Fish Waste: From Problem to Valuable Resource
Abstract
:1. Introduction
2. Fish Waste in the Circular Bioeconomy Era
3. Fish Byproducts as Source of High Added-Value Compounds
3.1. Collagen
Fish Collagen Applications
| Compound | Byproduct | Source | Applications | Activity/Concentration Used | Reference |
|---|---|---|---|---|---|
| Collagen | Skin | Tilapia | Cartilage tissue engineering | 2% weight/volume | [70] |
| Collagen/polycaprolactone | Scale | Oreochromis sp. | Cartilage tissue engineering | 12% weight/volume | [66] |
| Collagen/polycaprolactone | Skin | Paralichthys olivaceus | Bone regeneration | Not specified | [71] |
| Collagen | Scale | Oreochromis sp. | Tissue-engineeredoral mucosa | 1% weight/volume | [71,99] |
| Collagen/polyvinyl alcohol | Not specified | Oreochromis sp. | Human periodontal ligament fibroblasts (HPDLFs), gingival fibroblasts (HGFs) | Not specified | [72] |
| Collagen | Scales | Oreochromis niloticus | Bone regeneration | 0.3%, weight/volume | [73] |
| Collagen | Skin | Salmo salar | Bone tissue engineering | 0.2–2.5 mg/mL | [74] |
| Collagen | Cartilage | Prionace glauca Zeachara chilensis Bathyraja brachyurops | Bioscaffold | Not specified | [75] |
| Collagen/chitosan | Skin | Hypophthalmichthys molitrix | Skin regeneration | Not specified | [76] |
| Collagen | Skin | Oreochromis niloticus | Wound healing | 15 mg/mL | [78] |
| Collagen | Scale | Larimichthys crocea | Wound healing | 5–15% weight/volume | [82] |
| Collagen nanofibers | Skin | Oreochromis sp. | Skin regeneration, antibacterial | 30 mg/kg BW | [84] |
| Collagen | Skin | Oreochromis sp. | Wound healing | Not specified | [85] |
| Collagen | Scale | Ctenopharyngodon idellus | Wound healing | Not specified | [86] |
| Collagen | Skin | Lophius litulon | Antioxidant, wound healing | 1–8 mg/mL4 g/day | [87] |
| Collagen | Skin | Oreochromis sp. | Antioxidant | 2–7 mg/mL | [88] |
| Collagen/chitosan | Skin | Not specified | Antioxidant | 12–100 μg/mL | [89] |
| Collagen/gelatin | Skin | Priacanthus hamrur | Antioxidant, antibacterial | DPPH and ABTS 1 mg/mL Antibacterial 200 μg | [90] |
| Collagen | Swim bladders | Nibea japonica | Antioxidant | 12.5–50μg/mL | [91] |
| Collagen/gelatin | Cartilage | Carcharhinus albimarginatus | Antioxidant | 1–5 mg/mL | [94] |
| Collagen | Skin | Scomber japonicus | Antioxidant | 2.5–10 mg/mL | [93] |
| Collagen/gelatin | Scale | Katsuwonus pelamis | Antioxidant | 0.1–5 mg/mL | [100] |
| Collagen | Skin | Prionace glauca | Food packaging | 2.5% weight/weight | [97] |
| Collagen | Skin | Mustelus mustelus | Food packaging | 0.1% weight/volume | [98] |
3.2. Peptides
Fish Peptides Applications
| Compound | Byproduct | Source | Applications | Activity/ Concentration Used | Reference |
|---|---|---|---|---|---|
| SJGAP | Skin | Skipjack tuna (Katsuwonus pelamis) | Antimicrobial | MECs 3, 26, 4.8, 25, 2.7, 9, 16 μg/mL against B. subtilis, M. luteus, S. iniae, A. hydrophila, E. coli, V. parahaemolytics, C. albicans | [108] |
| YFGAP | Skin | Yellowfin tuna (Thunnus albacares) | Antimicrobial | MECs 1.2, 6.5, 17, 8, 3, 3.2 μg/mL against B. subtilis, M. luteus, S. iniae, A. hydrophila, E.coli, V. parahaemolytics | [109] |
| GKLNLFLSRLEILKLFVGA | Skin | Yellow catfish (Pelteobagrus fulvidraco) | Antimicrobial | MIC 2, 4, 16, 64 μg/mL against B. subtilis, S. aureus, E.coli, C. albicans | [110] |
| SIFIQRFTT, RKSGDPLGR, AKPGDGAGSGPR and GLPGLGPAGPK | Not specified | Scomber scombrus | Antibacterial | 200 μg/mL | [112] |
| N-KVEIVAINDPFIDL-C | Not specified | Scomber scombrus | Antibacterial | MIC 0.263, 0.131, 0.131, 0.263, 0.263 mMagainst L. acidophilus, L. ivanovi, L. monocytogenes, M. luteus, B. thetaiotaomicron | [113] |
| GWGSFFKKAAHVGKHVGKAALTHYL | Skin | Winter flounder (Pleuronectes americanus) | Antimicrobial | MIC 1.1–2.2, 4.4–8.8, 17.7–35.0, 2.2–3.3, 8.8–17.7, 17.7–35.0 μM against B. subtilis, P. haemolytica, S. aureus, E.coli, S. typhimurium (I and II), A. salmonicida | [111] |
| GPL, GPM | Skin | Theragra chalcogramma | Antihypertensive | IC50 = 2.6 and 17.1 μM | [115] |
| DPALATEPDPMPF | Not specified | Oreochromis niloticus | Antihypertensive | 1–20 μg/mL | [117] |
| FGASTRGA | Frame | Pollack (Theragra chalcogramma) | Antihypertensive | IC50 = 14.7 µM | [116] |
| GPEGPAGAR GETGPAGPAGAAGPAGPR | Skin | Oreochromis niloticus | Antihypertensive | 5 mg/mL | [118] |
| MVGSAPGVL, LGPLGHQ | Skin | Skate | Antihypertensive | IC50 = 3.09, 4.22 µM | [119] |
| GASSGMPG, LAYA | Skin | Pacific cod skin | Antihypertensive | IC50 = 6.9, 14.5 µM | [120] |
| Collagen peptides | Skin | Theragra chalcogramma | Antihypertensive | IC50 = 0.49 mg/mL | [122] |
| GIPGAP and APGAP | Skin | Raja clavata | Antihypertensive | IC50 =27.9, 170.2 μM | [123] |
| GY, VY, GF, VIY | Scale | Sea Bream | Antihypertensive | IC50 = 265, 16, 708, 7.5 µM | [124] |
| PGPLGLTGP, QLGFLGPR | Skin | Skate | Antihypertensive | IC50 = 95, 148 µM | [125] |
| GLPLNLP | Skin | Salmon (Oncorhynchus keta) | Antihypertensive | 18.7 µM | [126] |
| MIFPGAGGPEL | Frame | Sole (Limanda aspera) | Antihypertensive | 28.7 μg/ml | [127] |
| GDLGKTTTVSNWSPPKYKDTP | Frame | Tuna | Antihypertensive | 11.28 μM | [128] |
| Hydrolysates with MW between 1000 and 10,000 Da | Bone | Yellowtail | Antihypertensive, antioxidant | 1.9 mg/mL (ACE inhib.), ~10 mg/mL (DPPH) | [129] |
| Hydrolysates with MW ≤ 1000 Da | Bone | Yellowtail | Antihypertensive, antioxidant | 1.5 mg/mL (ACE inhib.), ~35 mg/mL (DPPH) | [129] |
| Hydrolysates with weight ̴ 30 KDa | Frame | Cod | Antioxidant, antihypertensive | ~20% (antiox.), ~40 mg protein/mL × 100 | [130] |
| Hydrolysates with weight ̴ 10 KDa | Frame | Cod | Antioxidant, antihypertensive | ~15% (antiox.), ~35 mg protein/mL × 100 | [130] |
| Hydrolysates with weight ̴ 5 KDa | Frame | Cod | Antioxidant, antihypertensive | ~40% (antiox.), ~20 mg protein/mL × 100 | [130] |
| Hydrolysates with weight ̴ 3 KDa | Frame | Cod | Antioxidant, antihypertensive | ~18% (antiox.), ~8 mg protein/mL × 100 | [130] |
| PYSFK, GFGPEL, VGGRP. | Skin | Grass carp (Ctenopharyngodon idella) | Antioxidant | DPPH radical 2.459, 3.634, 6.063 mM (DPPH), 3.563, 2.606, 4.241 mM (hydroxyl), 0.281, 0.530, 0.960 mM (ABTS) | [132] |
| AVGAT | Skin | Thornback ray | Antioxidant | 33% of activity at 3 mg/mL (DPPH) | [123] |
| DPALATEPDMPF | Skin | Nile tilapia (Oreochromis niloticus) | Antioxidant | 8.82 µM (DPPH), 7.56 µM (Hydroxyl) | [133] |
| PFGPD, PYGAKG, YGPM | Skin | Spanish mackerel Scomberomorous niphonius | Antioxidant | 0.80, 3.02, 0.72 mg/mL (DPPH), 0.81, 0.66, 0.88 mg/mL (hydroxyl), 0.91, 0.80, 0.73 (superoxide anion), 0.86, 1.07, 0.82 mg/mL (ABTS) | [134] |
| GSGGL, GPGGFI, FIGP | Skin | Blue leatherjacket (Navodon septentrionalis) | Antioxidant | 405 µg/mL (DPPH), 179 µg/mL (Hydroxyl); 194 µg/mL (DPPH), 89 µg/mL (Hydroxyl); 118 µg/mL (DPPH), 73 µg/mL (Hydroxyl) | [135] |
| GLFGPR GATGPQGPLGPR, VLGPF, QLGLGPV | Skin | Seabass (Lates calcarifer) | Antioxidant | 81.41, 10.4, 2.59, 0.50 mmol Trolox equivalents/µmol peptide (ABTS) | [136] |
| FDSGPAGVL, DGPLQAGQPGER | Skin | Jumbo squid (Dosidicus gigas) | Antioxidant | _ | [137] |
| PAGT | Skin | Amur sturgeon | Antioxidant | 5380 µg/mL (DPPH), 890 µg/mL (Hydroxyl), 8 µg/mL (ABTS) | [138] |
| EGL, YGDEY | Skin | Nile tilapia (Oreochromis niloticus) | Antioxidant | 4.61 µg/mL (Hydroxyl), 6.45 µg/mL (Hydroxyl) | [134] |
| LSGYGP | Skin | Tilapia (Oreochromis niloticus) | Antioxidant | 22.47 μg/mL | [140] |
| Hydrolysates | Skin | Snapper (Priacanthus macracanthus) | Antioxidant | _ | [141] |
| Hydrolysates | Skin | Snapper (Lutjanus vitta) | Antioxidant | _ | [142] |
| Hydrolysates | Skin | Sole | Antioxidant | _ | [143] |
| Hydrolysates | WB, Head, Gonads | Herring (Clupea harengus) | Antioxidant | _ | [144] |
| TCSP, TGGGNV | Skin | Cod (Gadus microcephalus) | Antihypertensive, Antioxidant | 81%, 68% at 500 µg/mL, 75% at 500 µg/mL (scavenging activity) for both | [145] |
| N-terminal RPDFDLEPPY | Frame | Sole (Limanda aspera) | Antioxidant | _ | [147] |
| Collagen/gelatin/peptides | Skin | Thunnus albacares | Antioxidant | 9–700 μg/mL | [92] |
| GLFGPR | Skin | Lates calcarifer | Antioxidant | 5, 10 mg/mL | [152] |
| HGPLGPL | Skin | Hoki (Johnius belengerii) | Antioxidant | 156.2 µM (DPPH) | [137] |
| GPRGTIGLVG, GPAGPAG and GFPSG | Scales | Pseudosciaena crocea | Antioxidant | IC50 (mg/mL): 0.293, 0.240, 0.107, (hydroxyl); 1.271, 0.675, 0.283 (DPPH); 0.463, 0.099, 0.151 (superoxide anion); 0.421, 0.309, 0.210 (ABTS). | [153] |
| HGPHGE, DGPKGH and MLGPFGPS | Scales | Katsuwonus pelamis | Antioxidant | EC50 mg/mL: 1.34, 0.54, 0.67 (DPPH) 1.03, 0.41, 0.74 (hydroxyl) 1.19, 0.71, 1.59 (superoxide anion) | [100] |
| GAEGFIF | Bone | Katsuwonus pelamis | Antioxidant | EC50 mg/mL: 0.57, 0.30 (DPPH); 0.25, 0.32 (hydroxyl) 0.52, 0.48 (superoxide anion) 0.41, 0.21 (ABTS) | [154] |
| GPE, GARGPQ and GFTGPPGNG | Cartilage | Sphyrna lewini | Antioxidant | EC50 mg/mL: 2.43, 2.66, 1.99 (DPPH); 0.28, 0.21, 0.15 (hydroxyl) 0.24, 0.18, 0.29 (ABTS); 0.10, 0.14, 0.11 (superoxide anion) | [155] |
| YGCC, DSSCSG, NNAEYYK and PAGNVR | Skin | Theragra chalcogramma | Antioxidant | IC50 = 7.63 μg/mL | [156] |
| NHRYDR | Skin | Horse Mackerel (Magalaspis cordyla) | Antioxidant | 72.3% (DPPH), 51.2% (Hydroxyl) | [157] |
| GNRGFACRHA | Skin | Crocker (Otolithes ruber) | Antioxidant | 79.6% (DPPH), 56.8% (Hydroxyl) | [157] |
| QGYRPLRGPEFL | Skin | Skate (Raja kenojei) | Neuroprotective | 24.26 μM | [158] |
| Collagen peptides | Skin | Salmon (Oncorhynchus keta) | Neuroprotective | 0.33, 1.0, 3.0 g/kg rat body | [159] |
| Collagen peptides | Skin | Salmon (Oncorhynchus keta) | Neuroprotective | 0.22%, 0.44% or 1.32% wt/wt diet | [160] |
| Collagen peptide drink | Not specified | Not specified | Antioxidant | 0.25% weight/volume | [162] |
| Hydrolyzed collagen | Not specified | Pangasius hypophthalmus | Skin elasticity | 10 g daily | [161] |
| EIGPSGGRGKPGKDGDAGPK, GFSGLDGAKGD | Skin | Cod | Matrix metalloproteinase inhibitory activity | 0.1 mg/mL | [163] |
| Collagen tripeptide | Skin | Sutchi catfish (Pangasius hypophthalmus) | Matrix metalloproteinase inhibitory, anti-photoaging | 167–333 mg/kg/day | [164] |
| Collagen hydrolysate | Not specified | Not specified | Osteoclastic differentiation of BMSCs | 0.2 mg/mL | [165] |
| SWFCP | Skin | Tuna | Adipocite differentiation | 0.5–1 mg/mL | [166] |
| FIMGLY | Cartilage | Raja porosa | Anticancer | IC50 = 4.81 mg/mL | [168] |
| Collagen hydrolysate | Skin | Aluterus monoceros | Anticancer/antidiabetic/wound healing | 0.05–1 mg/mL | [169] |
| Collagen peptides | Skin | Oncorhynchus keta | Bone regeneration | 1.125, 2.25 or 4.5 g kg−1 BW | [170] |
| Collagen peptide | Skin | Gadiformes species | Chondroprotective | 1 g/day | [171] |
| VLSGGTTMYASLYAE | Frame | Hoki (Johnius belengerii) | Calcium binding | - | [172] |
| VLSGGTTMAMYTLV | Frame | Pollack (Theragra chalcogramma) | Calcium binding | - | [173] |
| Phosphopeptide (FBP) | Bone | Hoki (Johnius belengerii) | Calcium binding | - | [174] |
| GPAGPHGPPGKDGR, AGPHGPPGKDGR, AGPAGPAGAR | Skin | Pacific cod | Iron-chelating | _ | [175] |
3.3. Chitin
| Compound | Byproduct | Source | Applications | Reference |
|---|---|---|---|---|
| Chitin | Scales | Cyprinus carpio I. | Not specified | [191] |
| Chitin, Chitosan | Scales | Labeo rohita | Not specified | [192] |
| Chitin, Chitosan | Scales | Labeo rohita | Not specified | [193] |
| Chitosan | Scales | Labeo rohita | Not specified | [194] |
| Chitin, Chitosan | Scales | Oreochromis niloticus | Not specified | [45] |
| Chitin | Scales | Chlorurus sordidus | Not specified | [197] |
| Chitin | Scales | Lutjanus argentimaculatus | Not specified | [197] |
| Chitin, Chitosan | Scales | Lutjanus sp. | Not specified | [198] |
| Chitosan | Scales | Anabas testudineus | Coagulation-flocculation treatment for iron removal | [199] |
Fish Chitin Applications
3.4. Oil
Fish Oil Applications
| Compound | By-product | Source | Applications | Activity | Reference |
|---|---|---|---|---|---|
| Oil | Viscera | LabeoRohita, Catla catla | Supplement in animal feeding | Reduction in cholesterol (9.2 to 16.6%) and in triglyceride (1.5 to 3.1%) | [248] |
| Cod liver oil | Liver | Cod fish | Supplement in bacterial growth media | 14.8 U/mL (lipase production) | [251] |
| Omega -3, -6, -7, -9 fatty acids | Head, tissues | Salmo salar | Antimicrobial | MIC: 25 and 12.5 (%v/v) | [252] |
| Tilapia oil | Viscera, fins, heads, skin, scales and mixed waste | Tilapia | Biodiesel | Not specified | [255] |
| Oil | Viscera | Centropomus Poeyi, Spondyliosoma cantharus, Scomberomorus cavalla, Eugerres plumieri | Biodiesel | Not specified | [256] |
| Fish Oil Methyl Ester (FOME) | Fish waste | Not specified | Biofuel | Not specified | [32] |
| Liver oil | Ray liver waste | Dasyatis pastinaca, Dasyatis violacea, Rhinoptera marginata | Antioxidant | IC50 0.92 to 2.1 mg/mL (DPPH) | [249] |
| Sulphated fatliquor | Fish waste | Not specified | Lubricant | Not specified | [48] |
3.5. Enzymes
Fish Enzyme Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- United Nations. Special Edition: Progress towards the Sustainable Development Goals. Available online: https://undocs.org/E/2019/68 (accessed on 17 September 2019).
- FAO. The State of World Fisheries and Acquaculture 2016. Available online: http://www.fao.org/publications/sofia/2016/en/#:~:text=The%20State%20of%20World’s%20Fisheries%20and%20Aquaculture%202016&text=As%20always%2C%20the%20scope%20is,%2C%20utilization%2C%20trade%20and%20consumption (accessed on 5 October 2020).
- FAO. The State of World Fisheries and Acquaculture 2018. Available online: http://www.fao.org/state-of-fisheries-aquaculture/2018/en (accessed on 5 November 2020).
- Lopes, C.; Antelo, L.T.; Franco-Uría, A.; Alonso, A.A.; Pérez-Martín, R. Valorisation of fish by-products against waste management treatments—Comparison of environmental impacts. Waste Manag. 2015, 46, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.Y.; Man, Y.B.; Wong, M.H. Use of food waste, fish waste and food processing waste for China’s aquaculture industry: Needs and challenge. Sci. Total Environ. 2018, 613, 635–643. [Google Scholar] [CrossRef]
- Stevens, J.R.; Newton, R.W.; Tlusty, M.; Little, D.C. The rise of aquaculture by-products: Increasing food production, value, and sustainability through strategic utilisation. Mar. Policy 2018, 90, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Beheshti Foroutani, M.; Parrish, C.C.; Wells, J.; Taylor, R.G.; Rise, M.L.; Shahidi, F. Minimizing marine ingredients in diets of farmed Atlantic salmon (Salmo salar): Effects on growth performance and muscle lipid and fatty acid composition. PLoS ONE 2018, 13, e0198538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, I.; Dauksas, E.; Remme, J.F.; Richardsen, R.; Løes, A.-K. Fish and fish waste-based fertilizers in organic farming—With status in Norway: A review. Waste Manag. 2020, 115, 95–112. [Google Scholar] [CrossRef]
- Guillen, J.; Holmes, S.J.; Carvalho, N.; Casey, J.; Dörner, H.; Gibin, M.; Mannini, A.; Vasilakopoulos, P.; Zanzi, A. A Review of the European Union Landing Obligation Focusing on Its Implications for Fisheries and the Environment. Sustainability 2018, 10, 900. [Google Scholar] [CrossRef] [Green Version]
- Shahidi, F.; Varatharajan, V.; Peng, H.; Senadheera, R. Utilization of marine by-products for the recovery of value-added products. J. Food Bioact. 2019, 6, 10–61. [Google Scholar] [CrossRef] [Green Version]
- Shavandi, A.; Hou, Y.; Carne, A.; McConnell, M.; Bekhit, A.E.-d.A. Marine waste utilization as a source of functional and health compounds. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2019; Volume 87, pp. 187–254. [Google Scholar]
- Mohan, S.V.; Varjani, S.; Pant, D.; Sauer, M.; Chang, J.S. Circular bioeconomy approaches for sustainability. Bioresour. Technol. 2020, 318, 124084. [Google Scholar] [CrossRef]
- Commission, E. A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment. Available online: https://ec.europa.eu/research/bioeconomy/pdf/ec_bioeconomy_strategy_2018.pdf (accessed on 17 September 2020).
- Coppola, D.; Oliviero, M.; Vitale, G.A.; Lauritano, C.; D’Ambra, I.; Iannace, S.; De Pascale, D. Marine Collagen from Alternative and Sustainable Sources: Extraction, Processing and Applications. Mar. Drugs 2020, 18, 214. [Google Scholar] [CrossRef] [Green Version]
- Arvanitoyannis, I.S.; Kassaveti, A. Fish industry waste: Treatments, environmental impacts, current and potential uses. Int. J. Food Sci. Technol. 2008, 43, 726–745. [Google Scholar] [CrossRef]
- Løes, A.-K.; Katsoulas, N.; Caceres, R.; de Cara, M.; Cirvilleri, G.; Kir, A.; Knebel, L.; Malinska, K.; Oudshoorn, F.; Raskin, B. Current use of Peat, Plastic and Fertiliser Inputs in Organic Horticultural and Arable Crops Across Europe; European Commission: Brussels, Belgium, 2018. [Google Scholar]
- Wu, Y.; Song, K. Anaerobic co-digestion of waste activated sludge and fish waste: Methane production performance and mechanism analysis. J. Clean. Prod. 2021, 279, 123678. [Google Scholar] [CrossRef]
- Choe, U.; Mustafa, A.M.; Lin, H.; Xu, J.; Sheng, K. Effect of bamboo hydrochar on anaerobic digestion of fish processing waste for biogas production. Bioresour. Technol. 2019, 283, 340–349. [Google Scholar] [CrossRef]
- Hou, Y.; Shavandi, A.; Carne, A.; Bekhit, A.A.; Ng, T.B.; Cheung, R.C.F.; Bekhit, A.E.-d.A. Marine shells: Potential opportunities for extraction of functional and health-promoting materials. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1047–1116. [Google Scholar] [CrossRef]
- Rustad, T.; Storrø, I.; Slizyte, R. Possibilities for the utilisation of marine by-products. Int. J. Food Sci. Technol. 2011, 46, 2001–2014. [Google Scholar] [CrossRef]
- Arnaud, C.; De Lamballerie, M.; Pottier, L. Effect of high pressure processing on the preservation of frozen and re-thawed sliced cod (Gadus morhua) and salmon (Salmo salar) fillets. High Press. Res. 2017, 38, 62–79. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Chamorro, S.; Brenes, A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: A review. Food Res. Int. 2015, 73, 204–212. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Benjakul, S. Proteolysis and Its Control Using Protease Inhibitors in Fish and Fish Products: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 496–509. [Google Scholar] [CrossRef]
- Adler-Nissen, J. Control of the proteolytic reaction and of the level of bitterness in protein hydrolysis processes. J. Chem. Technol. Biotechnol. Biotechnol. 2008, 34, 215–222. [Google Scholar] [CrossRef]
- Olsen, R.L.; Toppe, J.; Karunasagar, I. Challenges and realistic opportunities in the use of by-products from processing of fish and shellfish. Trends Food Sci. Technol. 2014, 36, 144–151. [Google Scholar] [CrossRef]
- Falch, E.; Sandbakk, M.; Aursand, M. On-board handling of marine by-products to prevent microbial spoilage, enzymatic reactions and lipid oxidation. In Maximising the Value of Marine By-Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 47–64. [Google Scholar]
- Guérard, F.; Sellos, D.; Le Gal, Y. Fish and Shellfish Upgrading, Traceability. In Tissue Engineering III: Cell—Surface Interactions for Tissue Culture; Springer: New York, NY, USA, 2005; Volume 96, pp. 127–163. [Google Scholar]
- Batista, I. By-catch, underutilized species and underutilized fish parts as food ingredients. In Maximising the Value of Marine By-Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 171–195. [Google Scholar]
- Galanakis, C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process. 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Ching-Velasquez, J.; Fernández-Lafuente, R.; Rodrigues, R.C.; Plata, V.; Rosales-Quintero, A.; Torrestiana-Sánchez, B.; Tacias-Pascacio, V.G. Production and characterization of biodiesel from oil of fish waste by enzymatic catalysis. Renew. Energy 2020, 153, 1346–1354. [Google Scholar] [CrossRef]
- Blanco, M.; Sotelo, C.G.; Pérez-Martín, R.I. New Strategy to Cope with Common Fishery Policy Landing Obligation: Collagen Extraction from Skins and Bones of Undersized Hake (Merluccius merluccius). Polymers 2019, 11, 1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakash, S.; Prabhahar, M.; Sendilvelan, S.; Venkatesh, R.; Singh, S.; Bhaskar, K. Experimental studies on the performance and emission characteristics of an automobile engine fueled with fish oil methyl ester to reduce environmental pollution. Energy Procedia 2019, 160, 412–419. [Google Scholar] [CrossRef]
- Prokopov, I.A.; Kovaleva, E.L.; Minaeva, E.D.; Pryakhina, E.A.; Savin, E.V.; Gamayunova, A.V.; Pozharitskaya, O.N.; Makarov, V.G.; Shikov, A.N. Animal-derived medicinal products in Russia: Current nomenclature and specific aspects of quality control. J. Ethnopharmacol. 2019, 240, 111933. [Google Scholar] [CrossRef]
- Kandyliari, A.; Mallouchos, A.; Papandroulakis, N.; Golla, J.P.; Lam, T.T.; Sakellari, A.; Karavoltsos, S.; Vasiliou, V.; Kapsokefalou, M. Nutrient Composition and Fatty Acid and Protein Profiles of Selected Fish By-Products. Foods 2020, 9, 190. [Google Scholar] [CrossRef] [Green Version]
- Esteban, M.; García, A.; Ramos, P.; Márquez, M. Evaluation of fruit–vegetable and fish wastes as alternative feedstuffs in pig diets. Waste Manag. 2007, 27, 193–200. [Google Scholar] [CrossRef]
- Vidotti, R.M.; Viegas, E.M.M.; Carneiro, D.J. Amino acid composition of processed fish silage using different raw materials. Anim. Feed. Sci. Technol. 2003, 105, 199–204. [Google Scholar] [CrossRef]
- Abbey, L.; Glover-Amengor, M.; Atikpo, M.O.; Atter, A.; Toppe, J. Nutrient content of fish powder from low value fish and fish byproducts. Food Sci. Nutr. 2017, 5, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Abuine, R.; Rathnayake, A.U.; Byun, H.-G. Biological activity of peptides purified from fish skin hydrolysates. Fish. Aquat. Sci. 2019, 22, 10. [Google Scholar] [CrossRef] [Green Version]
- Nasri, M. Bioactive Peptides from Fish Collagen Byproducts: A Review. In Byproducts from Agriculture and Fisheries: Adding Value for Food, Feed, Pharma, and Fuels; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 309–333. [Google Scholar]
- Lauritano, C.; Martínez, K.A.; Battaglia, P.; Granata, A.; De La Cruz, M.; Cautain, B.; Martín, J.; Reyes, F.; Ianora, A.; Guglielmo, L. First evidence of anticancer and antimicrobial activity in Mediterranean mesopelagic species. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Karkal, S.S.; Kudre, T.G. Valorization of fish discards for the sustainable production of renewable fuels. J. Clean. Prod. 2020, 275, 122985. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Venugopal, V. Enzymes from seafood processing waste and their applications in seafood processing. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 78, pp. 47–69. [Google Scholar]
- Granito, R.N.; Renno, A.C.M.; Yamamura, H.; De Almeida, M.C.; Ruiz, P.L.M.; Ribeiro, D.A. Hydroxyapatite from Fish for Bone Tissue Engineering: A Promising Approach. Int. J. Mol. Cell. Med. 2018, 7, 80–90. [Google Scholar] [PubMed]
- Alcalde, L.B.; Fonseca, G.G. Alkali process for chitin extraction and chitosan production from Nile tilapia (Oreochromis niloticus) scales. Lat. Am. J. Aquat. Res. 2016, 44, 683–688. [Google Scholar] [CrossRef]
- Bonilla-Méndez, J.R.; Hoyos-Concha, J.L. Methods of extraction, refining and concentration of fish oil as a source of omega-3 fatty acids. Corpoica Cienc. Tecnol. Agropecu. 2018, 19, 645–668. [Google Scholar]
- Huang, C.-Y.; Wu, C.-H.; Yang, J.-I.; Li, Y.-H.; Kuo, J.-M. Evaluation of iron-binding activity of collagen peptides prepared from the scales of four cultivated fishes in Taiwan. J. Food Drug Anal. 2015, 23, 671–678. [Google Scholar] [CrossRef] [Green Version]
- Saranya, R.; Selvi, A.T.; Jayapriya, J.; Aravindhan, R. Synthesis of Fat Liquor Through Fish Waste Valorization, Characterization and Applications in Tannery Industry. Waste Biomass Valorization 2020, 11, 6637–6647. [Google Scholar] [CrossRef]
- Jafari, H.; Lista, A.; Siekapen, M.M.; Ghaffari-Bohlouli, P.; Nie, L.; Alimoradi, H.; Shavandi, A. Fish Collagen: Extraction, Characterization, and Applications for Biomaterials Engineering. Polymers 2020, 12, 2230. [Google Scholar] [CrossRef] [PubMed]
- Müller, W.E.G. The Origin of Metazoan Complexity: Porifera as Integrated Animals. Integr. Comp. Biol. 2003, 43, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, K.H.; Miyata, T.; Rubin, A.L. Collagen as a Biomaterial. Annu. Rev. Biophys. Bioeng. 1974, 3, 231–253. [Google Scholar] [CrossRef]
- Karsdal, M. Biochemistry of Collagens, Laminins and Elastin: Structure, Function and Biomarkers; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Esposito, J.Y.; Cluzel, C.; Garrone, R.; Lethias, C. Evolution of collagens. Anat. Rec. 2002, 268, 302–316. [Google Scholar] [CrossRef]
- Mariod, A.; Fadul, H. Review: Gelatin, source, extraction and industrial applications. ACTA Sci. Pol. Technol. Aliment. 2013, 12, 135–147. [Google Scholar]
- Cole, C.; McGill, A. Effect of animal age and conditioning method on the conversion of bovine hide into gelatine. Int. J. Food Sci. Technol. 1988, 23, 525–529. [Google Scholar] [CrossRef]
- Kariduraganavar, M.Y.; Kittur, A.A.; Kamble, R.R. Polymer synthesis and processing. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–31. [Google Scholar]
- Edgar, S.; Hopley, B.; Genovese, L.; Sibilla, S.; Laight, D.; Shute, J. Effects of collagen-derived bioactive peptides and natural antioxidant compounds on proliferation and matrix protein synthesis by cultured normal human dermal fibroblasts. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- León-López, A.; Morales-Peñaloza, A.; Martínez-Juárez, V.M.; Vargas-Torres, A.; Zeugolis, D.I.; Aguirre-Álvarez, G. Hydrolyzed Collagen-Sources and Applications. Molecules 2019, 24, 4031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, C.; Wei, Q.; Ren, X. Selective Extraction of Collagen Peptides with High Purity from Cod Skins by Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2017, 5, 7220–7227. [Google Scholar] [CrossRef]
- Pallela, R.; Bhatnagar, I.; Venkatesan, J.; Shim, Y.; Kim, S. Application of Marine Collagen—Based Scaffolds in Bone Tissue Engineering. In Marine Biomaterials, 1st ed.; Kim, S.-K., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 519–528. [Google Scholar]
- Lim, Y.-S.; Ok, Y.-J.; Hwang, S.-Y.; Kwak, J.-Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sripriya, R.; Kumar, R. A Novel Enzymatic Method for Preparation and Characterization of Collagen Film from Swim Bladder of Fish Rohu (Labeo rohita). Food Nutr. Sci. 2015, 6, 1468–1478. [Google Scholar] [CrossRef] [Green Version]
- Subhan, F.; Ikram, M.; Shehzad, A.; Ghafoor, A. Marine Collagen: An Emerging Player in Biomedical applications. J. Food Sci. Technol. 2014, 52, 4703–4707. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, A.M.; Marques, A.P.; Silva, T.H.; Reis, R.L. Evaluation of the Potential of Collagen from Codfish Skin as a Biomaterial for Biomedical Applications. Mar. Drugs 2018, 16, 495. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.L.; Marques, A.L.P.; Martins, E.; Silva, T.H.; Reis, R.L. Cosmetic Potential of Marine Fish Skin Collagen. Cosmetics 2017, 4, 39. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Duan, L.; Li, Y.; She, Y.; Zhu, J.; Zhou, G.; Jiang, G.; Yang, Y. Nanofibrillar Decellularized Wharton’s Jelly Matrix for Segmental Tracheal Repair. Adv. Funct. Mater. 2020, 30, 1910067. [Google Scholar] [CrossRef]
- Shen, Y.; Tu, T.; Yi, B.; Wang, X.; Tang, H.; Liu, W.; Zhang, Y. Electrospun acid-neutralizing fibers for the amelioration of inflammatory response. Acta Biomater. 2019, 97, 200–215. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, G.; Cao, Y. Recent Progress in Cartilage Tissue Engineering—Our Experience and Future Directions. Engineering 2017, 3, 28–35. [Google Scholar] [CrossRef]
- Lin, L.; Xu, Y.; Li, Y.; Gong, X.; Wei, M.; Zhang, W.; Zhang, X.; Xu, Y. Nanofibrous Wharton’s jelly scaffold in combination with adipose-derived stem cells for cartilage engineering. Mater. Design 2020, 186, 108216. [Google Scholar] [CrossRef]
- Li, H.; Chen, R.; Jia, Z.; Wang, C.; Xu, Y.; Li, C.; Xia, H.; Meng, D. Porous fish collagen for cartilage tissue engineering. Am. J. Transl. Res. 2020, 12, 6107–6121. [Google Scholar]
- Suzuki, A.; Kato, H.; Kawakami, T.; Kodama, Y.; Shiozawa, M.; Kuwae, H.; Miwa, K.; Hoshikawa, E.; Haga, K.; Shiomi, A.; et al. Development of microstructured fish scale collagen scaffolds to manufacture a tissue-engineered oral mucosa equivalent. J. Biomater. Sci. Polym. Ed. 2020, 31, 578–600. [Google Scholar] [CrossRef]
- Zhou, T.; Zheng, K.; Sui, B.; Boccaccini, A.R.; Sun, J. In vitro evaluation of poly (vinyl alcohol)/collagen blended hydrogels for regulating human periodontal ligament fibroblasts and gingival fibroblasts. Int. J. Biol. Macromol. 2020, 163, 1938–1946. [Google Scholar] [CrossRef]
- Tang, J.; Saito, T. Biocompatibility of novel type I collagen purified from tilapia fish scale: An in vitro comparative study. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Hoyer, B.; Bernhardt, A.; Heinemann, S.; Stachel, I.; Meyer, M.; Gelinsky, M. Biomimetically Mineralized Salmon Collagen Scaffolds for Application in Bone Tissue Engineering. Biomacromolecules 2012, 13, 1059–1066. [Google Scholar] [CrossRef]
- Seixas, M.J.; Martins, E.; Reis, R.L.; Silva, T.H. Extraction and Characterization of Collagen from Elasmobranch Byproducts for Potential Biomaterial Use. Mar. Drugs 2020, 18, 617. [Google Scholar] [CrossRef]
- Cao, H.; Chen, M.M.; Liu, Y.; Liu, Y.Y.; Huang, Y.Q.; Wang, J.H.; Chen, J.D.; Zhang, Q.Q. Fish collagen-based scaffold containing PLGA microspheres for controlled growth factor delivery in skin tissue engineering. Colloids Surf. B Biointerfaces 2015, 136, 1098–1106. [Google Scholar] [CrossRef]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef] [Green Version]
- Ge, B.; Wang, H.; Li, J.; Liu, H.; Yin, Y.; Zhang, N.; Qin, S. Comprehensive Assessment of Nile Tilapia Skin (Oreochromis niloticus) Collagen Hydrogels for Wound Dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef] [Green Version]
- Elbialy, Z.I.; Atiba, A.; Abdelnaby, A.; Al-Hawary, I.I.; Elsheshtawy, A.; El-Serehy, H.A.; Abdel-Daim, M.M.; Fadl, S.E.; Assar, D.H. Collagen extract obtained from Nile tilapia (Oreochromis niloticus L.) skin accelerates wound healing in rat model via up regulating VEGF, bFGF, and α-SMA genes expression. BMC Vet. Res. 2020, 16, 352. [Google Scholar] [CrossRef]
- Madhumanchi, S.; Suedee, R.; Nakpheng, T.; Tinpun, K.; Temboot, P.; Srichana, T. Binding interactions of bacterial lipopolysaccharides to polymyxin B in an amphiphilic carrier ‘sodium deoxycholate sulfate’. Colloids Surf. B Biointerfaces 2019, 182, 110374. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Ao, H.; Long, T.; Zhou, J.; Tang, T.; Yue, B. Immobilizing bacitracin on titanium for prophylaxis of infections and for improving osteoinductivity: An in vivo study. Colloids Surf. B Biointerfaces 2017, 150, 183–191. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, X.; Li, S.; Zheng, Y.; Shi, X.; Li, F.; Guo, S.; Yang, J. Preparation of aminated fish scale collagen and oxidized sodium alginate hybrid hydrogel for enhanced full-thickness wound healing. Int. J. Biol. Macromol. 2020, 164, 626–637. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Sekar, S.; Meera, K.M.S.; Mukherjee, A.; Sastry, T.P.; Mandal, A.B. Fabrication of collagen scaffolds impregnated with sago starch capped silver nanoparticles suitable for biomedical applications and their physicochemical studies. Phys. Chem. Chem. Phys. 2014, 16, 20175–20183. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Sui, B.; Mo, X.; Sun, J. Multifunctional and biomimetic fish collagen/bioactive glass nanofibers: Fabrication, antibacterial activity and inducing skin regeneration in vitro and in vivo. Int. J. Nanomed. 2017, 12, 3495–3507. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, A.; Soliman, M.; Kotb, S.; Ali, M. Evaluation of fish skin as a biological dressing for metacarpal wounds in donkeys. BMC Vet. Res. 2020, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, H.; Zhang, X.; Chen, Z.; Zhao, D.; Ma, J. A comparative study of two porous sponge scaffolds prepared by collagen derived from porcine skin and fish scales as burn wound dressings in a rabbit model. Regen. Biomater. 2020, 7, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, J.; Tian, X.; Tang, Y.; Ding, G.; Yang, Z.; Jin, H. Pepsin-Soluble Collagen from the Skin of Lophius litulo: A Preliminary Study Evaluating Physicochemical, Antioxidant, and Wound Healing Properties. Mar. Drugs 2019, 17, 708. [Google Scholar] [CrossRef] [Green Version]
- Medina-Medrano, J.R.; Quiñones-Muñoz, T.A.; Arce-Ortíz, A.; Torruco-Uco, J.G.; Hernández-Martínez, R.; Lizardi-Jiménez, M.A.; Varela-Santos, E. Antioxidant Activity of Collagen Extracts Obtained from the Skin and Gills of Oreochromis sp. J. Med. Food 2019, 22, 722–728. [Google Scholar] [CrossRef]
- Hua, Y.; Ma, C.; Wei, T.; Zhang, L.; Shen, J. Collagen/Chitosan Complexes: Preparation, Antioxidant Activity, Tyrosinase Inhibition Activity, and Melanin Synthesis. Int. J. Mol. Sci. 2020, 21, 313. [Google Scholar] [CrossRef] [Green Version]
- Rajasree, S.R.; Gobalakrishnan, M.; Aranganathan, L.; Karthih, M. Fabrication and characterization of chitosan based collagen/gelatin composite scaffolds from big eye snapper Priacanthus hamrur skin for antimicrobial and anti oxidant applications. Mater. Sci. Eng. C 2020, 107, 110270. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, H.; Yang, F.; Jin, S.; Liu, C.; Zhang, L.; Huang, J.; Wang, S.; Yan, Z.; Cai, X.; et al. Physicochemical, antioxidant properties of giant croaker (Nibea japonica) swim bladders collagen and wound healing evaluation. Int. J. Biol. Macromol 2019, 138, 483–491. [Google Scholar] [CrossRef]
- Nurilmala, M.; Hizbullah, H.H.; Karnia, E.; Kusumaningtyas, E.; Ochiai, Y. Characterization and Antioxidant Activity of Collagen, Gelatin, and the Derived Peptides from Yellowfin Tuna (Thunnus albacares) Skin. Mar. Drugs 2020, 18, 98. [Google Scholar] [CrossRef] [Green Version]
- Asaduzzaman, A.K.M.; Getachew, A.T.; Cho, Y.-J.; Park, J.-S.; Haq, M.; Chun, B.-S. Characterization of pepsin-solubilised collagen recovered from mackerel (Scomber japonicus) bone and skin using subcritical water hydrolysis. Int. J. Biol. Macromol. 2020, 148, 1290–1297. [Google Scholar] [CrossRef]
- Jeevithan, E.; Bao, B.; Bu, Y.; Zhou, Y.; Zhao, Q.; Wu, W. Type II collagen and gelatin from silvertip shark (Carcharhinus albimarginatus) cartilage: Isolation, purification, physicochemical and antioxidant properties. Mar. Drugs 2014, 12, 3852–3873. [Google Scholar] [CrossRef] [Green Version]
- Shori, A.B.; Ming, K.S.; Baba, A.S. The effects of Lycium barbarum water extract and fish collagen on milk proteolysis and in vitro angiotensin I-converting enzyme inhibitory activity of yogurt. Biotechnol. Appl. Biochem. 2020. [Google Scholar] [CrossRef]
- Hall, J.E.; Granger, J.P.; do Carmo, J.M.; da Silva, A.A.; Dubinion, J.; George, E.; Hamza, S.; Speed, J.; Hall, M.E. Hypertension: Physiology and pathophysiology. Compr. Physiol. 2012, 2, 2393–2442. [Google Scholar]
- Liu, J.; Shibata, M.; Ma, Q.; Liu, F.; Lu, Q.; Shan, Q.; Hagiwara, T.; Bao, J. Characterization of fish collagen from blue shark skin and its application for chitosan- collagen composite coating to preserve red porgy (Pagrus major) meat. J. Food Biochem. 2020, 44, e13265. [Google Scholar] [CrossRef]
- Ben Slimane, E.; Sadok, S. Collagen from Cartilaginous Fish By-Products for a Potential Application in Bioactive Film Composite. Mar. Drugs 2018, 16, 211. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Kodama, Y.; Miwa, K.; Kishimoto, K.; Hoshikawa, E.; Haga, K.; Sato, T.; Mizuno, J.; Izumi, K. Manufacturing micropatterned collagen scaffolds with chemical-crosslinking for development of biomimetic tissue-engineered oral mucosa. Sci. Rep. 2020, 10, 22192. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.T.; Wang, Y.M.; Yang, X.R.; Zhao, Y.Q.; Chi, C.F.; Wang, B. Gelatin and Antioxidant Peptides from Gelatin Hydrolysate of Skipjack Tuna (Katsuwonus pelamis) Scales: Preparation, Identification and Activity Evaluation. Mar. Drugs 2019, 17, 565. [Google Scholar] [CrossRef] [Green Version]
- Fish Protein Hydrolysate Market Size, By Technology (Acid Hydrolysis, Enzymatic Hydrolysis), By Form (Powder, Paste, Liquid), By Source (Anchovy, Tilapia, Tuna, Sardine, Atlantic Salmon, Crustacean, Molluscs, Codfish), By Application (Animal Feed [Poultry {Broilers, Layers}, Swine, Calves, Aquaculture {Salmon, Trouts, Shrimps}, Equine], Pet Food [Cat, Dog], Food (Flavor Enhancers, Functional Food, Infant Formulation, Nutraceuticals, Sports Nutrition, Protein Supplements, Elderly Food Formulation, Clinicals), Cosmetics [By Protein Type {Collagen (Glycine, Hydroxylysine, Proline), Elastin, Keratin}], Agriculture [Fertilizers, Protection, Elicitor], Pharmaceutical), Industry Analysis Report, Regional Outlook, Application Development Potential, Price Trends, Competitive Market Share & Forecast, 2020–2026. Available online: https://www.gminsights.com/industry-analysis/fish-protein-hydrolysate-market (accessed on 5 December 2020).
- Giordano, D.; Costantini, M.; Coppola, D.; Lauritano, C.; Pons, L.N.; Ruocco, N.; Di Prisco, G.; Ianora, A.; Verde, C. Biotechnological Applications of Bioactive Peptides From Marine Sources. Adv. Microb. Physiol. 2018, 73, 171–220. [Google Scholar] [CrossRef]
- Harnedy, P.A.; Fitzgerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Urakova, I.N.; Pozharitskaya, O.N.; Demchenko, D.V.; Shikov, A.; Makarov, V.G. The biological activities of fish peptides and methods of their isolation. Russ. J. Mar. Biol. 2012, 38, 417–422. [Google Scholar] [CrossRef]
- Kim, S.-K.; Wijesekara, I. Development and biological activities of marine-derived bioactive peptides: A review. J. Funct. Foods 2010, 2, 1–9. [Google Scholar] [CrossRef]
- Halim, N.; Yusof, H.; Sarbon, N. Functional and bioactive properties of fish protein hydolysates and peptides: A comprehensive review. Trends Food Sci. Technol. 2016, 51, 24–33. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Rodríguez-Amado, I.; Sotelo, C.G.; Sanz, N.; Pérez-Martín, R.I.; Valcárcel, J. Production, Characterization, and Bioactivity of Fish Protein Hydrolysates from Aquaculture Turbot (Scophthalmus maximus) Wastes. Biomolecules 2020, 10, 310. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.-K.; Lee, M.J.; Go, H.-J.; Kim, Y.J.; Park, N.G. Antimicrobial function of the GAPDH-related antimicrobial peptide in the skin of skipjack tuna, Katsuwonus pelamis. Fish Shellfish Immunol. 2014, 36, 571–581. [Google Scholar] [CrossRef]
- Seo, J.-K.; Lee, M.J.; Go, H.-J.; Park, T.H.; Park, N.G. Purification and characterization of YFGAP, a GAPDH-related novel antimicrobial peptide, from the skin of yellowfin tuna, Thunnus albacares. Fish Shellfish Immunol. 2012, 33, 743–752. [Google Scholar] [CrossRef]
- Su, Y. Isolation and identification of pelteobagrin, a novel antimicrobial peptide from the skin mucus of yellow catfish (Pelteobagrus fulvidraco). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2011, 158, 149–154. [Google Scholar] [CrossRef]
- Cole, A.M.; Weis, P.; Diamond, G. Isolation and characterization of pleurocidin, an antimicrobial peptide in the skin secretions of winter flounder. J. Biol. Chem. 1997, 272, 12008-13. [Google Scholar] [CrossRef] [Green Version]
- Ennaas, N.; Hammami, R.; Beaulieu, L.; Fliss, I. Purification and characterization of four antibacterial peptides from protamex hydrolysate of Atlantic mackerel (Scomber scombrus) by-products. Biochem. Biophys. Res. Commun. 2015, 462, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Offret, C.; Fliss, I.; Bazinet, L.; Marette, A.; Beaulieu, L. Identification of A Novel Antibacterial Peptide from Atlantic Mackerel belonging to the GAPDH-Related Antimicrobial Family and Its In Vitro Digestibility. Mar. Drugs 2019, 17, 413. [Google Scholar] [CrossRef] [Green Version]
- Tejpal, C.; Vijayagopal, P.; Elavarasan, K.; Prabu, D.; Lekshmi, R.; Anandan, R.; Sanal, E.; Asha, K.; Chatterjee, N.; Mathew, S.; et al. Evaluation of pepsin derived tilapia fish waste protein hydrolysate as a feed ingredient for silver pompano (Trachinotus blochii) fingerlings: Influence on growth, metabolism, immune and disease resistance. Anim. Feed. Sci. Technol. 2021, 272, 114748. [Google Scholar] [CrossRef]
- Byun, H.-G.; Kim, S.-K. Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from Alaska pollack (Theragra chalcogramma) skin. Process. Biochem. 2001, 36, 1155–1162. [Google Scholar] [CrossRef]
- Je, J.-Y.; Park, P.-J.; Kwon, A.J.Y.; Kim, S.-K. A Novel Angiotensin I Converting Enzyme Inhibitory Peptide from Alaska Pollack (Theragra chalcogramma) Frame Protein Hydrolysate. J. Agric. Food Chem. 2004, 52, 7842–7845. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.-S.; Ngo, D.-H.; Kim, J.-A.; Ryu, B.; Kim, S.-K. An antihypertensive peptide from tilapia gelatin diminishes free radical formation in murine microglial cells. J. Agric. Food Chem. 2011, 59, 12193–12197. [Google Scholar] [CrossRef] [PubMed]
- Choonpicharn, S.; Tateing, S.; Jaturasitha, S.; Rakariyatham, N.; Suree, N.; Niamsup, H. Identification of bioactive peptide from Oreochromis niloticus skin gelatin. J. Food Sci. Technol. 2015, 53, 1222–1229. [Google Scholar] [CrossRef] [Green Version]
- Ngo, D.-H.; Kang, K.-H.; Ryu, B.; Vo, T.-S.; Jung, W.-K.; Byun, H.-G.; Kim, S.-K. Angiotensin-I converting enzyme inhibitory peptides from antihypertensive skate (Okamejei kenojei) skin gelatin hydrolysate in spontaneously hypertensive rats. Food Chem. 2015, 174, 37–43. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Ryu, B.; Kim, S.-K. Angiotensin- I- converting enzyme (ACE) inhibitory peptides from Pacific cod skin gelatin using ultrafiltration membranes. Process. Biochem. 2016, 51, 1622–1628. [Google Scholar] [CrossRef] [Green Version]
- Byun, H.-G.; Kim, S.-K. Structure and Activity of Angiotensin I Converting Enzyme Inhibitory Peptides Derived from Alaskan Pollack Skin. J. Biochem. Mol. Biol. 2002, 35, 239–243. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Harnedy, P.A.; Zhang, L.; Li, B.; Zhang, Z.; Hou, H.; Zhao, X.; FitzGerald, R.J. In vitro assessment of the multifunctional bioactive potential of Alaska pollock skin collagen following simulated gastrointestinal digestion. J. Sci. Food Agric. 2015, 95, 1514–1520. [Google Scholar] [CrossRef]
- Lassoued, I.; Mora, L.; Barkia, A.; Aristoy, M.C.; Nasri, M.; Toldrá, F. Bioactive peptides identified in thornback ray skin’s gelatin hydrolysates by proteases from Bacillus subtilis and Bacillus amyloliquefaciens. J. Proteom. 2015, 128, 8–17. [Google Scholar] [CrossRef]
- Fahmi, A.; Morimura, S.; Guo, H.; Shigematsu, T.; Kida, K.; Uemura, Y. Production of angiotensin I converting enzyme inhibitory peptides from sea bream scales. Process. Biochem. 2004, 39, 1195–1200. [Google Scholar] [CrossRef]
- Lee, J.K.; Jeon, J.-K.; Byun, H.-G. Effect of angiotensin I converting enzyme inhibitory peptide purified from skate skin hydrolysate. Food Chem. 2011, 125, 495–499. [Google Scholar] [CrossRef]
- Lee, J.K.; Jeon, J.-K.; Byun, H.-G. Antihypertensive effect of novel angiotensin I converting enzyme inhibitory peptide from chum salmon (Oncorhynchus keta) skin in spontaneously hypertensive rats. J. Funct. Foods 2014, 7, 381–389. [Google Scholar] [CrossRef]
- Jung, W.-K.; Mendis, E.; Je, J.-Y.; Park, P.-J.; Son, B.W.; Kim, H.C.; Choi, Y.K.; Kim, S.-K. Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2006, 94, 26–32. [Google Scholar] [CrossRef]
- Lee, S.-H.; Qian, Z.-J.; Kim, S.-K. A novel angiotensin I converting enzyme inhibitory peptide from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2010, 118, 96–102. [Google Scholar] [CrossRef]
- Ohba, R.; Deguchi, T.; Kishikawa, M.; Arsyad, F.; Morimura, S.; Kida, K. Physiological Functions of Enzymatic Hydrolysates of Collagen or Keratin Contained in Livestock and Fish Waste. Food Sci. Technol. Res. 2003, 9, 91–93. [Google Scholar] [CrossRef] [Green Version]
- Jeon, Y.-J.; Byun, H.-G.; Kim, S.-K. Improvement of functional properties of cod frame protein hydrolysates using ultrafiltration membranes. Process. Biochem. 1999, 35, 471–478. [Google Scholar] [CrossRef]
- Theodore, A.E.; Raghavan, S.; Kristinsson, H.G. Antioxidative activity of protein hydrolysates prepared from alkaline-aided channel catfish protein isolates. J. Agric. Food Chem. 2008, 56, 7459–7466. [Google Scholar] [CrossRef]
- Cai, L.; Wu, X.; Zhang, Y.; Li, X.; Ma, S.; Li, J. Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J. Funct. Foods 2015, 16, 234–242. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Qian, Z.-J.; Ryu, B.; Park, J.W.; Kim, S.-K. In vitro antioxidant activity of a peptide isolated from Nile tilapia (Oreochromis niloticus) scale gelatin in free radical-mediated oxidative systems. J. Funct. Foods 2010, 2, 107–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, X.; Zhuang, Y. Purification and characterization of novel antioxidant peptides from enzymatic hydrolysates of tilapia (Oreochromis niloticus) skin gelatin. Peptides 2012, 38, 13–21. [Google Scholar] [CrossRef]
- Chi, C.-F.; Wang, B.; Hu, F.-Y.; Wang, Y.-M.; Zhang, B.; Deng, S.-G.; Wu, C.-W. Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Res. Int. 2015, 73, 124–129. [Google Scholar] [CrossRef]
- Sae-Leaw, T.; Karnjanapratum, S.; O’Callaghan, Y.C.; O’Keeffe, M.B.; FitzGerald, R.J.; O’Brien, N.M.; Benjakul, S. Purification and identification of antioxidant peptides from gelatin hydrolysate of seabass skin. J. Food Biochem. 2017, 41, e12350. [Google Scholar] [CrossRef]
- Mendis, E.; Rajapakse, N.; Kim, S.K. Antioxidant properties of a radical-scavenging peptide purified from enzymatically prepared fish skin gelatin hydrolysate. J. Agric. Food Chem. 2005, 53, 581–587. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S.; Xu, X. Antioxidant and cryoprotective effects of Amur sturgeon skin gelatin hydrolysate in unwashed fish mince. Food Chem. 2015, 181, 295–303. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.T.; Byun, H.G.; Nam, K.S.; Joo, D.S.; Shahidi, F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J. Agric. Food Chem. 2001, 49, 1984–1989. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Zhuang, Y. Antiphotoaging effect and purification of an antioxidant peptide from tilapia (Oreochromis niloticus) gelatin peptides. J. Funct. Foods 2013, 5, 154–162. [Google Scholar] [CrossRef]
- Phanturat, P.; Benjakul, S.; Visessanguan, W.; Roytrakul, S. Use of pyloric caeca extract from bigeye snapper (Priacanthus macracanthus) for the production of gelatin hydrolysate with antioxidative activity. LWT Food Sci. Technol. 2010, 43, 86–97. [Google Scholar] [CrossRef]
- Khantaphant, S.; Benjakul, S. Comparative study on the proteases from fish pyloric caeca and the use for production of gelatin hydrolysate with antioxidative activity. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008, 151, 410–419. [Google Scholar] [CrossRef]
- Giménez, B.; Alemán, A.; Montero, P.; Gómez-Guillén, M.C. Antioxidant and functional properties of gelatin hydrolysates obtained from skin of sole and squid. Food Chem. 2009, 114, 976–983. [Google Scholar] [CrossRef] [Green Version]
- Sathivel, S.; Bechtel, P.J.; Babbitt, J.; Smiley, S.; Crapo, C.; Reppond, K.D.; Prinyawiwatkul, W. Biochemical and Functional Properties of Herring (Clupea harengus) Byproduct Hydrolysates. J. Food Sci. 2003, 68, 2196–2200. [Google Scholar] [CrossRef]
- Ngo, D.H.; Ryu, B.; Vo, T.S.; Himaya, S.W.; Wijesekara, I.; Kim, S.K. Free radical scavenging and angiotensin-I converting enzyme inhibitory peptides from Pacific cod (Gadus macrocephalus) skin gelatin. Int. J. Biol. Macromol. 2011, 49, 1110–1116. [Google Scholar] [CrossRef]
- Kim, S.Y.; Je, J.Y.; Kim, S.K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007, 18, 31–38. [Google Scholar] [CrossRef]
- Jun, S.-Y.; Park, P.-J.; Jung, W.-K.; Kim, S.-K. Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limanda aspera) frame protein. Eur. Food Res. Technol. 2004, 219, 20–26. [Google Scholar]
- Je, J.-Y.; Park, P.-J.; Kim, S.-K. Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res. Int. 2005, 38, 45–50. [Google Scholar] [CrossRef]
- Je, J.-Y.; Qian, Z.-J.; Byun, H.-G.; Kim, S.-K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process. Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Chen, X.-L.; Peng, M.; Li, J.; Tang, B.-L.; Shao, X.; Zhao, F.; Liu, C.; Zhang, X.-Y.; Li, P.-Y.; Shi, M.; et al. Preparation and functional evaluation of collagen oligopeptide-rich hydrolysate from fish skin with the serine collagenolytic protease from Pseudoalteromonas sp. SM9913. Sci. Rep. 2017, 7, 15716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.D.; Li, W.J.; Kong, S.Z.; Li, S.D.; Guo, J.Q.; Guo, M.H.; Cai, T.T.; Li, N.; Chen, R.Z.; Luo, R.Q.; et al. Protective effects of collagen polypeptide from tilapia skin against injuries to the liver and kidneys of mice induced by d-galactose. Biomed. Pharmacother. 2019, 117, 109204. [Google Scholar] [CrossRef]
- Sae-leaw, T.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N.M. Antioxidant, immunomodulatory and antiproliferative effects of gelatin hydrolysates from seabass (Lates calcarifer) skins. Int. J. Food Sci. Technol. 2016, 51, 1545–1551. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.M.; Chi, C.F.; Luo, H.Y.; Deng, S.G.; Ma, J.Y. Isolation and characterization of collagen and antioxidant collagen peptides from scales of croceine croaker (Pseudosciaena crocea). Mar. Drugs 2013, 11, 4641–4661. [Google Scholar] [CrossRef]
- Yang, X.-R.; Zhao, Y.-Q.; Qiu, Y.-T.; Chi, C.-F.; Wang, B. Preparation and Characterization of Gelatin and Antioxidant Peptides from Gelatin Hydrolysate of Skipjack Tuna (Katsuwonus pelamis) Bone Stimulated by in vitro Gastrointestinal Digestion. Mar. Drugs 2019, 17, 78. [Google Scholar] [CrossRef] [Green Version]
- Li, X.R.; Chi, C.F.; Li, L.; Wang, B. Purification and Identification of Antioxidant Peptides from Protein Hydrolysate of Scalloped Hammerhead (Sphyrna lewini) Cartilage. Mar. Drugs 2017, 15, 61. [Google Scholar] [CrossRef]
- Sun, L.; Chang, W.; Ma, Q.; Zhuang, Y. Purification of Antioxidant Peptides by High Resolution Mass Spectrometry from Simulated Gastrointestinal Digestion Hydrolysates of Alaska Pollock (Theragra chalcogramma) Skin Collagen. Mar. Drugs 2016, 14, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sampath Kumar, N.S.; Nazeer, R.A.; Jaiganesh, R. Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Amino Acids 2012, 42, 1641–1649. [Google Scholar] [CrossRef]
- Lee, J.K.; Li-Chan, E.C.Y.; Byun, H.-G. Characterization of β-secretase inhibitory peptide purified from skate skin protein hydrolysate. Eur. Food Res. Technol. 2015, 240, 129–136. [Google Scholar] [CrossRef]
- Xu, L.; Dong, W.; Zhao, J.; Xu, Y. Effect of Marine Collagen Peptides on Physiological and Neurobehavioral Development of Male Rats with Perinatal Asphyxia. Mar. Drugs 2015, 13, 3653–3671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pei, X.; Yang, R.; Zhang, Z.; Gao, L.; Wang, J.; Xu, Y.; Zhao, M.; Han, X.; Liu, Z.; Li, Y. Marine collagen peptide isolated from Chum Salmon (Oncorhynchus keta) skin facilitates learning and memory in aged C57BL/6J mice. Food Chem. 2010, 118, 333–340. [Google Scholar] [CrossRef]
- Evans, M.; Lewis, E.D.; Zakaria, N.; Pelipyagina, T.; Guthrie, N. A randomized, triple-blind, placebo-controlled, parallel study to evaluate the efficacy of a freshwater marine collagen on skin wrinkles and elasticity. J. Cosmet. Dermatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Hua, N.; Hsu, Y.-C.; Kan, K.-W.; Chen, J.-H.; Lin, Y.-H.; Lin, Y.-H.; Kuan, C.-M. Oral Collagen Drink for Antiaging: Antioxidation, Facilitation of the Increase of Collagen Synthesis, and Improvement of Protein Folding and DNA Repair in Human Skin Fibroblasts. Oxidative Med. Cell. Longev. 2020, 2020, 8031795. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Hou, H.; Fan, Y.; Yang, T.; Li, B. Identification of MMP-1 inhibitory peptides from cod skin gelatin hydrolysates and the inhibition mechanism by MAPK signaling pathway. J. Funct. Foods 2017, 33, 251–260. [Google Scholar] [CrossRef]
- Pyun, H.-B.; Kim, M.; Park, J.; Sakai, Y.; Numata, N.; Shin, J.-Y.; Shin, H.-J.; Kim, D.-U.; Hwang, J.-K. Effects of Collagen Tripeptide Supplement on Photoaging and Epidermal Skin Barrier in UVB-exposed Hairless Mice. Prev. Nutr. Food Sci. 2012, 17, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Sun, J. Osteogenically differentiated mesenchymal stem cells induced by hydrolyzed fish collagen maintain their immunomodulatory effects. Life Sci. 2019, 238, 116970. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Hur, J.; Ham, S.A.; Jo, Y.; Lee, S.; Choi, M.J.; Seo, H.G. Fish collagen peptide inhibits the adipogenic differentiation of preadipocytes and ameliorates obesity in high fat diet-fed mice. Int. J. Biol. Macromol. 2017, 104, 281–286. [Google Scholar] [CrossRef]
- Rajapakse, N.; Jung, W.-K.; Mendis, E.; Moon, S.-H.; Kim, S.-K. A novel anticoagulant purified from fish protein hydrolysate inhibits factor XIIa and platelet aggregation. Life Sci. 2005, 76, 2607–2619. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhao, Y.-Q.; Hu, F.-Y.; Chi, C.-F.; Wang, B. Anticancer Activity of a Hexapeptide from Skate (Raja porosa) Cartilage Protein Hydrolysate in HeLa Cells. Mar. Drugs 2016, 14, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, L.; Shakila, R.; Jeyasekaran, G. In vitro anti-cancer, anti-diabetic, anti-inflammation and wound healing properties of collagen peptides derived from unicorn leatherjacket (Aluterus monoceros) at different hydrolysis. Turk. J. Fish. Aquat. Sci. 2019, 19, 551–560. [Google Scholar]
- Xu, Y.; Han, X.; Li, Y. Effect of marine collagen peptides on long bone development in growing rats. J. Sci. Food Agric. 2010, 90, 1485–1491. [Google Scholar] [CrossRef]
- Ohnishi, A.; Osaki, T.; Matahira, Y.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Evaluation of the chondroprotective effects of glucosamine and fish collagen peptide on a rabbit ACLT model using serum biomarkers. J. Vet. Med. Sci. 2013, 75, 421–429. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.-K.; Kim, S.-K. Calcium-binding peptide derived from pepsinolytic hydrolysates of hoki (Johnius belengerii) frame. Eur. Food Res. Technol. 2007, 224, 763–767. [Google Scholar] [CrossRef]
- Jung, W.-K.; Karawita, R.; Heo, S.-J.; Lee, B.-J.; Kim, S.-K.; Jeon, Y.-J. Recovery of a novel Ca-binding peptide from Alaska Pollack (Theragra chalcogramma) backbone by pepsinolytic hydrolysis. Process. Biochem. 2006, 41, 2097–2100. [Google Scholar] [CrossRef]
- Jung, W.-K.; Park, P.-J.; Byun, H.-G.; Moon, S.-H.; Kim, S.-K. Preparation of hoki (Johnius belengerii) bone oligophosphopeptide with a high affinity to calcium by carnivorous intestine crude proteinase. Food Chem. 2005, 91, 333–340. [Google Scholar] [CrossRef]
- Wu, W.; Li, B.; Hou, H.; Zhang, H.; Zhao, X. Identification of iron-chelating peptides from Pacific cod skin gelatin and the possible binding mode. J. Funct. Foods 2017, 35, 418–427. [Google Scholar] [CrossRef]
- Chitosan Market Size, Share & Trends Analysis Report by Application (Pharmaceutical & Biomedical, Water Treatment, Cosmetics, Food & Beverage), by Region (APAC, North America, Europe, MEA), and Segment Forecasts, 2020–2027. Available online: https://www.researchandmarkets.com/reports/4076513/chitosan-market-size-share-and-trends-analysis (accessed on 5 December 2020).
- Chitin and Chitosan Derivatives—Global Market Trajectory & Analytics. Available online: https://www.researchandmarkets.com/reports/338576/chitin_and_chitosan_derivatives_global_market (accessed on 5 December 2020).
- Muñoz, I.; Rodríguez, C.; Gillet, D.; Moerschbacher, B.M. Life cycle assessment of chitosan production in India and Europe. Int. J. Life Cycle Assess. 2018, 23, 1151–1160. [Google Scholar] [CrossRef] [Green Version]
- Cheba, B.A. Chitin and Chitosan: Marine Biopolymers with Unique Properties and Versatile Applications. Glob. J. Biotechnol. Biochem. 2011, 6, 149–153. [Google Scholar]
- Shahidi, F.; Arachchi, J.K.V.; Jeon, Y.-J. Food applications of chitin and chitosans. Trends Food Sci. Technol. 1999, 10, 37–51. [Google Scholar] [CrossRef]
- Shahidi, F. Chitin and chitosan from marine by-products. In Maximising the Value of Marine By-Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 340–373. [Google Scholar]
- Airoldi, C. The weighty potentiality of nitrogenated basic centers in inorganic polymers and biopolymers for cation removal. Quím. Nova 2008, 31, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, H.; Yamamoto, H.; Kawashima, Y. Mucoadhesive nanoparticulate systems for peptide drug delivery. Adv. Drug Deliv. Rev. 2001, 47, 39–54. [Google Scholar] [CrossRef]
- Kato, Y.; Onishi, H.; Machida, Y. Application of chitin and chitosan derivatives in the pharmaceutical field. Curr. Pharm. Biotechnol. 2003, 4, 303–309. [Google Scholar] [CrossRef]
- Campana-Filho, S.P.; Britto, D.; Curti, E.; Abreu, F.R.; Cardoso, M.B.; Battisti, M.V.; Sim, P.C.; Goy, R.C.; Signini, R.; Lavall, R.L. Extraction, structures and properties of a- and b-chitin. Quím. Nova 2007, 30, 644–650. [Google Scholar] [CrossRef] [Green Version]
- Moura, C.; Muszinski, P.; Schmidt, C.; Almeida, J.; Pinto, L. Obtainment of chitin and production of chitosan from residues of shrimp and crab. Vetor 2006, 16, 37–45. [Google Scholar]
- Battisti, M.; Campana, S. Preparation and characterization of α-chitin and chitosan from the shells of Macrobrachium rosembergii. Quím. Nova 2007, 31, 2014–2019. [Google Scholar] [CrossRef] [Green Version]
- No, H.K.; Meyers, S.P. Preparation and Characterization of Chitin and Chitosan—A Review. J. Aquat. Food Prod. Technol. 1995, 4, 27–52. [Google Scholar] [CrossRef]
- Cohen, E. Chitin synthesis and inhibition: A revisit. Pest. Manag. Sci. 2001, 57, 946–950. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Carney, B.; Slater, J.; Brück, W. Mining marine shellfish wastes for bioactive molecules: Chitin and chitosan--Part A: Extraction methods. Biotechnol. J. 2008, 3, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Zaku, S.; Emmanuel, S.; Aguzue, O.; Thomas, S. Extraction and characterization of chitin; a functional biopolymer obtained from scales of common carp fish (Cyprinus carpio L.): A lesser known source. Afr. J. Food Sci. 2011, 5, 478–483. [Google Scholar]
- Kumari, S.; Rath, P.K. Extraction and Characterization of Chitin and Chitosan from (Labeo rohit) Fish Scales. Procedia Mater. Sci. 2014, 6, 482–489. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Rath, P.; Sri Hari Kumar, A.; Tiwari, T.N. Extraction and characterization of chitin and chitosan from fishery waste by chemical method. Environ. Technol. Innov. 2015, 3, 77–85. [Google Scholar] [CrossRef]
- Muslim, T.; Rahman, M.; Begum, H.; Rahman, M. Chitosan and Carboxymethyl Chitosan from Fish Scales of Labeo rohita. Dhaka Univ. J. Sci. 2013, 61. [Google Scholar] [CrossRef] [Green Version]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. J. Control. Release Off. J. Control. Release Soc. 2004, 100, 5–28. [Google Scholar] [CrossRef]
- Uawonggul, N.; Ruksakulpiwat, C. Study on dye-binding interactions of chitosan obtained from the fish scale of tilapia (Tilapia nilotica). In Proceedings of the 28th Congress on Science and Technology of Thailand, Bangkok, Thailand, 24–26 October 2002. [Google Scholar]
- Rumengan, I.; Suptijah, P.; Wullur, S.; Talumepa, A. Characterization of chitin extracted from fish scales of marine fish species purchased from local markets in North Sulawesi, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2017, 89, 012028. [Google Scholar] [CrossRef]
- Takarina, N.; Fanani, A. Characterization of chitin and chitosan synthesized from red snapper (Lutjanus sp.) scale’s waste. AIP Conf. Proc. 2017, 1862, 030108. [Google Scholar]
- Irawan, C.; Nata, I.; Putra, M.; Marisa, R.; Asnia, M.; Arifin, Y. Biopolymer of Chitosan from Fish Scales as Natural Coagulant for Iron—Contaminated Groundwater Treatment. J. Rekayasa Kim. Lingkung. 2018, 13, 93–99. [Google Scholar] [CrossRef]
- Yadav, M.; Goswami, P.; Paritosh, K.; Kumar, M.; Pareek, N.; Vivekanand, V. Seafood waste: A source for preparation of commercially employable chitin/chitosan materials. Bioresour. Bioprocess. 2019, 6, 8. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. Reactive Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Barriada, J.L.; Herrero, R.; Prada-Rodríguez, D.; de Vicente, M.E.S. Waste spider crab shell and derived chitin as low-cost materials for cadmium and lead removal. J. Chem. Technol. Biotechnol. 2007, 82, 39–46. [Google Scholar] [CrossRef]
- Yadav, A.V.; Bhise, S.B. Chitosan: A potential biomaterial effective against typhoid. Curr. Sci. 2004, 87, 1176–1178. [Google Scholar]
- Zheng, L.-Y.; Zhu, J.-F. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Benhabiles, M.S.; Salah, R.; Lounici, H.; Drouiche, N.; Goosen, M.F.A.; Mameri, N. Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocoll. 2012, 29, 48–56. [Google Scholar] [CrossRef]
- Choi, B.K.; Kim, K.Y.; Yoo, Y.J.; Oh, S.J.; Choi, J.H.; Kim, C.Y. In vitro antimicrobial activity of a chitooligosaccharide mixture against Actinobacillus actinomycetemcomitans and Streptococcus mutans. Int. J. Antimicrob. Agents 2001, 18, 553–557. [Google Scholar] [CrossRef]
- Eaton, P.; Fernandes, J.C.; Pereira, E.; Pintado, M.E.; Xavier Malcata, F. Atomic force microscopy study of the antibacterial effects of chitosans on Escherichia coli and Staphylococcus aureus. Ultramicroscopy 2008, 108, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Hasegawa, M.; Yagi, K.; Iwakawa, S.; Hirai, M. Chitosan Induces Apoptosis via Caspase-3 Activation in Bladder Tumor Cells. Jpn. J. Cancer Res. 2001, 92, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Qi, L.; Xu, Z.; Chen, M. In vitro and in vivo suppression of hepatocellular carcinoma growth by chitosan nanoparticles. Eur. J. Cancer 2007, 43, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.J.; Kim, S. Antitumor activity of chitosan oligosaccharides produced in ultrafiltration membrane reactor system. J. Microbiol. Biotechnol. 2002, 12, 503–507. [Google Scholar]
- Nagahama, H.; Nwe, N.; Jayakumar, R.; Koiwa, S.; Furuike, T.; Tamura, H. Novel biodegradable chitin membranes for tissue engineering applications. Carbohydr. Polym. 2008, 73, 295–302. [Google Scholar] [CrossRef]
- Yang, T.-L. Chitin-based Materials in Tissue Engineering: Applications in Soft Tissue and Epithelial Organ. Int. J. Mol. Sci. 2011, 12, 1936–1963. [Google Scholar] [CrossRef] [Green Version]
- Caner, C. The effect of edible eggshell coatings on egg quality and consumer perception. J. Sci. Food Agric. 2005, 85, 1897–1902. [Google Scholar] [CrossRef]
- Shankar, S.; Reddy, J.P.; Rhim, J.-W.; Kim, H.-Y. Preparation, characterization, and antimicrobial activity of chitin nanofibrils reinforced carrageenan nanocomposite films. Carbohydr. Polym. 2015, 117, 468–475. [Google Scholar] [CrossRef]
- Qingjie, S.; Zhang, S.; Yu, J.; Yang, J.; Xiong, L.; Sun, Q. Effects of chitin nano-whiskers on the antibacterial and physicochemical properties of maize starch films. Carbohydr. Polym. 2016, 147, 372–378. [Google Scholar] [CrossRef]
- Agulló, E.; Rodríguez, M.S.; Ramos, V.; Albertengo, L. Present and Future Role of Chitin and Chitosan in Food. Macromol. Biosci. 2003, 3, 521–530. [Google Scholar] [CrossRef]
- Georgantelis, D.; Blekas, G.; Katikou, P.; Ambrosiadis, I.; Fletouris, D.J. Effect of rosemary extract, chitosan and α-tocopherol on lipid oxidation and colour stability during frozen storage of beef burgers. Meat Sci. 2007, 75, 256–264. [Google Scholar] [CrossRef] [PubMed]
- Coma, V. Bioactive chitosan-based substances and films. In Current Research and Developments on Chitin and Chitosan in Biomaterial Science; Research Signpost: Thiruvananthapuram, India, 2008; pp. 21–51. [Google Scholar]
- ResearchAndMarkets Fish Oil Market by Species and Aquaculture, Animal Nutrition & Pet Food, Pharmaceuticals, Supplements & Functional Food and Others: Global Opportunity Analysis and Industry Forecast, 2021–2027. Available online: https://www.researchandmarkets.com/reports/5141668/fish-oil-market-by-species-and-aquaculture (accessed on 1 December 2020).
- Wijesundera, C.; Kitessa, S.; Abeywardena, M.; Bignell, W.; Nichols, P.D. Long-chain omega-3 oils: Current and future supplies, food and feed applications, and stability. Lipid Technol. 2011, 23, 55–58. [Google Scholar] [CrossRef]
- Jackson, A.; Newton, R.W. Project to Model the Use of Fisheries By-Product in the Production of Marine Ingredients, with Special Reference to the Omega 3 Fatty Acids EPA and DHA; University of Stirling: Stirling, UK, 2016. [Google Scholar]
- Kudre, T.G.; Bhaskar, N.; Sakhare, P.Z. Optimization and characterization of biodiesel production from rohu (Labeo rohita) processing waste. Renew. Energy 2017, 113, 1408–1418. [Google Scholar] [CrossRef]
- Ramakrishnan, V. Extraction of Proteins from Mackerel Fish Processing Waste Using Alcalase Enzyme. J. Bioproces. Biotechniq. 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.S.; Park, J.W. Mince from seafood processing by-product and surimi as food ingredients. In Maximising the Value of Marine By-Products; Shahidi, F., Ed.; Woodhead Publishing: Cambridge, UK, 2007; Chapter 9; pp. 196–228. [Google Scholar]
- Kim, S.-K.; Mendis, E. Bioactive compounds from marine processing byproducts—A review. Food Res. Int. 2006, 39, 383–393. [Google Scholar] [CrossRef]
- Khoddami, A.; Ariffin, A.; Bakar, J.; Mohd Ghazali, H. Quality and Fatty acid profile of the oil extracted from fish waste (head, intestine and liver) (Euthynnus affinis). Afr. J. Biotechnol. 2012, 11, 1683–1689. [Google Scholar] [CrossRef]
- Ashraf, S.A.; Adnan, M.; Patel, M.; Siddiqui, A.J.; Sachidanandan, M.; Snoussi, M.; Hadi, S. Fish-based Bioactives as Potent Nutraceuticals: Exploring the Therapeutic Perspective of Sustainable Food from the Sea. Mar. Drugs 2020, 18, 265. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Vasudevan Ramakrishnan, V.; Brooks, M.S.; Budge, S.M.; Dave, D. Fish Processing Wastes as a Potential Source of Proteins, Amino Acids and Oils: A Critical Review. J. Microb. Biochem. Technol. 2013, 5, 107–129. [Google Scholar]
- Kerton, F.M.; Liu, Y.; Omari, K.W.; Hawboldt, K. Green chemistry and the ocean-based biorefinery. Green Chem. 2013, 15, 860–871. [Google Scholar] [CrossRef] [Green Version]
- Linder, M.; Fanni, J.; Parmentier, M. Proteolytic extraction of salmon oil and PUFA concentration by lipases. Mar. Biotechnol. 2005, 7, 70–76. [Google Scholar] [CrossRef]
- Batista, I.; Ramos, C.; Mendonça, R.; Nunes, M.L. Enzymatic Hydrolysis of Sardine (Sardina pilchardus) By-products and Lipid Recovery. J. Aquat. Food Prod. Technol. 2009, 18, 120–134. [Google Scholar] [CrossRef]
- Araujo, J.; Sica, P.; Costa, C.; Márquez, M.C. Enzymatic Hydrolysis of Fish Waste as an Alternative to Produce High Value-Added Products. Waste Biomass Valorization 2020, 12, 847–855. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; de Diego, S.M.; Beltrán, S.; Jaime, I.; Sanz, M.T.; Rovira, J. Supercritical fluid extraction of fish oil from fish by-products: A comparison with other extraction methods. J. Food Eng. 2012, 109, 238–248. [Google Scholar] [CrossRef] [Green Version]
- Sarker, M.Z.I.; Selamat, J.; Habib, A.S.M.A.; Ferdosh, S.; Akanda, M.J.H.; Jaffri, J.M. Optimization of supercritical CO(2) extraction of fish oil from viscera of African Catfish (Clarias gariepinus). Int. J. Mol. Sci. 2012, 13, 11312–11322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuvendziev, S.; Lisichkov, K.; Zekovic, Z.; Marinkovski, M.; Musliu, Z. Supercritical fluid extraction of fish oil from common carp (Cyprinus carpio L.) tissues. J. Supercrit. Fluids 2017, 133, 528–534. [Google Scholar] [CrossRef]
- Jaiswal, K.; Jha, B.; Ramaswamy, A.P. Biodiesel production from discarded fish waste for sustainable clean energy development. J. Chem. Pharm. Sci. 2014, 6, 113–114. [Google Scholar]
- Chakraborty, K.; Joseph, D. Cooking and pressing is an effective and eco-friendly technique for obtaining high quality oil from Sardinella longiceps. Eur. J. Lipid Sci. Technol. 2015, 117, 837–850. [Google Scholar] [CrossRef]
- Crexi, V.; Monte, M.; Souza, L.; Pinto, L. Production and refinement of oil from carp (Cyprinus carpio) viscera. Food Chem. 2010, 119, 945–950. [Google Scholar] [CrossRef]
- Venkatesan, J.; Kim, S. Introduction to Seafood Processing By-Products; Springer: New York, NY, USA, 2014; pp. 1–9. [Google Scholar]
- Zampolli, A.; Bysted, A.; Leth, T.; Mortensen, A.; De Caterina, R.; Falk, E. Contrasting effect of fish oil supplementation on the development of atherosclerosis in murine models. Atherosclerosis 2006, 184, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-C.; Chaung, H.; Chung, M.-Y.; Huang, L.-T. Menhaden fish oil improves spatial memory in rat pups following recurrent pentylenetetrazole-induced seizures. Epilepsy Behav. 2006, 8, 516–521. [Google Scholar] [CrossRef]
- Rubio-Rodríguez, N.; Beltrán, S.; Jaime, I.; De Diego, S.M.; Sanz, M.T.; Carballido, J.R. Production of omega-3 polyunsaturated fatty acid concentrates: A review. Innov. Food Sci. Emerg. Technol. 2010, 11, 1–12. [Google Scholar] [CrossRef]
- Kosman, V.M.; Demchenko, D.V.; Pozharitskaya, O.N.; Shikov, A.N.; Selesneva, A.I.; Makarov, V.G.; Makarova, M.N. Cardioprotective effect of new functional food containing salmon oil with motherwort oil extract. Vopr. Pitan. 2016, 86, 58–63. [Google Scholar] [CrossRef]
- Narendran, R.; Frankle, W.G.; Mason, N.S.; Muldoon, M.F.; Moghaddam, B. Improved Working Memory but No Effect on Striatal Vesicular Monoamine Transporter Type 2 after Omega-3 Polyunsaturated Fatty Acid Supplementation. PLoS ONE 2012, 7, e46832. [Google Scholar] [CrossRef] [Green Version]
- Van De Rest, O.; Geleijnse, J.M.; Kok, F.J.; Van Staveren, W.A.; Dullemeijer, C.; Rikkert, M.O.; Beekman, A.T.; De Groot, C.P. Effect of fish oil on cognitive performance in older subjects: A randomized, controlled trial. Neurology 2008, 71, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Muhammed, M.; Domendra, D.; Muthukumar, S.; Sakhare, P.; Bhaskar, N. Effects of fermentatively recovered fish waste lipids on the growth and composition of broiler meat. Br. Poult. Sci. 2015, 56, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Sellami, M.; Ben Rebah, F.; Gargouri, Y.; Miled, N. Lipid composition and antioxidant activity of liver oils from ray species living in Tunisian coasts. Arab. J. Chem. 2018, 11, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Pozharitskaya, O.N.; Shikov, A.N.; Kosman, V.M.; Selezneva, A.I.; Urakova, I.N.; Makarova, M.N.; Makarov, V.G. Immunomodulatory and antioxidants properties of fixed combination of fish oil with plant extracts. Synergy 2015, 2, 19–24. [Google Scholar] [CrossRef]
- Esakkiraj, P.; Rajkumarbharathi, M.; Palavesam, A.; Immanuel, G. Lipase production by Staphylococcus epidermidis CMST-Pi 1 isolated from the gut of shrimp Penaeus indicus. Ann. Microbiol. 2010, 60, 37–42. [Google Scholar] [CrossRef]
- Inguglia, L.; Chiaramonte, M.; Di Stefano, V.; Schillaci, D.; Cammilleri, G.; Pantano, L.; Mauro, M.; Vazzana, M.; Ferrantelli, V.; Nicolosi, R.; et al. Salmo salar fish waste oil: Fatty acids composition and antibacterial activity. PeerJ 2020, 8, e9299. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control. 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Yahyaee, R.; Ghobadian, B.; Najafi, G. Waste fish oil biodiesel as a source of renewable fuel in Iran. Renew. Sustain. Energy Rev. 2013, 17, 312–319. [Google Scholar] [CrossRef]
- Martins, G.I.; Secco, D.; Rosa, H.A.; Bariccatti, R.A.; Dolci, B.D.; De Souza, S.N.M.; Santos, R.F.; Da Silva, T.R.B.; Gurgacz, F. Physical and chemical properties of fish oil biodiesel produced in Brazil. Renew. Sustain. Energy Rev. 2015, 42, 154–157. [Google Scholar] [CrossRef]
- Wisniewski, A.; Wiggers, V.; Simionatto, E.; Meier, H.; Barros, A.; Madureira, L. Biofuels from waste fish oil pyrolysis: Chemical composition. Fuel 2010, 89, 563–568. [Google Scholar] [CrossRef]
- Enzymes Market Size By Product (Proteases, Lipases, Carbohydrases [Amylases, Xylanases, Cellulases, Pectinases, Lactases], Polymerases & Nucleases, Phytases, Catalyses), By Application (Food & Beverage, Processed Food, Diary, Bakery, Confectionary), Industry Analysis Report, Regional Outlook, Growth Potential, Price Trends, Competitive Market Share & Forecast, 2018–2024. Available online: https://www.gminsights.com/industry-analysis/enzymes-market (accessed on 3 January 2021).
- Shahidi, F.; Kamil, Y.J. Enzymes from fish and aquatic invertebrates and their application in the food industry. Trends Food Sci. Technol. 2001, 12, 435–464. [Google Scholar] [CrossRef]
- Bruno, S.; Coppola, D.; di Prisco, G.; Giordano, D.; Verde, C. Enzymes from marine polar regions and their biotechnological applications. Mar. Drugs 2019, 17, 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Otín, C.; Bond, J.S. Proteases: Multifunctional enzymes in life and disease. J. Biol. Chem. 2008, 283, 30433–30437. [Google Scholar] [CrossRef] [Green Version]
- Sriket, C. Proteases in fish and shellfish: Role on muscle softening and prevention. Int. Food Res. J. 2014, 21, 433. [Google Scholar]
- Morrissey, M.; Okada, T. Marine enzymes from seafood by-products. In Maximising the Value of Marine By-Products; Elsevier: Amsterdam, The Netherlands, 2007; pp. 374–396. [Google Scholar]
- Gildberg, A.R.; Raa, J. Purification and characterization of pepsins from the arctic fish capelin (Mallotus villosus). Comp. Biochem. Physiol. A Comp. Physiol. 1983, 75, 337–342. [Google Scholar] [CrossRef]
- Gildberg, A. Aspartic proteinases in fishes and aquatic invertebrates. Comp. Biochem. Physiol. Part B Comp. Biochem. 1988, 91, 425–435. [Google Scholar] [CrossRef]
- Yazawa, K.; Numata, K. Recent advances in chemoenzymatic peptide syntheses. Molecules 2014, 19, 13755–13774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khangembam, B.K.; Chakrabarti, R. Trypsin from the digestive system of carp Cirrhinus mrigala: Purification, characterization and its potential application. Food Chem. 2015, 175, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-K.; Park, P.-J.; Kim, J.-B.; Shahidi, F. Purification and characterization of a collagenolytic protease from the filefish, Novoden modestrus. J. Biochem. Mol. Biol. 2002, 35, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Wong, H.; Schotz, M.C. The lipase gene family. J. Lipid Res. 2002, 43, 993–999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Yi, L.; Marek, P.; Iverson, B.L. Commercial proteases: Present and future. FEBS Lett. 2013, 587, 1155–1163. [Google Scholar] [CrossRef] [Green Version]
- Jabalia, N.; Mishra, P.; Chaudhary, N. Applications, challenges and future prospects of proteases: An overview. J. Agroecol. Nat. Resour. Manag. 2014, 1, 179–183. [Google Scholar]
- Sawant, R.; Nagendran, S. Protease: An enzyme with multiple industrial applications. World J. Pharm. Pharm. Sci. 2014, 3, 568–579. [Google Scholar]
- Jellouli, K.; Bougatef, A.; Daassi, D.; Balti, R.; Barkia, A.; Nasri, M. New alkaline trypsin from the intestine of grey triggerfish (Balistes capriscus) with high activity at low temperature: Purification and characterisation. Food Chem. 2009, 116, 644–650. [Google Scholar] [CrossRef]
- Fuchise, T.; Kishimura, H.; Sekizaki, H.; Nonami, Y.; Kanno, G.; Klomklao, S.; Benjakul, S.; Chun, B.-S. Purification and characteristics of trypsins from cold-zone fish, Pacific cod (Gadus macrocephalus) and saffron cod (Eleginus gracilis). Food Chem. 2009, 116, 611–616. [Google Scholar] [CrossRef]
- Gelman, A.; Mokady, S.; Cogan, U. The thermal properties of intestinal alkaline phosphatase of three kinds of deep-water fish. Comp. Biochem. Physiol. B Comp. Biochem. 1989, 94, 113. [Google Scholar]
- Simpson, B.; Haard, N. Cold-Adapted Enzymes from Fish; FAO: Rome, Italy, 1987. [Google Scholar]
- Svenning, R.; Stenberg, E.; Gildberg, A.; Nilsen, K. Biotechnological descaling of fish. Infofish Int. 1993, 6, 30–31. [Google Scholar]
- Gildberg, A. Enzymes and bioactive peptides from fish waste related to fish silage, fish feed and fish sauce production. J. Aquat. Food Prod. Technol. 2004, 13, 3–11. [Google Scholar] [CrossRef]
- Squires, J.; Haard, N.; Feltham, L. Pepsin isozymes from Greenland cod (Gadus ogac). 2. Substrate specificity and kinetic properties. Can. J. Biochem Cell Biol 1986, 65, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Gildberg, A. Recovery of proteinases and protein hydrolysates from fish viscera. Bioresour. Technol. 1992, 39, 271–276. [Google Scholar] [CrossRef]
- Haard, N.; Simpson, B. Proteases from aquatic organisms and their uses in the seafood industry. In Fisheries Processing; Springer: New York, NY, USA, 1994; pp. 132–154. [Google Scholar]
- Petersen, B.R. The impact of the enzymic hydrolysis process on recovery and use of proteins. In Enzymes and Food Processing; Springer: New York, NY, USA, 1981; pp. 149–175. [Google Scholar]
- Liu, J.; Lyu, F.; Zhou, X.; Wang, B.; Wang, X.; Ding, Y. Preparation of skipjack tuna (Katsuwonus pelamis) protein hydrolysate using combined controlled enzymatic hydrolysis and glycation for improved solubility and emulsifying properties. J. Food Nutr. Res. 2015, 3, 471–477. [Google Scholar]
- Han, X.-Q. Recovery of Digestive Enzymes from Atlantic Cod (Gadus morhua) and Their Utilization in Food Processing; Memorial University of Newfoundland: St. John’s, NL, Canada, 1993. [Google Scholar]
- Mohr, V. Enzymes technology in the meat and fish industries. Proc. Biochem. 1980, 15, 18–32. [Google Scholar]
- Vieira, G.H.; Martin, A.M.; Saker-Sampaiao, S.; Omar, S.; Goncalves, R.C. Studies on the enzymatic hydrolysis of Brazilian lobster (Panulirus spp.) processing wastes. J. Sci. Food Agric. 1995, 69, 61–65. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle proteins hydrolyzed with various alkaline proteases. J. Agric. Food Chem. 2000, 48, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Zamani, A.; Benjakul, S. Trypsin from unicorn leatherjacket (Aluterus monoceros) pyloric caeca: Purification and its use for preparation of fish protein hydrolysate with antioxidative activity. J. Sci. Food Agric. 2016, 96, 962–969. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S. Extraction and characterisation of pepsin-solubilised collagen from the skin of unicorn leatherjacket (Aluterus monocerous). Food Chem. 2010, 120, 817–824. [Google Scholar] [CrossRef]
- Arnesen, J.A.; Gildberg, A. Extraction of muscle proteins and gelatine from cod head. Process. Biochem. 2006, 41, 697–700. [Google Scholar] [CrossRef]
- Nalinanon, S.; Benjakul, S.; Visessanguan, W.; Kishimura, H. Tuna pepsin: Characteristics and its use for collagen extraction from the skin of threadfin bream (Nemipterus spp.). J. Food Sci. 2008, 73, C413–C419. [Google Scholar] [CrossRef]
- Tschersich, P.; Choudhury, G.S. Arrowtooth flounder (Atheresthes stomias) protease as a processing aid. J. Aquat. Food Prod. Technol. 1998, 7, 77–89. [Google Scholar] [CrossRef]
- Jeya Shakila, R.; Jeyasekaran, G. Fish processing byproducts: Quality Assessment and Applications. In Fish Processing By-Products: Quality Assessment and Application; Studium Press LLC.: New Delhi, India, 2014; pp. 107–135. [Google Scholar]
- Jeya Shakila, R.; Jeyasekaran, G. Protein recovery from fish processing discards. In Fish Processing By-Products: Quality Assessment and Application; Studium Press LLC.: New Delhi, India, 2015. [Google Scholar]
- Lie, Ø.; Lambertsen, G. Digestive lipolytic enzymes in cod (Gadus morrhua): Fatty acid specificity. Comp. Biochem. Physiol. Part B Comp. Biochem. 1985, 80, 447–450. [Google Scholar] [CrossRef]
- Díaz-López, M.; García-Carreño, F.L. Applications of fish and shellfish enzymes in food and feed products. In Seafood Enzymes; Routledge: London, UK, 2000; pp. 571–618. [Google Scholar]
- Sae-Leaw, T.; Benjakul, S. Lipase from liver of seabass (Lates calcarifer): Characteristics and the use for defatting of fish skin. Food Chem. 2018, 240, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kurtovic, I.; Marshall, S.N.; Zhao, X.; Simpson, B.K. Lipases from mammals and fishes. Rev. Fish. Sci. 2009, 17, 18–40. [Google Scholar] [CrossRef]
- Gildberg, A.R.; Olsen, R.L.; Bjarnason, J.B. Catalytic properties and chemical composition of pepsins from Atlantic cod (Gadus morhua). Comp. Biochem. Physiol. B Comp. Biochem. 1990, 96, 323–330. [Google Scholar] [CrossRef]
- Xu, R.; Wong, R.; Rogers, M.; Fletcher, G. Purification and characterization of acidic proteases from the stomach of the deepwater finfish orange roughy (Hoplostethus atlanticus). J. Food Biochem. 1996, 20, 31–48. [Google Scholar] [CrossRef]
- Lanes, O.; Guddal, P.H.; Gjellesvik, D.R.; Willassen, N.P. Purification and characterization of a cold-adapted uracil-DNA glycosylase from Atlantic cod (Gadus morhua). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 127, 399–410. [Google Scholar] [CrossRef]
- D’Adamo, I. Adopting a circular economy: Current practices and future perspectives. Soc. Sci. 2019, 8, 328. [Google Scholar] [CrossRef] [Green Version]
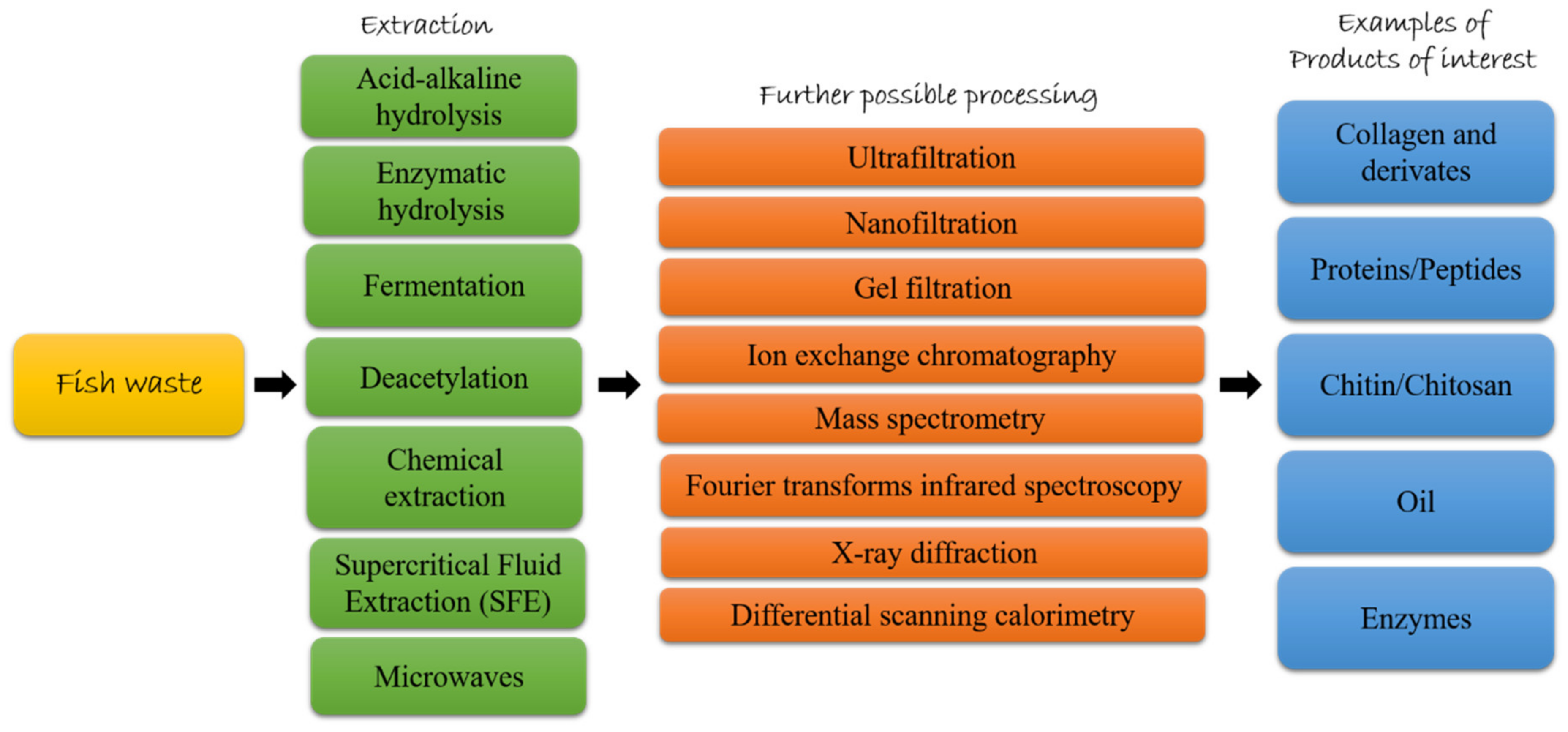
| Compound | Byproduct | Source | Applications | Reference |
|---|---|---|---|---|
| Pepsin | Stomach | Atlantic cod (Gadus morhua) | Caviar productionFish descaling | [298] |
| Pepsin | Viscera | cod | Silage production | [299] |
| Pepsin | Stomach | Orange roughy (Hoplostethus atlanticus) | Caviar production | [299] |
| Proteases | Pyloric ceca | Atlantic Salmon (Salmo salar) | Fish hydrolyzates production | [286] |
| Trypsin | Pyloric ceca | Unicorn leatherjacket (Aluterus monoceros) | Fish hydrolyzates production | [287] |
| Proteases | Intestine | Bluefin tuna (Thunnus thynnus) | Fish hydrolyzates production | [174] |
| Proteases | Stomach | Albacore tuna (Thunnus alalunga) | PSC extraction | [288] |
| Proteases | Stomach | Yellowfin tuna (Thunnus albacares) | PSC extraction | [288] |
| Uracil-DNA Glycosylase | Liver | Atlantic cod (Gadus morhua) | Molecular biology | [300] |
| Lipases | Intestine/Pyloric ceca | Atlantic cod (Gadus morhua) | Potential lipids synthesis | [294] |
| Lipases | Liver | Sea bass (Lates calcarifer) | Defatting of fish skin | [296] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. https://doi.org/10.3390/md19020116
Coppola D, Lauritano C, Palma Esposito F, Riccio G, Rizzo C, de Pascale D. Fish Waste: From Problem to Valuable Resource. Marine Drugs. 2021; 19(2):116. https://doi.org/10.3390/md19020116
Chicago/Turabian StyleCoppola, Daniela, Chiara Lauritano, Fortunato Palma Esposito, Gennaro Riccio, Carmen Rizzo, and Donatella de Pascale. 2021. "Fish Waste: From Problem to Valuable Resource" Marine Drugs 19, no. 2: 116. https://doi.org/10.3390/md19020116
APA StyleCoppola, D., Lauritano, C., Palma Esposito, F., Riccio, G., Rizzo, C., & de Pascale, D. (2021). Fish Waste: From Problem to Valuable Resource. Marine Drugs, 19(2), 116. https://doi.org/10.3390/md19020116










