Watanabe, Y.; et al. Akaterpin, a novel bioactive triterpene from the marine sponge Callyspongia sp. Tetrahedron Lett. 1997, 38, 1201–1202. [Google Scholar] [CrossRef]
- Jain, S.; Abraham, I.; Carvalho, P.; Kuang, Y.H.; Shaala, L.A.; Youssef, D.T.A.; Avery, M.A.; Chen, Z.S.; Sayed, K.A. Sipholane triterpenoids: Chemistry, reversal of ABCB1/P-glycoprotein-mediated multidrug resistance, and pharmacophore modeling. J. Nat. Prod. 2009, 72, 1291–1298. [Google Scholar] [CrossRef]
- Kashman, Y.; Yosief, T.; Carmeli, S. New triterpenoids from the red sea sponge Siphonochalina siphonella. J. Nat. Prod. 2001, 64, 175–180. [Google Scholar] [CrossRef]
- Carmely, S.; Kashman, Y. Neviotine-A, a new triterpene from the red sea sponge Siphonochalina siphonella. J. Org. Chem. 1986, 51, 784–788. [Google Scholar] [CrossRef]
- Ayyad, S.E.N.; Angawi, R.F.; Saqer, E.; Abdel-Lateff, A.; Badria, F.A. Cytotoxic neviotane triterpene-type from the red sea sponge Siphonochalina siphonella. Pharmacogn. Mag. 2014, 10, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Massarani, S.M.; El-Gamal, A.A.; Al-Said, M.S.; Al-Lihaibi, S.S.; Basoudan, O.A. In vitro cytotoxic, antibacterial and antiviral activities of triterpenes from the red sea sponge, Siphonochalina siphonella. Trop. J. Pharm. Res. 2015, 14, 33–40. [Google Scholar] [CrossRef] [Green Version]
- El-Beih, A.A.; El-Desoky, A.H.; Al-hammady, M.A.; Elshamy, A.I.; Hegazy, M.E.F.; Kato, H.; Tsukamoto, S. New inhibitors of RANKL-induced osteoclastogenesis from the marine sponge Siphonochalina siphonella. Fitoterapia 2018, 128, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Shirode, A.; Yacoub, S.; Barbo, A.; Sylvester, P.W.; Huntimer, E.; Halaweish, F.; Sayed, K.A. Biocatalysis of the anticancer sipholane triterpenoids. Planta Med. 2007, 73, 591–596. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Laphookhieo, S.; Shi, Z.; Fu, L.W.; Akiyama, S.I.; Chen, Z.S.; Youssef, D.T.A.; Soest, R.W.M.; Sayed, K.A. Reversal of P-glycoprotein-mediated multidrug resistance by sipholane triterpenoids. J. Nat. Prod. 2007, 70, 928–931. [Google Scholar] [CrossRef] [PubMed]
- Al-Lhaibi, S.S.; Abdel-Lateff, A.; Alarif, W.M.; Nogata, Y.; Ayyad, S.E.N.; Okino, T. Potent antifouling metabolites from red sea organisms. Asian J. Chem. 2015, 27, 2252–2256. [Google Scholar] [CrossRef]
- Carmely, S.; Kashman, Y. The sipholanes: A novel group of triterpenes from the marine sponge Siphonochalina siphonella. J. Org. Chem. 1983, 48, 3517–3525. [Google Scholar] [CrossRef]
- Carmely, S.; Loya, Y.; Kashman, Y. Siphonellinol, a new triterpene from the marine sponge Siphonochalina siphonella. Tetrahedron Lett. 1983, 24, 3673–3676. [Google Scholar] [CrossRef]
- Alam, P.; Alqahtani, A.S.; Husain, F.M.; Rehman, M.T.; Alajmi, M.F.; Noman, O.M.; Gamal, A.A.; Al-Massarani, S.M.; Khan, M.S. Siphonocholin isolated from red sea sponge Siphonochalina siphonella attenuates quorum sensing controlled virulence and biofilm formation. Saudi Pharm. J. 2020, 28, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Carmely, S.; Kashman, Y. The study of sipholanes by two-dimensional NMR spectroscopy. Magn. Reson. Chem. 1986, 24, 332–336. [Google Scholar] [CrossRef]
- Plisson, F.; Prasad, P.; Xiao, X.; Piggott, A.M.; Huang, X.C.; Khalil, Z.; Capon, R.J. Callyspongisines A-D: Bromopyrrole alkaloids from an australian marine sponge, Callyspongia sp. Org. Biomol. Chem. 2014, 12, 1579–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Huang, J.; Lin, X.; Zhang, Y.; Tao, H.; Liu, Y. A new diketopiperazine from the marine sponge Callyspongia species. Rec. Nat. Prod. 2016, 10, 117–121. [Google Scholar]
- Yang, B.; Hu, J.; Lei, H.; Chen, X.Q.; Zhou, X.F.; Liu, Y.H. Chemical constituents of marine sponge Callyspongia sp. from the south China sea. Chem. Nat. Compd. 2012, 48, 350–351. [Google Scholar] [CrossRef]
- Wang, G.Y.S.; Kuramoto, M.; Uemura, D.; Yamada, A.; Yamaguchi, K.; Yazawa, K. Three Novel Anti-mierofouling nitroalkyl pyridine alkaloids from the Okinawan marine sponge Callyspongia sp. Tetrahedron Lett. 1996, 37, 1813–1816. [Google Scholar] [CrossRef]
- Buchanan, M.S.; Carroll, A.R.; Addepalli, R.; Avery, V.M.; Hooper, J.N.A.; Quinn, R.J. Niphatoxin C, a cytotoxic tripyridine alkaloid from Callyspongia sp. J. Nat. Prod. 2007, 70, 2040–2041. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.M.; Ma, W.; Dong, J.D.; Zhou, X.F.; Xu, T.; Lee, K.J.; Yang, X.; Xu, S.H.; Liu, Y. A new 1,4-diazepine from south China sea marine sponge Callyspongia species. Molecules 2010, 15, 871–877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Dong, J.; Zhou, X.; Yang, X.; Lee, K.J.; Wang, L.; Zhang, S.; Liu, Y. Proline-containing dipeptides from a marine sponge of a Callyspongia Species. Helv. Chim. Acta. 2009, 92, 1112–1117. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, Y.; Gao, C.; Huang, R. A new diketopiperazine from South China Sea marine sponge Callyspongia sp. Nat. Prod. Res. 2014, 28, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Sperry, S.; Crews, P. A novel alkaloid from the indo-pacific sponge Clathria basilana. Tetrahedron Lett. 1996, 37, 2389–2390. [Google Scholar] [CrossRef]
- Gopichand, Y.; Schmitz, F.J. Two novel lactams from the marine sponge Halichondria melanodocia. J. Org. Chem. 1979, 44, 4995–4997. [Google Scholar] [CrossRef]
- Jayatilake, G.S.; Thornton, M.P.; Leonard, A.C.; Grimwade, J.E.; Baker, B.J. Metabolites from an antarctic sponge-associated bacterium, Pseudomonas aeruginosa. J. Nat. Prod. 1996, 59, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Adamczeski, M.; Reed, A.R.; Crews, P. New and known diketopiperazines from the Caribbean sponge, Calyx cf. Podatypa. J. Nat. Prod. 1995, 58, 201–208. [Google Scholar] [CrossRef]
- Gautschi, M.; Schmid, J.P.; Peppard, T.L.; Ryan, T.P.; Tuorto, R.M.; Yang, X. Chemical characterization of diketopiperazines in beer. J. Agric. Food Chem. 1997, 45, 3183–3189. [Google Scholar] [CrossRef]
- Stark, T.; Hofmann, T. Structures, sensory activity, and dose/response functions of 2,5-diketopiperazines in roasted cocoa nibs (Theobroma cacao). J. Agric. Food Chem. 2005, 53, 7222–7231. [Google Scholar] [CrossRef]
- Fdhila, F.; Vázquez, V.; Sánchez, J.L.; Riguera, R. DD-diketopiperazines: Antibiotics active against Vibrio anguillarum isolated from marine bacteria associated with cultures of Pecten maximus. J. Nat. Prod. 2003, 66, 1299–1301. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.W.; Feng, Y.; Shimizu, Y.; Pfeifer, T.A.; Wellington, C.; Hooper, J.N.A.; Quinn, R.J. ApoE secretion modulating bromotyrosine derivative from the australian marine sponge Callyspongia sp. Bioorg. Med. Chem. Lett. 2014, 24, 3537–3540. [Google Scholar] [CrossRef]
- Weller, D.D.; Stirchak, E.P.; Yokoyama, A. Preparation of oxygenated phenylacetic acids. J. Org. Chem. 1984, 49, 2061–2063. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Edrada-Ebel, R.A.; Mohamed, G.A.; Youssef, D.T.A.; Wray, V.; Proksch, P. Callyaerin G, a new cytotoxic cyclic peptide from the marine sponge Callyspongia aerizusa. Arkivoc 2008, 12, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Berer, N.; Rudi, A.; Goldberg, I.; Benayahu, Y.; Kashman, Y. Callynormine A, a new marine cyclic peptide of a novel class. Org. Lett. 2004, 6, 2543–2545. [Google Scholar] [CrossRef] [PubMed]
- Shaala, L.A.; Youssef, D.T.A.; Ibrahim, S.R.M.; Mohamed, G.A. Callyptide A, a new cytotoxic peptide from the red sea marine sponge Callyspongia species. Nat. Prod. Res. 2016, 30, 2783–2790. [Google Scholar] [CrossRef] [PubMed]
- Capon, R.J.; Ford, J.; Lacey, E.; Gill, J.H.; Heiland, K.; Friedel, T. Phoriospongin A and B: Two new nematocidal depsipeptides from the Australian marine sponges Phoriospongia sp. and Callyspongia bilamellata. J. Nat. Prod. 2002, 65, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Higuchi, K.; Murakami, N.; Tajima, H.; Aoki, S. Callystatin A, a potent cytotoxic polyketide from the marine sponge, Callyspongia truncata. Tetrahedron Lett. 1997, 38, 2859–2862. [Google Scholar] [CrossRef]
- Murakami, N.; Wang, W.; Aoki, M.; Tsutsui, Y.; Higuchi, K.; Aoki, S.; Kobayashi, M. Absolute stereostructure of Callystatin A, a potent cytotoxic polyketide from the marine sponge, Callyspongia truncata. Tetrahedron Lett. 1997, 38, 5533–5536. [Google Scholar] [CrossRef]
- Pham, C.D.; Hartmann, R.; Böhler, P.; Stork, B.; Wesselborg, S.; Lin, W.; Lai, D.; Proksch, P. Callyspongiolide, a cytotoxic macrolide from the marine sponge Callyspongia sp. Org. Lett. 2014, 16, 266–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshino, H.; Yoshihara, T.; Sakamura, S.; Shimanuki, T.; Sato, T.; Tajimi, A. Novel C-11 epoxy fatty acid from stromata of Epichloe typhina on Phleum pratense. Agric. Biol. Chem. 1989, 53, 2527–2528. [Google Scholar] [CrossRef]
- Mori, K.; Khlebnikov, V. Synthesis of (+)-dihydroactinidiolide, (+)- and (−)-actinidiolide, (+)- and (−)-loliolide as well as (+)- and (−)-epiloliolide. Liebigs Ann. Chem. 1993, 21, 77–82. [Google Scholar] [CrossRef]
- Huang, R.; Chen, Y.; Zhou, X.; Yang, X.; Liu, Y. A new n-acyl taurine from the South China Sea marine sponge Callyspongia sp. Chem. Nat. Compd. 2015, 51, 540–541. [Google Scholar] [CrossRef] [Green Version]
- Toth, S.I.; Schmitz, F.J. Two new cytotoxic peroxide-containing acids from a new guinea sponge, Callyspongia sp. J. Nat. Prod. 1994, 57, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskas, R.; Lidgard, R.O.; Murphy, P.T.; Wells, R.J.; Blount, J.F. Brominated tyrosine-derived metabolites from the sponge Ianthella basta. Aust. J. Chem. 1981, 34, 765–786. [Google Scholar] [CrossRef]
- Greve, H.; Kehraus, S.; Krick, A.; Kelter, G.; Maier, A.; Fiebig, H.H.; Wright, A.D.; Konig, G.M. Cytotoxic bastadin 24 from the australian sponge Ianthella quadrangulata. J. Nat. Prod. 2008, 71, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Jaspars, M.; Rali, T.; Laney, M.; Schatzman, R.C.; Diaz, M.C.; Schmitz, F.J.; Pordesimo, E.O.; Crews, P. The Search for inosine 5’-phosphate dehydrogenase (IMPDH) inhibitors from marine sponges. Evaluation of the bastadin alkaloids. Tetrahedron 1994, 50, 7367–7374. [Google Scholar] [CrossRef]
- López, S.; Fernández-Trillo, F.; Midón, P.; Castedo, L.; Saá, C. First stereoselective syntheses of (−)-siphonodiol and (−)-tetrahydrosiphonodiol, bioactive polyacetylenes from marine sponges. J. Org. Chem. 2005, 70, 6346–6352. [Google Scholar] [CrossRef] [PubMed]
- Díaz, Y.M.; Laverde, G.V.; Gamba, L.R.; Wandurraga, H.M.; Arévalo-Ferro, C.; Rodríguez, F.R.; Beltrán, C.D.; Hernández, L.C. Biofilm inhibition activity of compounds isolated from two Eunicea species collected at the Caribbean Sea. Rev. Bras. Farmacogn. 2015, 25, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Shi, Z.; Jain, S.; Kim, I.W.; Peng, X.X.; Abraham, I.; Youssef, D.T.A.; Fu, L.W.; Sayed, K.; Ambudkar, S.V.; Chen, Z.S. Sipholenol A, a marine-derived sipholane triterpene, potently reverses P-glycoprotein (ABCB1)-mediated multidrug resistance in cancer cells. Cancer Sci. 2007, 98, 1373–1380. [Google Scholar] [CrossRef]
- Ma, J.; Fu, G.; Wu, J.; Han, S.; Zhang, L.; Yang, M.; Yu, Y.; Zhang, M.; Lin, Y.; Wang, Y. 4-cholesten-3-one suppresses lung adenocarcinoma metastasis by regulating translocation of HMGB1, HIF1α and Caveolin-1. Cell Death Dis. 2016, 7, e2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villaseñor, I.M.; Angelada, J.; Canlas, A.P.; Echegoyen, D. Bioactivity studies on β-sitosterol and its glucoside. Phytother. Res. 2002, 16, 417–421. [Google Scholar] [CrossRef]
- Choi, S.; Kim, K.W.; Choi, J.S.; Han, S.T.; Park, Y.I.; Lee, S.K.; Kim, J.S.; Chung, M.H. Angiogenic activity of β-sitosterol in the ischaemia/reperfusion-damaged brain of mongolian gerbil. Planta Med. 2002, 68, 330–335. [Google Scholar] [CrossRef]
- Beltrame, F.L.; Pessini, G.L.; Doro, D.L.; Filho, B.P.D.; Bazotte, R.B.; Cortez, D.A.G. Evaluation of the antidiabetic and antibacterial activity of Cissus sicyoides. Braz. Arch. Biol. Technol. 2002, 45, 21–25. [Google Scholar] [CrossRef]
- Sen, A.; Dhavan, P.; Shukla, K.K.; Singh, S.; Tejovathi, G. Analysis of IR, NMR and antimicrobial activity of β-sitosterol isolated from Momordica charantia. Sci. Secur. J. Biotech. 2012, 1, 9–13. [Google Scholar]
- Kiprono, P.C.; Kaberia, F.; Keriko, J.M.; Karanja, J.N. The in vitro Anti-fungal and anti-bacterial activities of β-sitosterol from Senecio lyratus (Asteraceae). Verl. Z. Nat. 2000, 55, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Zeb, M.A.; Khan, S.U.; Rahman, T.U.; Sajid, M.; Seloni, S. Isolation and biological activity of β-sitosterol and stigmasterol from the roots of Indigofera heterantha. Pharm. Pharmacol. Int. J. 2017, 5, 204–207. [Google Scholar]
- Nirmal, S.A.; Pal, S.C.; Mandal, S.C.; Patil, A.N. Analgesic and anti-inflammatory activity of β-sitosterol isolated from Nyctanthes arbortristis leaves. Inflammopharmacology 2012, 20, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.B.; Nath, R.; Srivastava, N.; Shanker, K.; Kishor, K.; Bhargava, K.P. Anti-inflammatory and antipyretic activities of β-sitosterol. Planta Med. 1980, 39, 157–163. [Google Scholar] [CrossRef]
- Loizou, S.; Lekakis, I.; Chrousos, G.P.; Moutsatsou, P. β-Sitosterol exhibits anti-inflammatory activity in human aortic endothelial cells. Mol. Nutr. Food Res. 2010, 54, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Chai, J.W.; Kuppusamy, U.R.; Kanthimathi, M.S. Beta-sitosterol induces apoptosis in MCF-7 Cells. Malays. J. Biochem. Mol. Biol. 2008, 16, 28–30. [Google Scholar]
- Awad, A.B.; Holtz, R.L.; Cone, J.P.; Fink, C.S.; Chen, Y.C. Beta-sitosterol inhibits growth of HT-29 human colon cancer cells by activating the sphingomyelin cycle. Anticancer Res. 1998, 18, 471–473. [Google Scholar]
- Park, C.; Moon, D.O.; Rhu, C.H.; Choi, B.T.; Lee, W.H.; Kim, G.Y.; Choi, Y.H. β-sitosterol induces anti-proliferation and apoptosis in human leukemic U937 cells through activation of caspase-3 and induction of Bax/Bcl-2 ratio. Biol. Pharm. Bull. 2007, 30, 1317–1323. [Google Scholar] [CrossRef] [Green Version]
- Vundru, S.S.; Kale, R.K.; Singh, R.P. β-sitosterol induces G1 arrest and causes depolarization of mitochondrial membrane potential in breast carcinoma MDA-MB-231 cells. BMC Complement. Altern. Med. 2013, 13, 280. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chang, S.K.C.; Qu, G.; Li, T.; Cui, H. β-sitosterol inhibits cell growth and induces apoptosis in SGC-7901 human stomach cancer cells. J. Agric. Food Chem. 2009, 57, 5211–5218. [Google Scholar] [CrossRef]
- Holtz, R.L.; Fink, C.S.; Awad, A.B. β-sitosterol activates the sphingomyelin cycle and induces apoptosis in LNCaP human prostate cancer cells. Nutr. Cancer 1998, 32, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Sugano, M.; Morioka, H.; Ikeda, I. A Comparison of hypocholesterolemic activity of β-sitosterol and β-sitostanol in rats. J. Nutr. 1977, 107, 2011–2019. [Google Scholar] [CrossRef]
- Fraile, L.; Crisci, E.; Córdoba, L.; Navarro, M.A.; Osada, J.; Montoya, M. Immunomodulatory properties of beta-sitosterol in pig immune responses. Int. Immunopharmacol. 2012, 13, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Izumida, M.; Suga, K.; Ishibashi, F.; Kubo, Y. The spirocyclic imine from a marine benthic dinoflagellate, portimine, is a potent anti-human immunodeficiency virus type 1 therapeutic lead compound. Mar. Drugs 2019, 17, 495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tasdemir, D.; Mallon, R.; Greenstein, M.; Feldberg, L.R.; Kim, S.C.; Collins, K.; Wojciechowicz, D.; Mangalindan, G.C.; Concepcio, G.P.; Harper, M.K.; et al. Aldisine alkaloids from the philippine sponge Stylissa massa are potent inhibitors of mitogen-activated protein kinase kinase-1 (MEK-1). J. Med. Chem. 2002, 45, 529–532. [Google Scholar] [CrossRef]
- Ebada, S.S.; Linh, M.H.; Longeon, A.; Voogd, N.J.; Durieu, E.; Meijer, L.; Bourguet-Kondracki, M.L.; Singab, A.N.B.; Müller, W.E.G.; Proksch, P. Dispacamide E and other bioactive bromopyrrole alkaloids from two Indonesian marine sponges of the genus Stylissa. Nat. Prod. Res. 2015, 29, 231–238. [Google Scholar] [CrossRef]
- Meijer, L.; Thunnissen, A.M.W.H.; White, A.W.; Garnier, M.; Nikolic, M.; Tsai, L.H.; Walter, J.; Cleverley, K.E.; Salinas, P.C.; Wu, Y.Z.; et al. Inhibition of cyclin-dependent kinases, GSK-3β and CK1 by hymenialdisine, a marine sponge constituent. Chem. Biol. 2000, 7, 51–63. [Google Scholar] [CrossRef]
- Andrioli, W.J.; Santos, M.S.; Silva, V.B.; Oliveira, R.B.; Chagas-Paula, D.A.; Jorge, J.A.; Furtado, N.A.J.C.; Pupo, M.T.; Silva, C.H.T.P.; Naal, R.M.Z.G.; et al. δ-Lactam derivative from thermophilic soil fungus exhibits in vitro anti-allergic activity. Nat. Prod. Res. 2012, 26, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Yoshida, J.; Ide, N.; Sasaoka, T.; Yamaguchi, H.; Ono, K. Tetrahydro-β-carboline derivatives in aged garlic extract show antioxidant properties. J. Nutr. 2006, 136, 726–731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, K.; Geng, X.; Hashiguchi, M.; Suhara, H.; Fukunaga, S.; Yasutake, S.; Kondo, R.; Tsutsui, M.; Sato, I. Indole-3-carbaldehyde: A tyrosinase inhibitor from fungus YL185. J. Wood Sci. 2003, 49, 349–354. [Google Scholar] [CrossRef]
- Zeng, M.; Li, M.; Li, M.; Zhang, B.; Li, B.; Zhang, L.; Feng, W.; Zheng, X. 2-Phenylacetamide isolated from the seeds of Lepidium apetalum and its estrogen-like effects in vitro and in vivo. Molecules 2018, 23, 2293. [Google Scholar] [CrossRef] [Green Version]
- Takai, M.; Miyamoto, S.; Hattori, Y.; Tamura, S. Isolation of 2-phenylacetamide as a plant growth regulator produced by Actinomyces. Agric. Biol. Chem. 1963, 27, 876–877. [Google Scholar] [CrossRef]
- Cho, J.Y.; Moon, J.H.; Seong, K.Y.; Park, K.H. Antimicrobial Activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci. Biotechnol. Biochem. 1998, 62, 2273–2276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chong, K.P.; Rossall, S.; Atong, M. In vitro antimicrobial activity and fungitoxicity of syringic acid, caffeic acid and 4-hydroxybenzoic acid against Ganoderma Boninense. J. Agric. Sci. 2009, 1, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Peungvicha, P.; Temsiririrkkul, R.; Prasain, J.K.; Tezuka, Y.; Kadota, S.; Thirawarapan, S.S.; Watanabe, H. 4-hydroxybenzoic acid: A hypoglycemic constituent of aqueous extract of Pandanus odorus root. J. Ethnopharmacol. 1998, 62, 79–84. [Google Scholar] [CrossRef]
- Iizuka, H.; Adachi, R.; Koizumi, H.; Aoyagi, T.; Ohkawara, A.; Miura, Y. Effects of adenosine and 2’-deoxyadenosine on epidermal keratinocyte proliferation: Its relation to cyclic AMP formation. J. Investig. Dermatol. 1984, 82, 608–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.; Crossland, W.J.; Kulkarni, J.S.; Wakade, T.D.; Wakade, A.R. 2’-Deoxyadenosine causes cell death in embryonic chicken sympathetic ganglia and brain. Cell Tissue Res. 1999, 296, 281–291. [Google Scholar] [CrossRef]
- Zhang, S.; Rodriguez, L.M.L.; Leung, I.K.H.; Cook, G.M.; Harris, P.W.R.; Brimble, M.A. Total synthesis and conformational study of the anti-tubercular cyclic peptide callyaerin a bearing a rare rigidifying (z)-2,3- diaminoacrylamide moiety. Angew. Chem. Int. Ed. 2018, 57, 3631–3635. [Google Scholar] [CrossRef]
- Fogarty, S.; Ouyang, Y.; Li, L.; Chen, Y.C.; Rane, H.; Manoni, F.; Parra, K.J.; Rutter, J.; Harran, P.G. Callyspongiolide is a potent inhibitor of the vacuolar ATPase. J. Nat. Prod. 2020, 83, 3381–3386. [Google Scholar] [CrossRef]
- Zhang, H.J.; Hung, N.; Cuong, N.M.; Soejarto, D.D.; Pezzuto, J.M.; Fong, H.H.S.; Tan, G.T. Sesquiterpenes and butenolides, natural anti-HIV constituents from Litsea verticillata. Planta Med. 2005, 71, 452–457. [Google Scholar] [CrossRef]
- Xu, L.; Tao, X.; Gao, Y.; Zhang, W.; Meng, Y.; Li, C.; Jiang, M.; Ying, X. Cytotoxicity of hydroxydihydrobovolide and its pharmacokinetic studies in Portulaca oleracea L. extract. Braz. J. Pharm. Sci. 2017, 53, 1–9. [Google Scholar] [CrossRef]
- Hasegawa, T.; Yamada, K.; Shigemori, H.; Hasegava, K.; Miyamoto, K.; Ueda, J. Isolation and identification of a growth inhibitor from blue light-illuminated cress seedlings. Plant Growth Regul. 2002, 37, 45–47. [Google Scholar] [CrossRef]
- Elkhayat, E.S. Cytotoxic and antibacterial constituents from the roots of Sonchus oleraceus L. growing in Egypt. Pharmacogn. Mag. 2009, 5, 324–328. [Google Scholar] [CrossRef]
- Zajdel, S.M.; Graikou, K.; Głowniak, K.; Chinou, I. Chemical analysis of Penstemon campanulatus (Cav.) Willd.–Antimicrobial activities. Fitoterapia 2012, 83, 373–376. [Google Scholar] [CrossRef] [PubMed]
- Grabarczyk, M.; Wińska, K.; Mączka, W.; Potaniec, B.; Anioł, M. Loliolide–the most ubiquitous lactone. Folia Biol. Oecologica 2015, 11, 1–8. [Google Scholar] [CrossRef]
- Neergaard, J.S.; Rasmussen, H.B.; Stafford, G.I.; Staden, J.; Jäger, A.K. Serotonin transporter affinity of (−)-loliolide, a monoterpene lactone from Mondia whitei. S. Afr. J. Bot. 2010, 76, 593–596. [Google Scholar] [CrossRef] [Green Version]
- Ragasa, C.Y.; Agbayani, V.; Hernández, R.B.; Rideout, J.A. An Antimutagenic monoterpene from Malachra Fasciata (Malvaceae). Philipp. J. Sci. 1997, 126, 183–189. [Google Scholar]
- Yang, X.; Kang, M.C.; Lee, K.W.; Kang, S.M.; Lee, W.W.; Jeon, Y.J. Antioxidant activity and cell protective effect of loliolide isolated from Sargassum ringgoldianum subsp. coreanum. Algae 2011, 26, 201–208. [Google Scholar] [CrossRef]
- Kuniyoshi, M. Germination inhibitors from the brown alga Sargassum crassifolium (Phaeophyta, Sargassaceae). Bot. Mar. 1985, 28, 501–504. [Google Scholar] [CrossRef]
- Aoki, S.; Cho, S.H.; Ono, M.; Kuwano, T.; Nakao, S.; Kuwano, M.; Nakagawa, S.; Gao, J.Q.; Mayumi, T.; Shibuya, M.; et al. Bastadin 6, a spongean brominated tyrosine derivative, inhibits tumor angiogenesis by inducing selective apoptosis to endothelial cells. Anti-Cancer Drugs 2006, 17, 269–278. [Google Scholar] [CrossRef]
- Mathieu, V.; Wauthoz, N.; Lefranc, F.; Niemann, H.; Amighi, K.; Kiss, R.; Proksch, P. Cyclic versus hemi-bastadins. Pleiotropic anti-cancer effects: From apoptosis to anti-angiogenic and anti-migratory effects. Molecules 2013, 18, 3543–3561. [Google Scholar] [CrossRef] [PubMed]
- Niemann, H.; Lin, W.; Müller, W.E.G.; Kubbutat, M.; Lai, D.; Proksch, P. trimeric hemibastadin congener from the marine sponge Ianthella basta. J. Nat. Prod. 2013, 76, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Resuello, D.L.; Lirio, S.B.; Porto, A.E.; Macabeo, A.P.G.; Huang, H.Y.; Corpuz, M.J.A.T.; Villaflores, O.B. β-secretase 1 inhibitory activity and AMP-activated protein kinase activation of Callyspongia samarensis extracts. Nat. Prod. Res. 2018, 34, 525–529. [Google Scholar] [CrossRef] [PubMed]
- El-Damhougy, K.A.; Bashar, M.A.E.; El-Naggar, H.A.; Ibrahim, H.A.N.; Senna, F.M.A. GC-MS analysis of bioactive components of Callyspongia crassa (porifera) from gulf of aqaba red sea (egypt). Al Azhar Bull. Sci. 2017, 9, 111–118. [Google Scholar]
- Carballeira, N.M.; Pagán, M. New methoxylated fatty acids from the Caribbean sponge Callyspongia fallax. J. Nat. Prod. 2001, 64, 620–623. [Google Scholar] [CrossRef] [PubMed]
- Edrada, R.A.; Wray, V.; Berg, A.; Gräfe, U.; Brauers, G.; Proksch, P. Novel spiciferone derivatives from the fungus Drechslera hawaiiensis isolated from the marine sponge Callyspongia aerizusa. Verl. Z. Nat. 2000, 55, 218–221. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa, L.H.N.; de Araújo, R.D.; Sousa-Fontoura, D.; Menezes, F.G.; Araújo, R.M. Metabolities from Marine Sponges of the Genus Callyspongia: Occurrence, Biological Activity, and NMR Data. Mar. Drugs 2021, 19, 663. https://doi.org/10.3390/md19120663
de Sousa LHN, de Araújo RD, Sousa-Fontoura D, Menezes FG, Araújo RM. Metabolities from Marine Sponges of the Genus Callyspongia: Occurrence, Biological Activity, and NMR Data. Marine Drugs. 2021; 19(12):663. https://doi.org/10.3390/md19120663
Chicago/Turabian Stylede Sousa, Lucas Hilário Nogueira, Rusceli Diego de Araújo, Déborah Sousa-Fontoura, Fabrício Gava Menezes, and Renata Mendonça Araújo. 2021. "Metabolities from Marine Sponges of the Genus Callyspongia: Occurrence, Biological Activity, and NMR Data" Marine Drugs 19, no. 12: 663. https://doi.org/10.3390/md19120663
APA Stylede Sousa, L. H. N., de Araújo, R. D., Sousa-Fontoura, D., Menezes, F. G., & Araújo, R. M. (2021). Metabolities from Marine Sponges of the Genus Callyspongia: Occurrence, Biological Activity, and NMR Data. Marine Drugs, 19(12), 663. https://doi.org/10.3390/md19120663







