A Comparative Study on the Flocculation of Silica and China Clay with Chitosan and Synthetic Polyelectrolytes
Abstract
:1. Introduction
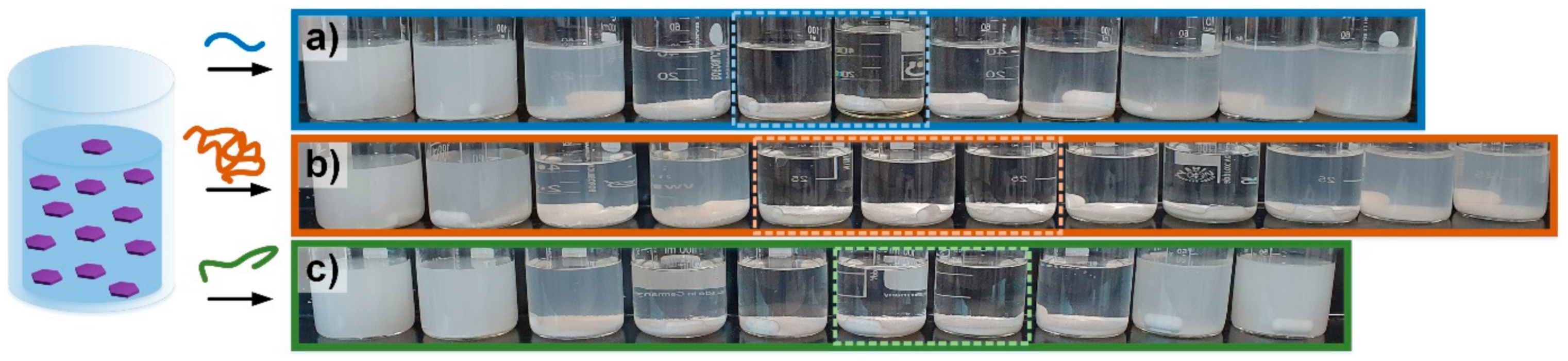
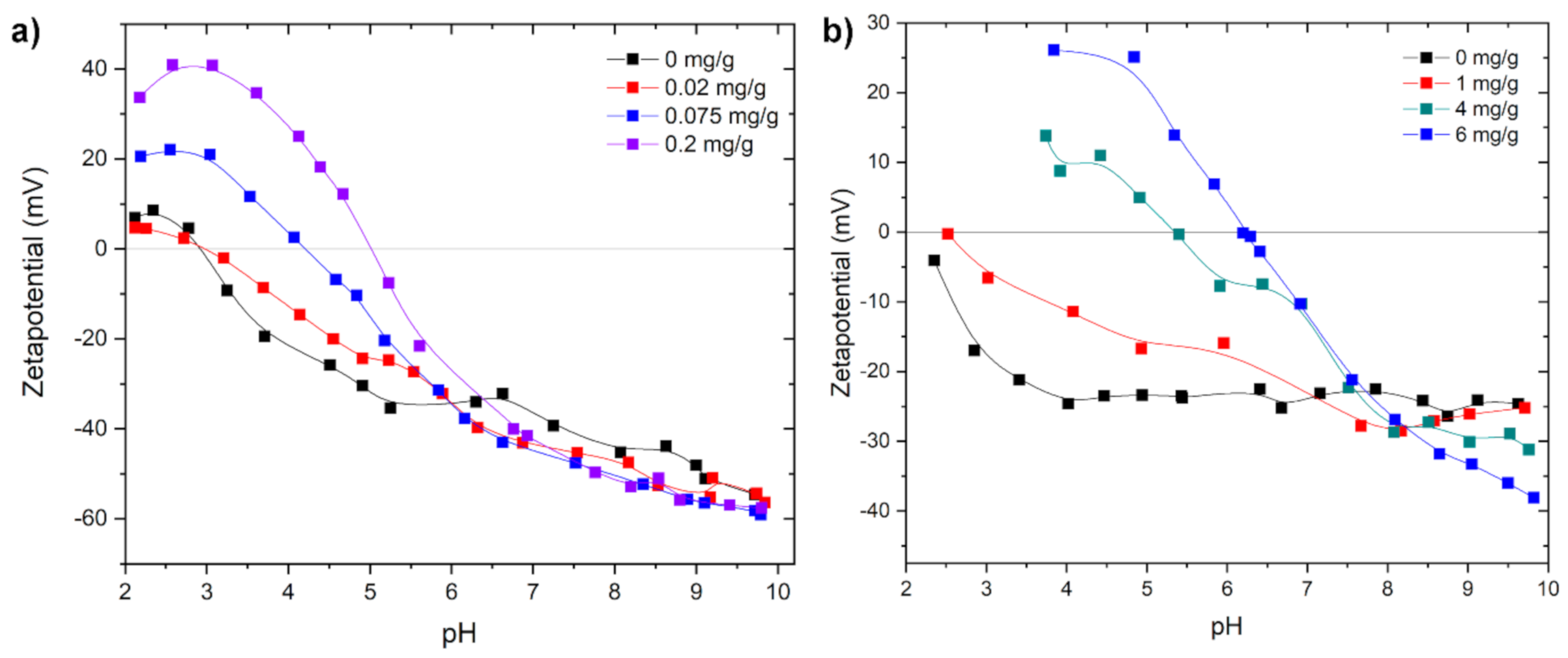
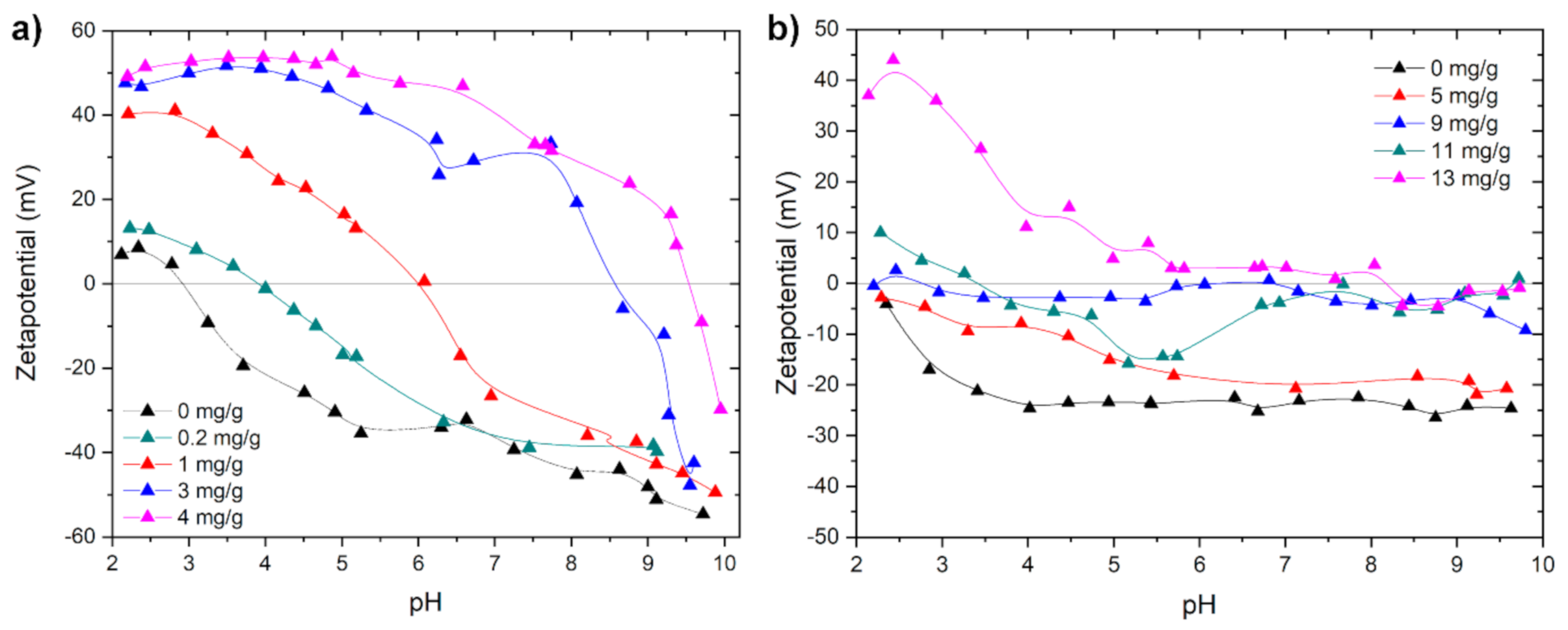
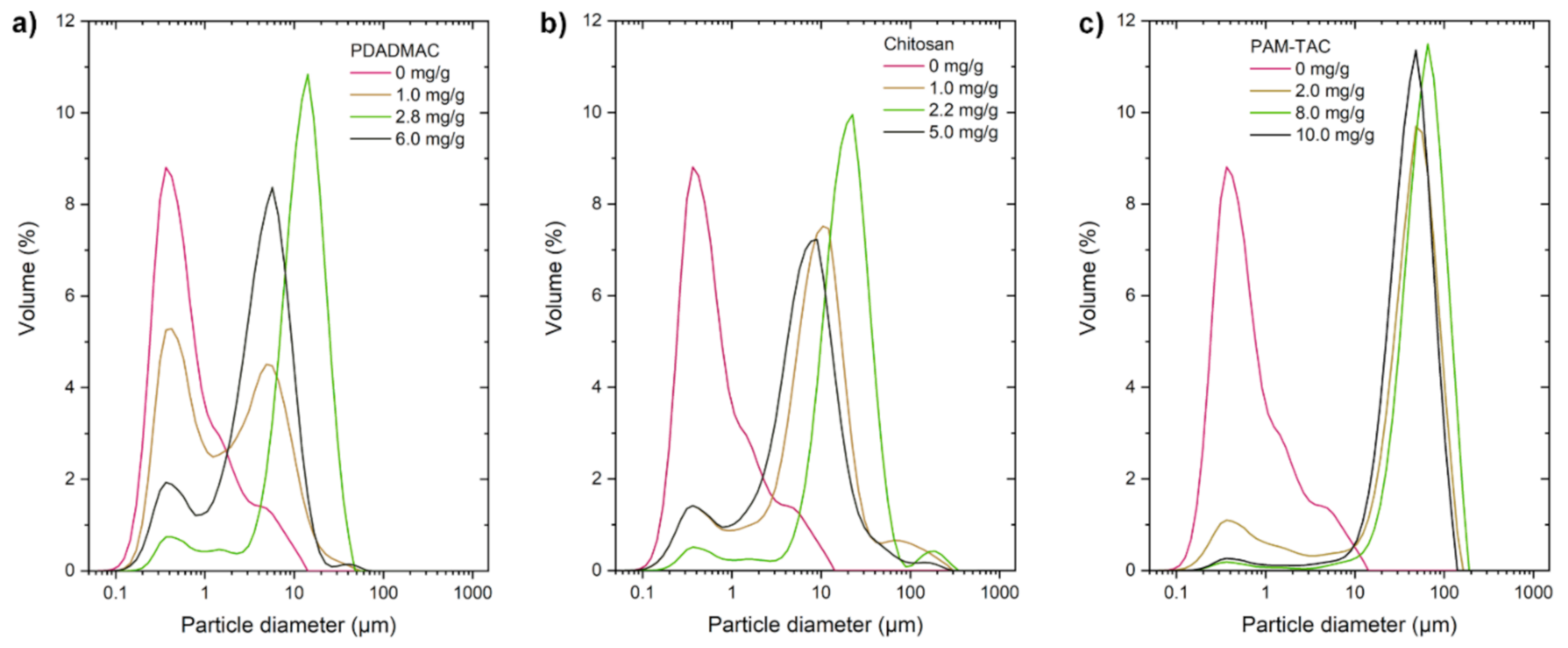
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Zetapotential and Particle Size Measurement in Dependence of the pH
3.2.2. Particle Charge Detection (PCD) Measurements
3.2.3. Turbidity Measurements
3.2.4. Particle Size Measurements
3.2.5. Nitrogen Sorption Measurements
3.2.6. Scanning Electron Microscopy (SEM)
3.3. Experiments
3.3.1. Preparation of the Polyelectrolyte Solutions
3.3.2. Flocculation Experiments
- (a)
- The following flocculants were added separately to a 50 mL silica suspension:
- PAM-TAC, 1 g/L: 0–4 mg/g
- 0 mg/g (pH 7.0), 0.2 mg/g (pH 6.8), 0.7 mg/g (pH 6.8), 1.0 mg/g (pH 6.9), 2.0 mg/g (pH 7.0), 3.0 mg/g (pH 6.9), 4.0 mg/g (pH 7.1).
- PDADMAC, 0.1 g/L: 0–2 mg/g
- 0 mg/g (pH 7.0), 0.015 mg/g (pH 7.2), 0.05 mg/g (pH 7.0), 0.15 mg/g (pH 7.0), 0.30 mg/g (pH 7.2), 1.0 mg/g (pH 7.5), 2.0 mg/g (pH 7.8).
- Chitosan, 0.1 g/L: 0–0.2 mg/g
- 0 mg/g (pH 7.0), 0.02 mg/g (pH 5.5), 0.025 mg/g (pH 5.4), 0.05 mg/g (pH 5.0), 0.075 mg/g (pH 4.9), 0.10 mg/g (pH 4.8), 0.20 mg/g (pH 4.4).
- (b)
- The following flocculants were added separately to a 50 mL china clay-suspension:
- PAM-TAC, 1 g/L: 0–13 mg/g
- 0 mg/g (pH 6.7), 5.0 mg/g (pH 7.3), 7.0 mg/g (pH 7.5), 9.0 mg/g (pH 7.4), 11.0 mg/g (pH 7.5), 13.0 mg/g (pH 7.5).
- PDADMAC, 1 g/L: 0–8 mg/g
- 0 mg/g (pH 6.7), 2.0 mg/g (pH 7.7), 4.0 mg/g (pH 7.7), 5.0 mg/g (pH 7.5), 6.0 mg/g (pH 7.6), 8.0 mg/g (pH 7.6).
- Chitosan, 1 g/L: 0–6 mg/g
- 0 mg/g (pH 6.6), 1.0 mg/g (pH 6.6), 2.0 mg/g (pH 5.9), 4.0 mg/g (pH 5.6), 6.0 mg/g (pH 4.6).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.S.; Robinson, J.; Chong, M.F. A review on application of flocculants in wastewater treatment. Process Saf. Environ. Prot. 2014, 92, 489–508. [Google Scholar] [CrossRef]
- Cainglet, A.; Tesfamariam, A.; Heiderscheidt, E. Organic polyelectrolytes as the sole precipitation agent in municipal wastewater treatment. J. Environ. Manag. 2020, 111002. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Dhuldhoya, N.C.; Merchant, U.C. Flocculants—an Ecofriendly Approach. J. Polym. Environ. 2006, 14, 195–202. [Google Scholar] [CrossRef]
- Bratby, J. Coagulation and Flocculation in Water and Wastewater Treatment, 3rd ed.; IWA Publishing: London, UK, 2016; ISBN 9781780407500. [Google Scholar]
- Kerr, J.L.; Lumsden, J.S.; Russell, S.K.; Jasinska, E.J.; Goss, G.G. Effects of anionic polyacrylamide products on gill histopathology in juvenile rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2014, 1552–1562. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.D.; Reinert, K.H.; Freeman, M.B. Aquatic Risk Assessment of Polymers. Environ. Sci. Technol. 1994, 28, 186A–192A. [Google Scholar] [CrossRef]
- Bolto, B.A. Soluble polymers in water purification. Prog. Polym. Sci. 1995, 20, 987–1041. [Google Scholar] [CrossRef]
- Škapa, S.; Vochozka, M. Waste energy recovery improves price competitiveness of artificial forage from rapeseed straw. Clean Technol. Environ. Policy 2019, 21, 1165–1171. [Google Scholar] [CrossRef]
- Stehel, V.; Maroušková, A.; Kolář, L. Techno-economic analysis of fermentation residues management places a question mark against current practices. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 721–726. [Google Scholar] [CrossRef]
- Sheng, P.; Dong, Y.; Vochozka, M. Analysis of Cost-Effective Methods to Reduce Industrial Wastewater Emissions in China. Water 2020, 12, 1600. [Google Scholar] [CrossRef]
- Krentz, D.-O.; Lohmann, C.; Schwarz, S.; Bratskaya, S.; Liebert, T.; Laube, J.; Heinze, T.; Kulicke, W.-M. Properties and Flocculation Efficiency of Highly Cationized Starch Derivatives. Starch Stärke 2006, 58, 161–169. [Google Scholar] [CrossRef]
- Yang, R.; Li, H.; Huang, M.; Yang, H.; Li, A. A review on chitosan-based flocculants and their applications in water treatment. Water Res. 2016, 59–89. [Google Scholar] [CrossRef]
- Wang, L.; Ma, F.; Qu, Y.; Sun, D.; Li, A.; Guo, J.; Yu, B. Characterization of a compound bioflocculant produced by mixed culture of Rhizobium radiobacter F2 and Bacillus sphaeicus F6. World J. Microbiol. Biotechnol. 2011, 27, 2559–2565. [Google Scholar] [CrossRef]
- Grenda, K.; Arnold, J.; Gamelas, J.A.F.; Cayre, O.J.; Rasteiro, M.G. Flocculation of silica nanoparticles by natural, wood-based polyelectrolytes. Sep. Purif. Technol. 2020, 231, 115888. [Google Scholar] [CrossRef]
- Weißpflog, J.; Boldt, R.; Kohn, B.; Scheler, U.; Jehnichen, D.; Tyrpekl, V.; Schwarz, S. Investigation of mechanisms for simultaneous adsorption of iron and sulfate ions onto chitosan with formation of orthorhombic structures. Colloids Surf. A Physicochem. Eng. Asp. 2020, 592, 124575. [Google Scholar] [CrossRef]
- Weißpflog, J.; Gündel, A.; Vehlow, D.; Steinbach, C.; Müller, M.; Boldt, R.; Schwarz, S.; Schwarz, D. Solubility and Selectivity Effects of the Anion on the Adsorption of Different Heavy Metal Ions onto Chitosan. Molecules 2020, 25, 2482. [Google Scholar] [CrossRef]
- Ghiorghita, C.-A.; Borchert, K.B.L.; Vasiliu, A.-L.; Zaharia, M.-M.; Schwarz, D.; Mihai, M. Porous thiourea-grafted-chitosan hydrogels: Synthesis and sorption of toxic metal ions from contaminated waters. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125504. [Google Scholar] [CrossRef]
- Mende, M.; Schwarz, D.; Steinbach, C.; Boldt, R.; Schwarz, S. The Influence of Salt Anions on Heavy Metal Ion Adsorption on the Example of Nickel. Materials 2018, 11, 373. [Google Scholar] [CrossRef] [Green Version]
- Bratskaya, S.Y.; Avramenko, V.A.; Sukhoverkhov, S.V.; Schwarz, S. Flocculation of Humic Substances and Their Derivatives with Chitosan. Colloid J. 2002, 64, 681–686. [Google Scholar] [CrossRef]
- Bratskaya, S.; Schwarz, S.; Chervonetsky, D. Comparative study of humic acids flocculation with chitosan hydrochloride and chitosan glutamate. Water Res. 2004, 38, 2955–2961. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, P.; Gradzielski, M.; Bustamante, H.; Vigneswaran, S. Progress, challenges, and opportunities in enhancing NOM flocculation using chemically modified chitosan: A review towards future development. Environ. Sci. Water Res. Technol. 2020, 6, 45–61. [Google Scholar] [CrossRef]
- Lasareva, E.V.; Parfenova, A.M.; Azovtseva, N.A. Formation of soil aggregates via clay flocculation with organic polyelectrolytes. Biointerface Res. Appl. Chem. 2020, 10, 5765–5771. [Google Scholar] [CrossRef]
- Divakaran, R.; Pillai, V.N.S. Mechanism of kaolinite and titanium dioxide flocculation using chitosan—Assistance by fulvic acids? Water Res. 2004, 38, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, D.; Ge, X.; Yan, M.; Yang, M. Flocculation of kaolin particles by two typical polyelectrolytes: A comparative study on the kinetics and floc structures. Colloids Surf. A Physicochem. Eng. Asp. 2006, 290, 288–294. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Deng, Y. Flocculation and reflocculation of clay suspension by different polymer systems under turbulent conditions. J. Colloid Interface Sci. 2004, 278, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Dryabina, S.S.; Rudenko, M.S.; Shulevich, Y.V.; Navrotskii, A.V.; Novakov, I.A. Specifics of kaolin dispersion flocculation due to a polyelectrolyte complex formation on particle surface. Colloid Polym. Sci. 2020, 298, 519–533. [Google Scholar] [CrossRef]
- Maruyama, H.; Seki, H.; Igi, A. Flocculation of quartz and kaolin by alginate-protamine complex. Biochem. Eng. J. 2020, 162, 107713. [Google Scholar] [CrossRef]
- Shakeel, A.; Safar, Z.; Ibanez, M.; van Paassen, L.; Chassagne, C. Flocculation of Clay Suspensions by Anionic and Cationic Polyelectrolytes: A Systematic Analysis. Minerals 2020, 10, 999. [Google Scholar] [CrossRef]
- Petzold, G.; Schwarz, S. Polyelectrolyte Complexes in Flocculation Applications. In Polyelectrolyte Complexes in the Dispersed and Solid State; Müller, M., Ed.; Springer: Berlin, Germany, 2014; pp. 25–65. ISBN 978-3-642-40745-1. [Google Scholar]
- Gregory, J.; Barany, S. Adsorption and flocculation by polymers and polymer mixtures. Adv. Colloid Interface Sci. 2011, 1–12. [Google Scholar] [CrossRef]
- Rothon, R. China Clay or Kaolin. In Fillers for Polymer Applications; Rothon, R., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 161–175. ISBN 978-3-319-28116-2. [Google Scholar]
- Domínguez-Robles, J.; Palenzuela, M.d.V.; Sánchez, R.; Loaiza, J.M.; Espinosa, E.; Rosal, A.; Rodríguez, A. Coagulation–Flocculation as an Alternative Way to Reduce the Toxicity of the Black Liquor from the Paper Industry: Thermal Valorization of the Solid Biomass Recovered. Waste Biomass Valor. 2020, 11, 4731–4742. [Google Scholar] [CrossRef]
- Maximova, N.; Österberg, M.; Koljonen, K.; Stenius, P. Lignin adsorption on cellulose fibre surfaces: Effect on surface chemistry, surface morphology and paper strength. Cellulose 2001, 8, 113–125. [Google Scholar] [CrossRef]
- Braguglia, C.M.; Mininni, G.; Rolle, E. Influence of anaerobic digestion on particle surface charge and optimal polymer dosage. Water Sci. Technol. 2006, 54, 43–50. [Google Scholar] [CrossRef]
- Bratskaya, S.; Schwarz, S.; Laube, J.; Liebert, T.; Heinze, T.; Krentz, O.; Lohmann, C.; Kulicke, W.-M. Effect of Polyelectrolyte Structural Features on Flocculation Behavior: Cationic Polysaccharides vs. Synthetic Polycations. Macromol. Mater. Eng. 2005, 290, 778–785. [Google Scholar] [CrossRef]
- Bratskaya, S.; Avramenko, V.; Schwarz, S.; Philippova, I. Enhanced flocculation of oil-in-water emulsions by hydrophobically modified chitosan derivatives. Colloids Surf. A Physicochem. Eng. Asp. 2006, 275, 168–176. [Google Scholar] [CrossRef]
- Mende, M.; Schwarz, S.; Petzold, G.; Jaeger, W. Destabilization of model silica dispersions by polyelectrolyte complex particles with different charge excess, hydrophobicity, and particle size. J. Appl. Polym. Sci. 2007, 103, 3776–3784. [Google Scholar] [CrossRef]
- Korhonen, M.H.J.; Rojas, O.J.; Laine, J. Effect of charge balance and dosage of polyelectrolyte complexes on the shear resistance of mineral floc strength and reversibility. J. Colloid Interface Sci. 2015, 448, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Petzold, G.; Rojas, R.; Goltzsche, C.; Lieske, A.; Jaeger, W. Einfluss der Molmasse auf die Flockungseffektivität von Poly-Diallyldimethylammoniumchlorid. Gwf Wasser Abwasser 2019, 10, 818–824. [Google Scholar]





| Sample | Molar Mass (g/mol) | Charge (meq/g) |
|---|---|---|
| PDADMAC | 37,000 | 6.60 |
| chitosan | 150,000–300,000 | 5.41 |
| PAM-TAC | ca. 6 mil. | 2.59 |
| silica | - | −0.0016 |
| china clay | - | −0.0243 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borchert, K.B.L.; Steinbach, C.; Schwarz, S.; Schwarz, D. A Comparative Study on the Flocculation of Silica and China Clay with Chitosan and Synthetic Polyelectrolytes. Mar. Drugs 2021, 19, 102. https://doi.org/10.3390/md19020102
Borchert KBL, Steinbach C, Schwarz S, Schwarz D. A Comparative Study on the Flocculation of Silica and China Clay with Chitosan and Synthetic Polyelectrolytes. Marine Drugs. 2021; 19(2):102. https://doi.org/10.3390/md19020102
Chicago/Turabian StyleBorchert, Konstantin B. L., Christine Steinbach, Simona Schwarz, and Dana Schwarz. 2021. "A Comparative Study on the Flocculation of Silica and China Clay with Chitosan and Synthetic Polyelectrolytes" Marine Drugs 19, no. 2: 102. https://doi.org/10.3390/md19020102
APA StyleBorchert, K. B. L., Steinbach, C., Schwarz, S., & Schwarz, D. (2021). A Comparative Study on the Flocculation of Silica and China Clay with Chitosan and Synthetic Polyelectrolytes. Marine Drugs, 19(2), 102. https://doi.org/10.3390/md19020102






