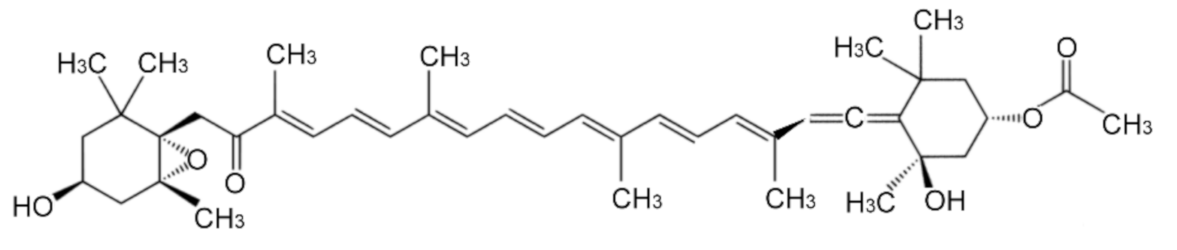
Cytoprotective Potential of Fucoxanthin in Oxidative Stress-Induced Age-Related Macular Degeneration and Retinal Pigment Epithelial Cell Senescence In Vivo and In Vitro
Abstract
:1. Introduction
2. Results
2.1. Fucoxanthin Pretrement Inhibits ROS Generation and Lipid Peroxidation in Sodium Iodate-Induced Retinal Degeneration Animal Model
2.2. Fucoxanthin Inhibits Cellular Senescence in Retinal Tissues of Sodium Iodate-Induced Retinal Degeneration In Vivo
2.3. Fucoxanthin Affects Oxidative Stress-Induced ROS Generation and Mitochondria Respiration
2.4. Fucoxanthin Protects ARPE-19 Cells from Hydrogen Peroxide-Induced Cellular Senescence and DNA Damage Response
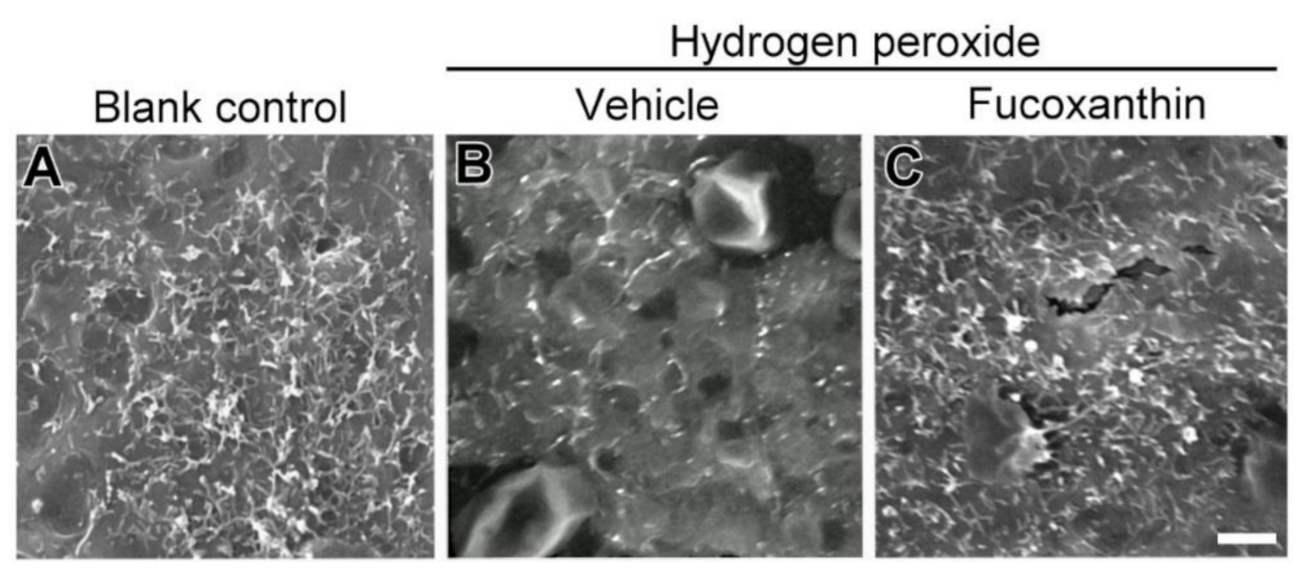
2.5. Fucoxanthin Promotes Cell Junction and Morphogenesis of Apical Microvilli
2.6. Fucoxanthin Protects Hydrogen Peroxide-Induced Degradation of Cytoskeleton Actin C and Disrupyion of Cell Junction
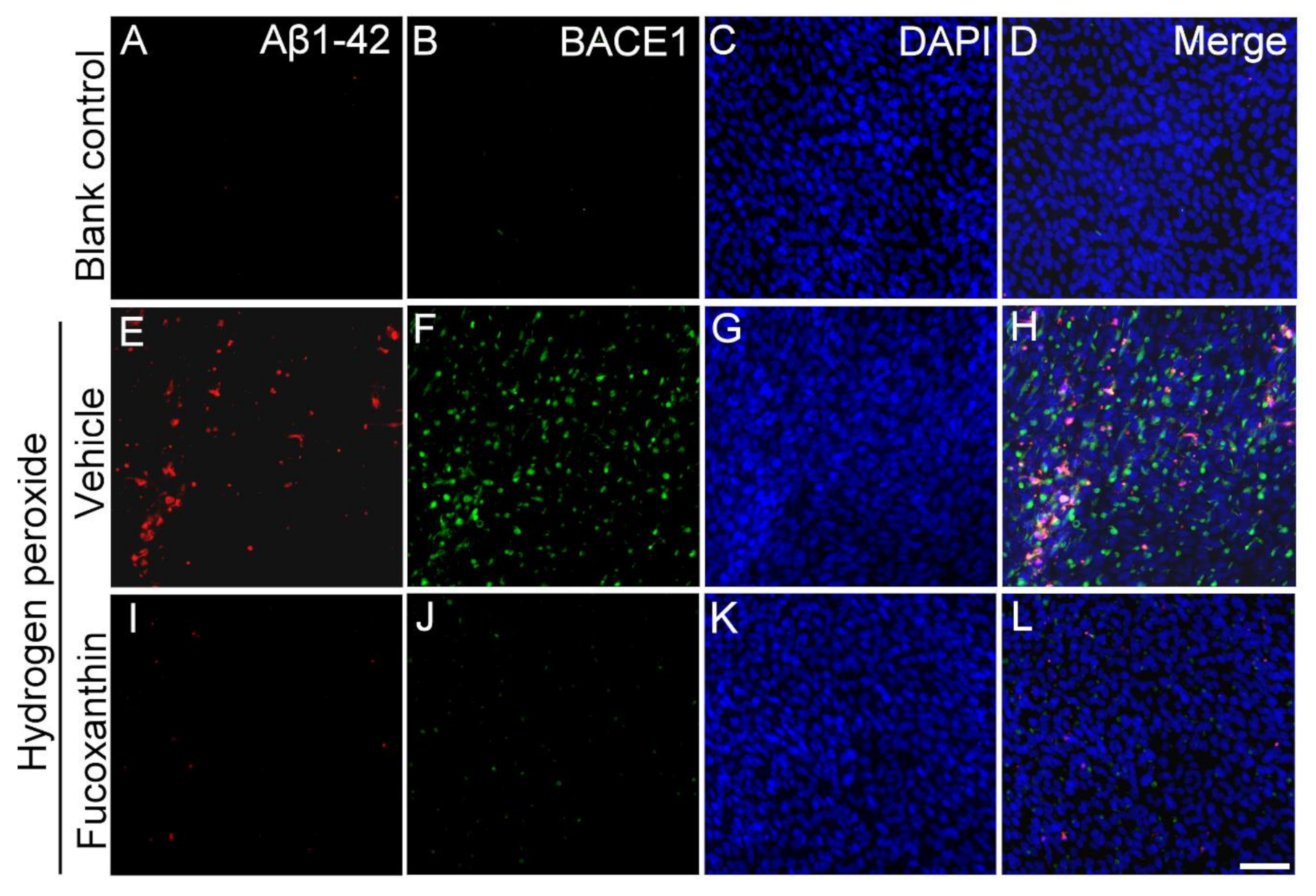
2.7. Fucoxanthin Inhibits Hydrogen Peroxide Exposure Up-Regulated Cellular Expressions of Aβ1-42 and Beta-Site Amyloid Precursor Protein-Cleaving Enzyme 1 (BACE1)
3. Discussion
4. Materials and Methods
4.1. Sodium Iodate-Induced Retinal Degeneration in Rat Model
4.2. Staining for SA b-Gal
4.3. ROS Generation
4.4. MDA Assay for Lipid Peroxidation
4.5. Cells and Treatments
4.6. MTT Assay for Mitochondrial Metabolic Rate
4.7. DNA Strand Damage Marker γ-H2AX
4.8. Scanning Electron Microscopy
4.9. Immunocytochemical Staining Assay
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, D.P. Redox theory of aging. Redox Biol. 2015, 5, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonilha, V.L. Age and disease-related structural changes in the retinal pigment epithelium. Clin. Ophthalmol. 2008, 2, 413–424. [Google Scholar] [CrossRef] [Green Version]
- Xia, C.H.; Liu, H.; Cheung, D.; Tang, F.; Chang, B.; Li, M.; Gong, X. NHE8 is essential for RPE cell polarity and photoreceptor survival. Sci. Rep. 2015, 5, 9358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Webster, S.H.; Rice, M.E.; Highman, B.; Von Oettingen, W.F. The toxicology of potassium and sodium iodates: Acute toxicity in mice. J. Pharmacol. Exp. Ther. 1957, 120, 171–178. [Google Scholar]
- Yang, Y.; Ng, T.K.; Ye, C.; Yip, Y.W.; Law, K.; Chan, S.O.; Pang, C.P. Assessing sodium iodate-induced outer retinal changes in rats using confocal scanning laser ophthalmoscopy and optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1696–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanus, J.; Anderson, C.; Sarraf, D.; Ma, J.; Wang, S. Retinal pigment epithelial cell necroptosis in response to sodium iodate. Cell Death Discov. 2016, 2, 16054. [Google Scholar] [CrossRef]
- Anderson, D.H.; Fisher, S.K. The relationship of primate foveal cones to the pigment epithelium. J. Ultrastruct. Res. 1979, 67, 23–32. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Hicks, D.; Hame, C.P. The retinal pigment epithelium in health and disease. Curr. Mol. Med. 2010, 10, 802–823. [Google Scholar] [CrossRef]
- Vinores, S.A.; Derevjanik, N.L.; Ozaki, H.; Okamoto, N.; Campochiaro, P.A. Cellular mechanisms of blood-retinal barrier dysfunction in macular edema. Doc. Ophthalmol. 1999, 97, 217–228. [Google Scholar] [CrossRef]
- De Smet, M.D.; Okada, A.A. Cystoid macular edema in uveitis. Dev. Ophthalmol. 2010, 47, 136–147. [Google Scholar] [PubMed]
- Dentchev, T.; Milam, A.H.; Lee, V.M.; Trojanowski, J.Q.; Dunaief, J.L. Amyloid-beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol. Vis. 2003, 9, 184–190. [Google Scholar] [PubMed]
- Masuda, N.; Tsujinaka, H.; Hirai, H.; Yamashita, M.; Ueda, T.; Ogata, N. Effects of concentration of amyloid β (Aβ) on viability of cultured retinal pigment epithelial cells. BMC Ophthalmol. 2019, 19, 70. [Google Scholar] [CrossRef] [Green Version]
- Ohno-Matsui, K. Parallel findings in age-related macular degeneration and Alzheimer’s disease. Prog. Retin. Eye Res. 2011, 30, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.; Zhu, P.; Verma, A.; Chakrabarty, P.; Rosario, A.M.; Golde, T.E.; Li, Q. Amyloid β peptides overexpression in retinal pigment epithelial cells via AAV-mediated gene transfer mimics AMD-like pathology in mice. Sci. Rep. 2017, 7, 3222. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, Y.; Liu, Y.; Li, C.; Wan, Q.; Yang, L.; Su, Y.; Cheng, Y.; Liu, C.; Wang, X.; et al. Reversed Senescence of Retinal Pigment Epithelial Cell by Coculture with Embryonic Stem Cell via the TGFβ and PI3K Pathways. Front. Cell Dev. Biol. 2020, 8, 588050. [Google Scholar] [CrossRef]
- Van Leeuwen, R.; Klaver, C.C.; Vingerling, J.R.; Hofman, A.; de Jong, P.T. Epidemiology of age-related maculopathy: A review. Eur. J. Epidemiol. 2003, 18, 845–854. [Google Scholar] [CrossRef]
- Malek, G.; Johnson, L.V.; Mace, B.E.; Saloupis, P.; Schmechel, D.E.; Rickman, D.W. Apolipoprotein E allele-dependent pathogenesis: A model for agerelated retinal degeneration. Proc. Natl. Acad. Sci. USA 2005, 102, 11900–11905. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Shi, Y.; Liu, X.; Zhang, H.; Gong, Y.; Gu, Q.; Wu, X.; Xu, X. A rat model for studying the biological effects of circulatng LDL in the choriocapillaris-BrM-RPE complex. Am. J. Pathol. 2012, 180, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Mitter, S.K.; Song, C.; Qi, X.; Mao, H.; Rao, H.; Akin, D.; Lewin, A.; Grant, M.; Dunn, W.J.; Ding, J.; et al. Dysregulated autophagy in the RPE is associated with increased susceptibility to oxidative stress and AMD. Autophagy 2014, 10, 1989–2005. [Google Scholar] [CrossRef] [Green Version]
- Imai, S.; Inokuchi, Y.; Nakamura, S.; Tsuruma, K.; Shimazawa, M.; Hara, H. Systemic administration of a free radical scavenger, edaravone, protects against light-induced photoreceptor degeneration in the mouse retina. Eur. J. Pharmacol. 2010, 642, 77–85. [Google Scholar] [CrossRef]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of antioxidant enzymes and small molecular weight antioxidants in the pathogenesis of age-related macular degeneration (AMD). Biogerontology 2013, 14, 461–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Mambro, V.M.; Borin, M.F.; Fonseca, M.J. Topical formulations with superoxide dismutase: Influence of formulation composition on physical stability and enzymatic activity. J. Pharm. Biomed. Anal. 2003, 32, 97–105. [Google Scholar] [CrossRef]
- Shamsi, F.A.; Chaudhry, I.A.; Boulton, M.E.; Al-Rajhi, A.A. L-Carnitine protects human retinal pigment epithelial cells from oxidative damage. Curr. Eye Res. 2007, 32, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Kikuchi, M.; Kubodera, A.; Kawakami, Y. Proton-donative antioxidant activity of fucoxanthin with 1,1-diphenyl-2-picrylhydrazyl (DPPH). Biochem. Mol. Biol. Int. 1997, 42, 361–370. [Google Scholar] [CrossRef]
- Morandi, A.C.; Molina, N.; Guerra, B.; Bolin, A.P.; Otton, R. Fucoxanthin in association with vitamin C acts as modulators of human neutrophil function. Eur. J. Nutr. 2013, 6, 779–792. [Google Scholar] [CrossRef]
- Chen, Y.C.; Cheng, C.Y.; Liu, C.T.; Sue, Y.M.; Chen, T.H.; Hsu, Y.H.; Hwang, P.A.; Chen, C.H. Alleviative effect of fucoxanthin-containing extract from brown seaweed Laminaria japonica on renal tubular cell apoptosis through upregulating Na+/H+ exchanger NHE1 in chronic kidney disease mice. J. Ethnopharmacol. 2018, 224, 391–399. [Google Scholar] [CrossRef]
- Karpiński, T.M.; Adamczak, A. Fucoxanthin―An Antibacterial Carotenoid. Antioxidants 2019, 8, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Zhang, H.; Liu, Y. Anti-Inflammatory and Apoptotic Signaling Effect of Fucoxanthin on Benzo(A)Pyrene-Induced Lung Cancer in Mice. J. Environ. Pathol. Toxicol. Oncol. 2019, 38, 239–251. [Google Scholar] [CrossRef]
- Chen, S.J.; Lee, C.J.; Lin, T.B.; Liu, H.J.; Huang, S.Y.; Chen, J.Z.; Tseng, K.W. Inhibition of Ultraviolet B-Induced Expression of the Proinflammatory Cytokines TNF-α and VEGF in the Cornea by Fucoxanthin Treatment in a Rat Model. Mar. Drugs 2016, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.J.; Lee, C.J.; Lin, T.B.; Peng, H.Y.; Liu, H.J.; Chen, Y.S.; Tseng, K.W. Protective Effects of Fucoxanthin on Ultraviolet B-Induced Corneal Denervation and Inflammatory Pain in a Rat Model. Mar. Drugs 2019, 17, 152. [Google Scholar] [CrossRef] [Green Version]
- Ambati, J.; Fowler, B.J. Mechanisms of age-related macular degeneration. Neuron 2012, 75, 26–39. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.; Neric, N.J.; Crabb, J.S.; Crabb, J.W.; Bhattacharya, S.K.; Rayborn, M.E.; Hollyfield, J.G.; Bonilha, V.L. Age-related changes in the retinal pigment epithelium (RPE). PLoS ONE 2012, 7, e38673. [Google Scholar] [CrossRef] [Green Version]
- Lu, Z.; Lin, V.; May, A.; Che, B.; Xiao, X.; Shaw, D.H.; Su, F.; Wang, Z.; Du, H.; Shaw, P.X. HTRA1 synergizes with oxidized phospholipids in promoting inflammation and macrophage infiltration essential for ocular VEGF expression. PLoS ONE 2019, 14, e0216808. [Google Scholar] [CrossRef]
- Tan, C.P.; Hou, Y.H. First evidence for the anti-inflammatory activity of fucoxanthin in high-fat-diet-induced obesity in mice and the antioxidant functions in PC12 cells. Inflammation 2014, 37, 443–450. [Google Scholar] [CrossRef]
- Aryan, N.; Betts-Obregon, B.S.; Perry, G.; Tsin, A.T. Oxidative Stress Induces Senescence in Cultured RPE Cells. Open Neurol. J. 2016, 10, 83–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Liu, Y.; Xie, J.; Huang, X.; Zhang, L.; Liu, H.; Li, L. Sirt3 mediates the protective effect of hydrogen in inhibiting ROS-induced retinal senescence. Free Radic. Biol. Med. 2019, 135, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, S.G.; Lin, H.; Godley, B.F.; Boulton, M.E. Mitochondrial DNA damage and its potential role in retinal degeneration. Prog. Retin. Eye Res. 2008, 27, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.L.; Lukas, T.J.; Yuan, M.; Neufeld, A.H. Increased mitochondrial DNA damage and down-regulation of DNA repair enzymes in aged rodent retinal pigment epithelium and choroid. Mol. Vis. 2008, 14, 644–651. [Google Scholar] [PubMed]
- Blasiak, J.; Glowacki, S.; Kauppinen, A.; Kaarniranta, K. Mitochondrial and nuclear DNA damage and repair in age-related macular degeneration. Int. J. Mol. Sci. 2013, 14, 2996–3010. [Google Scholar] [CrossRef] [Green Version]
- Rottenberg, H.; Hoek, J.B. The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore. Aging Cell. 2017, 16, 943–955. [Google Scholar] [CrossRef] [Green Version]
- Ferrington, D.A.; Ebeling, M.C.; Kapphahn, R.J.; Terluk, M.R.; Fisher, C.R.; Polanco, J.R.; Roehrich, H.; Leary, M.M.; Geng, Z.; Dutton, J.R.; et al. Altered bioenergetics and enhanced resistance to oxidative stress in human retinal pigment epithelial cells from donors with age-related macular degeneration. Redox Biol. 2017, 13, 55–265. [Google Scholar]
- Golestaneh, N.; Chu, Y.; Xiao, Y.Y.; Stoleru, G.L.; Theos, A.C. Dysfunctional autophagy in RPE, a contributing factor in age-related macular degeneration. Cell Death Dis. 2017, 8, e2537. [Google Scholar] [CrossRef]
- Brown, E.E.; DeWeerd, A.J.; Ildefonso, C.J.; Lewin, A.S.; Ash, J.D. Mitochondrial oxidative stress in the retinal pigment epithelium (RPE) led to metabolic dysfunction in both the RPE and retinal photoreceptors. Redox Biol. 2019, 24, 101201. [Google Scholar] [CrossRef]
- Mao, Y.; Finnemann, S.C. Live Imaging of LysoTracker-Labelled Phagolysosomes Tracks Diurnal Phagocytosis of Photoreceptor Outer Segment Fragments in Rat RPE Tissue Ex Vivo. Adv. Exp. Med. Biol. 2016, 85, 717–773. [Google Scholar]
- Miyoshi, J.; Takai, Y. Structural and functional associations of apical junctions with cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 670–691. [Google Scholar] [CrossRef] [Green Version]
- Shen, L. Tight junctions on the move: Molecular mechanisms for epithelial barrier regulation. Ann. N. Y. Acad. Sci. 2012, 1258, 9–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha-Vaz, J.; Bernardes, R.; Lobo, C. Blood-retinal barrier. Eur. J. Ophthalmol. 2011, 21, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell. Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Sun., J.; Huang, P.; Liang, J.; Li, J.; Shen, M.; She, X.; Feng, Y.; Luo, X.; Liu, T.; Sun, X. Cooperation of Rel family members in regulating Aβ1-40-mediated proinflammatory cytokine secretion by retinal pigment epithelial cells. Cell. Death Dis 2017, 8, e3115. [Google Scholar] [CrossRef] [Green Version]
- Lynn, S.A.; Keeling, E.; Munday, R.; Gabha, G.; Griffiths, H.; Lotery, A.J.; Ratnayaka, J.A. The complexities underlying age-related macular degeneration: Could amyloid beta play an important role? Neural. Regen. Res. 2017, 12, 538–548. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-J.; Lin, T.-B.; Peng, H.-Y.; Liu, H.-J.; Lee, A.-S.; Lin, C.-H.; Tseng, K.-W. Cytoprotective Potential of Fucoxanthin in Oxidative Stress-Induced Age-Related Macular Degeneration and Retinal Pigment Epithelial Cell Senescence In Vivo and In Vitro. Mar. Drugs 2021, 19, 114. https://doi.org/10.3390/md19020114
Chen S-J, Lin T-B, Peng H-Y, Liu H-J, Lee A-S, Lin C-H, Tseng K-W. Cytoprotective Potential of Fucoxanthin in Oxidative Stress-Induced Age-Related Macular Degeneration and Retinal Pigment Epithelial Cell Senescence In Vivo and In Vitro. Marine Drugs. 2021; 19(2):114. https://doi.org/10.3390/md19020114
Chicago/Turabian StyleChen, Shiu-Jau, Tzer-Bin Lin, Hsien-Yu Peng, Hsiang-Jui Liu, An-Sheng Lee, Cheng-Hsien Lin, and Kuang-Wen Tseng. 2021. "Cytoprotective Potential of Fucoxanthin in Oxidative Stress-Induced Age-Related Macular Degeneration and Retinal Pigment Epithelial Cell Senescence In Vivo and In Vitro" Marine Drugs 19, no. 2: 114. https://doi.org/10.3390/md19020114
APA StyleChen, S.-J., Lin, T.-B., Peng, H.-Y., Liu, H.-J., Lee, A.-S., Lin, C.-H., & Tseng, K.-W. (2021). Cytoprotective Potential of Fucoxanthin in Oxidative Stress-Induced Age-Related Macular Degeneration and Retinal Pigment Epithelial Cell Senescence In Vivo and In Vitro. Marine Drugs, 19(2), 114. https://doi.org/10.3390/md19020114





