Potential of Exopolysaccharide from Porphyridium marinum to Contend with Bacterial Proliferation, Biofilm Formation, and Breast Cancer
Abstract
:1. Introduction
2. Results
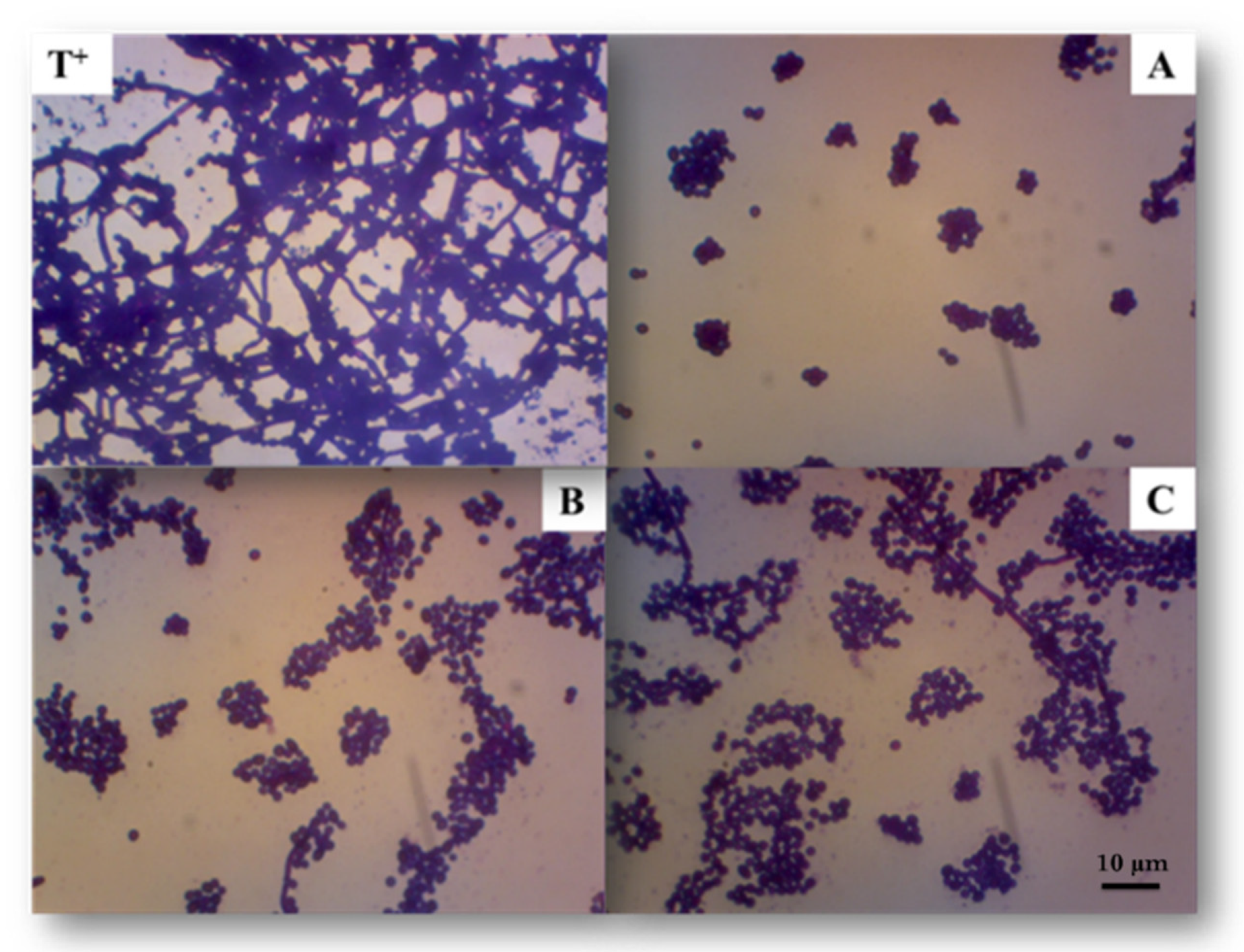
2.1. Production of Different Molar Masses Exopolysaccharides by HPH
2.2. Structural and Physico-Chemical Characterization of EPS Samples
2.3. Biological Activities
2.3.1. Antibacterial and Anti-Candida Albicans Activities
2.3.2. Inhibition of Biofilm Formation by Candida Albicans ATCC 10231
2.3.3. T1 Mammary Carcinoma Cells Sensitivity towards EPS Samples
3. Discussion
4. Materials and Methods
4.1. Extraction of Exopolysaccharides
4.2. Preparation of Different-MW Exopolysaccharides
- EPS-0C for the undegraded sample,
- EPS-2C for the polysaccharide after two cycles,
- EPS-5C for the polysaccharide after five cycles.
4.3. Exopolysaccharides Characterization
4.3.1. Total Sugars, Neutral Sugars, Uronic Acids, Sulfate and Proteins Content
4.3.2. Monosaccharide Composition of EPS
4.3.3. Molar Masses Determination
4.3.4. Infrared Spectrometry Footprint (FTIR)
4.3.5. Shear Flow Behavior of EPS Samples
4.4. Biological Activities
4.4.1. Antibacterial Activity
4.4.2. Anti-Candida Albicans Activity
4.4.3. Inhibition of Biofilm Formation
4.4.4. Cytotoxicity Evaluation
4.4.5. Antiproliferative Effect of Different MW-EPS on Breast Cancer Cells
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gargouch, N.; Karkouch, I.; Elleuch, J.; Elkahoui, S.; Michaud, P.; Abdelkafi, S.; Laroche, C.; Fendri, I. Enhanced B-phycoerythrin production by the red microalga Porphyridium marinum: A powerful agent in industrial applications. Int. J. Biol. Macromol. 2018, 120, 2106–2114. [Google Scholar] [CrossRef] [PubMed]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ji, L.; Shi, Q.; Wu, H.; Fan, J. Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium sp. Bioresour. Technol. 2019, 292, 122048. [Google Scholar] [CrossRef]
- Gaignard, C.; Gargouch, N.; Dubessay, P.; Delattre, C.; Pierre, G.; Laroche, C.; Fendri, I.; Abdelkafi, S.; Michaud, P. New horizons in culture and valorization of red microalgae. Biotechnol. Adv. 2019, 37, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Geresh, S.; Arad, S.; Levy-Ontman, O.; Zhang, W. Isolation and characterization of poly- and oligosaccharides from the red microalga Porphyridium sp. Carbohydr. Res. 2009, 344, 343–349. [Google Scholar] [CrossRef]
- Roussel, M.; Villay, A.; Delbac, F.; Michaud, P.; Laroche, C.; Roriz, D.; El Alaoui, H.; Diogon, M. Antimicrosporidian activity of sulphated polysaccharides from algae and their potential to control honeybee nosemosis. Carbohydr. Polym. 2015, 133, 213–220. [Google Scholar] [CrossRef]
- Soanen, N.; Da Silva, E.; Gardarin, C.; Michaud, P.; Laroche, C. Improvement of exopolysaccharide production by Porphyridium marinum. Bioresour. Technol. 2016, 213, 231–238. [Google Scholar] [CrossRef]
- Villay, A.; de Filippis, F.L.; Picton, L.; LeCerf, D.; Vial, C.; Michaud, P. Comparison of polysaccharide degradations by dynamic high-pressure homogenization. Food Hydrocoll. 2012, 27, 278–286. [Google Scholar] [CrossRef]
- Porto, B.C.; Cristianini, M. Effect of dynamic high pressure on emulsifying and encapsulant properties of cashew tree gum. Carbohydr. Polym. 2018, 186, 350–357. [Google Scholar] [CrossRef]
- Fenoradosoa, T.A.; Laroche, C.; Delattre, C.; Dulong, V.; Lecerf, D.; Picton, L.; Michaud, P. Rheological behavior and non-enzymatic degradation of a sulphated galactan from Halymenia durvillei (Halymeniales, Rhodophyta). Appl. Biochem. Biotechnol. 2012, 167, 1303–1313. [Google Scholar] [CrossRef]
- Geresh, S.; Adin, I.; Yarmolinsky, E.; Karpasas, M. Characterization of the extracellular polysaccharide of Porphyridium sp.: Molecular weight determination and rheological properties. Carbohyd. Polym. 2002, 50, 183–189. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Kyomugasho, C.; Van Looveren, N.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Molecular and rheological characterization of different cell wall fractions of Porphyridium cruentum. Carbohydr. Polym. 2018, 195, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Gaignard, C.; Macao, V.; Gardarin, C.; Rihouey, C.; Picton, L.; Michaud, P.; Laroche, C. The red microalga Flintiella sanguinaria as a new exopolysaccharide producer. J. Appl. Phycol. 2018, 30, 2803–2814. [Google Scholar] [CrossRef]
- Capek, P.; Matulováa, M.; Combourieu, B. The extracellular proteoglycan produced by Rhodella grisea. Int. J. Biol. Macromol. 2008, 43, 390–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Percival, E.; Foyle, A.R. The extracellular polysaccharides of Porphyridium cruentum and Porphyridium aerugineum. Carbohydr. Res. 1979, 72, 165–176. [Google Scholar] [CrossRef]
- Medina-Cabrera, E.V.; Rühmann, B.; Schmid, J.; Sieber, V. Characterization and comparison of Porphyridium sordidum and Porphyridium purpureum concerning growth characteristics and polysaccharide production. Algal Res. 2020, 49, 101931. [Google Scholar] [CrossRef]
- Monsoor, M.A.; Kalapathy, U.; Proctor, A. Determination of polygalacturonic acid content in pectin extracts by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2001, 74, 233–238. [Google Scholar] [CrossRef]
- Gómez-Ordóñez, E.; Rupérez, P. FTIR-ATR spectroscopy as a tool for polysaccharide identification in edible brown and red seaweeds. Food Hydrocoll. 2011, 25, 1514–1520. [Google Scholar] [CrossRef]
- Chopin, T.; Kerin, B.F.; Mazerolle, R. Phyccolloid chemistry as taxonomic indicator of phylogeny in the Gigartinales, Rhodophyceae: A review and current developments using fourrier transform infrared diffuse reflectance spectroscopy. Phycol. Res. 1999, 47, 167–188. [Google Scholar] [CrossRef]
- Deng, J.; Shi, J.J.; Li, X.Z.; Liu, H.M. Soluble polysaccharides isolation and characterization from rabbit eye blueberry (Vaccinium ashei) fruits. Bioresources 2013, 8, 405–419. [Google Scholar]
- Tomar, S.; Adaganti, S.Y. Production of ethanol using Calliandra shrub by hydrothermal pretreatment method. Int. J. Curr. Eng. Tech. 2013, 3, 1921–1924. [Google Scholar]
- Li, G.Y.; Luo, Z.C.; Yuan, F.; Yu, X.B. Combined process of high-pressure homogenization and hydrothermal extraction for the extraction of fucoidan with good antioxidant properties from Nemacystus decipients. Food Bioprod. Process. 2017, 106, 35–42. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Medina-Cabrera, E.V.; Gansbiller, M.; Rühmann, B.; Schmid, J.; Sieber, V. Rheological characterization of Porphyridium sordidum and Porphyridium purpureum exopolysaccharides. Carbohydr. Polym. 2021, 253, 117237. [Google Scholar] [CrossRef]
- Geresh, S.; Arad, S.M. The extracellular polysaccharides of the red microalgae: Chemistry and rheology. Bioresour. Technol. 1991, 38, 195–201. [Google Scholar] [CrossRef]
- Eteshola, E.; Karpasas, M.; Arad, S.M.; Gottlieb, M. Red microalga exopolysaccharides: 2. Study of the rheology, morphology and thermal gelation of aqueous preparations. Acta Polym. 1998, 49, 549–556. [Google Scholar] [CrossRef]
- Balti, R.; Balc’h, R.L.; Brodu, N.; Gilbert, M.; Le Gouic, B.; Le Gall, S.; Sinquin, C.; Massé, A. Concentration and purification of Porphyridium cruentum exopolysaccharides by membrane filtration at various cross-flow velocities. Process Biochem. 2018, 74, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Matsui, S.M.; Muizzudin, N.; Arad, S.M.; Marenus, K. Sulfated polysaccharides from red microalgae anti-inflammatory properties in vitro and in vivo. Appl. Biochem. Biotechnol. 2003, 104, 13–22. [Google Scholar] [CrossRef]
- Tannin-Spitz, T.; Bergman, M.; Van-Moppes, D.; Grossman, S.; Arad, S.M. Antioxidant activity of the polysaccharide of the red microalga Porphyridium sp. J. Appl. Phycol. 2005, 17, 215–222. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulphated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef] [Green Version]
- Guzmán-Murillo, M.A.; Ascencio, F. Anti-adhesive activity of sulphated exopolysaccharides of microalgae on attachment of the red sore disease-associated bacteria and Helicobacter pylori to tissue culture cells. Lett. Appl. Microbiol. 2000, 30, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Huheihel, M.; Ishanu, V.; Tal, J.; Arad, S.M. Activity of Porphyridium sp. polysaccharide against herpes simplex viruses in vitro and in vivo. J. Biochem. Biophys. Methods 2002, 48, 189–200. [Google Scholar] [CrossRef]
- Arad, S.M.; Levy-Ontman, O. Red microalgal cell-wall polysaccharides: Biotechnological aspects. Curr. Opin. Biotech. 2010, 21, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Gardeva, E.; Toshkova, R.; Minkova, K.; Gigova, L. Cancer Protective Action of Polysaccharide, Derived from Red Microalga Porphyridium cruentum—A Biological Background. Biotechnol. Biotech. Equip. 2009, 23, 783–787. [Google Scholar] [CrossRef]
- Sun, L.; Wang, L.; Zhou, Y. Immunomodulation and antitumor activities of different molecular-weight polysaccharides from Porphyridium cruentum. Carbohydr. Polym. 2012, 87, 1206–1210. [Google Scholar] [CrossRef]
- Chen, D.; Wu, X.Z.; Wen, Z.Y. Sulfated polysaccharides and immune response: Promoter or inhibitor? Panminerva Med. 2008, 177–183. [Google Scholar]
- Zhou, G.F.; Sheng, W.X.; Yao, W.D.; Wang, C.H. Effect of low molecular λ-carrageenan from Chondrus ocellatus on antitumor H-22 activity of 5-Fu. Pharmacol. Res. 2006, 53, 129–134. [Google Scholar] [CrossRef]
- Zhu, X.L.; Chen, A.F.; Lin, Z.B. Ganoderma lucidum polysaccharides enhance the function of immunological effector cells in immunosuppressed mice. J. Ethnopharmacol. 2007, 111, 219–226. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, Y.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef]
- Valaperta, R.; Tejada, M.R.; Frigerio, M.; Moroni, A.; Ciulla, E.; Cioffi, S.; Capelli, P.; Costa, E. Staphylococcus aureus nosocomial infections: The role of a rapid and low-cost characterization for the establishment of a surveillance system. New Microbiol. 2010, 33, 223–232. [Google Scholar]
- Zeaki, N.; Johler, S.; Skandamis, P.N.; Schelin, J. The Role of Regulatory Mechanisms and Environmental Parameters in Staphylococcal Food Poisoning and Resulting Challenges to Risk Assessment. Front. Microbiol. 2019, 10, 1307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Tabbene, O.; Slimene, I.B.; Bouabdallah, F.; Mangoni, M.L.; Urdaci, M.; Limam, F. Production of Anti-Methicillin-Resistant Staphylococcus Activity from Bacillus subtilis sp. Strain B38 Newly Isolated from Soil. Appl. Biochem. Biotechnol. 2009, 157, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.Y.; Yuan, Z.L.; Hu, D.D.; Hu, G.H.; Zhang, R.L.; Zhong, H.; Yan, L.; Jiang, Y.Y.; Su, J.; Wang, Y. Effect of loureirin A against Candida albicans biofilms. Chin. J. Nat. Med. 2019, 17, 616–623. [Google Scholar] [CrossRef]
- Vale-Silva, L.A.; Buchta, V.; Valentova, E. Effect of sub-inhibitory concentration of some established and experimental antifungal compounds on the germ tube formation in Candida albicans. Folia Microbiol. 2007, 52, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Ariyachet, C.; Solis, N.V.; Liu, Y.; Prasadarao, N.V.; Filler, S.G.; McBride, A.E. SR-Like RNA-binding protein Slr1 affects Candida albicans filamentation and virulence. Infect. Immun. 2013, 81, 267–1276. [Google Scholar] [CrossRef] [Green Version]
- Borghi, E.; Morace, G.; Borgo, F.; Rajendran, R.; Sherry, L.; Nile, C.; Ramage, G. New strategic insights into managing fungal biofilms. Front. Microbiol. 2015, 6, 1077. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, A.M.; de Morais, R.M. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci. 2014, 101, 56–63. [Google Scholar] [CrossRef]
- Doghri, I.; Lavaud, J.; Dufour, A.; Bazire, A.; Lanneluc, I.; Sablé, S. Cell-bound exopolysaccharides from an axenic culture of the intertidal mudflat Navicula phyllepta diatom affect biofilm formation by benthic bacteria. J. Appl. Phycol. 2017, 29, 165–177. [Google Scholar] [CrossRef]
- Vijayabaskar, P.; Vaseela, N.; Thirumaran, G. Potential antibacterial and antioxidant properties of a sulfated polysaccharide from the brown marine algae Sargassum swartzii. Chin. J. Nat. Med. 2012, 10, 421–428. [Google Scholar] [CrossRef]
- Amorim, R.; Rodrigues, J.; Holanda, M.; Quinderé, A.; Paula, R.; Melo, V.M.M.; Benevides, N.M.B. Antimicrobial effect of a crude sulfated polysaccharide from the red seaweed Gracilaria ornate. Braz. Arch. Biol. Technol. 2012, 55, 171–181. [Google Scholar] [CrossRef]
- Costa, E.; Silva, S.; Tavaria, F.; Pintado, M. Antimicrobial and Antibiofilm Activity of Chitosan on the Oral Pathogen Candida albicans. Pathogens 2014, 3, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Cobrado, L.; Silva-Dias, A.; Azevedo, M.M.; Pina-Vaz, C.; Rodrigues, A.G. In vivo antibiofilm effect of cerium, chitosan and hamamelitannin against usual agents of catheter-related bloodstream infections. J. Antimicrob. Chemother. 2013, 68, 126–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- No, H.K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Silva, L.P.; Britto, D.; Seleghim, M.H.R.; Assis, O.B.G. In vitro activity of water-soluble quaternary chitosan chloride salt against. E coli. World J. Microbiol. Biotechnol. 2010, 26, 2089–2092. [Google Scholar] [CrossRef]
- Laroche, C.; Delattre, C.; Mati-Baouche, N.; Salah, R.; Ursu, A.V.; Moulti-Mati, F.; Michaud, P.; Pierre, G. Bioactivity of Chitosan and Its Derivatives. Curr. Org. Chem. 2018, 22, 641–667. [Google Scholar] [CrossRef]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.L.; Yang, D.Z.; Li, Z.; Yao, K.D. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar]
- Monsigny, M.; Petit, C.; Roche, A.C. Colorimetric determination of neutral sugars by a resorcinol sulfuric acid micromethod. Anal. Biochem. 1988, 175, 525–530. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Montreuil, J.; Spick, G.; Chosson, A.; Segard, E.; Scheppler, N. Methods of study of the structure of glycoproteins. J. Pharm. Belg. 1963, 18, 529–546. [Google Scholar] [PubMed]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–269. [Google Scholar] [CrossRef]
- Azaiez, S.; Ben Slimene, I.; Karkouch, I.; Essid, R.; Jallouli, S.; Djebali, N.; Elkahoui, S.; Limam, F.; Tabbene, O. Biological control of the soft rot bacterium Pectobacterium carotovorum by Bacillus amyloliquefaciens strain Ar10 producing glycolipid-like compounds. Microbiol. Res. 2018, 217, 23–33. [Google Scholar] [CrossRef]
- Grieco, P.; Carotenuto, A.; Auriemma, L.; Limatola, A.; Di Maro, S.; Merlino, F.; Mangoni, M.L.; Luca, V.; Di Grazia, A.; Campiglia, P.; et al. Novel α-MSH peptide analogues with broad spectrum antimicrobial activity. PLoS ONE 2013, 8, e61614. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.; Yip, H.K.; Samaranayake, Y.H.; Yau, J.Y.; Samaranayake, L.P. Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J. Clin. Microbiol. 2003, 41, 2961–2967. [Google Scholar] [CrossRef] [Green Version]
- Medjeldi, S.; Bouslama, L.; Benabdallah, A.; Essid, R.; Haou, S.; Elkahoui, S. Biological activities, and phytocompounds of northwest Algeria Ajuga iva (L) extracts: Partial identification of the antibacterial fraction. Microb. Pathog. 2018, 121, 173–178. [Google Scholar] [CrossRef]





| Samples | % Recovery | Mn (kDa) | Mw (kDa) | Rg (nm) | Rh (nm) | [ƞ] mL/g |
|---|---|---|---|---|---|---|
| EPS-0C | 8.5 | 890 (±35%) | 1400 (± 40%) | 150 (± 5%) | 67 (± 5.1) | 1480 (± 1%) |
| EPS-2C | 73 | 400 (± 0.7%) | 550 (± 2%) | 43 (± 2%) | 26 (± 0.5) | 230 (± 0.4%) |
| EPS-5C | 72 | 340 (± 0.6%) | 550 (± 4%) | 41 (± 3%) | 21 (± 0.6) | 155 (± 0.5%) |
| EPS Samples | |||
|---|---|---|---|
| EPS-0C | EPS-2C | EPS-5C | |
| Uronic acids (%) | 22 ± 0.1 | 22 ± 0.1 | 21 ± 0.2 |
| Sulfates (%) | 9.2 ± 0.3 | 8.7 ± 0.8 | 9.2 ± 0.7 |
| Xylose (%) | 47 | 47 | 44 |
| Galactose (%) | 25 | 25 | 29 |
| Glucose (%) | 20 | 19 | 20 |
| Fucose (%) | 1 | 1 | 1 |
| Arabinose (%) | 2 | 2 | 1 |
| Glucuronic acid (%) | 5 | 5 | 4 |
| Strains | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| EPS-0C | EPS-2C | EPS-5C | Cefazolin | Amphotericin B | |
| Gram (−) Bacteria | |||||
| Escherichia coli ATCC 25922 | 62.5 | 2500 | 2500 | 8 | - |
| Salmonella Enteritidis | 125 | 2500 | - | 4 | - |
| Gram (+) Bacteria | |||||
| Staphylococcus aureus ATCC 29213 | 125 | 1250 | 2500 | 1 | - |
| Staphylococcus Methicilin Resistant (SMR) | 1000 | - | - | 512 | - |
| Yeast | |||||
| Candida albicans ATCC 10231 | - | - | - | - | 0.125 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gargouch, N.; Elleuch, F.; Karkouch, I.; Tabbene, O.; Pichon, C.; Gardarin, C.; Rihouey, C.; Picton, L.; Abdelkafi, S.; Fendri, I.; et al. Potential of Exopolysaccharide from Porphyridium marinum to Contend with Bacterial Proliferation, Biofilm Formation, and Breast Cancer. Mar. Drugs 2021, 19, 66. https://doi.org/10.3390/md19020066
Gargouch N, Elleuch F, Karkouch I, Tabbene O, Pichon C, Gardarin C, Rihouey C, Picton L, Abdelkafi S, Fendri I, et al. Potential of Exopolysaccharide from Porphyridium marinum to Contend with Bacterial Proliferation, Biofilm Formation, and Breast Cancer. Marine Drugs. 2021; 19(2):66. https://doi.org/10.3390/md19020066
Chicago/Turabian StyleGargouch, Nesrine, Fatma Elleuch, Ines Karkouch, Olfa Tabbene, Chantal Pichon, Christine Gardarin, Christophe Rihouey, Luc Picton, Slim Abdelkafi, Imen Fendri, and et al. 2021. "Potential of Exopolysaccharide from Porphyridium marinum to Contend with Bacterial Proliferation, Biofilm Formation, and Breast Cancer" Marine Drugs 19, no. 2: 66. https://doi.org/10.3390/md19020066
APA StyleGargouch, N., Elleuch, F., Karkouch, I., Tabbene, O., Pichon, C., Gardarin, C., Rihouey, C., Picton, L., Abdelkafi, S., Fendri, I., & Laroche, C. (2021). Potential of Exopolysaccharide from Porphyridium marinum to Contend with Bacterial Proliferation, Biofilm Formation, and Breast Cancer. Marine Drugs, 19(2), 66. https://doi.org/10.3390/md19020066







