Anti-Inflammatory Mechanisms of Fucoidans to Treat Inflammatory Diseases: A Review
Abstract
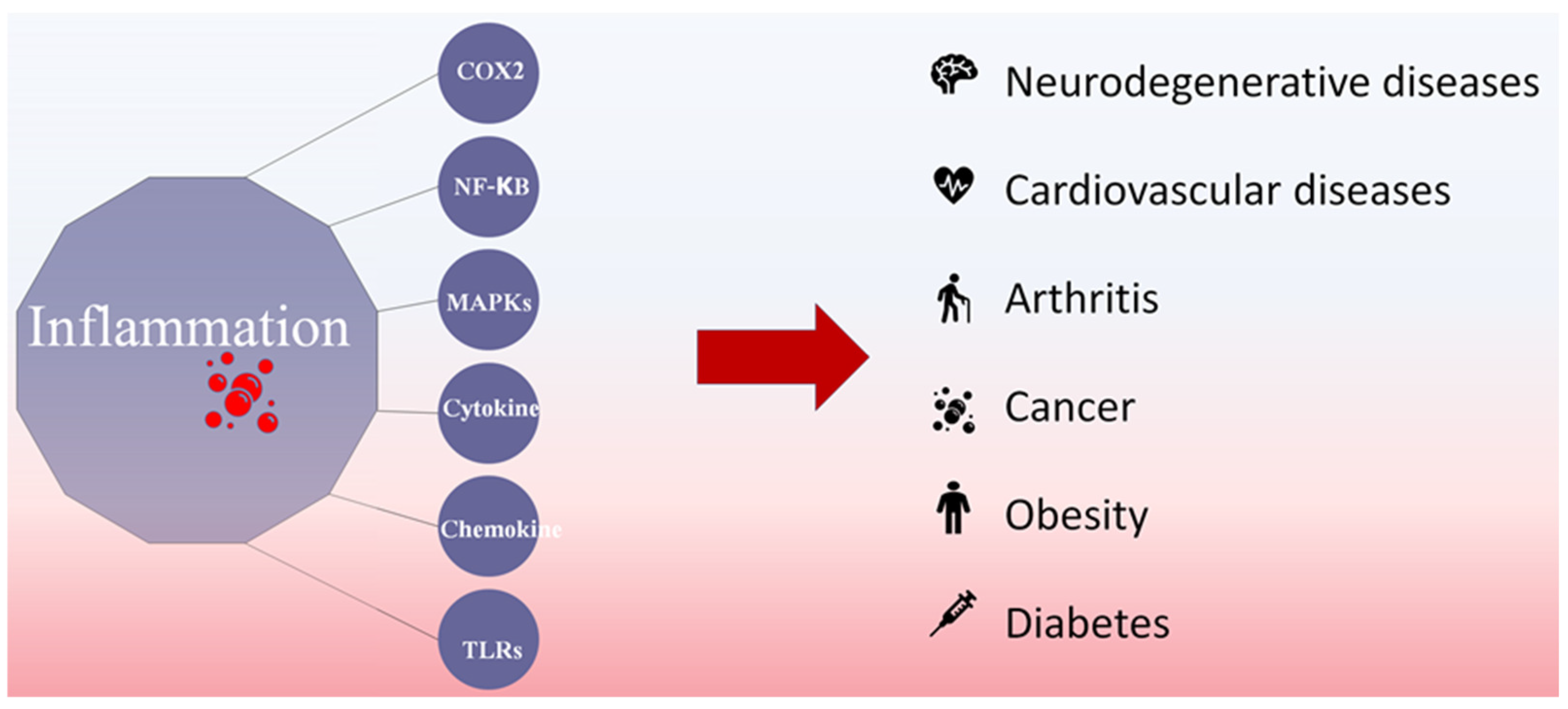
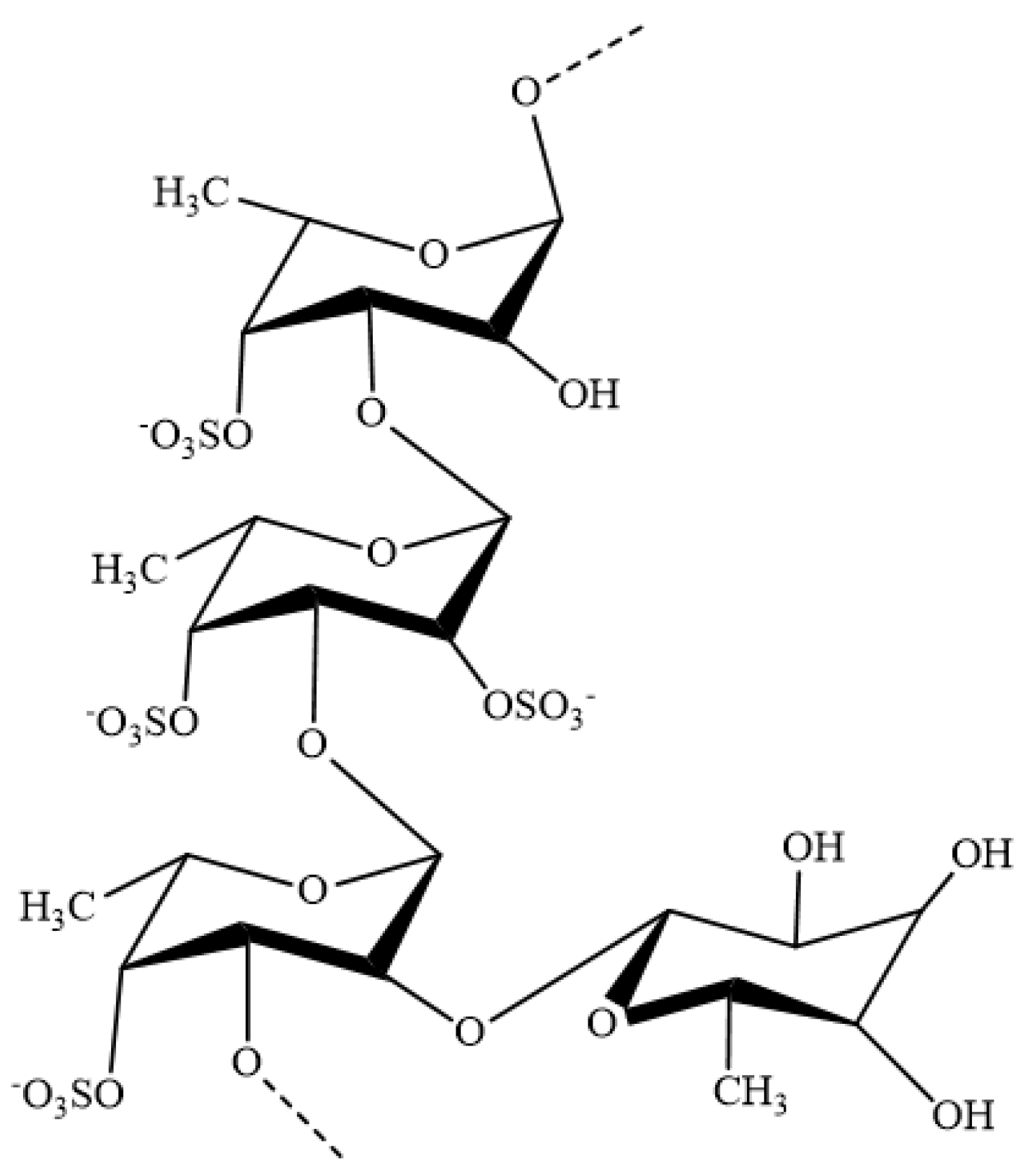
:1. Introduction
2. Anti-Inflammatory Mechanisms of Fucoidans
2.1. COX2 Inhibition of Fucoidan
2.2. NF-κB Inhibition of Fucoidan
2.3. MAPK Inhibition of Fucoidan
2.4. Cytokine Secretion Modulators
2.5. Chemokine Inhibition of Fucoidan
2.6. JAK-STAT Inhibition of Fucoidan
2.7. TLRs’ Inhibition of Fucoidan
2.8. Keap1/NRF2 Stimulation Properties Reported from Fucoidans
3. Material and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kotas, M.E.; Medzhitov, R. Homeostasis, inflammation, and disease susceptibility. Cell 2015, 160, 816–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Zhou, Q.G.; Chen, Y.Y.; Guo, A.Z.; Hu, C.M. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef]
- Kundu, J.K.; Surh, Y.J. Inflammation: Gearing the journey to cancer. Mutat Res. 2008, 659, 15–30. [Google Scholar] [CrossRef]
- Zayed, A.; Ulber, R. Fucoidans: Downstream Processes and Recent Applications. Mar. Drugs 2020, 18, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullón, B.; Gagaoua, M.; Barba, F.J.; Gullón, P.; Zhang, W.; Lorenzo, J.M. Seaweeds as promising resource of bioactive compounds: Overview of novel extraction strategies and design of tailored meat products. Trends Food Sci. Technol. 2020, 100, 1–18. [Google Scholar] [CrossRef]
- Menshova, R.V.; Shevchenko, N.M.; Imbs, T.I.; Zvyagintseva, T.N.; Malyarenko, O.S.; Zaporoshets, T.S.; Besednova, N.N.; Ermakova, S.P. Fucoidans from Brown Alga Fucus evanescens: Structure and Biological Activity. Front. Mar. Sci. 2016, 3. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Park, Y.-J.; Jeon, Y.-J.; Ryu, B. Bioactivities of the edible brown seaweed, Undaria pinnatifida: A review. Aquaculture 2018, 495, 873–880. [Google Scholar] [CrossRef]
- Fitton, J.H. Therapies from fucoidan; multifunctional marine polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, S.; Shi, W.; Qi, X.; Song, W.; Mou, J.; Yang, J. Structure characterization, antioxidant and immunoregulatory properties of a novel fucoidan from the sea cucumber Stichopus chloronotus. Carbohydr. Polym. 2020, 231, 115767. [Google Scholar] [CrossRef]
- Olatunji, O. Fucoidan. In Aquatic Biopolymers: Understanding Their Industrial Significance and Environmental Implications; Springer International Publishing: Cham, Switzerland, 2020; pp. 95–120. [Google Scholar] [CrossRef]
- Siahaan, E.A.; Pangestuti, R.; Munandar, H.; Kim, S.-K. Cosmeceuticals Properties of Sea Cucumbers: Prospects and Trends. Cosmetics 2017, 4, 26. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, H.R.; Biller, P.; Ross, A.B.; Adams, J.M.M. The seasonal variation of fucoidan within three species of brown macroalgae. Algal Res. 2017, 22, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Sanjeewa, K.K.A.; Lee, J.S.; Kim, W.S.; Jeon, Y.J. The potential of brown-algae polysaccharides for the development of anticancer agents: An update on anticancer effects reported for fucoidan and laminaran. Carbohydr. Polym. 2017, 177, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Hashemi Goradel, N.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell. Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Levy, B. Success of prostaglandin E2 in structure–function is a challenge for structure-based therapeutics. Proc. Natl. Acad. Sci. USA 2003, 100, 8609–8611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.Y.; Pillinger, M.H.; Abramson, S.B. Prostaglandin E2 synthesis and secretion: The role of PGE2 synthases. Clin. Immunol. 2006, 119, 229–240. [Google Scholar] [CrossRef]
- Maneix, L.; Servent, A.; Poree, B.; Ollitrault, D.; Branly, T.; Bigot, N.; Boujrad, N.; Flouriot, G.; Demoor, M.; Boumediene, K.; et al. Up-regulation of type II collagen gene by 17beta-estradiol in articular chondrocytes involves Sp1/3, Sox-9, and estrogen receptor alpha. J. Mol. Med. 2014, 92, 1179–1200. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Majid, M.; Haq, I.U.; Khan, M.R.; Kim, S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef]
- Garcia, C.V.; Lamas-Vazquez, M.; Blanco, F.; Dominguez, H.; Meijide-Failde, R. Impact of different fucoidans on pathological pathways activated in osteoarthritic articular cells. Osteoarthr. Cartil. 2019, 27, S147–S148. [Google Scholar] [CrossRef] [Green Version]
- Jeong, J.-W.; Hwang, S.J.; Han, M.H.; Lee, D.-S.; Yoo, J.S.; Choi, I.-W.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Kim, G.-Y.; et al. Fucoidan inhibits lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages and zebrafish larvae. Mol. Cell. Toxicol. 2017, 13, 405–417. [Google Scholar] [CrossRef]
- Park, J.; Cha, J.D.; Choi, K.M.; Lee, K.Y.; Han, K.M.; Jang, Y.S. Fucoidan inhibits LPS-induced inflammation in vitro and during the acute response in vivo. Int. Immunopharmacol. 2017, 43, 91–98. [Google Scholar] [CrossRef]
- Azab, K.S.; Meky, N.H.; El-Deghidy, E.A.M.; Azoz, G. Response of COX2/PGE2 Inflammatory Pathway to Brown Seaweed Extract in Rats Exposed to Gamma Radiation. World J. Nucl. Sci. Technol. 2017, 7, 189–205. [Google Scholar] [CrossRef] [Green Version]
- Jayawardena, T.U.; Fernando, I.P.S.; Lee, W.W.; Sanjeewa, K.K.A.; Kim, H.S.; Lee, D.S.; Jeon, Y.J. Isolation and purification of fucoidan fraction in Turbinaria ornata from the Maldives; Inflammation inhibitory potential under LPS stimulated conditions in in-vitro and in-vivo models. Int. J. Biol. Macromol. 2019, 131, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Shanura Fernando, I.P.; Asanka Sanjeewa, K.K.; Samarakoon, K.W.; Kim, H.-S.; Gunasekara, U.K.D.S.S.; Park, Y.-J.; Abeytunga, D.T.U.; Lee, W.W.; Jeon, Y.-J. The potential of fucoidans from Chnoospora minima and Sargassum polycystum in cosmetics: Antioxidant, anti-inflammatory, skin-whitening, and antiwrinkle activities. J. Appl. Phycol. 2018, 30, 3223–3232. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Sanjeewa, K.K.A.; Samarakoon, K.W.; Lee, W.W.; Kim, H.S.; Kang, N.; Ranasinghe, P.; Lee, H.S.; Jeon, Y.J. A fucoidan fraction purified from Chnoospora minima; a potential inhibitor of LPS-induced inflammatory responses. Int. J. Biol. Macromol. 2017, 104, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, Z.; Nie, K. Structural characterization of a novel polysaccharide from Sargassum thunbergii and its antioxidant and anti-inflammation effects. PLoS ONE 2019, 14, e0223198. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Oh, J.Y.; Jayawardena, T.U.; Jeon, Y.J.; Ryu, B. Anti-inflammatory and anti-melanogenesis activities of sulfated polysaccharides isolated from Hizikia fusiforme: Short communication. Int. J. Biol. Macromol. 2020, 142, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Pozharitskaya, O.N.; Obluchinskaya, E.D.; Shikov, A.N. Mechanisms of Bioactivities of Fucoidan from the Brown Seaweed Fucus vesiculosus L. of the Barents Sea. Mar. Drugs 2020, 18, 275. [Google Scholar] [CrossRef]
- Chapman, N.R.; Rocha, S.; Adcock, I.M.; Perkins, N.D. NF-κB Function in Inflammation, Cellular Stress and Disease. In Sensing, Signaling and Cell Adaptation; Storey, K.B., Storey, J.M., Eds.; Elsevier: Amsterdam, Netherlands, 2002; Volume 3, pp. 61–73. [Google Scholar]
- Biswal, S. Molecular Imaging of Rheumatoid Arthritis and Osteoarthritis. In Arthritis in Color; Bruno, M.A., Mosher, T.J., Gold, G.E., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2009; pp. 193–213. [Google Scholar] [CrossRef]
- Berthiaume, J.M.; Kirk, J.A.; Ranek, M.J.; Lyon, R.C.; Sheikh, F.; Jensen, B.C.; Hoit, B.D.; Butany, J.; Tolend, M.; Rao, V.; et al. Pathophysiology of Heart Failure and an Overview of Therapies. In Cardiovascular Pathology; Buja, L.M., Butany, J., Eds.; Academic Press: San Diego, CA, USA, 2016; pp. 271–339. [Google Scholar] [CrossRef]
- Lund, A.K. Oxidants and Endothelial Dysfunction. In Comprehensive Toxicology; McQueen, C.A., Ed.; Elsevier: Oxford, UK, 2010; pp. 243–274. [Google Scholar] [CrossRef]
- Bai, X.; Li, M.; Wang, X.; Chang, H.; Ni, Y.; Li, C.; He, K.; Wang, H.; Yang, Y.; Tian, T.; et al. Therapeutic potential of fucoidan in the reduction of hepatic pathology in murine schistosomiasis japonica. Parasit Vectors 2020, 13, 451. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Ren, D.; Song, Y.; Wu, L.; He, Y.; Peng, Y.; Zhou, H.; Liu, S.; Cong, H.; Zhang, Z.; et al. Gastric protective activities of fucoidan from brown alga Kjellmaniella crassifolia through the NF-kappaB signaling pathway. Int. J. Biol. Macromol. 2020, 149, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, L.; Pan, Q.; Jin, X. Effects of fucoidan on NF-κB-mediated inflammatory response in rats with acute myocardial infarction. Int. J. Clin. Exp. Med. 2019, 12, 12781–12787. [Google Scholar]
- Zheng, Y.; Liu, T.; Wang, Z.; Xu, Y.; Zhang, Q.; Luo, D. Low molecular weight fucoidan attenuates liver injury via SIRT1/AMPK/PGC1alpha axis in db/db mice. Int. J. Biol. Macromol. 2018, 112, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Hai-Lan, C.; Hong-Lian, T.; Jian, Y.; Manling, S.; Heyu, F.; Na, K.; Wenyue, H.; Si-Yu, C.; Ying-Yi, W.; Ting-Jun, H. Inhibitory effect of polysaccharide of Sargassum weizhouense on PCV2 induced inflammation in mice by suppressing histone acetylation. Biomed. Pharmacother. 2019, 112, 108741. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H.; Luo, P.; Li, Y. Production Inhibition and Excretion Promotion of Urate by Fucoidan from Laminaria japonica in Adenine-Induced Hyperuricemic Mice. Mar. Drugs 2018, 16, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, M.; Liang, H.; Ji, X.; Liu, Y.; Ge, Y.; Hou, L.; Sun, T. Fucoidan prevent murine autoimmune diabetes via suppression TLR4-signaling pathways, regulation DC/Treg induced immune tolerance and improving gut microecology. Nutr. Metab. 2019, 16, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, V.T.; Ko, S.C.; Oh, G.W.; Heo, S.Y.; Jeon, Y.J.; Park, W.S.; Choi, I.W.; Choi, S.W.; Jung, W.K. Anti-inflammatory effects of sodium alginate/gelatine porous scaffolds merged with fucoidan in murine microglial BV2 cells. Int. J. Biol. Macromol. 2016, 93, 1620–1632. [Google Scholar] [CrossRef] [PubMed]
- Asanka Sanjeewa, K.K.; Jayawardena, T.U.; Kim, H.S.; Kim, S.Y.; Shanura Fernando, I.P.; Wang, L.; Abetunga, D.T.U.; Kim, W.S.; Lee, D.S.; Jeon, Y.J. Fucoidan isolated from Padina commersonii inhibit LPS-induced inflammation in macrophages blocking TLR/NF-kappaB signal pathway. Carbohydr. Polym. 2019, 224, 115195. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Kim, S.Y.; Kim, H.S.; Ahn, G.; Jee, Y.; Jeon, Y.J. In vitro and in vivo anti-inflammatory activities of high molecular weight sulfated polysaccharide; containing fucose separated from Sargassum horneri: Short communication. Int. J. Biol. Macromol. 2018, 107, 803–807. [Google Scholar] [CrossRef]
- Stansfield, W.E.; Ranek, M.; Pendse, A.; Schisler, J.C.; Wang, S.; Pulinilkunnil, T.; Willis, M.S. The Pathophysiology of Cardiac Hypertrophy and Heart Failure. In Cellular and Molecular Pathobiology of Cardiovascular Disease; Willis, M.S., Homeister, J.W., Stone, J.R., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 51–78. [Google Scholar] [CrossRef]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [Green Version]
- Che, N.; Ma, Y.; Xin, Y. Protective Role of Fucoidan in Cerebral Ischemia-Reperfusion Injury through Inhibition of MAPK Signaling Pathway. Biomol. Ther. 2017, 25, 272–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choo, G.-S.; Lee, H.-N.; Shin, S.-A.; Kim, H.-J.; Jung, J.-Y. Anticancer Effect of Fucoidan on DU-145 Prostate Cancer Cells through Inhibition of PI3K/Akt and MAPK Pathway Expression. Mar. Drugs 2016, 14, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjeewa, K.K.A.; Jayawardena, T.U.; Kim, S.-Y.; Kim, H.-S.; Ahn, G.; Kim, J.; Jeon, Y.-J. Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-κB and MAPK pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Wu, G.-J.; Shiu, S.-M.; Hsieh, M.-C.; Tsai, G.-J. Anti-inflammatory activity of a sulfated polysaccharide from the brown alga Sargassum cristaefolium. Food Hydrocoll. 2016, 53, 16–23. [Google Scholar] [CrossRef]
- Nie, M.; Wang, Y.; Lu, Y.; Yuan, Y.; Liu, Y.; Li, X. Protective effects of fucoidan against hyperoxic lung injury via the ERK signaling pathway. Mol. Med. Rep. 2018, 17, 1813–1818. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Sun, J.; Song, B.; Zhang, L.; Shao, Q.; Liu, Y.; Yuan, D.; Zhang, Y.; Qu, X. Fucoidan inhibits CCL22 production through NF-kB pathway in M2 macrophages: A potential therapeutic strategy for cancer. Sci. Rep. 2016, 6, 35855. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.J.; Lin, M.H.; Kuo, T.C.; Chou, C.M.; Mi, F.L.; Cheng, C.H.; Lin, C.W. Fucoidan from Laminaria japonica exerts antitumor effects on angiogenesis and micrometastasis in triple-negative breast cancer cells. Int. J. Biol. Macromol. 2020, 149, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef]
- AlKahtane, A.A.; Abushouk, A.I.; Mohammed, E.T.; ALNasser, M.; Alarifi, S.; Ali, D.; Alessia, M.S.; Almeer, R.S.; AlBasher, G.; Alkahtani, S.; et al. Fucoidan alleviates microcystin-LR-induced hepatic, renal, and cardiac oxidative stress and inflammatory injuries in mice. Environ. Sci. Pollut. Res. Int. 2019, 145, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Phan, N.N.; Lu, W.J.; Ngoc Hieu, B.T.; Lin, Y.C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Xu, J.; Ge, K.; Tian, Q.; Zhao, P.; Guo, Y. Anti-inflammatory effect of low molecular weight fucoidan from Saccharina japonica on atherosclerosis in apoE-knockout mice. Int. J. Biol. Macromol. 2018, 118, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pei, L.; Liu, H.; Qv, K.; Xian, W.; Liu, J.; Zhang, G.J.I.J.C.E.P. Fucoidan attenuates atherosclerosis in LDLR-/-mice through inhibition of inflammation and oxidative stress. Int. J. Clin. Exp. Pathol. 2016, 9, 6896–6904. [Google Scholar]
- Kim, Y.I.; Oh, W.S.; Song, P.H.; Yun, S.; Kwon, Y.S.; Lee, Y.J.; Ku, S.K.; Song, C.H.; Oh, T.H. Anti-photoaging effects of low molecular-weight fucoidan on ultraviolet B-irradiated mice. Mar. Drugs 2018, 16, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Zhu, W.; Wang, T.; Jin, L.; Liu, T.; Li, X.; Guan, Z.; Jiang, Z.; Meng, X.; Wang, J.; et al. Low molecule weight fucoidan mitigates atherosclerosis in ApoE (-/-) mouse model through activating multiple signal pathway. Carbohydr. Polym. 2019, 206, 110–120. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Costa Lima, S.A.; Reis, S. Development of methotrexate loaded fucoidan/chitosan nanoparticles with anti-inflammatory potential and enhanced skin permeation. Int. J. Biol. Macromol. 2019, 124, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ryu, J.M.; Han, Y.S.; Zia, M.F.; Kwon, H.Y.; Noh, H.; Han, H.J.; Lee, S.H. Fucoidan improves bioactivity and vasculogenic potential of mesenchymal stem cells in murine hind limb ischemia associated with chronic kidney disease. J. Mol. Cell. Cardiol. 2016, 97, 169–179. [Google Scholar] [CrossRef]
- Kim, H.M.; Ahn, C.; Kang, B.T.; Kang, J.H.; Jeung, E.B.; Yang, M.P. Fucoidan suppresses excessive phagocytic capacity of porcine peripheral blood polymorphonuclear cells by modulating production of tumor necrosis factor-alpha by lipopolysaccharide-stimulated peripheral blood mononuclear cells. Res. Vet. Sci. 2018, 118, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Wang, L.; Fu, X.; Duan, D.; Jeon, Y.J.; Xu, J.; Gao, X. In vitro and in vivo anti-inflammatory activities of a fucose-rich fucoidan isolated from Saccharina japonica. Int. J. Biol. Macromol. 2020, 156, 717–729. [Google Scholar] [CrossRef]
- Dutot, M.; Grassin-Delyle, S.; Salvator, H.; Brollo, M.; Rat, P.; Fagon, R.; Naline, E.; Devillier, P. A marine-sourced fucoidan solution inhibits Toll-like-receptor-3-induced cytokine release by human bronchial epithelial cells. Int. J. Biol. Macromol. 2019, 130, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, K.; Li, S.; Liu, T.; Wang, F.; Xia, Y.; Lu, J.; Zhou, Y.; Guo, C. Pretreatment with Fucoidan from Fucus vesiculosus protected against ConA-Induced acute liver Injury by inhibiting both intrinsic and extrinsic apoptosis. PLoS ONE 2016, 11, e0152570. [Google Scholar] [CrossRef] [PubMed]
- Herath, K.; Kim, H.J.; Kim, A.; Sook, C.E.; Lee, B.Y.; Jee, Y. The Role of Fucoidans Isolated from the Sporophylls of Undaria pinnatifida against Particulate-Matter-Induced Allergic Airway Inflammation: Evidence of the Attenuation of Oxidative Stress and Inflammatory Responses. Molecules 2020, 25, 2869. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.M.; Liu, P.Y.; Chen, Y.A.; Tseng, H.Y.; Shen, P.C.; Hwang, P.A.; Hsu, H.L. Oligo-Fucoidan prevents IL-6 and CCL2 production and cooperates with p53 to suppress ATM signaling and tumor progression. Sci. Rep. 2017, 7, 11864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopplin, G.; Rokstad, A.M.; Mélida, H.; Bulone, V.; Skjåk-Bræk, G.; Aachmann, F.L. Structural Characterization of Fucoidan from Laminaria hyperborea: Assessment of Coagulation and Inflammatory Properties and Their Structure–Function Relationship. ACS Appl. Bio Mater. 2018, 1, 1880–1892. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Hu, S.; Wang, J.; Li, S.; Jiang, W. Fucoidan from Acaudina molpadioides protects pancreatic islet against cell apoptosis via inhibition of inflammation in type 2 diabetic mice. Food Sci. Biotechnol. 2016, 25, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, K.; Feng, J.; Murakami, H.; Nagata, S.; Watling, D.; Rogers, N.C.; Stark, G.R.; Kerr, I.M.; Ihle, J.N. Jak1 plays an essential role for receptor phosphorylation and Stat activation in response to granulocyte colony-stimulating factor. Blood 1997, 90, 597–604. [Google Scholar] [CrossRef]
- Qi, Z.; Yin, F.; Lu, L.; Shen, L.; Qi, S.; Lan, L.; Luo, L.; Yin, Z. Baicalein reduces lipopolysaccharide-induced inflammation via suppressing JAK/STATs activation and ROS production. Inflamm. Res. 2013, 62, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Q.; Li, S.; Dai, W.; Feng, J.; Wu, L.; Liu, T.; Chen, K.; Xia, Y.; Lu, J.; et al. The natural product fucoidan ameliorates hepatic ischemia-reperfusion injury in mice. Biomed. Pharmacother. 2017, 94, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Pan, H.F.; Shao, S.L.; Xu, X.M. Anti-tumor and anti-angiogenic effects of Fucoidan on prostate cancer: Possible JAK-STAT3 pathway. BMC Complement. Altern. Med. 2017, 17, 378. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol 2014, 5, 461. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.Q.; Wei, J.G.; Tu, M.J.; Gu, J.G.; Zhang, W. Fucoidan Alleviates Acetaminophen-Induced Hepatotoxicity via Oxidative Stress Inhibition and Nrf2 Translocation. Int. J. Mol. Sci. 2018, 19, 4050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, M.J.; Chung, H.S. Fucoidan reduces oxidative stress by regulating the gene expression of HO1 and SOD1 through the Nrf2/ERK signaling pathway in HaCaT cells. Mol. Med. Rep. 2016, 14, 3255–3260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhao, W.; Wang, L.; Chen, Y.; Zhang, H.; Wang, T.; Yang, X.; Xing, F.; Yan, J.; Fang, X. Protective Effects of Fucoidan against Hydrogen Peroxide-Induced Oxidative Damage in Porcine Intestinal Epithelial Cells. Animals 2019, 9, 1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Seaweed | Tested Organism | Effect | Reference |
|---|---|---|---|
| Chnoospora minima | LPS-activated RAW 264.7 macrophages | COX2 inhibition | [27] |
| Chnoospora minima and Sargassum polycystum | LPS-activated RAW 264.7 macrophages | Inhibit LPS-induced PGE2 secretion | [26] |
| Commercial grade fucoidan | Male Wistar albino rats | COX2 inhibition | [24] |
| Commercial grade fucoidans | BALB/c mice and RAW 264.7 macrophages | Inhibit LPS-induced COX2 secretion | [23] |
| Fucus vesiculosus, Undaria pinnatifida, and Macrocystis pyrifera | Chondrocytes and Synoviocytes | Inhibit IL-1β-induced COX2 secretion | [21] |
| Fucus vesiculosis | RAW 264.7 macrophages and zebrafish embryos | Inhibit LPS-induced PGE2 secretion | [22] |
| Hikizia fusiforme | RAW 264.7 macrophages | COX2 inhibition | [29] |
| Sargassum thunbergii | RAW 264.7 macrophages | Downregulation of LPS-activated COX2 expression | [28] |
| Turbinaria ornate | RAW 264.7 macrophages and zebrafish embryos | COX2 inhibition | [25] |
| Undaria pinnatifida | Rabbit articular chondrocytes | Downregulation of COX2 observed in chondrocytes | [20] |
| Undaria pinnatifida | Arthritic rats | Downregulation of COX2 | [20] |
| Seaweed | Tested Organism | Effect | Reference |
|---|---|---|---|
| Commercial grade fucoidan | NOD diabetic mice | NF-κB p65 inhibition | [41] |
| Commercial fucoidan | Ischemia–reperfusion injured rats | Inhibition of phosphorylation of ERK, JNK, and p38 | [47] |
| Commercial fucoidan | M2 macrophages | NF-κB p65 inhibition | [52] |
| Commercial fucoidan | Sprague Dawley (SD) rats | Inhibition of phosphorylation of ERK, JNK, and p38 | [47] |
| Fucus vesiculosus | Balb/c mice model | Inhibition of phosphorylation of ERK | [51] |
| Hizikia fusiforme | murine microglial BV2 cells | NF-κB p65 inhibition | [42] |
| Kjellmaniella crassifolia | Wistar rat | Inhibition of aspirin-induced NF-κB activation via stabilization of IκB-α | [36] |
| Laminaria Japonica | SPF SD rats | Downregulated IκB degradation | [37] |
| Laminaria japonica | diabetic db/db mice | Downregulated NF-κB degradation | [38] |
| Laminaria japonica | Hyperuricemic mice | NF-κB p65 inhibition | [40] |
| Laminaria japonica | MDA-MB-231 and HCC1806 cells | Inhibition of phosphorylation of ERK, JNK, and p38 | [53] |
| Padina commersonii | RAW 264.7 cells | Inhibition of phosphorylation of IKK and subsequent phosphorylation of NF-κB-p65 and p50 | [43] |
| Sargassum cristaefolium | RAW 264.7 cells | Inhibition of phosphorylation of ERK, JNK, and p38 | [50] |
| Sargassum horneri | RAW 264.7 cells | LPS-activated IKβ-α phosphorylation | [44] |
| Sargassum horneri | RAW 264.7 cells | Inhibition of phosphorylation of ERK and JNK | [43] |
| Sargassum weizhouense | Kunming inbred mice | NF-κB p65 inhibition | [39] |
| Undaria pinnatifida | DU-145 cancer cells | Inhibition of phosphorylation of ERK and p38 | [48] |
| Seaweed | Tested Organism | Effect | Reference |
|---|---|---|---|
| Ascophyllum nodosum | Bronchial epithelial cells | Inhibition of IL-1β, IL-6, and TNF-α | [66] |
| Chnoospora minima | RAW 264.7 cells | Inhibition of IL-1β, IL-6, and TNF-α | [27] |
| Commercial fucoidan | Mesenchymal stem cell | Inhibit TNF-α | [63] |
| Ecklonia cava | HR-1 hairless mice | Inhibition of UV-B-exposed IL-1β production | [60] |
| Fucus vesiculosus | RAW 264.7 cells | Inhibition of IL-1β, IL-6, and TNF-α | [22] |
| Fucus vesiculosus | THP1 monocytes | Inhibition of IL-1β, IL-6, and TNF-α | [62] |
| Fucus vesiculosus | Peripheral polymorphonuclear cells | Inhibition of TNF-α | [64] |
| Fucus vesiculosus | BALB/C mice | Inhibition of TNF-α | [67] |
| Laminaria Japonica | Swiss albino mice | Inhibition of IL-1β, IL-6, and TNF-α | [56] |
| Laminaria Japonica | LDLR-/- mice | Inhibition of IL-1β, IL-6, and TNF-α | [59] |
| Laminaria Japonica | SPF SD rats | Inhibition of IL-6 and TNF-α | [37] |
| Saccharina japonica | Atherosclerotic mice | Inhibition of IL-6 | [58] |
| Saccharina japonica | ApoE-knockout (-/-) mice | Inhibition of IL-1 expression | [61] |
| Sargassum hemiphyllum | C57BL/6 mice model | Inhibition of IL-1 expression | [60] |
| Sargassum hemiphyllum | Caco-2 cell | Inhibition of IL-1β and TNF-α | [57] |
| Sargassum thunbergii | RAW 264.7 cells | Inhibition of IL-1β, IL-6, and TNF-α | [28] |
| Undaria pinnatifida | Balb/c mice | Inhibition of IL-4 | [68] |
| Seaweed | Tested Organism | Effect | Reference |
|---|---|---|---|
| * Acaudina molpadioides | C57BL/6J mice | CCL3 inhibiton | [71] |
| Ascophyllum nodosum | Bronchial epithelial cells | CCL5, CCL22, CXCL1, CXCL5, and CXCL8 inhibiton | [66] |
| Commercial fucoidan | Athymic nude mice | p-JAK and p-STAT3 inhibition | [75] |
| Commercial fucoidan | HCT 116 cells | CCL2/MCP-1 inhibiton | [69] |
| Commercial fucoidan | M2 macrophages | CCL2, CCL4, CCL5 and CCL22 inhibiton | [52] |
| Fucus vesiculosis | Male BALB/C mice | p-JAK2 and p-STAT1 inhibition | [74] |
| Fucus vesiculosis | BALB/C mice | p-JAK2 and p-STAT1 inhibition | [67] |
| Laminaria hyperborea | Human whole blood | CXCL10 and CCL5 inhibiton | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanjeewa, K.K.A.; Herath, K.H.I.N.M.; Yang, H.-W.; Choi, C.S.; Jeon, Y.-J. Anti-Inflammatory Mechanisms of Fucoidans to Treat Inflammatory Diseases: A Review. Mar. Drugs 2021, 19, 678. https://doi.org/10.3390/md19120678
Sanjeewa KKA, Herath KHINM, Yang H-W, Choi CS, Jeon Y-J. Anti-Inflammatory Mechanisms of Fucoidans to Treat Inflammatory Diseases: A Review. Marine Drugs. 2021; 19(12):678. https://doi.org/10.3390/md19120678
Chicago/Turabian StyleSanjeewa, Kalu K. Asanka, Kalahe H. I. N. M. Herath, Hye-Won Yang, Cheol Soo Choi, and You-Jin Jeon. 2021. "Anti-Inflammatory Mechanisms of Fucoidans to Treat Inflammatory Diseases: A Review" Marine Drugs 19, no. 12: 678. https://doi.org/10.3390/md19120678
APA StyleSanjeewa, K. K. A., Herath, K. H. I. N. M., Yang, H.-W., Choi, C. S., & Jeon, Y.-J. (2021). Anti-Inflammatory Mechanisms of Fucoidans to Treat Inflammatory Diseases: A Review. Marine Drugs, 19(12), 678. https://doi.org/10.3390/md19120678








