Antiviral Potential of Algal Metabolites—A Comprehensive Review
Abstract
:1. Introduction
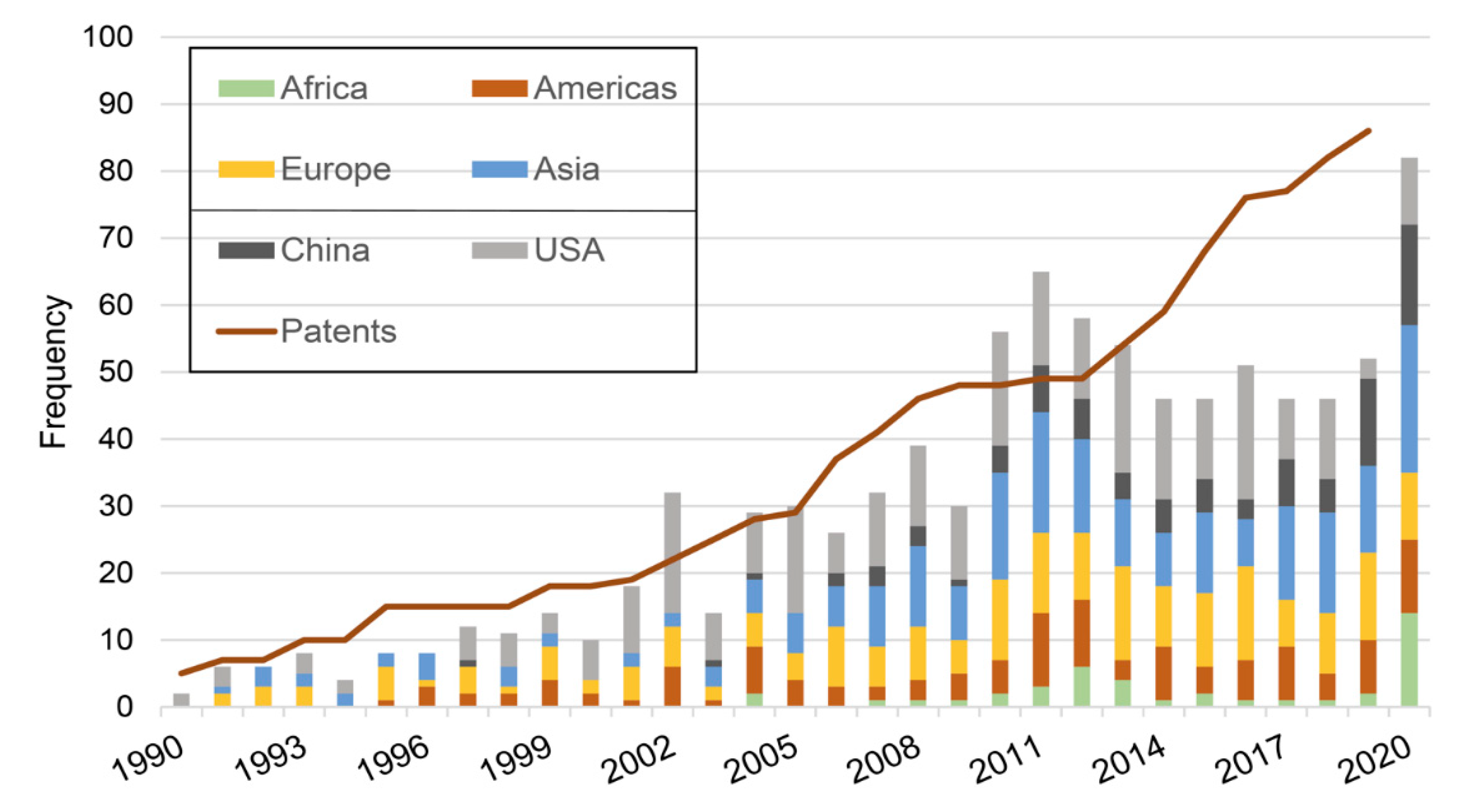
2. Bibliometric Analysis
2.1. Progression Overtime
2.2. Main Trends: Organisms/Molecules/Diseases
2.3. Aquaculture and Agriculture
3. Polysaccharides
3.1. Fucoidans
3.2. Carrageenans
3.3. Ulvans
3.4. Spirulan
4. Lectins
4.1. Griffithsin
4.2. Cyanovirin-n
4.3. Scytovirin
4.4. Microvirin
5. Diterpenes
6. Clinical Trials
7. Patent Analysis
8. Materials and Methods
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Breitbart, M.; Rohwer, F. Here a virus, there a virus, everywhere the same virus? Trends Microbiol. 2005, 13, 278–284. [Google Scholar] [CrossRef]
- Loutfy, M.R.; Wu, W.; Letchumanan, M.; Bondy, L.; Antoniou, T.; Margolese, S.; Zhang, Y.; Rueda, S.; McGee, F.; Peck, R.; et al. Systematic review of HIV transmission between heterosexual serodiscordant couples where the HIV-positive partner is fully suppressed on antiretroviral therapy. PLoS ONE 2013, 8, e55747. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Sperle, I.; Maticic, M.; Wiessing, L. A systematic review of hepatitis C virus treatment uptake among people who inject drugs in the European Region. BMC Infect. Dis. 2014, 14, S16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murrell, S.; Wu, S.-C.; Butler, M. Review of dengue virus and the development of a vaccine. Biotechnol. Adv. 2011, 29, 239–247. [Google Scholar] [CrossRef]
- Sehrawat, S.; Kumar, D.; Rouse, B.T. Herpesviruses: Harmonious pathogens but relevant cofactors in other diseases? Front. Cell. Infect. Microbiol. 2018, 8, 177. [Google Scholar] [CrossRef]
- Jacob, S.T.; Crozier, I.; Fischer, W.A.; Hewlett, A.; Kraft, C.S.; Vega, M.-A.D.L.; Soka, M.J.; Wahl, V.; Griffiths, A.; Bollinger, L.; et al. Ebola virus disease. Nat. Rev. Dis. Primers 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World health organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- Li, X.; Qian, H.; Miyamoto, F.; Naito, T.; Kawaji, K.; Kajiwara, K.; Hattori, T.; Matsuoka, M.; Watanabe, K.; Oishi, S.; et al. A simple, rapid, and sensitive system for the evaluation of anti-viral drugs in rats. Biochem. Biophys. Res. Commun. 2012, 424, 257–261. [Google Scholar] [CrossRef]
- De Clercq, E. Strategies in the design of antiviral drugs. Nat. Rev. Drug Discov. 2002, 1, 13–25. [Google Scholar] [CrossRef]
- Piwowar, A.; Harasym, J. The importance and prospects of the use of algae in agribusiness. Sustainability 2020, 12, 5669. [Google Scholar] [CrossRef]
- Rajauria, G. Chapter 15—Seaweeds: A sustainable feed source for livestock and aquaculture. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 389–420. [Google Scholar]
- Ścieszka, S.; Klewicka, E. Algae in food: A general review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3538–3547. [Google Scholar] [CrossRef]
- Yasuhara-Bell, J.; Lu, Y. Marine compounds and their antiviral activities. Antivir. Res. 2010, 86, 231–240. [Google Scholar] [CrossRef]
- Dmytryk, A.; Tuhy, Ł. Algae as source of pharmaceuticals. In Prospects and Challenges in Algal Biotechnology; Tripathi, B., Kumar, D., Eds.; Springer: Singapore, 2017. [Google Scholar]
- Vo, T.-S.; Kim, S.-K. Potential Anti-HIV agents from marine resources: An overview. Mar. Drugs 2010, 8, 2871–2892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wang, S.X.; Guan, H.S. The antiviral activities and mechanisms of marine polysaccharides: An overview. Mar. Drugs 2012, 10, 2795–2816. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xing, M.C.; Cao, Q.; Ji, A.G.; Liang, H.; Song, S.L. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raposo, M.F.D.; de Morais, A.M.B.; de Morais, R. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [Green Version]
- Dinesh, S.; Menon, T.; Hanna, L.E.; Suresh, V.; Sathuvan, M.; Manikannan, M. In vitro anti-hiv-1 activity of fucoidan from Sargassum swartzii. Int. J. Biol. Macromol. 2016, 82, 83–88. [Google Scholar] [CrossRef]
- Thuy, T.T.; Ly, B.M.; Van, T.T.; Quang, N.V.; Tu, H.C.; Zheng, Y.; Seguin-Devaux, C.; Mi, B.; Ai, U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015, 115, 122–128. [Google Scholar] [CrossRef]
- Trinchero, J.; Ponce, N.M.A.; Cordoba, O.L.; Flores, M.L.; Pampuro, S.; Stortz, C.A.; Salomon, H.; Turk, G. Antiretroviral activity of fucoidans extracted from the brown seaweed Adenocystis utricularis. Phytother. Res. 2009, 23, 707–712. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef] [Green Version]
- Feldman, S.C.; Reynaldi, S.; Stortz, C.A.; Cerezo, A.S.; Damonte, E.B. Antiviral properties of fucoidan fractions from Leathesia difformis. Phytomedicine 1999, 6, 335–340. [Google Scholar] [CrossRef]
- Ponce, N.M.A.; Pujol, C.A.; Damonte, E.B.; Flores, M.L.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003, 338, 153–165. [Google Scholar] [CrossRef]
- Rabanal, M.; Ponce, N.M.A.; Navarro, D.A.; Gomez, R.M.; Stortz, C.A. The system of fucoidans from the brown seaweed dictyota dichotoma: Chemical analysis and antiviral activity. Carbohydr. Polym. 2014, 101, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, K.; Nakano, T.; Hashimoto, M.; Kanekiyo, K.; Hayashi, T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008, 8, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Krylova, N.V.; Ermakova, S.P.; Lavrov, V.F.; Leneva, I.A.; Kompanets, G.G.; Iunikhina, O.V.; Nosik, M.N.; Ebralidze, L.K.; Falynskova, I.N.; Silchenko, A.S.; et al. The comparative analysis of antiviral activity of native and modified fucoidans from brown algae Fucus evanescens in vitro and in vivo. Mar. Drugs 2020, 18, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, G.L.; Yu, G.L.; Wang, W.; Zhao, X.L.; Zhang, J.Z.; Ewart, S.H. Properties of polysaccharides in several seaweeds from Atlantic Canada and their potential anti-influenza viral activities. J. Ocean Univ. China 2012, 11, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.H.; Zhang, X.H.; Miao, Y.; Zhou, Y.; Shi, J.; Yan, M.X.; Chen, A.J. Studies on antiviral and immuno-regulation activity of low molecular weight fucoidan from laminaria japonica. J. Ocean Univ. China 2018, 17, 705–711. [Google Scholar] [CrossRef]
- Mandal, P.; Mateu, C.G.; Chattopadhyay, K.; Pujol, C.A.; Damonte, E.B.; Ray, B. Structural features and antiviral activity of sulphated fucans from the brown seaweed Cystoseira indica. Antivir. Chem. Chemother. 2007, 18, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Witvrouw, M.; DeClercq, E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen. Pharmacol. Vasc. Syst. 1997, 29, 497–511. [Google Scholar] [CrossRef]
- Iqbal, M.; McCauley, J.W.; Flick-Smith, H. Interactions of bovine viral diarrhoea virus glycoprotein Erns with cell surface glycosaminoglycans. J. Gen. Virol. 2000, 81, 451–459. [Google Scholar] [CrossRef]
- Kim, H.; Lim, C.Y.; Lee, D.B.; Seok, J.H.; Kim, K.H.; Chung, M.S. Inhibitory effects of Laminaria japonica fucoidans against noroviruses. Viruses 2020, 12, 997. [Google Scholar] [CrossRef]
- Damonte, E.B.; Matulewicz, M.C.; Cerezo, A.S. Sulfated seaweed polysaccharides as antiviral agents. Curr. Med. Chem. 2004, 11, 2399–2419. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wu, J.D.; Zhang, X.S.; Hao, C.; Zhao, X.L.; Jiao, G.L.; Shan, X.D.; Tai, W.J.; Yu, G.L. Inhibition of influenza a virus infection by fucoidan targeting viral neuraminidase and cellular EGFR pathway. Sci. Rep. 2017, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Peng, H.R.; Wang, Q.L.; Liu, Z.Q.; Dong, X.P.; Wen, C.R.; Ai, C.Q.; Zhang, Y.J.; Wang, Z.F.; Zhu, B.W. Inhibitory activities of marine sulfated polysaccharides against SARS-CoV-2. Food Funct. 2020, 11, 7415–7420. [Google Scholar] [CrossRef] [PubMed]
- Kwon, P.S.; Oh, H.; Kwon, S.-J.; Jin, W.; Zhang, F.; Fraser, K.; Hong, J.J.; Linhardt, R.J.; Dordick, J.S. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discov. 2020, 6. [Google Scholar] [CrossRef]
- Synytsya, A.; Bleha, R.; Synytsya, A.; Pohl, R.; Hayashi, K.; Yoshinaga, K.; Nakano, T.; Hayashi, T. Mekabu fucoidan: Structural complexity and defensive effects against avian influenza a viruses. Carbohydr. Polym. 2014, 111, 633–644. [Google Scholar] [CrossRef]
- Hayashi, K.; Lee, J.B.; Nakano, T.; Hayashi, T. Anti-Influenza a virus characteristics of a fucoidan from sporophyll of Undaria pinnatifida in mice with normal and compromised immunity. Microbes Infect. 2013, 15, 302–309. [Google Scholar] [CrossRef]
- Shikov, A.N.; Flisyuk, E.V.; Obluchinskaya, E.D.; Pozharitskaya, O.N. Pharmacokinetics of marine-derived drugs. Mar. Drugs 2020, 18, 557. [Google Scholar] [CrossRef]
- Richards, C.; Williams, N.A.; Fitton, J.H.; Stringer, D.N.; Karpiniec, S.S.; Park, A.Y. Oral fucoidan attenuates lung pathology and clinical signs in a severe influenza a mouse model. Mar. Drugs 2020, 18, 246. [Google Scholar] [CrossRef]
- O’Keefe, B.R.; Giomarelli, B.; Barnard, D.L.; Shenoy, S.R.; Chan, P.K.S.; McMahon, J.B.; Palmer, K.E.; Barnett, B.W.; Meyerholz, D.K.; Wohlford-Lenane, C.L.; et al. Broad-Spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family coronaviridae. J. Virol. 2010, 84, 2511–2521. [Google Scholar] [CrossRef] [Green Version]
- Ishag, H.Z.A.; Li, C.; Huang, L.; Sun, M.X.; Wang, F.J.; Ni, B.; Malik, T.; Chen, P.Y.; Mao, X. Griffithsin inhibits Japanese encephalitis virus infection in vitro and in vivo. Arch. Virol. 2013, 158, 349–358. [Google Scholar] [CrossRef]
- Meuleman, P.; Albecka, A.; Belouzard, S.; Vercauteren, K.; Verhoye, L.; Wychowski, C.; Leroux-Roels, G.; Palmer, K.E.; Dubuisson, J. Griffithsin has antiviral activity against hepatitis C virus. Antimicrob. Agents Chemother. 2011, 55, 5159–5167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nixon, B.; Stefanidou, M.; Mesquita, P.M.M.; Fakioglu, E.; Segarra, T.; Rohan, L.; Halford, W.; Palmer, K.E.; Herold, B.C. Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J. Virol. 2013, 87, 6257–6269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levendosky, K.; Mizenina, O.; Martinelli, E.; Jean-Pierre, N.; Kizima, L.; Rodriguez, A.; Kleinbeck, K.; Bonnaire, T.; Robbiani, M.; Zydowsky, T.M.; et al. Griffithsin and carrageenan combination to target herpes simplex virus 2 and human papillomavirus. Antimicrob. Agents Chemother. 2015, 59, 7290–7298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.C.; Emau, P.; Jiang, Y.H.; Tian, B.P.; Morton, W.R.; Gustafson, K.R.; Boyd, M.R. Cyanovirin-N gel as a topical microbicide prevents rectal transmission of shiv89.6p in macaques. AIDS Res. Hum. Retrovir. 2003, 19, 535–541. [Google Scholar] [CrossRef]
- Tsai, C.C.; Emau, P.; Jiang, Y.H.; Agy, M.B.; Shattock, R.J.; Schmidt, A.; Morton, W.R.; Gustafson, K.R.; Boyd, M.R. Cyanovirin-N inhibits AIDS virus infections in vaginal transmission models. AIDS Res. Hum. Retrovir. 2004, 20, 11–18. [Google Scholar] [CrossRef]
- Lagenaur, L.A.; Swedek, I.; Lee, P.P.; Parks, T.P. Robust vaginal colonization of macaques with a novel vaginally disintegrating tablet containing a live biotherapeutic product to prevent HIV infection in women. PLoS ONE 2015, 10, e0122730. [Google Scholar] [CrossRef]
- Garrison, A.R.; Giomarelli, B.G.; Lear-Rooney, C.M.; Saucedo, C.J.; Yellayi, S.; Krumpe, L.R.H.; Rose, M.; Paragas, J.; Bray, M.; Olinger, G.G.; et al. The cyanobacterial lectin scytovirin displays potent in vitro and in vivo activity against Zaire Ebola virus. Antivir. Res. 2014, 112, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Barros, C.D.; Garrido, V.; Melchiades, V.; Gomes, R.; Gomes, M.W.L.; Teixeira, V.L.; Paixao, I. Therapeutic efficacy in balb/c mice of extract from marine alga Canistrocarpus cervicornis (Phaeophyceae) against herpes simplex virus type 1. J. Appl. Phycol. 2017, 29, 769–773. [Google Scholar] [CrossRef]
- Van De Velde, F.; Knutsen, S.H.; Usov, A.I.; Rollema, H.S.; Cerezo, A.S. 1h and 13c high resolution NMR spectroscopy of carrageenans: Application in research and industry. Trends Food Sci. Technol. 2002, 13, 73–92. [Google Scholar] [CrossRef]
- McHugh, D.J. Production and Utilization of Products from Commercial Seaweeds; Food and Agriculture Organization of the United Nations: Rome, Italy, 1987.
- Imeson, A.P. 7—Carrageenan and furcellaran. In Handbook of Hydrocolloids, 2nd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 164–185. [Google Scholar]
- Carlucci, M.J.; Ciancia, M.; Matulewicz, M.C.; Cerezo, A.S.; Damonte, E.B. Antiherpetic activity and mode of action of natural carrageenans of diverse structural types. Antivir. Res. 1999, 43, 93–102. [Google Scholar] [CrossRef]
- Talarico, L.B.; Damonte, E.B. Interference in dengue virus adsorption and uncoating by carrageenans. Virology 2007, 363, 473–485. [Google Scholar] [CrossRef] [Green Version]
- Rashid, N.N.; Yusof, R.; Rothan, H.A. Antiviral and virucidal activities of sulphated polysaccharides against Japanese encephalitis virus. Trop. Biomed. 2020, 37, 713–721. [Google Scholar]
- Girond, S.; Crance, J.M.; Vancuyckgandre, H.; Renaudet, J.; Deloince, R. Antiviral activity of carrageenan on Hepatitis-A virus-replication in cell-culture. Res. Virol. 1991, 142, 261–270. [Google Scholar] [CrossRef]
- Gonzalez, M.E.; Alarcon, B.; Carrasco, L. Polysaccharides as antiviral Agents—Antiviral activity of carrageenan. Antimicrob. Agents Chemother. 1987, 31, 1388–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolender, A.A.; Pujol, C.A.; Damonte, E.B.; Cerezo, A.S.; Matulewicz, M.C. Sulfation of kappa-carrageenan and antiviral activity. An. Asoc. Quim. Argent. 1998, 86, 304–311. [Google Scholar]
- Carlucci, M.J.; Scolaro, L.A.; Damonte, E.B. Inhibitory action of natural carrageenans on herpes simplex virus infection of mouse astrocytes. Chemotherapy 1999, 45, 429–436. [Google Scholar] [CrossRef]
- Baba, M.; Snoeck, R.; Pauwels, R.; De Clercq, E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 1988, 32, 1742–1745. [Google Scholar] [CrossRef] [Green Version]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Müller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef] [Green Version]
- Desftischer, P.; Talarico, L.; Noseda, M.; Pitabguimaraes, S.; Damonte, E.; Duarte, M. Chemical structure and antiviral activity of carrageenans from meristiella gelidium against herpes simplex and dengue virus. Carbohydr. Polym. 2006, 63, 459–465. [Google Scholar] [CrossRef]
- Piccini, L.E.; Carro, A.C.; Quintana, V.M.; Damonte, E.B. Antibody-Independent and dependent infection of human myeloid cells with dengue virus is inhibited by carrageenan. Virus Res. 2020, 290, 198150. [Google Scholar] [CrossRef]
- Grassauer, A.; Weinmuellner, R.; Meier, C.; Pretsch, A.; Prieschl-Grassauer, E.; Unger, H. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol. J. 2008, 5, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reunov, A.; Nagorskaya, V.; Lapshina, L.; Yermak, I.; Barabanova, A. Effect of κ/ß-carrageenan from red alga Tichocarpus crinitus (Tichocarpaceae) on infection of detached tobacco leaves with tobacco mosaic virus. J. Plant Dis. Prot. 2004, 111, 165–172. [Google Scholar] [CrossRef]
- Maguire, R.A.; Zacharopoulos, V.R.; Phillips, D.M. Carrageenan-Based nonoxynol-9 spermicides for prevention of sexually transmitted infections. Sex. Transm. Dis. 1998, 25, 494–500. [Google Scholar] [CrossRef]
- Carlucci, M.J.; Scolaro, L.A.; Noseda, M.D.; Cerezo, A.S.; Damonte, E.B. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antivir. Res. 2004, 64, 137–141. [Google Scholar] [CrossRef]
- Hamasuna, R.; Eizuru, Y.; Shishime, Y.; Minamishima, Y. Protective effect of carrageenan against murine cytomegalovirus-infection in mice. Antivir. Chem. Chemother. 1993, 4, 353–360. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 20. [Google Scholar] [CrossRef]
- Lopes, N.; Ray, S.; Espada, S.F.; Bomfim, W.A.; Ray, B.; Faccin-Galhardi, L.C.; Linhares, R.E.C.; Nozawa, C. Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. Int. J. Biol. Macromol. 2017, 102, 605–612. [Google Scholar] [CrossRef]
- Chiu, Y.H.; Chan, Y.L.; Li, T.L.; Wu, C.J. Inhibition of Japanese encephalitis virus infection by the sulfated polysaccharide extracts from Ulva lactuca. Mar. Biotechnol. 2012, 14, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Briseno, J.A.; Cruz-Suarez, L.E.; Sassi, J.F.; Ricque-Marie, D.; Zapata-Benavides, P.; Mendoza-Gamboa, E.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. Sulphated polysaccharides from Ulva clathrata and Cladosiphon okamuranus seaweeds both inhibit viral attachment/entry and cell-cell fusion, in NDV infection. Mar. Drugs 2015, 13, 697–712. [Google Scholar] [CrossRef]
- Moran-Santibanez, K.; Cruz-Suarez, L.E.; Ricque-Marie, D.; Robledo, D.; Freile-Pelegrin, Y.; Pena-Hernandez, M.A.; Rodriguez-Padilla, C.; Trejo-Avila, L.M. Synergistic effects of sulfated polysaccharides from Mexican seaweeds against measles virus. Biomed. Res. Int. 2016, 8502123. [Google Scholar] [CrossRef] [Green Version]
- Hardouin, K.; Bedoux, G.; Burlot, A.S.; Donnay-Moreno, C.; Berge, J.P.; Nyvall-Collen, P.; Bourgougnon, N. Enzyme-Assisted extraction (EAE) for the production of antiviral and antioxidant extracts from the green seaweed Ulva armoricana (Ulvales, Ulvophyceae). Algal Res. 2016, 16, 233–239. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Chen, X.; Liu, X.; Zhang, F.; Hu, L.; Yue, Y.; Li, K.; Li, P. Characterization and comparison of the structural features, immune-modulatory and anti-avian influenza virus activities conferred by three algal sulfated polysaccharides. Mar. Drugs 2015, 14, 4. [Google Scholar] [CrossRef]
- Pujol, C.A.; Ray, S.; Ray, B.; Damonte, E.B. Antiviral activity against dengue virus of diverse classes of algal sulfated polysaccharides. Int. J. Biol. Macromol. 2012, 51, 412–416. [Google Scholar] [CrossRef]
- Chi, Y.Z.; Zhang, M.F.; Wang, X.; Fu, X.J.; Guan, H.S.; Wang, P. Ulvan lyase assisted structural characterization of ulvan from Ulva pertusa and its antiviral activity against vesicular stomatitis virus. Int. J. Biol. Macromol. 2020, 157, 75–82. [Google Scholar] [CrossRef]
- Hayashi, K.; Hayashi, T.; Kojima, I. A natural sulfated polysaccharide, calcium spirulan, isolated from Spirulina platensis: In vitro and ex vivo evaluation of anti-herpes simplex virus and anti-human immunodeficiency virus activities. AIDS Res. Hum. Retrovir. 1996, 12, 1463–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, T.; Hayashi, K.; Maeda, M.; Kojima, I. Calcium spirulan, an inhibitor of enveloped virus replication, from a blue-green alga Spirulina platensis. J. Nat. Prod. 1996, 59, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Rechter, S.; Konig, T.; Auerochs, S.; Thulke, S.; Walter, H.; Dornenburg, H.; Walter, C.; Marschall, M. Antiviral activity of Arthrospira-Derived spirulan-like substances. Antivir. Res. 2006, 72, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.B.; Hou, X.L.; Hayashi, K.; Hayashi, T. Effect of partial desulfation and oversulfation of sodium spirulan on the potency of anti-herpetic activities. Carbohydr. Polym. 2007, 69, 651–658. [Google Scholar] [CrossRef]
- Mader, J.; Gallo, A.; Schommartz, T.; Handke, W.; Nagel, C.H.; Gunther, P.; Brune, W.; Reich, K. Calcium spirulan derived from Spirulina platensis inhibits herpes simplex virus 1 attachment to human keratinocytes and protects against herpes labialis. J. Allergy Clin. Immunol. 2016, 137, 197–203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coelho, L.; Silva, P.M.D.; Lima, V.L.D.; Pontual, E.V.; Paiva, P.M.G.; Napoleao, T.H.; Correia, M.T.D. Lectins, interconnecting proteins with biotechnological/pharmacological and therapeutic applications. Evid. Based Complement. Altern. Med. 2017, 1594074. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.A.; Ramessar, K.; O’Keefe, B.R. Antiviral lectins: Selective inhibitors of viral entry. Antivir. Res. 2017, 142, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Mazalovska, M.; Kouokam, J.C. Lectins as promising therapeutics for the prevention and treatment of HIV and other potential coinfections. BioMed Res. Int. 2018, 3750646. [Google Scholar] [CrossRef]
- Singh, R.S.; Walia, A.K. Lectins from red algae and their biomedical potential. J. Appl. Phycol. 2018, 30, 1833–1858. [Google Scholar]
- Besednova, N.; Zaporozhets, T.; Kuznetsova, T.; Makarenkova, I.; Fedyanina, L.; Kryzhanovsky, S.; Malyarenko, O.; Ermakova, S. Metabolites of seaweeds as potential agents for the prevention and therapy of influenza infection. Mar. Drugs 2019, 17, 373. [Google Scholar] [CrossRef] [Green Version]
- Besednova, N.N.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Makarenkova, I.D.; Smolina, T.P.; Fedyanina, L.N.; Kryzhanovsky, S.P.; Zaporozhets, T.S. Marine algae metabolites as promising therapeutics for the prevention and treatment of HIV/AIDS. Metabolites 2019, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Lee, C. Griffithsin, a highly potent broad-spectrum antiviral lectin from red algae: From discovery to clinical application. Mar. Drugs 2019, 17, 567. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; O’Keefe, B.R.; Sowder, R.C.; Bringans, S.; Gardella, R.; Berg, S.; Cochran, P.; Turpin, J.A.; Buckheit, R.W.; McMahon, J.B.; et al. Isolation and characterization of griffithsin, a novel HIV-Inactivating protein, from the red alga Griffithsia sp. J. Biol. Chem. 2005, 280, 9345–9353. [Google Scholar] [CrossRef] [Green Version]
- Emau, P.; Tian, B.; O’Keefe, B.R.; Mori, T.; McMahon, J.B.; Palmer, K.E.; Jiang, Y.; Bekele, G.; Tsai, C.C. Griffithsin, a potent HIV entry inhibitor, is an excellent candidate for Anti-HIV microbicide. J. Med. Primatol. 2007, 36, 244–253. [Google Scholar] [CrossRef]
- Banerjee, K.; Michael, E.; Eggink, D.; van Montfort, T.; Lasnik, A.B.; Palmer, K.E.; Sanders, R.W.; Moore, J.P.; Klasse, P.J. Occluding the mannose moieties on human immunodeficiency virus type 1 gp120 with griffithsin improves the antibody responses to both proteins in mice. AIDS Res. Hum. Retrovir. 2012, 28, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Nguyen, K.; LiWang, P.J. Griffithsin retains anti-hiv-1 potency with changes in gp120 glycosylation and complements broadly neutralizing antibodies pgt121 and pgt126. Antimicrob. Agents Chemother. 2020, 64, 17. [Google Scholar] [CrossRef] [Green Version]
- Zeitlin, L.; Pauly, M.; Whaley, K.J. Second-Generation HIV microbicides: Continued development of griffithsin. Proc. Natl. Acad. Sci. USA 2009, 106, 6029–6030. [Google Scholar] [CrossRef] [Green Version]
- Crakes, K.R.; Herrera, C.; Morgan, J.L.; Olstad, K.; Hessell, A.J.; Ziprin, P.; LiWang, P.J.; Dandekar, S. Efficacy of silk fibroin biomaterial vehicle for in vivo mucosal delivery of griffithsin and protection against HIV and SHIV infection ex vivo. J. Int. AIDS Soc. 2020, 23, 12. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, P.; Albecka, A.; Belouzard, S.; Vercauteren, K.; Verhoye, L.; Wychowski, C.; Leroux-Roels, G.; Palmer, K.E.; Dubuisson, J. The lectin griffithsin has antiviral activity against hepatitis C virus in vitro and in vivo. J. Hepatol. 2012, 56, S335–S336. [Google Scholar] [CrossRef]
- Lo, M.K.; Spengler, J.R.; Krumpe, L.R.H.; Welch, S.R.; Chattopadhyay, A.; Harmon, J.R.; Coleman-McCray, J.D.; Scholte, F.E.M.; Hotard, A.L.; Fuqua, J.L.; et al. Griffithsin inhibits nipah virus entry and fusion and can protect syrian golden hamsters from lethal nipah virus challenge. J. Infect. Dis. 2020, 221, S480–S492. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava-Ranjan, P.; Lo, M.K.; Chatterjee, P.; Flint, M.; Nichol, S.T.; Montgomery, J.M.; O’Keefe, B.R.; Spiropoulou, C.F. Hantavirus infection is inhibited by griffithsin in cell culture. Front. Cell. Infect. Microbiol. 2020, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Millet, J.K.; Seron, K.; Labitt, R.N.; Danneels, A.; Palmer, K.E.; Whittaker, G.R.; Dubuisson, J.; Belouzard, S. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antivir. Res. 2016, 133, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.X.; Xu, W.; Gu, C.J.; Cai, X.; Qu, D.; Lu, L.; Xie, Y.H.; Jiang, S.B. Griffithsin with a broad-spectrum antiviral activity by binding glycans in viral glycoprotein exhibits strong synergistic effect in combination with a pan-coronavirus fusion inhibitor targeting sars-cov-2 spike s2 subunit. Virol. Sin. 2020, 35, 857–860. [Google Scholar] [CrossRef]
- Giomarelli, B.; Schumacher, K.M.; Taylor, T.E.; Sowder, R.C.; Hartley, J.L.; McMahon, J.B.; Mori, T. Recombinant production of Anti-HIV protein, griffithsin, by auto-induction in a fermentor culture. Protein Expr. Purif. 2006, 47, 194–202. [Google Scholar] [CrossRef]
- O’Keefe, B.R.; Vojdani, F.; Buffa, V.; Shattock, R.J.; Montefiori, D.C.; Bakke, J.; Mirsalis, J.; d’Andrea, A.L.; Hume, S.D.; Bratcher, B.; et al. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc. Natl. Acad. Sci. USA 2009, 106, 6099–6104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vafaee, Y.; Alizadeh, H. Heterologous production of recombinant Anti-HIV microbicide griffithsin in transgenic lettuce and tobacco lines. Plant Cell Tissue Organ Cult. 2018, 135, 85–97. [Google Scholar] [CrossRef]
- Petrova, M.I.; van den Broek, M.F.L.; Spacova, I.; Verhoeven, T.L.A.; Balzarini, J.; Vanderleyden, J.; Schols, D.; Lebeer, S. Engineering Lactobacillus rhamnosus gg and gr-1 to express HIV-Inhibiting griffithsin. Int. J. Antimicrob. Agents 2018, 52, 599–607. [Google Scholar] [CrossRef]
- Yang, H.T.; Li, J.; Patel, S.K.; Palmer, K.E.; Devlin, B.; Rohan, L.C. Design of poly(lactic-co-glycolic acid) (PLGA) nanoparticles for vaginal co-delivery of griffithsin and dapivirine and their synergistic effect for HIV prophylaxis. Pharmaceutics 2019, 11, 184. [Google Scholar] [CrossRef] [Green Version]
- Tyo, K.M.; Duan, J.H.; Kollipara, P.; dela Cerna, M.V.C.; Lee, D.; Palmer, K.E.; Steinbach-Rankins, J.M. Ph-Responsive delivery of griffithsin from electrospun fibers. Eur. J. Pharm. Biopharm. 2019, 138, 64–74. [Google Scholar] [CrossRef]
- Lal, M.; Lai, M.S.; Ugaonkar, S.; Wesenberg, A.; Kizima, L.; Rodriguez, A.; Levendosky, K.; Mizenina, O.; Fernandez-Romero, J.; Zydowsky, T. Development of a vaginal fast-dissolving insert combining griffithsin and carrageenan for potential use against sexually transmitted infections. J. Pharm. Sci. 2018, 107, 2601–2610. [Google Scholar] [CrossRef]
- Girard, L.; Birse, K.; Holm, J.B.; Gajer, P.; Humphrys, M.S.; Garber, D.; Guenthner, P.; Noel-Romas, L.; Abou, M.; McCorrister, S.; et al. Impact of the griffithsin Anti-HIV microbicide and placebo gels on the rectal mucosal proteome and microbiome in non-human primates. Sci. Rep. 2018, 8, 13. [Google Scholar] [CrossRef]
- Boyd, M.R.; Gustafson, K.R.; McMahon, J.B.; Shoemaker, R.H.; Okeefe, B.R.; Mori, T.; Gulakowski, R.J.; Wu, L.; Rivera, M.I.; Laurencot, C.M.; et al. Discovery of Cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob. Agents Chemother. 1997, 41, 1521–1530. [Google Scholar] [CrossRef] [Green Version]
- Gustafson, K.R.; Sowder, R.C.; Henderson, L.E.; Cardellina, J.H.; McMahon, J.B.; Rajamani, U.; Pannell, L.K.; Boyd, M.R. Isolation, primary sequence determination, and disulfide bond structure of Cyanovirin-N, an Anti-HIV (human immunodeficiency virus) protein from the cyanobacterium Nostoc ellipsosporum. Biochem. Biophys. Res. Commun. 1997, 238, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Bewley, C.A.; Gustafson, K.R.; Boyd, M.R.; Covell, D.G.; Bax, A.; Clore, G.M.; Gronenborn, A.M. Solution structure of Cyanovirin-N, a potent HIV-Inactivating protein. Nat. Struct. Biol. 1998, 5, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Dey, B.; Lerner, D.L.; Lusso, P.; Boyd, M.R.; Elder, J.H.; Berger, E.A. Multiple antiviral activities of Cyanovirin-N: Blocking of human immunodeficiency virus type 1 gp120 interaction with cd4 and coreceptor and inhibition of diverse enveloped viruses. J. Virol. 2000, 74, 4562–4569. [Google Scholar] [CrossRef]
- Mori, T.; Boyd, M.R. Cyanovirin-N, a potent human immunodeficiency virus-inactivating protein, blocks both cd4-dependent and cd4-independent binding of soluble gp120 (sgp120) to target cells, inhibits scd4-induced binding of sgp120 to cell-associated cxcr4, and dissociates bound sgp120 from target cells. Antimicrob. Agents Chemother. 2001, 45, 664–672. [Google Scholar]
- Yang, F.; Bewley, C.A.; Louis, J.M.; Gustafson, K.R.; Boyd, M.R.; Gronenborn, A.M.; Clore, G.M.; Wlodawer, A. Crystal structure of Cyanovirin-N, a potent HIV-Inactivating protein, shows unexpected domain swapping. J. Mol. Biol. 1999, 288, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Bewley, C.A.; Otero-Quintero, S. The potent Anti-HIV protein Cyanovirin-N contains two novel carbohydrate binding sites that selectively bind to man(8) d1d3 and man(9) with nanomolar affinity: Implications for binding to the HIV envelope protein gp120. J. Am. Chem. Soc. 2001, 123, 3892–3902. [Google Scholar] [CrossRef]
- Matei, E.; Basu, R.; Furey, W.; Shi, J.; Calnan, C.; Aiken, C.; Gronenborn, A.M. Structure and glycan binding of a new Cyanovirin-N homolog. J. Biol. Chem. 2016, 291, 18967–18976. [Google Scholar] [CrossRef] [Green Version]
- O’Keefe, B.R.; Smee, D.F.; Turpin, J.A.; Saucedo, C.J.; Gustafson, K.R.; Mori, T.; Blakeslee, D.; Buckheit, R.; Boyd, M.R. Potent anti-influenza activity of Cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 2003, 47, 2518–2525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrientos, L.G.; O’Keefe, B.R.; Bray, M.; Anthony, S.; Gronenborn, A.M.; Boyd, M.R. Cyanovirin-N binds to the viral surface glycoprotein, gp(1,2) and inhibits infectivity of ebola virus. Antivir. Res. 2003, 58, 47–56. [Google Scholar] [CrossRef]
- Huskens, D.; Vermeire, K.; Vanderneulebroucke, E.; Balzarini, J.; Schols, D. Safety concerns for the potential use of Cyanovirin-N as a microbicidal anti-hiv agent. Int. J. Biochem. Cell Biol. 2008, 40, 2802–2814. [Google Scholar] [CrossRef] [PubMed]
- Colleluori, D.M.; Tien, D.; Kang, F.R.; Pagliei, T.; Kuss, R.; McCormick, T.; Watson, K.; McFadden, K.; Chaiken, I.; Buckheit, R.W.; et al. Expression, purification, and characterization of recombinant Cyanovirin-N for vaginal Anti-HIV microbicide development. Protein Expr. Purif. 2005, 39, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Trivedi, J.; Mitra, D. High yield production of recombinant Cyanovirin-N (antiviral lectin) exhibiting significant Anti-HIV activity, from a rationally selected Escherichia coli strain. Process Biochem. 2020, 93, 1–11. [Google Scholar] [CrossRef]
- Pusch, O.; Boden, D.; Hannify, S.; Lee, F.; Tucker, L.D.; Boyd, M.R.; Wells, J.M.; Ramratnam, B. Bioengineering lactic acid bacteria to secrete the HIV-1 virucide cyanovirin. JAIDS 2005, 40, 512–520. [Google Scholar] [CrossRef]
- Pusch, O.; Kalyanaraman, R.; Tucker, L.D.; Wells, J.M.; Ramratnam, B.; Boden, D. An Anti-HIV microbicide engineered in commensal bacteria: Secretion of HIV-1 fusion inhibitors by lactobacilli. AIDS 2006, 20, 1917–1922. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, H.; Sheervalilou, R.; Zarghami, N. An update of the recombinant protein expression systems of Cyanovirin-N and challenges of preclinical development. BioImpacts 2018, 8, 139–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sexton, A.; Drake, P.M.; Mahmood, N.; Harman, S.J.; Shattock, R.J.; Ma, J.K.C. Transgenic plant production of Cyanovirin-N, an hiv microbicide. FASEB J. 2006, 20, 356–358. [Google Scholar] [CrossRef]
- Sexton, A.; Harman, S.; Shattock, R.J.; Ma, J.K.C. Design, expression, and characterization of a multivalent, combination HIV microbicide. FASEB J. 2009, 23, 3590–3600. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Huang, D.E.; Chen, W.; Guo, C.W.; Wei, B.; Wu, C.C.; Peng, Z.; Fan, J.; Hou, Z.B.; Fang, Y.S.; et al. Linker-Extended native Cyanovirin-N facilitates pegylation and potently inhibits HIV-1 by targeting the glycan ligand. PLoS ONE 2014, 9, e86455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bokesch, H.R.; O’Keefe, B.R.; McKee, T.C.; Pannell, L.K.; Patterson, G.M.L.; Gardella, R.S.; Sowder, R.C.; Turpin, J.; Watson, K.; Buckheit, R.W.; et al. A potent novel Anti-HIV protein from the cultured cyanobacterium Scytonema varium. Biochemistry 2003, 42, 2578–2584. [Google Scholar] [CrossRef]
- Alexandre, K.B.; Gray, E.S.; Lambson, B.E.; Moore, P.L.; Choge, I.A.; Mlisana, K.; Karim, S.S.; McMahon, J.; O’Keefe, B.; Chikwamba, R.; et al. Mannose-Rich glycosylation patterns on HIV-1 subtype c gp120 and sensitivity to the lectins, griffithsin, Cyanovirin-N and scytovirin. Virology 2010, 402, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, K.B.; Gray, E.S.; Mufhandu, H.; McMahon, J.B.; Chakauya, E.; O’Keefe, B.R.; Chikwamba, R.; Morris, L. The lectins griffithsin, Cyanovirin-N and scytovirin inhibit HIV-1 binding to the dc-sign receptor and transfer to cd4(+) cells. Virology 2012, 423, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Takebe, Y.; Saucedo, C.J.; Lund, G.; Uenishi, R.; Hase, S.; Tsuchiura, T.; Kneteman, N.; Ramessar, K.; Tyrrell, D.L.J.; Shirakura, M.; et al. Antiviral lectins from red and blue-green algae show potent in vitro and in vivo activity against hepatitis C virus. PLoS ONE 2013, 8, e64449. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, A.S.; Lima, A.R.J.; de Souza, R.C.; Santos, A.S.; Vianez, J.L.D.G.; Goncalves, E.C. Anti-Dengue virus activity of scytovirin and evaluation of point mutation effects by molecular dynamics and binding free energy calculations. Biochem. Biophys. Res. Commun. 2017, 490, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Wash, R.; Soria, C.D. True blood: Dengue virus evolution. Nat. Rev. Microbiol. 2015, 13, 662. [Google Scholar] [CrossRef]
- Xiong, C.Y.; O’Keefe, B.R.; Botos, I.; Wlodawer, A.; McMahon, J.B. Overexpression and purification of scytovirin, a potent, novel Anti-HIV protein from the cultured cyanobacterium Scytonema varium. Protein Expr. Purif. 2006, 46, 233–239. [Google Scholar] [CrossRef]
- Janahi, E.M.A.; Haque, S.; Akhter, N.; Wahid, M.; Jawed, A.; Mandal, R.K.; Lohani, M.; Areeshi, M.Y.; Almalki, S.; Das, S.; et al. Bioengineered intravaginal isolate of Lactobacillus plantarum expresses algal lectin scytovirin demonstrating anti-hiv-1 activity. Microb. Pathog. 2018, 122, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Huskens, D.; Ferir, G.; Vermeire, K.; Kehr, J.C.; Balzarini, J.; Dittmann, E.; Schols, D. Microvirin, a novel α(1,2)-mannose-specific lectin isolated from Microcystis aeruginosa, has Anti-HIV-1 activity comparable with that of Cyanovirin-N but a much higher safety profile. J. Biol. Chem. 2010, 285, 24845–24854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahzad-ul-Hussan, S.; Gustchina, E.; Ghirlando, R.; Clore, G.M.; Bewley, C.A. Solution structure of the monovalent lectin microvirin in complex with man α(1-2)man provides a basis for Anti-HIV activity with low toxicity. J. Biol. Chem. 2011, 286, 20788–20796. [Google Scholar] [CrossRef] [Green Version]
- Parajuli, B.; Acharya, K.; Bach, H.C.; Parajuli, B.; Zhang, S.Y.; Smith, A.B.; Abrams, C.F.; Chaiken, I. Restricted HIV-1 env glycan engagement by lectin-reengineered DAVEI protein chimera is sufficient for lytic inactivation of the virus. Biochem. J. 2018, 475, 931–957. [Google Scholar] [CrossRef] [Green Version]
- Min, Y.Q.; Duan, X.C.; Zhou, Y.D.; Kulinich, A.; Meng, W.; Cai, Z.P.; Ma, H.Y.; Liu, L.; Zhang, X.L.; Voglmeir, J. Effects of microvirin monomers and oligomers on hepatitis C virus. Biosci. Rep. 2017, 37, 9. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.Y.; Li, H.; Zhao, Z.S.; Xia, X.; Li, B.; Zhang, J.R.; Yan, X.J. Diterpenes from the marine algae of the genus Dictyota. Mar. Drugs 2018, 16, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- vonRanke, N.L.; Ribeiro, M.M.J.; Miceli, L.A.; de Souza, N.P.; Abrahim-Vieira, B.A.; Castro, H.C.; Teixeira, V.L.; Rodrigues, C.R.; Souza, A.M.T. Structure-Activity relationship, molecular docking, and molecular dynamic studies of diterpenes from marine natural products with Anti-HIV activity. J. Biomol. Struct. Dyn. 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Pereira, H.S.; Leao-Ferreira, L.R.; Moussatche, N.; Teixeira, V.L.; Cavalcanti, D.N.; Costa, L.J.; Diaz, R.; Frugulhetti, I. Antiviral activity of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis against human immunodeficiency virus type 1 (hiv-1). Antivir. Res. 2004, 64, 69–76. [Google Scholar] [CrossRef]
- Pereira, H.D.S.; Leao-Ferreira, L.R.; Moussatche, N.; Teixeira, V.L.; Cavalcanti, D.N.; da Costa, L.J.; Diaz, R.; Paixao, L.C.P.; Frugulhetti, I. Effects of diterpenes isolated from the Brazilian marine alga Dictyota menstrualis on hiv-1 reverse transcriptase. Planta Med. 2005, 71, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Cirne-Santos, C.C.; Barros, C.D.; Esteves, P.O.; Gomes, M.W.L.; Gomes, R.D.P.; Cavalcanti, D.N.; Obando, J.M.C.; Ramos, C.J.B.; Villaca, R.C.; Teixeira, V.L.; et al. Antiviral activity against chikungunya virus of diterpenes from the seaweed Dictyota menstrualis. Rev. Bras. Farmacogn. Braz. J. Pharmacogn. 2020, 30, 709–714. [Google Scholar] [CrossRef]
- Cirne-Santos, C.C.; Teixeira, V.L.; Castello-Branco, L.R.; Frugulhetti, I.; Bou-Habib, D.C. Inhibition of HIV-1 replication in human primary cells by a dolabellane diterpene isolated from the marine algae Dictyota pfaffii. Planta Med. 2006, 72, 295–299. [Google Scholar] [CrossRef]
- Pardo-Vargas, A.; Oliveira, I.D.; Stephens, P.R.S.; Cirne-Santos, C.C.; Paixao, I.; Ramos, F.A.; Jimenez, C.; Rodriguez, J.; Resende, J.; Teixeira, V.L.; et al. Dolabelladienols A-C, new diterpenes isolated from Brazilian brown alga Dictyota pfaffii. Mar. Drugs 2014, 12, 4247–4259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens, P.R.S.; Cirne-Santos, C.C.; De Souza Barros, C.; Teixeira, V.L.; Carneiro, L.A.D.; Amorim, L.D.S.C.; Ocampo, J.S.P.; Castello-Branco, L.R.R.; De Palmer Paixão, I.C.N. Diterpene from marine brown alga Dictyota friabilis as a potential microbicide against HIV-1 in tissue explants. J. Appl. Phycol. 2017, 29, 775–780. [Google Scholar] [CrossRef]
- Cheng, S.; Zhao, M.; Sun, Z.; Yuan, W.; Zhang, S.; Xiang, Z.; Cai, Y.; Dong, J.; Huang, K.; Yan, P. Diterpenes from a Chinese collection of the brown alga Dictyota plectens. J. Nat. Prod. 2014, 77, 2685–2693. [Google Scholar] [CrossRef]
- Soares, A.R.; Abrantes, J.L.; Souza, T.M.L.; Fontes, C.F.L.; Pereira, R.C.; Frugulhetti, I.; Teixeira, V.L. In vitro antiviral effect of meroditerpenes isolated from the Brazilian seaweed Stypopodium zonale (Dictyotales). Planta Med. 2007, 73, 1221–1224. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, J.P.; Pereira, R.C.; Abrantes, J.L.; dos Santos, C.C.C.; Rebello, M.A.; Frugulhetti, I.; Teixeira, V.L. In vitro antiviral diterpenes from the Brazilian brown alga Dictyota pfaffii. Planta Med. 2004, 70, 856–860. [Google Scholar] [CrossRef]
- Abrantes, J.L.; Barbosa, J.; Cavalcanti, D.; Pereira, R.C.; Fontes, C.F.L.; Teixeira, V.L.; Souza, T.M.L.; Paixao, I.C.P. The effects of the diterpenes isolated from the Brazilian brown algae Dictyota pfaffii and Dictyota menstrualis against the herpes simplex type-1 replicative cycle. Planta Med. 2010, 76, 339–344. [Google Scholar] [CrossRef] [Green Version]
- Vallim, M.A.; Barbosa, J.E.; Cavalcanti, D.N.; De-Paula, J.C.; da Silva, V.; Teixeira, V.L.; Paixao, I. In vitro antiviral activity of diterpenes isolated from the Brazilian brown alga Canistrocarpus cervicornis. J. Med. Plants Res. 2010, 4, 2379–2382. [Google Scholar]
- Cirne-Santos, C.C.; Barros, C.D.; de Oliveira, M.C.; Rabelo, V.W.H.; Azevedo, R.C.; Teixeira, V.L.; Ferreira, D.F.; Paixao, I. In vitro studies on the inhibition of replication of zika and chikungunya viruses by dolastane isolated from seaweed Canistrocarpus cervicornis. Sci. Rep. 2020, 10, 10. [Google Scholar] [CrossRef]
- Cirne-Santos, C.C.; Barros, C.D.; Gomes, M.W.L.; Gomes, R.; Cavalcanti, D.N.; Obando, J.M.C.; Ramos, C.J.B.; Villaca, R.C.; Teixeira, V.L.; Paixao, I. In vitro antiviral activity against zika virus from a natural product of the Brazilian brown seaweed Dictyota menstrualis. Nat. Prod. Commun. 2019, 14, 7. [Google Scholar] [CrossRef] [Green Version]
- Magnan, S.; Tota, J.E.; El-Zein, M.; Burchell, A.N.; Schiller, J.T.; Ferenczy, A.; Tellier, P.P.; Coutlee, F.; Franco, E.L.; Grp, C.S. Efficacy of a carrageenan gel against transmission of cervical HPV (catch): Interim analysis of a randomized, double-blind, placebo-controlled, phase 2b trial. Clin. Microbiol. Infect. 2019, 25, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Perino, A.; Consiglio, P.; Maranto, M.; De Franciscis, P.; Marci, R.; Restivo, V.; Manzone, M.; Capra, G.; Cucinella, G.; Calagna, G. Impact of a new carrageenan-based vaginal microbicide in a female population with genital HPV-Infection: First experimental results. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6744–6752. [Google Scholar]
- National Library of Medicine (US). Identifier Nct02354144, Lubricant Investigation in Men to Inhibit Transmission of HPV Infection (Limit-HPV); National Library of Medicine (US): Bethesda, MD, USA, 2000. Available online: https://clinicaltrials.gov/ct2/show/NCT02354144 (accessed on 29 January 2021).
- Graf, C.; Bernkop-Schnürch, A.; Egyed, A.; Koller, C.; Prieschl-Grassauer, E.; Morokutti-Kurz, M. Development of a nasal spray containing xylometazoline hydrochloride and iota-carrageenan for the symptomatic relief of nasal congestion caused by rhinitis and sinusitis. Int. J. Gen. Med. 2018, 11, 275–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedland, B.; Keller, M.J.; Creasy, G.; Fernandez-Romero, J.; Teleshova, N.; Plagianos, M.; Ray, L.; Ugaonkar, S.; Alami, M.; O’Keefe, B.R.; et al. Griffithsin administered vaginally for 14 days is well-tolerated, with Anti-HIV activity up to 8 hours post dose in the first-in-human trial. AIDS Res. Hum. Retrovir. 2018, 34, 187. [Google Scholar]
- ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (U.S.). 29 February 2000. Identifier Nct04032717, Griffithsin-Based Rectal Microbicide for Prevention of Viral Entry (Prevent). Available online: https://clinicaltrials.gov/ct2/show/NCT04032717 (accessed on 25 July 2019).
- Skoler-Karpoff, S.; Ramjee, G.; Ahmed, K.; Altini, L.; Plagianos, M.G.; Friedland, B.; Govender, S.; De Kock, A.; Cassim, N.; Palanee, T.; et al. Efficacy of carraguard for prevention of HIV infection in women in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1977–1987. [Google Scholar] [CrossRef]
- Whitehead, S.J.; McLean, C.; Chaikummao, S.; Braunstein, S.; Utaivoravit, W.; van de Wijgert, J.H.; Mock, P.A.; Siraprapasiri, T.; Friedland, B.A.; Kilmarx, P.H.; et al. Acceptability of carraguard vaginal microbicide gel among HIV-Infected women in Chiang Rai, Thailand. PLoS ONE 2011, 6, e14831. [Google Scholar] [CrossRef] [Green Version]
- Coggins, C. Preliminary safety and acceptability of a carrageenan gel for possible use as a vaginal microbicide. Sex. Transm. Infect. 2000, 76, 480–483. [Google Scholar] [CrossRef]
- Altini, L.; Blanchard, K.; Coetzee, N.; de Kock, A.; Elias, C.; Ellertson, C.; Friedland, B.; Hoosen, A.; Jones, H.E.; Kilmarx, P.H.; et al. Expanded safety and acceptability of the candidate vaginal microbicide Carraguard® in South Africa. Contraception 2010, 82, 563–571. [Google Scholar]
- Martin, S.; Blanchard, K.; Manopaiboon, C.; Chaikummao, S.; Schaffer, K.; Friedland, B.; Kilmarx, P.H. Carraguard® acceptability among men and women in a couples study in Thailand. J. Womens Health 2010, 19, 1561–1567. [Google Scholar] [CrossRef]
- Elias, C.J.; Coggins, C.; Alvarez, F.; Brache, V.; Fraser, I.S.; Lacarra, M.; Lähteenmäkl, P.; Massai, R.; Mishell, D.R.; Phillips, D.M.; et al. Colposcopic evaluation of a vaginal gel formulation of iota-carrageenan. Contraception 1997, 56, 387–389. [Google Scholar] [CrossRef]
- Kilmarx, P.H.; Van De Wijgert, J.H.H.M.; Chaikummao, S.; Jones, H.E.; Limpakarnjanarat, K.; Friedland, B.A.; Karon, J.M.; Manopaiboon, C.; Srivirojana, N.; Yanpaisarn, S.; et al. Safety and acceptability of the candidate microbicide Carraguard in Thai Women. JAIDS J. Acquir. Immune Defic. Syndr. 2006, 43, 327–334. [Google Scholar] [CrossRef]
- Ludwig, M.; Enzenhofer, E.; Schneider, S.; Rauch, M.; Bodenteich, A.; Neumann, K.; Prieschl-Grassauer, E.; Grassauer, A.; Lion, T.; Mueller, C.A. Efficacy of a carrageenan nasal spray in patients with common cold: A randomized controlled trial. Respir. Res. 2013, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Fazekas, T.; Eickhoff, P.; Pruckner, N.; Vollnhofer, G.; Fischmeister, G.; Diakos, C.; Rauch, M.; Verdianz, M.; Zoubek, A.; Gadner, H.; et al. Lessons learned from a double-blind randomised placebo-controlled study with a iota-carrageenan nasal spray as medical device in children with acute symptoms of common cold. BMC Complement. Altern. Med. 2012, 12, 8. [Google Scholar] [CrossRef] [Green Version]
- Teas, J.; Irhimeh, M.R. Dietary algae and HIV/AIDS: Proof of concept clinical data. J. Appl. Phycol. 2012, 24, 575–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Compound | Organism | Viruses | Model | Activity |
|---|---|---|---|---|
| Fucoidan | Undaria pinnatifida | Avian influenza A virus | Mice | Decreased viral replication, while augmenting the production of antibodies [39] |
| Avian influenza A virus | Mice | Reduced viral replication, weight loss and mortality. Increased life span. Induced no viral resistance [40] | ||
| Influenza A (H1N1) virus | Mice | Improved clinical signs of ill-health and reduced gross lung pathology [42] | ||
| Kjelmaniella crassifolia | Influenza A virus | Mice | Intranasal administration markedly improved survival and decreased viral titers. Induced no viral resistance [36] | |
| Fucus evanescens | Herpes simplex virus-2 | Mice | Intraperitoneal administration protected reduced lethal infection by 50% [28] | |
| Griffithsin | Griffithsia sp. (Rhodophyta) | SARS-CoV (Coronaviridae) | Mice | High viral dose—100% survival compared to 30% in control group [43] |
| Japanese encephalitis virus (Flaviviridae) | Mice | Lethal viral dose—100% survival compared to 0% in control group [44] | ||
| Hepatitis C virus | Mice | Viral load below detection in infected mice [45] | ||
| Herpes simplex virus-2 | Mice | Significantly protected mice from vaginal infection (0/5 treated vs 3/5 control) [46] | ||
| Herpes simplex virus-2/Human papilloma virus | Mice | Significantly protected mice from HSV-2 vaginal infection and HPV-16 [47] | ||
| Cyanovirin-n | Nostoc ellipsosporum (Cyanobacteria) | HIV-1 | Macaques | Treated with topical gel were resistant to a chimera of HIV viruses [48,49] |
| HIV-1 | Macaques | 63% reduction of HIV transmission when dosed vaginally with a Lactobacillus expressing CV-N [50] | ||
| Scytovirin | Scytonema varium (Cyanobacteria) | Ebola virus | Mice | Prevented the death of most infected mice [51] |
| Diterpene | Canistrocarpus cervicornis (Dictyotales) | Herpes simplex virus | Mice | Significantly less lesions when treated with a 2% diterpene ointment [52] |
| Compound | Organism | Intervention | Condition | Main Sponsor | Date | Outcome |
|---|---|---|---|---|---|---|
| Griffithsin | Griffithsia sp. (Rhodophyta) | Q-Griffithsin (Q-GRFT) enema | HIV Prevention | National Institute of Allergy and Infectious Diseases (USA) | 2021 | On-going [163] |
| Griffithsin Gel | HIV Infection | Population Council | 2018 | Griffithsin and carrageenan in a gel formulation are safe for vaginal use for up to 14 days with potent anti-HIV activity in cell-based assays and cervical explants [162] | ||
| Carrageenan | Red seaweed (Rhodophyta) | Carraguard (PC-515) | HIV Prevention | Population Council | 2007 | This study did not show Carraguard’s efficacy in prevention of vaginal transmission of HIV [164] |
| 2004 | Daily Carraguard vaginal gel use was highly acceptable in this population of HIV-infected women [165]. | |||||
| 2003 | Vaginal use of the gel did not cause significant adverse effects in a small number of low-risk, sexually abstinent women [166] | |||||
| 2002 | Carraguard was not associated with more vaginal, cervical or external genital irritation than placebo and it was acceptable when used approximately 3.5 times per week, including during sex [167] | |||||
| 2002 | Men and women found the gel acceptable and thought that it should be made available if it is found to be safe and effective [168] | |||||
| 1997 | Use of 5 mL of a 2% gel formulation of n-carrageenan was not associated with significant irritation of the vaginal epithelium when administered once daily in the absence of sexual intercourse [169] | |||||
| HIV & HsV infections | Population Council | 2001 | Carraguard can safely be used an average of four times per week with or without sex and is acceptable to women [170] | |||
| Coldamaris (with Carragelose) | Symptoms of common cold | Marinomed Biotechnologie GmbH (Austria) | 2015 | Direct local administration of carrageenan with nasal sprays reduced the duration of cold symptoms. A significant reduction of viral load in the nasal wash fluids of patients confirmed similar findings from earlier trials in children and adults [171] | ||
| St. Anna Children’s Hospital (Austria) | 2011 | Administration of carrageenan nasal spray in children as well as in adults increased viral clearance and reduced relapses of symptoms [172] | ||||
| Carrashield | Human papillomavirus infection | Canadian Institute of Health Research (Canada) | 2009 | Trial’s interim analysis suggests that using a carrageenan-based lubricant gel can reduce the risk of genital HPV infections in women [158] | ||
| Carvir | Human papillomavirus infection | University of Palermo (Italy) | 2019 | Carvir vulvovaginal microbicide gel is safe and well-tolerated [159] | ||
| Carrageenan-based gel | Human Papillomavirus Infection | McGill University (Canada) | 2020 | On-going [160] | ||
| Spirulan | Arthrospira platensis (cyanobacteria) | Spirulan containing cream | HsV-1 infection | Leibniz Institute for Experimental Virology (Germany) | 2016 | Prophylactic effect against Herpes labialis was superior to that of acyclovir cream [85] |
| Algal Extract | Undaria and Arthrospira | Extract | HIV Infection | University of South Carolina (USA) | 2008 | Undaria, Arthrospira and a combination of both were non-toxic and over time may improve clinical endpoints of HIV/AIDS [173] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagarete, A.; Ramos, A.S.; Puntervoll, P.; Allen, M.J.; Verdelho, V. Antiviral Potential of Algal Metabolites—A Comprehensive Review. Mar. Drugs 2021, 19, 94. https://doi.org/10.3390/md19020094
Pagarete A, Ramos AS, Puntervoll P, Allen MJ, Verdelho V. Antiviral Potential of Algal Metabolites—A Comprehensive Review. Marine Drugs. 2021; 19(2):94. https://doi.org/10.3390/md19020094
Chicago/Turabian StylePagarete, António, Ana Sofia Ramos, Pål Puntervoll, Michael J. Allen, and Vítor Verdelho. 2021. "Antiviral Potential of Algal Metabolites—A Comprehensive Review" Marine Drugs 19, no. 2: 94. https://doi.org/10.3390/md19020094






